Abstract
Background:
With a large array of disease modifying therapies (DMTs) for relapsing-remitting MS (RRMS), identifying the optimal treatment option for the individual patient is challenging and switching of immunotherapies is often required. The objective of this study was to systematically investigate reasons for DMT switching in patients on immunotherapies for mild/moderate MS, and provide real-life insights into currently applied therapeutic strategies.
Methods:
This noninterventional, cross-sectional study (ML29913) at 50 sites in Germany included RRMS patients on therapies for mild/moderate MS who switched immunotherapy in the years 2014–2017. The key outcome variable was the reason to switch, as documented in the medical charts, based on failure of current therapy, cognitive decline, adverse events (AEs), patient wish, or a woman’s wish to become pregnant. Expectations of the new DMT and patients’ assessment of the decision maker were also recorded.
Results:
The core analysis population included 595 patients, with a mean age of 41.6 years, of which 69.7% were female. More than 60% of patients had at least one relapse within 12 months prior to the switch. The main reasons to switch DMT were failure of current therapy (53.9%), patient wish (22.4%), and AEs (19.0%). Most patients (54.3%) were switched within DMTs for mild/moderate MS; only 43.5% received a subsequent DMT for active/highly active MS. While clinical and outcome-oriented aspects were the most frequently mentioned expectations of the new DMT for physicians, aspects relating to quality of life played a major role for patients.
Conclusions:
Our data indicate suboptimal usage of DMTs, including monoclonal antibodies, for active/highly active MS in German patients. This illustrates the medical need for DMTs combining high efficacy, low safety risk, and low therapy burden.
Keywords: disease modifying therapies, multiple sclerosis, real-world data, relapsing-remitting multiple sclerosis, treatment switch
Introduction
The pathophysiological hallmarks of multiple sclerosis (MS) call for vigilant monitoring of subclinical disease activity and early effective intervention to control inflammation, prevent axonal/neuronal loss, and optimise long-term outcomes for patients.1,2 In the absence of curative treatments, the therapeutic landscape of MS disease modifying therapies (DMTs) is continuously growing.2,3 Agents for the treatment of mild/moderate relapsing-remitting MS (RRMS) include injectable beta interferons (IFN-β-1a, IFN-β-1b) and glatiramer acetate preparations as well as the oral DMTs dimethyl fumarate and teriflunomide.2 More potent available options include fingolimod, and monoclonal antibodies (alemtuzumab, natalizumab, ocrelizumab*).2 At the time this study was conducted, 13 drugs were approved for the treatment of MS in the European Union (EU).4
The heterogeneity of the disease, and the diversity of available DMTs, contribute to the challenge of selecting the DMT that best meets an individual patient’s needs, including effectiveness, acceptable risk/benefit ratio, and convenience.5 In the absence of predictive biomarkers, neurologists have to adopt a stepwise, often time-consuming, optimisation approach, including switching DMTs during the disease course.6,7
Reasons for switching a DMT may include lack of efficacy, adverse events (AEs), and inadequate patient adherence.7 Only few real-life studies have investigated the reasons for switching DMTs in detail. A recent multicentre, retrospective, Italian study by Sacca and colleagues, with data from 2954 newly diagnosed patients with RRMS collected from 2010 through 2017, found that 48% of patients switched therapy within the 3 years of observation.8 Poor efficacy was the predominant cause for switching, more frequently in patients treated with first-line injectable therapies than with second-line treatments such as fingolimod and natalizumab.8 The North American Research Committee on Multiple Sclerosis (NARCOMS), a large, voluntary, patient-driven MS registry, conducted a supplemental survey with 308 patients with RRMS to investigate the reasons for switching DMTs: physicians’ recommendation was most frequently indicated as the main reason to switch (24.5% of patients), whereby perceived lack of efficacy was stated by only 13.7% of patients.9 However, the physicians’ perspective was not documented in the NARCOMS survey, and the answer ‘physicians’ recommendation’ certainly includes failure of therapy as an underlying reason to switch DMT. Furthermore, fewer treatment options were available at the time of the NARCOMS survey, which was based on DMT switches in 2010/2011.9
The retrospective German TYPIC survey published in 2011 with 7896 patients on basic immune therapies (IFN-β or glatiramer acetate) found a strong association between relapse rate in the previous 12 months and the likelihood of switching.10
Specific switch reasons have not yet been investigated systematically in real-world outpatient settings in Germany. We conducted this noninterventional, retrospective study to analyse the prevalence of reasons in RRMS patients for switching from a DMT for mild/moderate MS in the diversified treatment landscape in Germany. We also asked patients and physicians for their expectations of the postswitch DMT. Furthermore, in the context of emerging treatment options, we aimed to gain insights into currently applied therapeutic strategies.
Patients and methods
Study design and patients
This noninterventional, cross-sectional study (Roche ML29913) was conducted between March 2016 and September 2017 at 50 sites in Germany, comprising mainly office-based neurologists (n = 37), but also medical centres (n = 13). In this study, the switch from a DMT for mild/moderate MS [defined as beta interferons (IFN-β-1a, IFN-β-1b), glatiramer acetate, dimethyl fumarate, or teriflunomide] to any other DMT (including the EU label second-line options fingolimod, natalizumab, mitoxantrone, alemtuzumab) was investigated. The study protocol was approved by the Ethics Committee of Friedrich-Alexander University Erlangen-Nürnberg, Germany (approval number 26_16B), and the study was conducted in accordance with the guidelines for good pharmacoepidemiological practice and the applicable laws and regulations.
Adult patients with RRMS who switched from a DMT for mild/moderate MS (as defined above) to another DMT were eligible for the study. According to the original study protocol, the last switch must have occurred within 12 months before signature of contract for each site. An amendment from 13 September 2016 prolonged this time period to 24 months. This amendment was introduced to address slow recruitment, and to include more patients who switched from dimethyl fumarate (European Medicine Agency approval of Tecfidera in January 2014). All treatments were required to conform to the standard of care and the current respective summaries of product characteristics. Patients were recruited during routine site visits. There was only one visit per patient, which included collection of patient information and signature of informed consent, as well as the completion of the patient questionnaire. To minimise inclusion bias, treating physicians were required to document patients in the order in which the routine visits took place. Information regarding the treatment before and after the switch, as well as reasons for switching (including specification of AEs) were recorded retrospectively using medical charts. In addition, expectations of the new DMT, as well as patients’ assessment of the decision maker for the switch (physician, patient, joint decision) were collected from physician and patient questionnaires in the cross-sectional part of the study.
Outcome measures
The key outcome variable was the reason to switch DMT, as documented in medical charts based on failure of therapy [magnetic resonance imaging (MRI) activity or relapses, increased disability, or fatigue], cognitive decline, AEs, patient’s wish (worsening of symptoms, new symptoms, increased impairment, poor tolerability, inconvenient application form, other), a woman’s wish to become pregnant, and other. Fatigue was included as a criterion for failure of therapy because it is a frequently reported, disabling symptom in patients with MS. Fatigue often precedes Expanded Disability Status Scale (EDSS) and cognitive decline and thus is informative for early therapy optimisation. Multiple answers were possible. Additionally, data regarding the main reason leading to a switch were collected. Here, only one answer was possible.
Further outcome measures included physicians’ expectations concerning the new DMT at time of switch (three answers possible from the following options: relevance of drug characteristics with regard to prevention of relapses, prevention of new lesions, effects on progression of disability, good tolerability, less extensive pharmacovigilance monitoring, convenient application form, costs), patients’ expectations (two answers possible from the following options: relevance of drug characteristics with regard to good effectiveness, effects on progression of disability, good tolerability, convenient application form) and patients’ assessment of who made the decision for the switch. Prespecified subgroup analyses by EDSS score (low EDSS score of 0–3.5, and high EDSS score of ⩾4) were performed for reasons to switch as well as for physicians’ and patients’ expectations concerning the new DMT.
Data from patient charts regarding reasons to switch and baseline characteristics were recorded retrospectively via electronic case report form (eCRF). Data from physicians’ and patients’ questionnaires about their expectations regarding therapy and decision making were entered by the study personnel into the eCRF.
AEs leading to a switch from a DMT for mild/moderate MS to another DMT were analysed retrospectively. In addition, AEs occurring or emerging during the time from signing the informed consent to completing the questionnaires were documented in the eCRF (data not shown).
Statistics
Statistical analysis was based on the core analysis population (CAP), that is, all enrolled patients with valid data. Missing values were not replaced. The analysis of this study was exploratory, and used primarily descriptive statistical methods. Inferential methods (e.g. confidence intervals) were used in selected analyses, and were interpreted in an exploratory manner. Categorical variables were displayed by absolute and relative frequencies (percentages). Percentages for categorical variables were based on all nonmissing values (=100%). For reasons to switch, two-sided 95% confidence intervals (Wilson scores) were reported. Continuous variables were summarised with number of observations, mean, standard deviation (SD), median, minimum, maximum, and the 25% and 75% percentiles. Due to the exploratory nature of this study, no adjustment for multiplicity was foreseen.
Sample size justification
The intended sample size of 600 patients was chosen for reasons of feasibility rather than statistical considerations. Based on a two-sided 95% confidence level, and assuming each response category as a separate, binomially distributed variable, this intended sample size allows an estimation of the proportion of each reason to switch with a precision of at least 4% (i.e. half width of the 95% confidence interval ⩽4%) using normal approximation, respectively.
Results
Patient population
In this study of 607 patients screened across 51 centres in Germany, 600 patients were enrolled. Of these, 406 patients entered the study before the protocol amendment from 13 September 2016, and 194 patients after the amendment, which prolonged the maximum retrospective time period for the documented switch from 12 months to 24 months. Major protocol deviations led to the exclusion of five patients from the final analysis; thus, the CAP included 595 patients (Figure 1).
Figure 1.
Patient selection.
CAP, core analysis population; DMT, disease modifying therapy.
Baseline patient and disease characteristics
Baseline patient and disease characteristics are listed in Table 1. Patients were 69.7% female, and the mean age of patients (± SD) was 41.6 ± 10.9 years (range 18–76 years). More than 60% of patients had one or more relapses within 12 months prior to the switch. A low EDSS score of 0–3.5 was documented for 82.2% of patients with available EDSS score, with a higher EDSS score of ⩾4 for 17.8% of patients (Table 1). Before switching, 53.1% of patients were on IFN-β preparations, 20.7% on glatiramer acetate, 19.2% on dimethyl fumarate, and 7.1% on teriflunomide. The percentage of patients that already had one or more former immunotherapy switches was 57.8%, while 42.2% had not changed their DMT before (Table 1). The mean time from the latest switch until the study visit was 14.6 ± 10.1 (SD) months. The mean treatment duration was 35.3 ± 40.8 (SD) months, with the last DMT before the switch, and 12.7 ± 9.6 (SD) months with the first DMT after the switch.
Table 1.
Baseline patient and disease characteristics.
| Characteristics | |
|---|---|
| Age in years, mean (±SD) (n = 595) | 41.6 (10.9) |
| Range in years | 18–76 |
| Age group, n (%) (n = 595) | |
| ⩽30 years | 106 (17.8) |
| 31–45 years | 262 (44.0) |
| ⩾46 years | 227 (38.2) |
| Gender, n (%) (n = 595) | |
| Male | 180 (30.3) |
| Female | 415 (69.7) |
| Time since first MS diagnosis in years, mean (±SD) (n = 594) | 8.7 (6.7) |
| Range in years | 0–43 |
| Time since first MS symptoms in years, mean (±SD) (n = 590) | 10.3 (7.7) |
| Range in years | 0–45 |
| Number of relapses within the last 24 months before switcha, n (%) (n = 593) | |
| 0 | 165 (27.8) |
| 1 | 200 (33.7) |
| 2 | 153 (25.8) |
| ⩾3 | 75 (12.6) |
| Number of relapses within the last 12 months before switcha, n (%) (n = 594) | |
| 0 | 224 (37.7) |
| 1 | 260 (43.8) |
| 2 | 86 (14.5) |
| ⩾3 | 24 (4.0) |
| Last MRI before switcha, n (%) (n = 594) | |
| Time point within ⩽1 year | 454 (76.4) |
| Within ⩾1 year to <2 years | 72 (12.1) |
| Within ⩾2 years | 53 (8.9) |
| None | 15 (2.5) |
| EDSS score before switcha, n (%) (n = 569) | |
| 0 to 3.5 | 468 (82.2) |
| ⩾4 | 101 (17.8) |
| Incidences of the previous switchesa, n (%) (n = 595) | |
| 1–3 | 328 (55.1) |
| ⩾4 | 16 (2.7) |
| None | 251 (42.2) |
Retrospective data collection.
EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; MS, multiple sclerosis; SD, standard deviation.
Reasons for switching DMT for mild/moderate MS
As reason for switching DMT (multiple reasons possible) the answer reported most frequently according to the medical charts was failure of therapy [56.0% (95% CI: 52.0–59.9%) of patients], followed by patient wish [28.7% (95% CI: 25.3–32.5%) of patients] and AEs [21.0% (95% CI: 17.9–24.4%) of patients] (Supplementary Figure S1). Similar results were obtained when asking only for the main reason to switch (Figure 2). For more than half of the patients [53.9% (95% CI: 49.9–58.0%)], the main reason to switch DMT for mild-to-moderate MS was failure of therapy, which included MRI activity or relapses, increased disability and fatigue. Patient wish was documented as reason to change the medication for 22.4% (95% CI: 19.0–25.7%) of patients and AEs for 19.0% (95% CI: 15.8–22.1%) of patients.
Figure 2.
Main reason to switch DMT. Data were recorded retrospectively using medical charts.
AE, adverse event; CI, confidence interval; DMT, disease modifying therapy.
In a subgroup analysis by EDSS, failure of therapy was a more prevalent reason to switch DMT in the EDSS score subgroup ⩾4 than in the EDSS score subgroup 0–3.5 (62.4% versus 53.8%) (Supplementary Figure 2).
DMT disposition and switch mode
While 54.3% of patients were switched between platform therapies (IFN-β preparations, dimethyl fumarate, glatiramer acetate, teriflunomide), 43.5% of patients received a DMT with an EU label for active/highly active MS (alemtuzumab, fingolimod, natalizumab, mitoxantrone) (Figure 3a). Overall frequencies of DMTs before and after the switch are provided in Figure 3b (for all patients) and 3c (for patients with main reason to switch being failure of therapy). Before switching, most patients were given IFN-β preparations, followed by glatiramer acetate, dimethyl fumarate, and teriflunomide. The most prevalent single DMT after the switch was fingolimod (27.4% of all patients and 43.3% of patients who switched due to failure of therapy), followed by dimethyl fumarate (22.5%) in all patients and natalizumab (20.2%) in patients who switched due to failure of therapy, respectively (Figure 3b,c). The highest proportion of all patients switched from IFN-β preparations to dimethyl fumarate (14.6%), followed by 11.6% of patients who switched from IFN-β preparations to fingolimod (Supplementary Figure S3). The most prevalent switch mode was from injected to oral drugs (49.1% of patients) (Supplementary Figure S4).
Figure 3.

Switch-related characteristics. Therapeutic nature of the switch (a). Overall frequencies of DMTs before and after the switch for all patients (b) and for patients with main reason to switch being failure of therapy (c).
aOther medications: 12× daclizumab, 1× rituximab.
bOther medications: 5× daclizumab, 1× rituximab.
Alem, alemtuzumab; DMF, dimethyl fumarate; DMT, disease modifying therapy; Fingo, fingolimod; Glat, glatiramer acetate; IFN, interferon; Mitox, mitoxantrone; NTZ, natalizumab; Teri, teriflunomide.
Adverse events
Overall, in 21.2% of patients at least one AE occurred that led to a switch (135 AEs were recorded in 126 patients). Of note, one acute episode of MS was recorded as an AE; for this patient the reason to switch documented in the eCRF was ‘failure of therapy’. A relationship to the DMT was indicated for 18.8% of patients. No serious AE was documented that resulted in a switch. AEs leading to a switch were most frequently of the system organ class (SOC) ‘general disorders and administration site conditions’ [5.9% (35/595) patients with 37 events], followed by AEs of the SOC ‘skin and subcutaneous tissue disorders’ [4.0% (24/595) patients with 25 events]. Among 316 IFN-pretreated patients, only 1 instance of fatigue was reported as a switch-related AE. Switch-related AEs by SOC observed in the CAP (Supplementary Figure S5) were consistent with known safety profiles of the DMTs IFN-β 1a and 1b, dimethyl fumarate, glatiramer actetate and teriflunomide.11–20
Decision maker and expectations of DMT at time of switch
The majority of patients (83.5%) reported that they decided jointly with their treating physician to switch their DMT (Figure 4a). For 11.9% of patients, the switch was driven by patient demand and for 5.4% of patients, the switch was recommended by the physician (Figure 4a).
Figure 4.
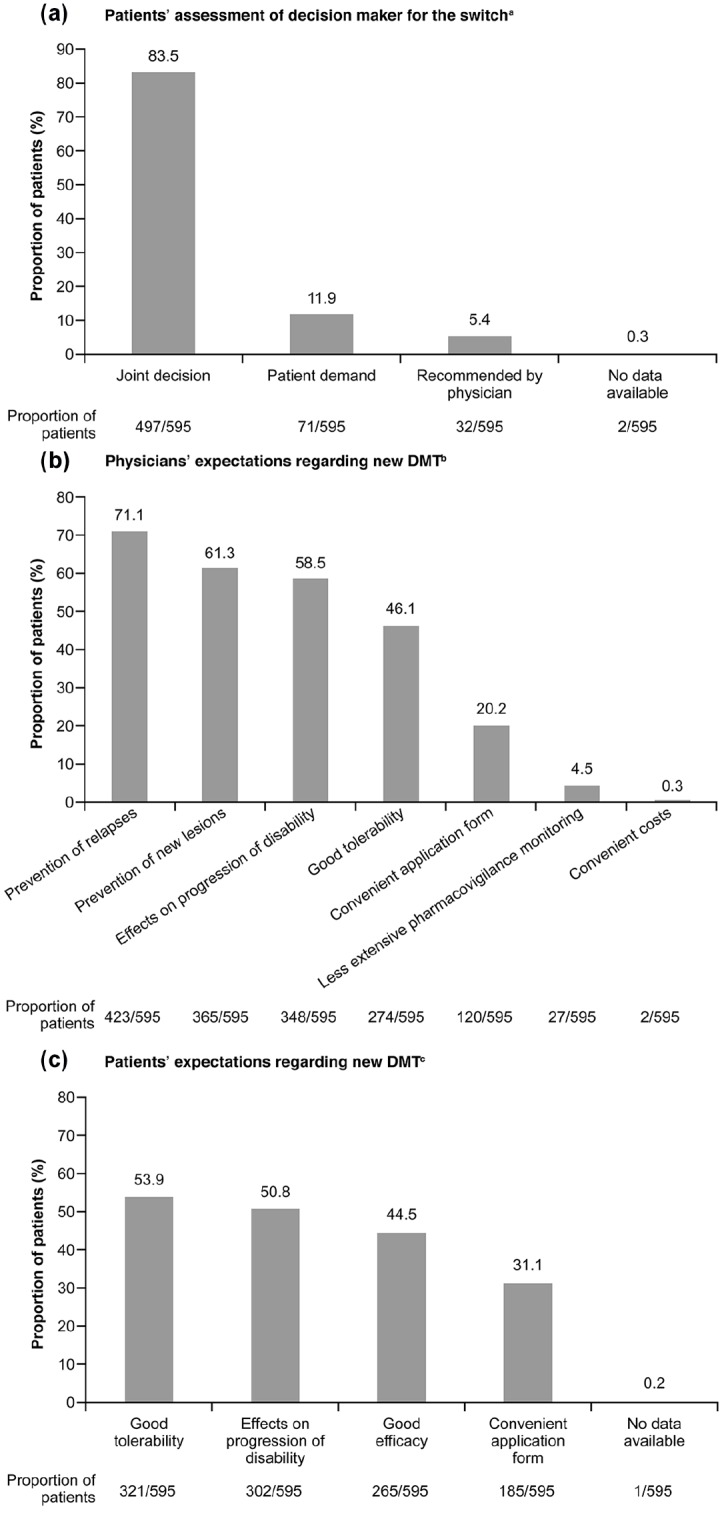
Decision maker and expectations regarding new DMT. (a) Patients’ assessment of decision maker for the switch. (b) Physicians’ expectations regarding new DMT. (c) Patients’ expectations regarding new DMT.
aData were recorded using patient questionnaires. Multiple answers per patient were possible.
bData were recorded using physician questionnaires. Up to three answers were possible. Answer options: relevance of drug characteristics with regard to prevention of relapses, prevention of new lesions, effects on progression of disability, good tolerability, less extensive pharmacovigilance monitoring, convenient application form, costs.
cData were recorded using patient questionnaires. Up to two answers were possible. Answer options: relevance of drug characteristics with regard to good effectiveness, effects on progression of disability, good tolerability, convenient application form.
DMT, disease modifying therapy.
When the treating physicians were asked about their expectations concerning their choice of medication after the switch, the most prevalent criterion was prevention of relapses (for 71.1% of patients) (Figure 4b). Prevention of new lesions (for 61.3% of patients), and effects on the progression of disability (for 58.5% of patients) were further aspects frequently mentioned by physicians. Physicians expected the new DMT to be well-tolerated in the case of 46.1% of patients and conveniently applicable in the case of 20.2% of patients (Figure 4b).
The results were different when patients were asked about their main expectations concerning the new DMT. Good tolerability was most frequently mentioned (53.9% of patients), followed by effects on progression of disability (50.8% of patients), good effectiveness (44.5% of patients) and convenient application form (31.1% of patients) (Figure 4c).
For patients with an EDSS score of 0–3.5 (n = 468), the most prevalent physicians’ expectation regarding the new DMT was prevention of relapses, while for patients with an EDSS score ⩾4 (n = 101) effects on the progression of disability was most frequently mentioned (Supplementary Figure S6a). Patients with an EDSS score of 0–3.5 most frequently expected good tolerability, while patients with an EDSS score ⩾4 most frequently expected effects on the progression of disability (Supplementary Figure S6b).
Discussion
This study presents a systematic overview of the reasons for switching DMTs for RRMS in clinical routine in Germany, and switch-related expectations both from the physicians’ and the patients’ perspectives. Although failure of therapy was the main reason (53.9% of patients) as well as the most frequently mentioned reason (56.0% of patients) to switch the DMT, the proportion of patients who switched to a DMT with a label for active/highly active MS was only 43.5%. Of the 321 documented cases switched for failure on previous DMTs, 28.9% remained on therapies for mild–moderate MS after the switch. When patients were switched to DMTs for active/highly active MS, fingolimod (27.4% of patients) was robustly preferred over monoclonal antibodies (natalizumab: 12.6% of patients, alemtuzumab: 3.2% of patients) although fingolimod and monoclonal antibodies are equally recommended by the European guidelines,21 and monoclonal antibodies may be more effective than fingolimod in the prevention of relapses.22 In line with the eligibility criteria limiting documented cases to patients with a recent switch of DMT, disease activity was high in our cohort. Relapses within the last 12 and 24 months were observed in 62.3% and 72.2% of patients, respectively.
In summary, findings reveal a suboptimal usage of DMTs for active/highly active MS, including therapeutic antibodies, and thus a relevant gap between current clinical practice and ideal disease management of MS. Similar observations were made in recent population-based studies. In the Italian real-life study by Sacca and colleagues, switches due to inefficacy were more frequent than switches due to intolerance/safety, and at 3 years from first therapy, the rate of switches between first-line therapies for mild or moderate MS was approximately twice the rate of switches to fingolimod or natalizumab.8 The German TYPIC survey was published in 2011 when the only approved therapies for highly active RRMS were natalizumab and mitoxantrone.10 In this survey, about one-third of patients had relapses during the last 12 months, and approximately one-quarter were eligible for DMTs for active/highly active MS by measurable disease activity; however, escalating current DMTs for mild/moderate MS to more potent therapies was considered for only about 20% of patients.10
Together with our study, these recent findings prompt further exploration of why treatment approaches did not change significantly since the TYPIC survey. Conditions for early and efficacious intervention in MS may have become more favourable in the meantime: the recently revised McDonald criteria allow for an earlier diagnosis of MS (McDonald criteria 2017),23 more treatment options exist, and the efficacy of intervention with a DMT for active/highly active MS was proven in phase III trials for example, comparing alemtuzumab versus IFN-β 1a.24,25 In addition, the concept of no evidence of disease activity (NEDA) has become an important therapeutic goal for physicians, which involves early treatment optimisation with escalation therapies.1,5
The treatment landscape is currently changing, and injectable DMTs for mild/moderate RRMS may gradually be replaced by newer oral DMTs such as dimethyl fumarate and teriflunomide.5 In our study, this is reflected by the DMT frequencies before and after the switch. The proportion of patients receiving IFN-β preparations or glatiramer acetate declined substantially, while the prevalence of dimethyl fumarate or teriflunomide increased. Accordingly, with regard to the route of administration before and after the switch, the most prevalent switch mode was from injectables to oral drugs (49.1% of patients).
While safety concerns contribute to the reluctance of physicians to increase use of DMTs for active/highly active MS, another important factor bidirectionally influencing choice of treatment is shared decision-making between patient and physician. A joint decision led to the switch of the DMT in 83.5% of cases in the current study. Aspects relating to quality of life played a major role for patients (good tolerability 53.9%; effects on the progression of disability 50.8%), while clinical and outcome-oriented aspects were the most frequently mentioned expectations for physicians (prevention of relapses 71.1%; prevention of new lesions 61.3%; effects on progression of disability 58.5%). This difference in expectations was particularly pronounced in the majority of patients with a lesser degree of disability (EDSS score 0–3.5), while patients with an EDSS ⩾4 agreed with their physicians that prevention of progression of disability was the main switch-related expectation.
The treatment decision is also influenced by the convenience of administration. A convenient application form of the new DMT was expected by 31.1% of patients and physicians expected the new DMT to be conveniently applicable for 20.2% of patients. These results are in line with other surveys that were conducted in Germany showing that patients prefer oral to injectable treatments, and that the route of administration, as well as the treatment frequency, strongly influence the patients’ preference for a given DMT.26,27
It is not uncommon for patients to discontinue DMTs, for example due to tolerability concerns.7 In fact, a long-term adherence study reported a discontinuation rate of 46%.28 In the therapy satisfaction in patients with relapsing-remitting multiple sclerosis (THEPA-MS) survey, including 3312 patients with MS in Germany, improvements regarding side effects and convenience of treatment were found to be particularly promising approaches to increase adherence.29 Current findings illustrate that the individual patient’s expectations have to be adequately considered in the decision-making process to ensure long-term satisfaction and adherence.
The German healthcare system and national treatment guidelines provide much freedom for therapeutic decisions in the management of MS. There are no access hurdles to all approved MS DMTs in Germany. Guidelines from the German Society of Neurology (DGN) mandate certain criteria for breakthrough disease activity to guide switching to more potent DMTs.30
While being a timely and systematic investigation of switch reasons in Germany, the study at hand has limitations. These include the noninterventional, and thus noncontrolled, design based on retrospective data collection from patient charts. Medical charts may be subject to bias caused by heterogeneities in patients, or treatments and data collection across centres. Furthermore, accompanying MRI data of lesion activity before and after the switch were not evaluated. Nevertheless, the patient baseline characteristics in our study show that the cohort represented a typical patient population with RRMS requiring treatment optimisation. The group of involved neurologists, covering various institutional and office-based settings, is representative of the German healthcare system. Our data thus provide recent and relevant real-life information on currently applied therapies and switch strategies, revealing an often-conservative treatment approach to MS in Germany.
The limited use of DMTs for active/highly active MS indicates a persisting medical need for a DMT that combines high efficacy, low safety risk and low burden of therapy.
Given the currently available array of DMTs, more evidence-based data on treatment sequencing and long-term data on newer DMTs, focusing on patient relevant items and long-term clinical outcomes would greatly support better identification of the optimal DMT in clinical routine.5
Supplemental Material
Supplemental material, Supplementary_File_S1 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supplementary_File_S2 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supplementary_File_S3 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supplementary_File_S4 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supplementary_File_S5 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supplementary_File_S6 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Acknowledgments
We would like to thank all patients, their families, investigators and nurses who contributed to this study. Medical writing assistance (drafting of the manuscript, implementation of revisions made by the authors) was provided by Physicians World Europe GmbH (Mannheim, Germany) and AMS Advanced Medical Services GmbH (Mannheim, Germany), financially supported by Roche Pharma AG.
Not approved at time of study conduct
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This research was funded by Roche Pharma AG, Grenzach-Wyhlen, Germany.
Conflict of interest statement: All authors received support for their contribution to this study as well as medical writing support for the development of this manuscript from Roche Pharma AG, Grenzach-Wyhlen, Germany.
RAL received compensation for activities with Almirall, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis Pharma, Roche and TEVA as well as research support from Biogen, Merck, and Novartis. MM received compensation for activities with Almirall, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, and TEVA. EO received honoraria for studies from Abbvie, Allergan, Bayer, Biogen, Genzyme, Merck & Co., Merck Serono, Merz, Novartis, Roche, Sanofi, TEVA, UCB, and Zambon. PO received compensation for activities with Bayer, Biogen, Genzyme, Merck, Novartis, Roche, and TEVA. NR has no additional disclosures. MS received honoraria for lectures, studies and consulting from Merck, Sanofi, Novartis, and Genzyme. KTW received honoraria for lectures, studies, and consultancy from Almirall, Bayer, Biogen, Genzyme, Merck Serono, Novartis, Roche, Sanofi, and TEVA. TZ received reimbursements for participation in scientific advisory boards from Almirall, Bayer Healthcare, Biogen, Celgene, Novartis, Merck Serono, TEVA, Genzyme, and Roche; he also received speaker honoraria from Almirall, Bayer Healthcare, Biogen, Celgene, Genzyme, Novartis, Roche, TEVA, and research support from Bayer Healthcare, BAT, Biogen, Genzyme, Novartis, and TEVA. AM, SHS, JAK are current employees of Roche Pharma AG. VZ is a current employee of F. Hoffmann-La Roche.
ORCID iDs: Tjalf Ziemssen  https://orcid.org/0000-0001-8799-8202
https://orcid.org/0000-0001-8799-8202
Ralf A. Linker  https://orcid.org/0000-0002-8740-3106
https://orcid.org/0000-0002-8740-3106
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mathias Mäurer, Klinikum Würzburg Mitte, Standort Juliusspital, Würzburg, Germany.
Klaus Tiel-Wilck, Neurologisches Facharztzentrum Berlin, Berlin, Germany.
Eckard Oehm, Group practice for Neurology, Psychiatry and Psychotherapy, Freiburg, Germany.
Nils Richter, Group practice for Neurology, Düsseldorf, Germany.
Michael Springer, Neurological practice Dr. Springer, Pirmasens, Germany.
Patrick Oschmann, Klinikum Bayreuth, Bayreuth, Germany.
Arndt Manzel, Roche Pharma AG, Grenzach-Wyhlen, Germany.
Stefanie Hieke-Schulz, Roche Pharma AG, Grenzach-Wyhlen, Germany.
Vera Zingler, F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Julia A. Kandenwein, Roche Pharma AG, Grenzach-Wyhlen, Germany
Tjalf Ziemssen, Universitätsklinikum Carl Gustav Carus, Centre for Clinical Neuroscience, Dresden, Germany.
Ralf A. Linker, Neurologische Klinik der Universität Regensburg, Universitätsstraße 84, Regensburg, 93053, Germany; Klinik für Neurologie der Universität Regensburg, Regensburg, Germany.
References
- 1. Ziemssen T, Derfuss T, de Stefano N, et al. Optimizing treatment success in multiple sclerosis. J Neurol 2016; 263: 1053–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burton JM, Freedman MS. The shifting landscape of disease-modifying therapies for relapsing multiple sclerosis. J Neuroophthalmol 2018; 38: 210–216. [DOI] [PubMed] [Google Scholar]
- 3. Menzin J, Caon C, Nichols C, et al. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm 2013; 19: S24–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Multiple Sclerosis Platform. MS facts | MS treatments. http://www.emsp.org/about-ms/ms-treatments/ (accessed 23 October 2018).
- 5. Grand’Maison F, Yeung M, Morrow SA, et al. Sequencing of disease-modifying therapies for relapsing-remitting multiple sclerosis: a theoretical approach to optimizing treatment. Curr Med Res Opin 2018; 34: 1419–1430. [DOI] [PubMed] [Google Scholar]
- 6. Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron 2018; 97: 742–768. [DOI] [PubMed] [Google Scholar]
- 7. Miller AE. Switching or discontinuing disease-modifying therapies for multiple sclerosis. Continuum (Minneap Minn) 2016; 22: 851–863. [DOI] [PubMed] [Google Scholar]
- 8. Sacca F, Lanzillo R, Signori A, et al. Determinants of therapy switch in multiple sclerosis treatment-naive patients: a real-life study. Mult Scler 2019; 25: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 9. Salter AR, Marrie RA, Agashivala N, et al. Patient perspectives on switching disease-modifying therapies in the NARCOMS registry. Patient Prefer Adherence 2014; 8: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mäurer M, Dachsel R, Domke S, et al. Health care situation of patients with relapsing-remitting multiple sclerosis receiving immunomodulatory therapy: a retrospective survey of more than 9000 German patients with MS. Eur J Neurol 2011; 18: 1036–1045. [DOI] [PubMed] [Google Scholar]
- 11. Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The multiple sclerosis collaborative research group (MSCRG). Ann Neurol 1996; 39: 285–294. [DOI] [PubMed] [Google Scholar]
- 12. Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon β-1a treatment regimens in MS: the EVIDENCE trial. Neurology 2002; 59: 1496–1506. [DOI] [PubMed] [Google Scholar]
- 13. The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43: 655–661. [DOI] [PubMed] [Google Scholar]
- 14. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 15. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 16. Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology 1995; 45: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 17. Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol 2001; 49: 290–297. [PubMed] [Google Scholar]
- 18. Khan O, Rieckmann P, Boyko A, et al. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann Neurol 2013; 73: 705–713. [DOI] [PubMed] [Google Scholar]
- 19. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 20. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13: 247–256. [DOI] [PubMed] [Google Scholar]
- 21. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol 2018; 25: 215–237. [DOI] [PubMed] [Google Scholar]
- 22. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017; 16: 271–281. [DOI] [PubMed] [Google Scholar]
- 23. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 24. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 26. Utz KS, Hoog J, Wentrup A, et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: a choice-based conjoint analysis. Ther Adv Neurol Disord 2014; 7: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Becker V, Heeschen V, Schuh K, et al. Patient satisfaction and healthcare services in specialized multiple sclerosis centres in Germany. Ther Adv Neurol Disord 2018; 11: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Portaccio E, Zipoli V, Siracusa G, et al. Long-term adherence to interferon beta therapy in relapsing-remitting multiple sclerosis. Eur Neurol 2008; 59: 131–135. [DOI] [PubMed] [Google Scholar]
- 29. Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing-remitting multiple sclerosis: the THEPA-MS survey. Ther Adv Neurol Disord 2016; 9: 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deutsche Gesellschaft für Neurologie (DGN). Diagnose und Therapie der Multiplen Sklerose Entwicklungsstufe: S2e Stand: April 2012, Ergänzung August 2014 Gültig bis: 2017, Gültigkeit der Leitlinie nach Überprüfung durch das Leitliniensekretariat verlängert bis 29.9.2017; AWMF-Registernummer: 030/050, http://www.awmf.org/leitlinien/detail/ll/030-050.html (2015, accessed 23 July 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_File_S1 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental material, Supplementary_File_S2 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental material, Supplementary_File_S3 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental material, Supplementary_File_S4 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental material, Supplementary_File_S5 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders
Supplemental material, Supplementary_File_S6 for Reasons to switch: a noninterventional study evaluating immunotherapy switches in a large German multicentre cohort of patients with relapsing-remitting multiple sclerosis by Mathias Mäurer, Klaus Tiel-Wilck, Eckard Oehm, Nils Richter, Michael Springer, Patrick Oschmann, Arndt Manzel, Stefanie Hieke-Schulz, Vera Zingler, Julia A. Kandenwein, Tjalf Ziemssen and Ralf A. Linker in Therapeutic Advances in Neurological Disorders




