Abstract
Background:
In this work, we aimed to establish a clinical target in the management of knee osteoarthritis (KOA) and to propose good clinical practice (GCP) statements for carrying out a treat-to-target strategy.
Methods:
A steering committee of seven experts had formulated a provisional set of recommendations that were exposed for discussion and modification to a technical expert panel (TEP) of 25 multidisciplinary experts from Europe, North America, South America and Asia. The level of evidence and strength of each recommendation was discussed. The TEP formulated overarching principles and GCP statements based on the level of agreement for each item with a vote using a 10-point numerical scale.
Results:
Two overarching principles and 10 GCP statements were formulated by the TEP. These GCP statements suggest: treatment should achieve clinical improvement bringing the patient to the Patient Acceptable Symptom State (PASS); pharmacological and nonpharmacological treatment should begin as early as possible, with an early diagnosis of symptomatic KOA; the patient should be evaluated every 3–6 months; risk factors of KOA progression should be identified and managed with patients at the beginning of the treatment and monitored regularly; treatment should be adapted according to patient phenotype and disease severity; healthy lifestyle must be promoted and monitored. The level of agreement average ranged from 8.7 to 9.6 on scale.
Conclusions:
The proposed overarching principles and GCP statements have the aim of involving patients, general practitioners and multidisciplinary specialists in sharing a therapeutic treat-to-target strategy for KOA management based on the best evidence and expert opinions.
Keywords: knee osteoarthritis, NSAIDs, osteoarthritis, outcome research, treatment
Introduction
Osteoarthritis (OA) is a chronic disease that results in joint dysfunction and hypomobility, evolving to joint failure and consequently, prosthetic replacement.1,2 Moreover, the hypomobility due to OA can complicate other pathologies, such as diabetes mellitus (DM) and cardiopathy, and increases mortality in other comorbidities.3
There are several types of pharmacological and nonpharmacological therapeutic interventions available for treating OA.4–6 As per the current recommended guidelines, some of them are still controversial.7,8 Even though many comparative studies have been conducted, predominantly against placebo, the ideal therapy for OA has not been identified, since OA is a multifactorial disease with different targets. This leads to a multimodal intervention that can vary according to different stages of disease and clinical subsets of OA patients.9–12
Trials and cohort studies on OA have investigated different outcomes such as pain, articular function or delay of radiological progression. However, the ideal clinical target to be achieved in real-world OA management has never been proposed. The ‘treat to target’ concept is based on identification and specific definition of appropriate treatment targets, using available evidence, increasing the chance of not missing an effective therapy in a heterogeneous population. In contrast, the use of the treat-to-target strategy has significantly improved the pathology management in rheumatoid arthritis (RA).13,14
Given its success in RA, the need to develop a treat-to-target strategy to be applied in knee OA (KOA) can be summarized by two fundamental justifications: the difficulty in identifying specific therapeutic targets to be used as a guide in patient follow up (specific disease markers are lacking), and above all, the absence of a uniform and shared therapeutic management algorithm in the treatment of patients suffering from KOA.
During the last International Symposium on Intra-Articular Treatment (ISIAT), held in Prague in October 2017, a multidisciplinary international technical expert panel (TEP) proposed a treat-to-target strategy for KOA to improve management of patients. ISIAT was supported by the presence of European Patients’ Academy representatives of OA patients.
Methods
Working group and first step: A steering committee consisting of seven experts (MA, BRR, CX, HBG, PRJ, RR, ME) was selected based on their expertise in treating OA, participation in clinical trials and development of consensus statements. These experts comprise rheumatologists, orthopaedic practitioners and medical rehabilitation doctors who are specialists dealing with OA in their daily clinical practice. Moreover, these experts are established scientists who have been conducting clinical trials and research during their careers in OA resulting in publishing articles in established internationally recognized journals. A comprehensive systematic literature (SLR) review was performed as a mandatory initial step for a shared consensus on the definition of treat-to-target and operative procedures.
The following questions were formulated as the basis of the search:
(1) Is there a reported strategy to treat on target for KOA?
(2) What are the most commonly used outcome measures of efficacy/effectiveness, safety and adherence in clinical trials and observational studies in patients with KOA?
(3) What are the cut-off levels of pain, function and quality of life or combined indices used at entry in clinical trials on KOA?
Two expert librarians performed the literature research in Medline (PubMed) and EMBASE. Inclusion criteria key words were ‘Knee OA’, ‘Randomized Controlled Trials (RCT)’ and ‘cohort’. All other articles retrieved not satisfying inclusion criteria were excluded (e.g. other SLRs). After the literature review, the steering committee formulated a provisional set of recommendations.
Second step: The provisional recommendations were subject to discussions and modifications by 25 experts (14 rheumatologists, 6 orthopaedists, 3 physiatrists, 1 epidemiologist and 1 patient representative) from Europe, North and Latin America and Asia during the ISIAT 2017 meeting (Prague, October 2017). After discussion, the TEP framed the overarching principles and recommendations.
Third step: Subsequently, the group discussed and updated these items before voting. They were asked to rate the level of agreement for each item using a 10-point numerical rating scale where 1 = do not agree at all and 10 = agree completely.
The scores were pooled to generate a mean agreement value for each of the principles and recommendations and the strength of agreement was classified according to the three proposed ranges: strong if the mean score was at least 7; moderate if the mean score was greater than 3 and less than 7; and weak if the mean score was no more than 3. The results were presented in a final face-to-face meeting in April 2018.
Results
Comprehensive systematic literature review
The final SLR included 1467 articles as detailed in the flowchart (Figure 1). The answers to the search questions are the following:
Figure 1.
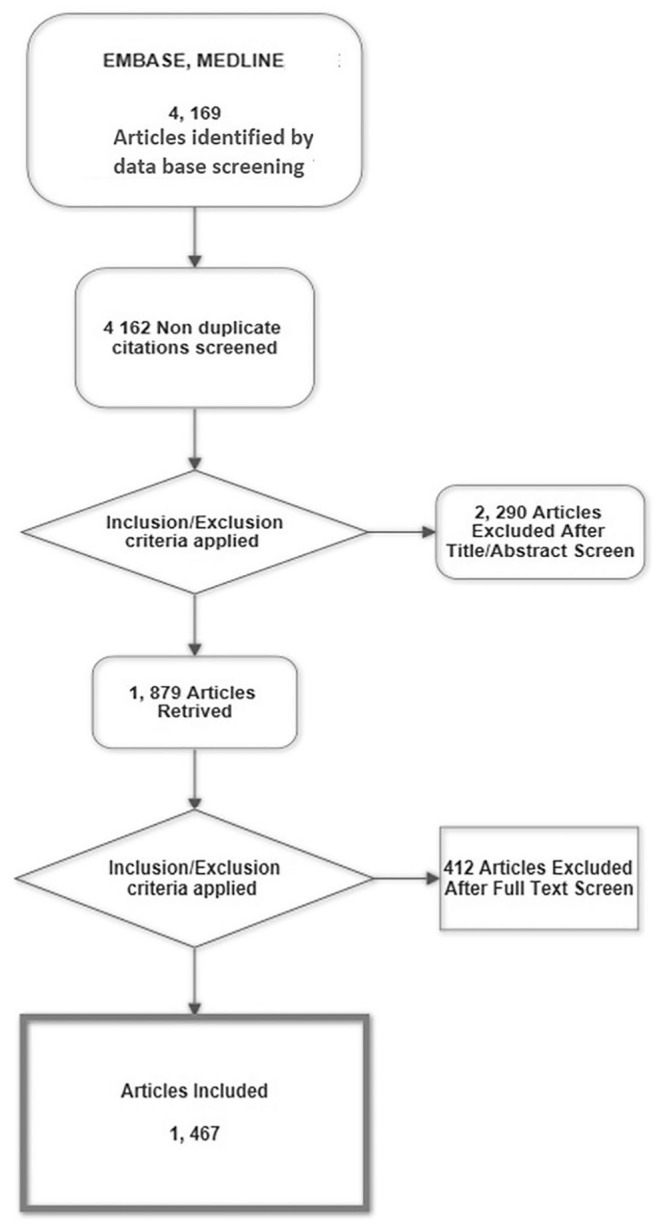
Flow chart of the systemic literature search.
A comprehensive search was performed in Medline and EMBASE databases. Inclusion was limited to cohort and randomised clinical studies of individuals with KOA.
KOA, knee osteoarthritis.
(1) no article was found to report a ‘treat to target’ strategy for KOA;
(2) the most commonly used outcome measures of efficacy/effectiveness in clinical trials and cohort observational studies involving patients with KOA were Western Ontario and McMaster Universities Arthritis Index complete (WOMAC) score (51.87%) and the Visual Analogue Scale (VAS) for pain (44.99%);
(3) outcome measures of safety and adherence were reported as adverse events (AEs) or serious adverse events (SAEs; 16.43%) and adherence (1.64%);
(4) the cut-off levels of outcome measures used for inclusion in clinical trials on KOA were VAS pain ⩾ 4, Numeric Rating Scale (NRS) ⩾ 4 and Lequesne’s Algofunctional Index (LFI) ⩾ 4.
The final statements proposed by the TEP encompassing two overarching principles and 10 GCP statements and their level of agreement are reported in Table 1.
Table 1.
GCP statements and level of agreement.
| GCP statements | Level of consensus | Distribution of ratings |
Average ± SD | Median | Range | ||
|---|---|---|---|---|---|---|---|
| ⩽3 | 4–6 | ⩾7 | |||||
| (1) The primary target for treatment of knee OA should be a clinical improvement, bringing the patient to the PASS | Strongly in favour | 0 | 1 | 24 | 8.7 ± 1.3 | 9 | 6–10 |
| (2) Treatment should begin as early as possible with the diagnosis of symptomatic OA, and include pharmacological and nonpharmacological treatment | Unanimously in favour | 0 | 0 | 25 | 9.3 ± 1 | 10 | 7–10 |
| (3) All patients should be encouraged to maintain a healthy weight and adopt regular and appropriate physical activity | Unanimously in favour | 0 | 0 | 25 | 9.2 ± 1 | 10 | 7–10 |
| (4) The management should be evaluated every 3–6 months (depending on the patient symptoms) until the desired target is reached and continued thereafter | Unanimously in favour | 0 | 0 | 25 | 9 ± 1.1 | 9 | 7–10 |
| (5) Documenting measures of pain, function, physical and mental state, and consumption of painkillers (analgesics, NSAIDs, etc.) regularly, to monitor clinical improvement, adherence, tolerability and safety is recommended | Strongly in favour | 1 | 0 | 24 | 8.7 ± 1.6 | 10 | 3–10 |
| (6) The patient has to be appropriately informed about the treatment options and a shared decision should be made | Unanimously in favour | 0 | 0 | 25 | 9.4 ± 1 | 10 | 7–10 |
| (7) Modifiable risk factors of OA progression should be identified and managed with patients at the beginning of the treatment and monitored regularly | Unanimously in favour | 0 | 0 | 25 | 9.4 ± 1 | 10 | 7–10 |
| (8) Comorbidities and concomitant treatments should be systematically screened and managed | Unanimously in favour | 0 | 0 | 25 | 9.3 ± 1 | 10 | 7–10 |
| (9) The treatment should be adapted according to patient phenotype and disease severity | Strongly in favour | 0 | 1 | 24 | 9.1 ± 1.3 | 10 | 5–10 |
| (10) Surgical options should be considered for the appropriate patients | Strongly in favour | 0 | 1 | 24 | 9.4 ± 1.1 | 10 | 6–10 |
Numerical details show the degree of agreement rated from 0 to 10 and level of consensus is defined as strong and unanimous for each individual point.
GCP, good clinical practice; NSAIDs, nonsteroidal anti-inflammatory drugs; OA, osteoarthritis; PASS, Patient Acceptable Symptom State; SD, standard deviation.
Final statements
Overarching principles
(1) The treatment of KOA must be based on a shared decision between patient and physician.
A well-informed patient is able to participate in shared decision making. Being aware of the options in pharmacological, nonpharmacological and complimentary treatments and by weighing the benefits and risks, the patient may choose the most appropriate management. This also might boost self-confidence and confidence in his/her doctor. In addition, best knowledge and best information will encourage the patient to discuss a change in the current treatment. Additionlly, the identification and management of modifiable risk factors related to KOA should be shared and planned with the patient.
(2) The primary goal of treating the patient with KOA is to maximize long-term health-related quality of life through control of symptoms, prevention of evolution of structural damage, improvement of mobility and self-management.
The KOA treatment should be based on decisions that offer a good quality of life to the patient. The treatment should be personalized to suit the particular lifestyle of each individual patient and specific needs (work, sports, daily activities, leisure). The treating team of health practitioners (HPs) should work in cooperation with the patient to foster his/her well-being. Treatment should control symptoms, disease flares/relapses, maximize function, and avoid long-term structural damage and disabilities. The patient should be encouraged to acquire self-management techniques, and these should include adoption of a healthy lifestyle, cognitive and behaviour skills.15
GCP statements
(1) The primary target for treatment of KOA should be a clinical improvement, bringing the patient to the Patient Acceptable Symptom State (PASS).
In daily clinical practice, physicians frequently ask two questions to assess the effectiveness of a treatment: ‘Are you feeling better?’ and ‘Are you feeling good?’. Unsurprisingly, it has been demonstrated that patients prioritize on feeling good than on feeling better.16 The PASS is a clinically relevant cut-off that allows assessment of clinical status of an individual patient, at a given time, by classifying the patient as being in ‘an acceptable state’ (score ⩽ PASS threshold) or not (score > the PASS). In other words, PASS can be defined as the highest level of different symptoms [e.g. pain, Patient’s Global Assessment (PGA), functional improvements] beyond which patients consider themselves well.17 Thus, it can be considered a clinically relevant treatment target. It is an absolute value (satisfactory or not), not a change. In 2005, Dougados and colleagues published one of the first prospective studies evaluating PASS against the three main patient-reported outcomes (PROs) used in clinical trials in knee and hip OA: VAS pain, VAS patient global assessment and WOMAC function score. They demonstrated the robustness of this index in the evaluation of the patient affected by KOA and in particular, the centrality of the patient’s role in considering disease activity because the definition of the PASS is anchored to the personal experience of the patient (satisfaction and adaptation to symptoms).17 Patients with KOA considered their state satisfactory (PASS threshold) if their pain score was less than 32.3 mm on the 0–100 mm VAS and the PASS estimates were similar (scores of approximately 33), considering both knee and hip OA for these reported outcomes.17 Similar results were demonstrated in other published studies,18,19 underlining the study conducted by Bellamy and coworkers, showing the importance of country-specific PASS. On the basis of the previous published results that Conrozier and colleagues considered, in patients affected by KOA treated with viscosupplementation, PASS threshold ⩽ 4/10 for WOMAC pain, <4 for patients’ global assessment of pain, and <5/10 for WOMAC function, demonstrated the utility of the PASS in patient evaluation.16 In accordance with this last study, we proposed considering a pain cut off of 4 (PASS + ⩽4/10) as threshold of the therapeutic target to reach in patients affected by KOA.
(2) Treatment should begin as early as possible with the diagnosis of symptomatic OA and include pharmacological and nonpharmacological treatment.
Early management of KOA is recommended by several guidelines15,20,21 and supported by the National Public Health Agenda for Osteoarthritis, the Centres for Disease Control and Prevention and the Arthritis Foundation.22 The rationale for this approach relies on the hypothesis that early interventions might modify the course of the disease including patho-anatomy and clinical features of KOA.
Improved understanding of disease pathogenesis and advances in the investigation of biomarkers could increase the ability to diagnose early OA and to manage clinical and functional consequences. Even though pharmacological agents play a key role in symptom relief, there is a growing interest in disease-modifying agents in KOA that might delay disease progression.
(3) All patients should be encouraged to maintain a healthy weight and adopt regular and appropriate physical activity.
Therapeutic exercises, particularly low-impact aerobic training, aquatic exercise and strengthening are recommended by several guidelines, as both core treatment and first-line conservative approach for KOA-related pain and disability.15,20,21 Pain must be controlled to encourage regular physical activity. Changes of appropriate lifestyle should be encouraged as soon as possible, and regular weight control should be included through the introduction of a balanced diet that needs to consider existing comorbidities (e.g. DM, hypercholesterolaemia, hypertension).
(4) The management should be evaluated every 3–6 months (depending on the patient symptoms) until the desired target is reached and continued thereafter.
As observed for other joint diseases such as RA, it has been demonstrated that close monitoring of patient compliance is an important strategy in patient management. In particular, pharmacological and nonpharmacological treatments should be scrupulously followed.13 Symptom control can be fast acting [e.g. nonsteroidal anti-inflammatory drugs (NSAIDs) or analgesics] or slow acting [e.g. nonpharmacological treatments, symptomatic slow-acting drugs of OA (SYSADOAs), exercise or weight loss]. The suggested 3–6-month follow up is a reasonable period to achieve the therapeutic target. Thus, it is important to highlight that the use of SYSADOAs for the management of KOA is not recommended practice in North America and the UK, and is still not recommended by the Osteoarthritis Research Society International (OARSI) and remains controversial, but without doubt, it is supported by various clinical trials and positive experiences in clinical practice.
Once target is achieved, regular monitoring over time is a fundamental principle in the management of KOA, as it is a chronic disease. The periodic assessment of the patient’s disease status allows an effective evaluation of both compliance and effectiveness of the selected therapeutic strategies.
(5) Documenting measures of pain, function, physical and mental state, and consumption of painkillers (analgesics, NSAIDs, etc.) regularly to monitor clinical improvement, adherence, tolerability and safety is recommended.
Different types of PROs are used in clinical trials of KOA. A core set of three clinical measures were specified in the Outcome Measures in Rheumatology Clinical Trials (OMERACT) III Conference and ratified by the 1996 OARSI Task Force OA Clinical Trial Guidelines. This core set is generally based on evaluation of pain, physical function and patient global assessment. Pain can be evaluated on a five-point Likert scale (e.g. none, mild, moderate, severe, very severe), 11-point (0–10) NRS or on a 100 mm VAS but also, single questions can be used. It is also possible to use a part of some tools with multi-items, such as the Health Assessment Questionnaire (HAQ), or multiconcept, such as the WOMAC pain subscale, or multidimensional measures. Physical function/disability can be measured on a Likert-type scale, NRS or VAS, or multidimensional tools with a physical function subscale (WOMAC physical function subscale, the knee injury and Osteoarthritis Outcome Score function subscale) and the HAQ disability index (HAQ-DI/Improved HAQ). The third domain, the PGA status, is usually measured on a rating scale (Likert, NRS or VAS).23
The regular monitoring of these domains is essential for evaluating the disease evolution over time, for evaluating the effectiveness of the therapeutic choices and for monitoring the patient’s drug compliance and tolerability.
(6) The patient has to be appropriately informed about the treatment options and a shared decision should be made.
The physician and the patient should discuss the condition of the disease, and the former should explain in detail the benefits of the chosen treatment and its side effects. The doctor must listen to the concerns and worries of the patient and address him/her in lay language. By this method, the patient and his doctor can codecide on the most appropriate treatment. Moreover, a scrupulous information will raise patient’s awareness and aid him/her in early recognition of side effects.
(7) Modifiable risk factors of OA progression should be identified and managed with patients at the beginning of the treatment and monitored regularly.
The management of modifiable OA risk factors such as weight loss and regular resistance-training exercises is a fundamental element in the management of the patient.
Risk factors of OA are supported by recent results drawn from the Osteoarthritis Initiative (OAI) and the CHECK studies, which confirm the relevance of modifiable risk factors such as overweight in the development of KOA.24,25
In particular, among the OAI patient population, overweight was identified as a risk factor for developing bone-marrow lesions and joint effusions.26 The data from CHECK also suggest that body mass index (BMI) may play a role in the reduction of knee range of movement and in overall activities in patients affected by KOA.25 Cross-sectional and longitudinal studies on KOA have consistently demonstrated a linear association between overweight and obesity as measured by BMI and waist circumference, and the prevalence and incidence of knee KOA (RR: 2.4), with obesity identified as the main modifiable risk factor.27–32 At a 10-year follow up in a population aged 24–76 years, the incidence of KOA was 7.3% [confidence interval (CI) 5.7–9.0] and a high BMI (>30) was significantly associated with KOA[odds ratio (OR) 2.81; 95%CI 1.32–5.96].33 Obesity may also impact physical activity, disease progression [relative risk (RR): 2.6] and disease severity, especially in terms of lifetime expectancy and survival of total knee replacement.34,35 Weight loss may improve pain, function and physical activity.36,37
Weight loss is a main task in overweight and obese KOA patients either by diet with exercise or medical intervention including bariatric surgery in patients with morbid obesity. A weight loss over 5% may improve symptoms and function.36 This effect is observed even in patients with advanced stage of the disease.38 Long-term maintenance of weight loss in patients with KOA is difficult to achieve and dietary advice including low-energy diet may be helpful. Obesity is also associated with sleep apnoea and steatohepatitis, which might complicate surgery options in these patients.39
(8) Comorbidities and concomitant treatments should be systematically screened and managed.
Although treatment options and medications that can help relieve OA symptoms, primarily pain, are widely in use, it is very important to consider comorbidities before choosing how to manage KOA. The metabolic syndrome (MetS), has been observed more frequently in patients with KOA compared with the non-OA population (59% versus 23 %).40 The presence of metabolic diseases seems to have a cumulative and negative effect on the incidence and the progression of KOA.41–43 Together with age, physical inactivity induced by OA disability and low-grade inflammation associated with KOA, MetS may contribute to increase cardiovascular (CV) disease and CV-related mortality.3,44
There is also a controversial association between type 2 DM and KOA.45–48
The concerns about an association between atherosclerosis and OA arise from several positive studies.28 A recent meta-analysis shows that the risk of ischemic heart disease for OA patients was 1.78 (95% CI 1.18–2.69) and the risk of heart failure was 2.80 (95% CI 2.25–3.49) as compared with non-OA patients.44 This CV risk in KOA is mainly driven by impairment of physical activity, since this risk was not observed in patients with hand OA.49
In these elderly and overweight populations with KOA, concomitant treatment should be carefully monitored. Several drugs used in KOA can induce side effects that might be more severe due to associated comorbidities. Thus, given the different safety profiles, the choice of NSAIDs, traditional or coxibs, should be based on individual patient risk factors.50 The reputation of acetaminophen as a safe drug has been challenged. Long-term use and high dose of acetaminophen can induce hepatic, digestive (RR 1.14–1.31) and cardiovascular AEs (RR 1.36).51–53 Before performing intra-articular injections, concomitant anticoagulants should be considered, while corticosteroid injections may aggravate DM and hypertension frequently associated with KOA.40,54 In this elderly polymedicated population, drug–drug interactions are more frequent, and NSAIDs are particularly at risk.55 In the elderly population, the use of NSAIDs should be carefully monitored in patients taking antihypertensive drugs, such as inhibitors of angiotensin-converting enzyme and diuretics, due to the increased risk of renal failure.56,57 NSAIDs should also be carefully considered in patients with CV risk on antithrombotic drugs (i.e. low-dose aspirin, clopidogrel) due to the risk of gastrointestinal bleeding, especially for traditional NSAIDs.5,58–60 The highest average number of severe/contraindicated alerts per use was observed for NSAIDs in an elderly population taking antithrombotic drugs.53 Similarly, the association between NSAIDs and anticoagulant therapy should be restricted.5 In these cases, topical NSAIDs might be preferred because no serious gastrointestinal or renal AEs were observed in trials or in the general population.61 The cumulative dose of acetaminophen used in other clinical conditions, may expose patients with KOA to greater gastrointestinal, hepatic or CV side effects 52,53 For these reasons, the benefit/risk ratio of any treatments in KOA patients, including screening for comorbidities and concomitant treatments for those comorbidities, should be regularly and systematically evaluated.
(9) The treatment should be adapted according to patient phenotype and disease severity.
The complexity of factors involved in the development and progression of OA makes it impossible to offer a standardized treatment for all individuals. There is a great deal of heterogeneity between patients, since OA pathophysiology comprises mechanical, inflammatory, metabolic, post-traumatic, molecular, genetic, epigenetic and psychological alterations, among others;62 each factor acting alone, or in combination. Similarly, there is great variation among individuals regarding disease trajectory, with some evolving rapidly, while others remain stable for long periods of time.12,63
Several authors have proposed different OA phenotypes based on clinical or imaging findings.9–12 A recent systematic review identified distinct groups of variables that can be explored for a better definition of the phenotypes in OA.9 Therefore, adapting the treatment according to patient phenotype and disease severity is essential for adequately treating OA patients and for achieving the best results.
(10) Surgical options should be considered for the appropriate patients.
Knee arthroplasty should be considered when both pharmacological and nonpharmacological treatments have failed. Arthroscopic lavage is not recommended for patients with degenerative pathology.64
Total knee arthroplasty remains a valid option in patients who have advanced degenerative changes in the knee and are symptomatically severe. Such patients must be medically screened to assess their fitness for surgery before arthroplasty is offered.65
Other surgical treatments, such as osteotomy and realignment procedures, may also be considered in appropriate patients after discussing the risks, rewards and longevity of these procedures.
Agreement
With regard to the consensus vote, both for overarching principles and GCP statements, the results show a high degree of agreement with average values no lower than 8.7 and with a level of consensus that oscillates between strong and unanimous for each individual point. The numerical details are reported in Table 2.
Table 2.
Overarching principles.
| Overarching principles | Level of consensus | Distribution of ratings |
Average ± SD | Median | Range | ||
|---|---|---|---|---|---|---|---|
| ⩽3 | 4–6 | ⩾7 | |||||
| (1) The treatment of knee OA must be based on a shared decision between patient and physician | Unanimous favour | 0 | 0 | 25 | 9.6 ± 0.9 | 10 | 7–10 |
| (2) The primary goal of treating the patient with knee OA is to maximize long-term health-related quality of life through control of symptoms, prevention of evolution of structural damage, improvement of mobility and self-management | Strong in favour | 0 | 2 | 23 | 9.1 ± 1.4 | 10 | 5–10 |
Numerical details show the degree of agreement rated from 0 to 10 and level of consensus is defined as strong and unanimous for each individual point.
OA, osteoarthritis; SD, standard deviation.
Discussion
To our knowledge, this is the first proposal for a treat-to-target strategy in KOA. This international interdisciplinary TEP has tried to formulate a series of GCP statements that could be of guidance for treat-to-target strategies in the management of KOA. At present, the treat-to-target strategy represents a different approach to OA management, focusing on reaching an easy and acceptable clinical target in patients rather than adopting the best ideal therapy for OA. It is also a valid contribution to KOA management, taking into account the chronicity, progression of disease and the frequent association of comorbidities that complicate the management of the patient.
The TEP’s final work consisted of two overarching principles and 10 GCP statements pertaining to the implementation of a treat-to-target strategy in the management of the patient with KOA. The overarching principles express two fundamental concepts: the importance of sharing therapeutic choices between the doctor and patient, and defining the goal of these therapeutic choices. Sharing the therapeutic strategies with the patient is the first step to ensure the patient’s compliance to choices made. If the patient does not believe and does not share the management options recommended by the physician, it will be difficult to succeed in achieving the target. The second overarching principle focuses on the objectives: the physician must explain the complexity of a chronic pathology such as KOA, emphasizing the need to monitor the patient at different levels.
The following 10 statements express more in detail the indications useful in achieving what is expressed in the overarching principles.
OA lacks a diagnosis and target biomarker, even though for many years now, research has been focused on resolving this gap. Our aim is to introduce a new approach for the management of OA, consisting of a treat-to-target concept based on SLR and expert consensus. This paper introduces PASS as a target to achieve in the management of KOA. The first suggests the use of a specific clinimetric indicator for monitoring the disease with PASS. The PASS introduces the concept of well-being or remission of symptoms and has been demonstrated a clinically relevant outcome for the patient, even though it needs further research to evaluate the robustness, temporal consistency, and age and sex dependency of the preliminary results. Dougados and coworkers were among the first to investigate the usefulness of this outcome in the evaluation of patients affected by various pathologies including KOA.17–19 The evaluation of the patient’s symptoms allows the analysis of the patient on different levels, as expressed in the overarching principles, reaffirming the centrality of the patient’s opinion. For this purpose, still with some limits,66 PASS can be an easy and appropriate outcome as a clinical target of the patient with KOA. PASS was also considered the most reliable and simple PRO for decision of retreatment with hyaluronic acid, by the experts of the European Viscosupplementation Consensus group.67
The proposed second GCP statement emphasizes the use of pharmacological and nonpharmacological treatments in the management of the patient with KOA as reported by several international guidelines.15,68–70 Since a clear characterization and definition of early OA stages is lacking, in 2016, an international panel of 29 physicians promoted by the Italian Society of Rheumatology, representing national societies of rheumatology of several European countries, proposed establishing an agreed clinical definition of early symptomatic KOA and simple criteria for the referral of patients with suspected KOA at the initial symptomatic stage of the disease, before the onset of radiological damage.70 The early management of KOA, aiming to slow its course with pharmacological and nonpharmacological strategies, could be the starting point for the application of a treat-to-target strategy for KOA.
In the third GCP statement the modification of lifestyle in patients affected by KOA is highlighted. Conservative nonpharmacological strategies, particularly exercise, are recommended in the management of OA (e.g. aerobic, strengthening, aquatic and Tai Chi exercise). Obviously, the optimal exercise regimen should be determined and individualized on the basis of the stage of the disease, patient preference, comorbidities and accessibility.71
A recent systematic review and meta-analysis provided evidence about the effectiveness of land-based exercise for people with KOA reducing joint pain and improving physical function and quality of life over the short term and for at least 2–6 months after the treatment.72 Recently, the European League Against Rheumatism (EULAR) realized specific guidelines for the management of physical exercise in patients with KOA.73 In the modification of lifestyle, the management of the weight and pain should also be considered, especially in elderly patients.74
Statement number 4 suggests timing for the evaluation of the patient over time according to a follow up consistent with what has already been proposed in other pathologies such as RA.13 The fundamental concept is that considering the chronicity of the pathology and the multilevel therapeutic approach, a regular and prolonged follow up is necessary in order to better evaluate the patient and to maintain the results over time.
In GCP statement 5, the various ‘domains’ to precisely evaluate the patient are defined. However, these domains clearly demonstrate the complexity of the KOA patient who requires a regular follow up which should be part of the treat-to-target strategy.
Along with painkillers, a multimodal approach should be chosen. When the diagnosis of symptomatic KOA is made, this includes the early use of nonpharmacological and pharmacological treatments,75 targeting inflammation, preventing sensitization and transition to chronic pain. This may ensure maximization of the beneficial effects of therapy and may delay disease progression.
However, awareness of treatment potential side effects is mandatory. In fact, NSAIDs are associated with a risk of gastrointestinal and CV AEs. International guidelines such as those of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases or EULAR recommendations can guide the physicians in identifying patients at risk of significant CV or gastrointestinal side effects.76 Recently, CV risk with NSAIDs has been challenged, and it was proposed that all NSAIDs (tradional and coxibs) can induce short- or long-term CV events, including death. With coxibs, the risk is due to continuous use for more than 30 days, whereas for other NSAIDs, a heightened myocardial infarction risk can occur within 7 days.77 For a short-term use, opioids such as tramadol may be considered for severely symptomatic KOA patients. The appropriate selection of the patient to be subjected to this kind of therapy remains crucial.76
Statement 6 may appear redundant, as it is addressed in the first overarching principle. The TEP considered it appropriate to include this statement to reiterate the importance of the shared treatment choice between physician and patient. It also emphasizes a very important concept of giving complete information about the therapeutic choices to the patient. To assure patient compliance and safety, it is not enough to share the choices, but is also pivotal that the patient understands the characteristics and purpose of the therapeutic choices.
The risk factors are discussed in the next GCP statement. In KOA, the management of the patient risk factors is fundamental in improving the efficacy of the treatment: weight loss is the key aim in overweight and obese KOA patients. This may be achieved either by diet and exercise or medical intervention, including bariatric surgery, especially in patients who are morbidly obese.36,37,78 In these patients, sleep apnoea and steatohepatitis, usually associated with obesity, may complicate a surgical option.30
There are rising concerns in recent studies of an association between atherosclerosis and OA. A recent meta-analysis shows that the risk of ischaemic heart disease in OA patients was 1.78 (95% CI 1.18–2.69) and the risk of heart failure was 2.80 (95% CI 2.25–3.49) as compared with nonosteoarthritic patients.44 This CV risk in KOA is mainly driven by poor physical activity.49 Moreover, according to a recent systematic review,79 high-quality studies suggest that progressive resistance training improves overall physical performance in patients with early KOA, and both strength training and self-management are effective for pain relief, functional improvement and suitable for middle-aged adults with early KOA.80
The eighth statement emphasizes the management of comorbidities. Obesity, dyslipidaemia, CV disease and DM can significantly affect the patient’s general condition. While some meta-analyses, which include studies of patients with high BMI,45,46 have found a significant and positive association between the two diseases, with an increased risk of 25%, more recent studies disputed these meta-analyses, as they reported no association between DM and KOA.48 Despite uncertain data, the consensus suggests the management of KOA should now include the control of all comorbidities included in MetS to improve the quality of life and decrease the CV risk. Thus, the presence of any concomitant pathologies needs to be evaluated when discussing the options of management with the patient. Therefore, the treatment and the monitoring of comorbidity are crucial during the regular review of the patient, as is the modification of daily lifestyle, if applicable.
The importance of personalization of therapy based on the characteristics of each patient is discussed in GCP statement 9. The focus is on the presence of different KOA phenotypes seen in clinical practice. As described in these recommendations, the disease phenotype of the KOA should be considered in the evaluation of the patient. Several authors have proposed different OA phenotypes based on different parameters (clinical or imaging findings),11,62 and their precise identification can influence the efficacy of therapy. According to OA aetiological and molecular/biological events of the disease, a classification of OA into three subsets has been suggested. It is based on the main underlying pathophysiological mechanisms: type I, or genetically determined; type II, or oestrogen-hormone dependent; and type III, or age-related OA.81 Moreover, the progression of disease can change one phenotype into another and so they appear interchangeable.82,83 A recent review summarizes a set of variables suggesting the existence of five different clinical phenotypes of KOA.10 Other studies underlined importance of distinguishing clinical phenotype of KOA based on pain sensitization, psychological distress, radiographic severity, body mass index (BMI), muscle strength, inflammation and comorbidities,9 also, taking into consideration sex, metabolic abnormalities and pattern of cartilage damage.84 Treatment options for distinct clinical subsets of KO are still controversial. In addition, a special focus on recognizing early KOA is relevant. Langworthy and colleagues reported on the younger military population with increased risk of KOA; they highlighted the importance of viscosupplementation and multimodal approach including exercise plans, physical therapy, and weight loss, analgesics or NSAIDs on request.85
In the early phases of disease, a wide spectrum of predictors of evaluation should be considered. Meniscal change, bone-marrow oedema, synovitis, and infrapatellar fat pad (Hoffa’s fat pad) synovitis are initially involved in the degenerative process and have been found to be predictive of radiographic change within 4 years.84 However, still, there is no uniform characterization of the phenotypes that remain at present a topic of discussion in the scientific community.
In the last statement, there is focus on the indication of arthroplasty in patients with advanced degenerative changes and severe symptoms not responsive to pharmacological and nonpharmacological strategies.64 Preoperative screening and a careful discussion with patients regarding indications, procedures and patient expectations should be performed to those offered arthroplasty.65 Reconstructive surgical treatment strategies with the aim of forming a repair tissue or unloading joint compartments with articular cartilage damage can be also proposed. These surgical reconstructive techniques improving joint function could postpone the need for joint replacement; however, since no definitive data are available, an accurate discussion with the patient must be carried out.
One of the methodological limitations of this TEP is the absence in the working group of general practitioner (GP) representatives. Considering the fundamental role of the GP in managing the patient affected by KOA, involving these professionals in developing a strategy for treat-to-target in KOA to guarantee the success of what is proposed is essential. Similar projects and work plans involving patients, specialists and GPs are needed to ensure the best possible care for the patient.
Research agenda
In order to improve the ‘treat-to-target strategy’ this TEP also proposed the following research agenda to improve the customization of treatment (e.g. different treatments according to age):
(1) to investigate specific biomarkers to monitor disease;
(2) to determine the best type of physical activity according to patient phenotype;
(3) to study concomitant interactions with other diseases in patients affected by KOA;
(4) to establish the limits of acceptable BMI;
(5) to identify the subset/phenotype of patients (e.g. bone-marrow lesions);
(6) to characterize the pain phenotype of each patient;
(7) to explore the new horizons of informatics in the long-term management of OA patients (applications, telematic communications, etc.).
Conclusion
After the success exhibited by the ‘treat-to-target strategy’ in RA, this TEP suggests it is the time to consider the use of a treat-to-target strategy in the management of KOA. The experts suggest providing both pharmacological and nonpharmacological therapeutic choices shared between physicians and patients, with regular evaluation of efficacy and tolerability over time. PASS can represent a valid outcome as a clinical target in the evaluation of the evolution of the patient’s disease status. The proposed overarching principles and GCP statements are aimed at involving patients, physician specialists and GPs in sharing a therapeutic strategy for KOA and management based on the aim of achieving and maintaining a clinical and acceptable status for OA patients over a long time.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Abiogen Pharma Spa supported all the costs of the meeting of the authors, including travel and subsistence. No honoraria were paid to the participants. Abiogen Pharma Spa had no input in the writing or editing of the paper.
ORCID iD: Natasa Isailovic  https://orcid.org/0000-0002-9819-8822
https://orcid.org/0000-0002-9819-8822
Contributor Information
Alberto Migliore, Rheumatology Unit, San Pietro Fatebenefratelli Hospital, Rome, Italy.
Gianfranco Gigliucci, Rheumatology Unit, San Pietro Fatebenefratelli Hospital, Rome, Italy.
Liudmila Alekseeva, Department of Metabolic Diseases of Bone and Joints, VA Nasonova Research Institute of Rheumatology, Moscow, Russian Federation.
Sachin Avasthi, Department of Emergency Medicine, Dr Ram Manohar Lohia Hospital, Lucknow, India.
Raveendhara R Bannuru, Centre for Treatment Comparison and Integrative Analysis Division of Rheumatology, Tufts Medical Centre, Boston, MA, USA.
Xavier Chevalier, Unit of Rheumatology, Henri Mondor Hospital, Créteil, France.
Thierry Conrozier, Service de Rhumatologie, Hôpital Nord Franche, Belfort, France.
Sergio Crimaldi, Chirurgia Ortopedica Mininvasiva e Nuove Tecnologie, Humanitas Research Hospital, Castellanza, Italy.
Nemanja Damjanov, Institute of Rheumatology, University of Belgrade Medical School, Belgrade, Serbia.
Gustavo Constantino de Campos, Department of Orthopaedics and Traumatology, University of Campinas, São Paulo, Brazil.
Demirhan Diracoglu, Department of Physical Medicine and Rehabilitation Division of Pain Medicine, Istanbul University, Istanbul, Turkey.
Gabriel Herrero-Beaumont, Joint and Bone Research Unit, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Giovanni Iolascon, Department of Medical and Surgical Specialties and Dentistry, University of Campania ‘L Vanvitelli’, Caserta, Italy.
Ruxandra Ionescu, Department of Internal Medicine and Rheumatology Sf. Maria Hospital, University of Medicine and Pharmacy ‘Carol Davila’, Bucharest, Romania.
Natasa Isailovic, Division of Rheumatology and Clinical Immunology, Humanitas Research Hospital, Via A. Manzoni 56, Rozzano, Milan 20089, Italy.
Jörg Jerosch, Orthopaedic Department, Johanna Etienne Hospital, Neuss, Germany.
Jorge Lains, Physical Rehabilitation Medicine Department, Rovisco Pais Medical and Rehabilitation Centre, Tocha, Portugal.
Emmanuel Maheu, Rheumatology Department, AP-HP, Saint-Antoine Hospital, Paris, France.
Souzi Makri, EUPATI Graduate and Patient Advocate, Brussels, Belgium.
Natalia Martusevich, Department of Rheumatology, Belorussian State Medical University, Minsk, Belarus.
Marco Matucci Cerinc, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Mihaela Micu, Second Rehabilitation Department, Rehabilitation Clinical Hospital, Cluj-Napoca, Romania.
Karel Pavelka, Institute of Rheumatology, Prague, Czech Republic.
Robert J Petrella, Department of Family Medicine, School of Kinesiology University Western Ontario, Ontario, Canada.
Umberto Tarantino, Department of Orthopaedics and Traumatology, ‘Policlinico Tor Vergata’ Foundation, Rome, Italy.
Raghu Raman, Academic Department of Orthopaedics, Hull and East Yorkshire NHS Trust Castle Hill Hospital, Cottingham, UK.
References
- 1. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81: 646–656. [PMC free article] [PubMed] [Google Scholar]
- 2. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 3. Nuesch E, Dieppe P, Reichenbach S, et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 2011; 342: d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 5. Bruyère O, Honvo G, Veronese N, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. Epub ahead of print 30 April 2019. DOI: 10.1016/j.semarthrit.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 6. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013; 21: 571–576. [DOI] [PubMed] [Google Scholar]
- 7. Migliore A, Bizzi E, Herrero-Beaumont J, et al. The discrepancy between recommendations and clinical practice for viscosupplementation in osteoarthritis: mind the gap! Eur Rev Med Pharmacol Sci 2015; 19: 1124–1129. [PubMed] [Google Scholar]
- 8. Maheu E, Bannuru RR, Herrero-Beaumont G, et al. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: results of an extensive critical literature review. Semin Arthritis Rheum. Epub ahead of print 19 June 2018. DOI: 10.1016/j.semarthrit.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 9. Deveza LA, Melo L, Yamato TP, et al. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartilage 2017; 25: 1926–1941. [DOI] [PubMed] [Google Scholar]
- 10. Dell’Isola A, Allan R, Smith SL, et al. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2016; 17: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van der Esch M, Knoop J, Van der Leeden M, et al. Clinical phenotypes in patients with knee osteoarthritis: a study in the Amsterdam osteoarthritis cohort. Osteoarthritis Cartilage 2015; 23: 544–549. [DOI] [PubMed] [Google Scholar]
- 12. Karsdal MA, Bihlet A, Byrjalsen I, et al. OA phenotypes, rather than disease stage, drive structural progression–identification of structural progressors from 2 phase III randomized clinical studies with symptomatic knee OA. Osteoarthritis Cartilage 2015; 23: 550–558. [DOI] [PubMed] [Google Scholar]
- 13. Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target recommendations of an international task force. Ann Rheum Dis 2010; 69: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016; 75: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22: 363–388. [DOI] [PubMed] [Google Scholar]
- 16. Conrozier T, Monet M, Lohse A, et al. Getting better or getting well? The patient acceptable symptom state (PASS) better predicts patient’s satisfaction than the decrease of pain, in knee osteoarthritis subjects treated with viscosupplementation. Cartilage. Epub ahead of print 11 August 2017. DOI: 10.1177/1947603517723072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 2005; 64: 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dougados M, Moore A, Yu S, et al. Evaluation of the patient acceptable symptom state in a pooled analysis of two multicentre, randomised, double-blind, placebo-controlled studies evaluating lumiracoxib and celecoxib in patients with osteoarthritis. Arthritis Res Ther 2007; 9: R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tubach F, Ravaud P, Martin-Mola E, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res (Hoboken) 2012; 64: 1699–1707. [DOI] [PubMed] [Google Scholar]
- 20. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013; 21: 577–579. [DOI] [PubMed] [Google Scholar]
- 21. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64: 465–474. [DOI] [PubMed] [Google Scholar]
- 22. Arthritis Foundation/Centers for Disease Control and Prevention. A national public health agenda for osteoarthritis, http://www.cdc.gov/arthritis/docs/OAagenda.pdf (2010, accessed 13 March 2018).
- 23. McAlindon TE, Driban JB, Henrotin Y, et al. OARSI clinical trials recommendations: design, conduct, and reporting of clinical trials for knee osteoarthritis. Osteoarthritis Cartilage 2015; 23: 747–760. [DOI] [PubMed] [Google Scholar]
- 24. Driban JB, McAlindon TE, Amin M, et al. Risk factors can classify individuals who develop accelerated knee osteoarthritis: data from the osteoarthritis initiative. J Orthop Res 2018; 36: 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bastick AN, Wesseling J, Damen J, et al. Defining knee pain trajectories in early symptomatic knee osteoarthritis in primary care: 5-year results from a nationwide prospective cohort study (CHECK). Br J Gen Pract 2016; 66: e32–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lo GH, McAlindon TE, Niu J, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2009; 17: 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teichtahl AJ, Wang Y, Wluka AE, et al. Obesity and knee osteoarthritis: new insights provided by body composition studies. Obesity 2008; 16: 232–240. [DOI] [PubMed] [Google Scholar]
- 28. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham study. Ann Intern Med 1988; 109: 18–24. [DOI] [PubMed] [Google Scholar]
- 29. Eaton CB. Obesity as a risk factor for osteoarthritis: mechanical versus metabolic. Med Health R I 2004; 84: 201–204. [PubMed] [Google Scholar]
- 30. Kulkarnia K, Karssiensa T, Kumarb V, et al. Obesity and osteoarthritis. Maturitas 2016; 89: 22–28. [DOI] [PubMed] [Google Scholar]
- 31. Duclos M. Osteoarthritis, obesity and type 2 diabetes: the weight of waist circumference. Ann Physical Rehabilitation Med 2016; 59: 157–160. [DOI] [PubMed] [Google Scholar]
- 32. Grotle M, Hagen KB, Natvig B, et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord 2008; 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum 2000; 43: 995–1000. [DOI] [PubMed] [Google Scholar]
- 34. Lohmander LS, Gerhardsson de, Verdier M, Rollof J, et al. Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis 2009; 68: 490–496. [DOI] [PubMed] [Google Scholar]
- 35. Nicholls AS, Kiran A, Javaid MK, et al. Change in body mass index during middle age affects risk of total knee arthoplasty due to osteoarthritis: a 19-year prospective study of 1003 women. Knee 2012; 19: 316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Christensen R, Bartels EM, Astrup A, et al. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2007; 66: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richette P, Poitou C, Garnero P, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis 2011; 70: 139–144. [DOI] [PubMed] [Google Scholar]
- 38. Gudbergsen H, Boesen M, Lohmander LS, et al. Weight loss is effective for symptomatic relief in obese subjects with knee osteoarthritis independently of joint damage severity assessed by high-field MRI and radiography. Osteoarthritis Cartilage 2012; 20: 495–502. [DOI] [PubMed] [Google Scholar]
- 39. Christensen P, Henriksen M, Bartels EM, et al. Long-term weight-loss maintenance in obese patients with knee osteoarthritis: a randomized trial. Am J Clin Nutr 2017; 106: 755–763. [DOI] [PubMed] [Google Scholar]
- 40. Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med 2009; 121: 9–20. [DOI] [PubMed] [Google Scholar]
- 41. Yoshimura N, Muraki S, Oka H, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012; 20: 1217–1226. [DOI] [PubMed] [Google Scholar]
- 42. Li H, George DM, Jaarsma RL, et al. Metabolic syndrome and components exacerbate osteoarthritis symptoms of pain, depression and reduced knee function. Ann Transl Med 2016; 4: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baudart P, Louati K, Marcelli C, et al. Association between osteoarthritis and dyslipidaemia: a systematic literature review and meta-analysis. RMD Open 2017; 3: e000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hall AJ, Stubbs B, Mamas MA, et al. Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. Eur J Prev Cardiol 2016; 23: 938–946. [DOI] [PubMed] [Google Scholar]
- 45. Louati K, Vidal C, Berenbaum F, et al. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 2015; 1: e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Williams MF, London DA, Husni EM, et al. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. J Diabetes Complicat 2016; 30: 944–950. [DOI] [PubMed] [Google Scholar]
- 47. Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: what are the links? Diabetes Res Clin Pract 2016; 122: 198–206. [DOI] [PubMed] [Google Scholar]
- 48. Nielen JTH, Emans PJ, Dagnelie PC, et al. Severity of diabetes mellitus and total hip or knee replacement: a population-based case-control study. Medicine (Baltimore) 2016; 95: e3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kluzek S, Sanchez-Santos MT, Leyland KM, et al. Painful knee but not hand osteoarthritis is an independent predictor of mortality over 23 years follow-up of a population-based cohort of middle-aged women. Ann Rheum Dis 2016; 75: 1749–1756. [DOI] [PubMed] [Google Scholar]
- 50. Coxib and traditional NSAID Trialists’ (CNT) Collaboration, Bhala N, Emberson J, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Richette P. How safe is acetaminophen in rheumatology? Joint Bone Spine 2014; 81: 4–5. [DOI] [PubMed] [Google Scholar]
- 52. Roberts E, Delgado Nunes V, Buckner S, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis 2016; 75: 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doherty M, Hawkey C, Goulder M, et al. A randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/paracetamol in community-derived people with knee pain. Ann Rheum Dis 2011; 70: 1534–1541. [DOI] [PubMed] [Google Scholar]
- 54. Nguyen C, Rannou F. The safety of intra-articular injections for the treatment of knee osteoarthritis: a critical narrative review. Expert Opin Drug Saf 2017; 16: 897–902. [DOI] [PubMed] [Google Scholar]
- 55. Schneider KL, Kastenmüller K, Weckbecker K, et al. Potential drug-drug interactions in a cohort of elderly, polymedicated primary care patients on antithrombotic treatment. Drugs Aging. 2018; 35: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang X, Donnan PT, Bell S, et al. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol 2017; 18: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hsu CC, Wang H, Hsu YH, et al. Use of nonsteroidal anti-inflammatory drugs and risk of chronic kidney disease in subjects with hypertension: nationwide longitudinal cohort study. Hypertension 2015; 66: 524–533. [DOI] [PubMed] [Google Scholar]
- 58. Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011; 342: c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016; 375: 2519–2529. [DOI] [PubMed] [Google Scholar]
- 60. Reed GW, Abdallah MS, Shao M, et al. Effect of aspirin coadministration on the safety of celecoxib, naproxen, or ibuprofen. J Am Coll Cardiol 2018; 71: 1741–1751. [DOI] [PubMed] [Google Scholar]
- 61. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med 2018; 52: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Castaneda S, Roman-Blas JA, Largo R, et al. Osteoarthritis: a progressive disease with changing phenotypes. Rheumatology 2014; 53: 1–3. [DOI] [PubMed] [Google Scholar]
- 63. Collins JE, Katz JN, Dervan EE, et al. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2014; 22: 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ 2017; 357: j1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wylde V, Dieppe P, Hewlett S, et al. Total knee replacement: is it really an effective procedure for all? Knee 2007; 356: 417–423. [DOI] [PubMed] [Google Scholar]
- 66. Mahler EAM, Boers N, Bijlsma JWJ, et al. Patient acceptable symptom state in knee osteoarthritis patients succeeds across different patient-reported outcome measures assessing physical function, but fails across other dimensions and rheumatic diseases. J Rheumatol 2018; 45: 122–127. [DOI] [PubMed] [Google Scholar]
- 67. Raman R, Henrotin Y, Chevalier X, et al. Decision algorithms for the retreatment with viscosupplementation in patients suffering from knee osteoarthritis: recommendations from the EUROpean VIScosupplementation COnsensus Group (EUROVISCO). Cartilage. Epub ahead of print 1 February 2017. DOI: 10.1177/1947603517693043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010; 18: 4764–4799. [DOI] [PubMed] [Google Scholar]
- 69. Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013; 72: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 70. Migliore A, Scirè CA, Carmona L, et al. The challenge of the definition of early symptomatic knee osteoarthritis: a proposal of criteria and red flags from an international initiative promoted by the Italian society for rheumatology. Rheumatol Int 2017; 37: 1227–1236. [DOI] [PubMed] [Google Scholar]
- 71. Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport 2011; 14: 4–9. [DOI] [PubMed] [Google Scholar]
- 72. Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev 2015; 4: Cd004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rausch Osthoff AK, Niedermann K, Braun J, et al. EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis 2018; 77: 1251–1260. [DOI] [PubMed] [Google Scholar]
- 74. Quintrec JL, Verlhac B, Cadet C, et al. Physical exercise and weight loss for hip and knee osteoarthritis in very old patients: a systematic review of the literature. Open Rheumatol J 2014; 8: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Khan M, Adili A, Winemaker M, et al. Management of osteoarthritis of the knee in younger patients. CMAJ 2018; 190: E72–E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pelletier JP, Martel-Pelletier J, Rannou F, et al. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016; 45(Suppl. 4): S22–S27. [DOI] [PubMed] [Google Scholar]
- 77. Bally M, Beauchamp ME, Abrahamowicz M, et al. Risk of acute myocardial infarction with real-world NSAIDs depends on dose and timing of exposure. Pharmacoepidemiol Drug Saf 2018; 27: 69–77. [DOI] [PubMed] [Google Scholar]
- 78. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013; 310: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Iolascon G, Gimigliano F, Moretti A, et al. Early osteoarthritis: how to define, diagnose, and manage. A systematic review. Eur Geriatr Med 2017; 8: 383–396. [Google Scholar]
- 80. Farr JN, Going SB, McKnight PE, et al. Progressive resistance training improves overall physical activity levels in patients with early osteoarthritis of the knee: a randomized controlled trial. Phys Ther 2010; 90: 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Herrero-Beaumont G, Roman-Blas JA, Castaneda S, et al. Primary osteoarthritis no longer primary: three subsets with distinct etiological, clinical, and therapeutic characteristics. Semin Arthritis Rheum 2009; 39: 71–80. [DOI] [PubMed] [Google Scholar]
- 82. Bruyère O, Cooper C, Arden N, et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging 2015; 32: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roman-Blas JA, Bizzi E, Largo R, et al. An update on the up and coming therapies to treat osteoarthritis, a multifaceted disease. Expert Opin Pharmacother 2016; 17: 1745–1756. [DOI] [PubMed] [Google Scholar]
- 84. Nelson FRT. The value of phenotypes in knee osteoarthritis research. Open Orthop J 2018; 12: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Langworthy MJ, Nelson F, Owens BD. Viscosupplementation for treating osteoarthritis in the military population. Mil Med 2014; 179: 815–820. [DOI] [PubMed] [Google Scholar]


