Abstract
Background. Malaria is an important health and economic burden in sub-Saharan Africa. Conventional economic evaluations typically consider only direct costs to the health care system and government budgets. This paper quantifies the potential impact of malaria vaccination on the wider economy, using Ghana as an example. Methods. We used a computable general equilibrium model of the Ghanaian economy to estimate the macroeconomic impact of malaria vaccination in children under the age of 5, with a vaccine efficacy of 50% against clinical malaria and 20% against malaria mortality. The model considered changes in demography and labor productivity, and projected gross domestic product (GDP) over a time frame of 30 years. Vaccine coverage ranging from 20% to 100% was compared with a baseline with no vaccination. Results. Malaria vaccination with 100% coverage was projected to increase the GDP of Ghana over 30 years by US$6.93 billion (in 2015 prices) above the baseline without vaccination, equivalent to an increase in annual GDP growth of 0.5%. Projected GDP per capita would increase in the first year due to immediate reductions in time lost from work by adults caring for children with malaria, then decrease for several years as reductions in child mortality increase the number of dependent children, then show a sustained increase after Year 11 due to long-term productivity improvements in adults resulting from fewer malaria episodes in childhood. Conclusion. Investing in improving childhood health by vaccinating against malaria could result in substantial long-term macroeconomic benefits when these children enter the workforce as adults. These macroeconomic benefits are not captured by conventional economic evaluations and constitute an important potential benefit of vaccination.
Keywords: economic growth, general equilibrium, Ghana, human capital, malaria vaccine, public health
Introduction
Malaria remains an important public health burden in sub-Saharan Africa, with an estimated 407,000 malaria deaths in 2016.1 The clinical symptoms of malaria range from nonspecific mild febrile illness to life-threatening disease with coma, respiratory distress, severe anemia, or shock.2 In addition to established preventive malaria interventions, such as insecticide-treated nets, indoor residual spraying, and seasonal malaria chemoprevention, the RTS,S malaria vaccine candidate is now under evaluation after having been piloted with children (0-5 years of age) in moderate- to high-transmission settings in sub-Saharan Africa as recommended by the World Health Organization. Decisions on country-level vaccine introduction will need to take into account the potential public health impact of vaccination, together with an evaluation of its potential budgetary and economic impact.
Commonly used forms of economic evaluation such as cost-effectiveness analysis,3–5 budget impact analysis,6–8 and budget optimization analysis9 are typically limited to considering direct costs to the healthcare system and governmental budgets.10 Analyses of this type for the RTS,S vaccine are presented elsewhere.11–16 However, many of the costs of disease are borne at household level. For example, households may have to pay for treatment and transport to a clinic, and may lose income if a parent has to take time away from work to care for a child with malaria. A study in Ghana, Kenya, and Tanzania published in 2013 estimated that 55% to 70% of the costs of an episode of malaria were borne by households.17 It is possible to include some of these household costs, such as lost income and out-of-pocket expenditure on treatment and transport, in economic evaluations that take a societal perspective. However, even this does not capture the effect of changes in household behavior on the wider economy.
Malaria indirectly effects economic growth because households contribute to the economy by providing labor to firms, by producing goods and services, and by consuming goods and services. When a parent has to take time away from work to care for a child with malaria, not only does the affected household lose income, the employer also loses the value of the lost work time. Furthermore, the household will have less to spend on consumption, thereby reducing the income of individuals and firms from which the household would otherwise have bought goods or services.
Other examples of indirect effects are reduced investment in education per child, reduced educational attainment resulting from missed schooldays due to illness, lower skills due to impaired cognitive development, lower household savings, and reductions in tourism and foreign direct investment.18 Cross-country regression analysis using data from 1965 to 1990 estimated that countries with intensive malaria had an economic growth that was 1.3% lower per person per year than countries without malaria, after taking into account factors such as initial income level, overall health, and tropical location.19
Conventional economic evaluations (such as partial equilibrium models) do not account for indirect effects of the disease on the wider economy, and therefore provide only a partial view of the economic benefit of interventions to reduce disease.10,20–23 Computable general equilibrium (CGE) models offer a promising approach to exploring these wider economic effects.24 CGE models consider different, but interrelated, elements of the economy including households, government, production sectors (such as manufacturing, agriculture, and transport), capital, labor, and foreign trade. The economic relationships between them is calibrated using a social accounting matrix for national income and input–output data by sector.25 Contrary to other methods, general equilibrium can account for wider changes that result from behavior adjustments (e.g., consumption and production) of all key economic agents. For this reason, this approach is highly suitable to estimate the impact of a positive productivity shock after reducing a widespread disease.
CGE models have been applied to simulate the economic impact of antimicrobial resistance26 and pandemic influenza.25,27,28 In our paper, we explore the broader economic impact of malaria vaccination on the Ghanaian economy. (An early draft of this paper was catalogued by an ISPOR conference.)29 The impact of malaria vaccination was measured by considering the economic impacts of changes in 1) malaria-related child mortality, 2) short-term productivity due to caregiving for a child with malaria (which alters the contribution of a malaria-affected household to the economy and the resources available for household consumption). Finally, 3) long-term labor productivity resulting from impaired cognitive development and missed schooling due to malaria in childhood.
Methods
Model Structure
We developed a CGE model that includes the effects of malaria on demography and labor productivity. The core CGE model considers a range of economic agents in Ghana, including the government, households, firms, and the rest of the world. The economic behavior and interactions of each agent were modelled using standard preference functional forms, based on established microeconomic theory and computational methods. The model was numerically simulated using the computer program GAMS and its MPSGE solver.30,31 GAMS is one of the most commonly used software environment for applied CGE modelling. (See www.gams.com for further information.)
The economy was assumed a small open economy. Firms select the combination of labor, goods, and service inputs required to produce their output of goods and/or services and maximize their profits. Households maximize their utility by offering their labor to firms, consuming goods and services, and saving from their income. The model finds the equilibrium at which prices of all goods and services are such that the quantity supplied equals the quantity demanded across all sectors. (We provide the full model description in the online supplementary appendix of this paper, including the GAMS/MPSGE code.)
The model’s equations are calibrated to the 2007 social accounting matrix (SAM) of Ghana in Breisinger et al.32,33 This is a table expressed in terms of incomes and expenditures (i.e., a double entry accounting method), which is now a standard approach to calibrate functional form to real-life data.34 The SAM is subsequently updated to 2015 US dollars by chaining the consumer price index and exchange rate.
The SAM provides measures for three labor skill-types (self-employed, skilled, and unskilled), capital, and land, and 90 types of household characterized by administrative district, rural or urban location, and income level. We link the administrative districts to five ecological zones with differences in malaria incidence. Furthermore, we assume that the government provided a fixed level of goods and services to the population based on tax revenues.
The model projects 30 years forwards, a period selected because it is long enough to capture effects on the adult labor force of improved health in childhood.
Demographic Model
Population demographics over the model time horizon were modelled using the existing DemProj demographic model from the Spectrum Policy Modeling System of the Health Policy Project, which projects population size and composition based on fertility, mortality, and migration.35 The model was adapted to account for malaria-specific mortality and regional variations in Ghana. It was assumed that any changes in demographic parameters such as migration and fertility rates were not affected by any interventions to prevent malaria.
Impact of Malaria
The effect of malaria was taken into account in the model in three ways. First, the demographic model included the specific effect of malaria on child mortality, estimated by combining projected clinical malaria episodes with a case-fatality rate. Second, for each episode of malaria occurring in a child under the age of 5, the model estimated the immediate productivity loss resulting from adult caregivers losing time from work. Third, for children exposed to several episodes of malaria during childhood, the model considered long-term reductions in their productivity as adults resulting from missed schooling, greater susceptibility to other health problems, and cognitive impairment. Children were assumed to enter the labor force at the age of 15 years.
Note that episodes of malaria occurring in adults cause productivity losses due to absence from work or loss in productivity while at work. However, since we assume that the vaccine is provided only to children, its effect cancels out between scenarios.
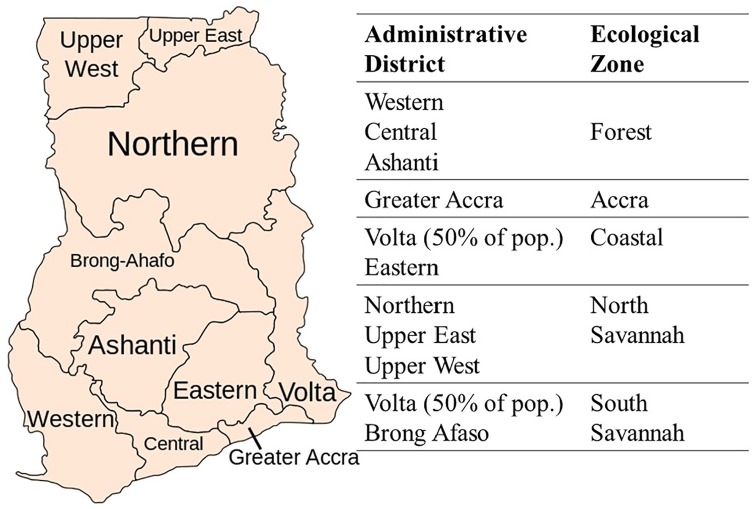
Malaria episodes were based on regional malaria epidemiology corresponding to five ecological zones with differences in malaria incidence. These are presented in Figure 1. The occurrence of malaria episodes ranged from 0 to a maximum of 9, and was modelled using a Poisson distribution, with a distribution mean equal to the mean baseline number of clinical malaria episodes in each zone (Table 1).
Figure 1.
Mapping administrative districts onto malaria ecological zones.
Note: The figure shows the link between administrative regions and malaria ecological zones.
Table 1.
Key Input Data Used in the Model
| Parameter | Value | Source |
|---|---|---|
| Total number of working days per year | 235 | Authors’ assumption |
| Range of malaria episodes per person-year | 0–9 | Authors’ assumption |
| Event distribution | Poisson | 37 |
| Baseline mean number of malaria episodes per year | Age year 0–4, 5–64 | |
| Nationala | 1.0, 0.5 | 38 |
| Regionalb | 39 | |
| Accra | 0.53, 0.27 | |
| Coast | 0.86, 0.43 | |
| Forest | 1.62, 0.81 | |
| North Savannah | 1.29, 0.65 | |
| South Savannah | 0.69, 0.35 | |
| Adult productivity loss for child’s episode of malaria | ||
| Days absent from work to provide care | 2 | 40–47 |
| Productivity loss when absent | 100% | Authors’ assumption |
| Long-term productivity loss in adulthood resulting from malaria in early childhood | ||
| Days at work but with compromised skills | 235 | Authors’ assumption |
| Productivity loss with ≤1 episode of childhood malaria | 0% | Authors’ assumption using48–50 |
| Productivity loss with 2 episodes of childhood malaria | 10% | Authors’ assumption using48–50 |
| Productivity loss with ≥3 episodes of childhood malaria | 25% | Authors’ assumption using48–50 |
Asante et al. (2011)38 find 1.3 primary-case-definition episodes per person-years in the first 18 months of life in Ghana. As this study applies episodes for 0 to 4 years of life, and children aged 18 months to 4 years of age have relatively fewer episodes of malaria, we assume 1.0 episode per person-year from 0 to 4 years. Without academic evidence on adult episodes of malaria across regions of Ghana, authors assume 50% fewer episodes for adults than children.
Episodes of malaria occurring in children resulted in productivity losses when an adult had to take time away from work to care for the sick child (Table 1). Furthermore, individuals experiencing multiple episodes of malaria as children were assumed to experience long-term productivity losses throughout their working lives as adults due to lower skills or compromised health. In people with two episodes of childhood malaria, this was modelled as a 10% reduction in productivity, and in those with three or more episodes as a 25% reduction in productivity, based on published literature (Table 1).
Impact of Malaria Interventions
The projected impact of a malaria vaccination program was evaluated by running a baseline model simulation with no vaccination program (i.e., with existing malaria interventions only). This was then compared with intervention scenarios introducing a malaria vaccination program that would have a protective effect for children under the age of 5, therefore assuming that five birth cohorts have been immunized. We assumed a range of coverage levels, starting at 20% coverage and increasing to 100% coverage in increments of 20 percentage points. The vaccine efficacy in the model was assumed to be 20% against mortality and 50% efficacy against clinical malaria episodes. The model did not account for herd effects, or any impact on the effectiveness of other interventions.
The effect of vaccination on mortality and demographics was estimated using the Lives Saved tool (LiST) from the Spectrum Policy Modeling System of the Health Policy Project.35 This tool estimates the impact of each level of vaccination coverage on childhood mortality rates, and the DemProj tool simultaneously uses this information to generate the resulting demographic population projections. The effect of malaria vaccination would be expected to reduce childhood mortality, thereby increasing the number of surviving children. Fertility rates are projected to decline in the demographic component of the model. We did not assume additional fertility reduction that would be indirectly caused by the reduction of malaria mortality in children with the vaccine.
The effect of vaccination on productivity losses due to malaria episodes was estimated by reducing the number of baseline clinical malaria episodes by 50% (vaccine efficacy) in the proportion of children under the age of 5 covered in each vaccination scenario. The model assumed no changes in the number of malaria episodes in adults, since the modelled vaccination program targeted only children under the age of 5. Therefore, the immediate change in labor productivity due to malaria episodes would reflect only the change in the amount of time lost by adult caregivers.
The effect of vaccination on long-term productivity losses was modelled as a reduction in the proportion of children experiencing two or more malaria episodes, resulting in a lower proportion of young adults entering the workforce with impaired skills due to childhood malaria.
It was assumed that the malaria vaccine costs would be funded by international programs such as the Global Alliance for Vaccines and Immunization (GAVI), which would be consistent with the malaria vaccine pilot implementation.36
The primary model output was gross domestic product (GDP) per capita over time for the different vaccine coverage levels modelled, expressed in 2015 US dollars (US$). The model can also calculate household revenues across different socioeconomic groups and for urban versus rural households.
Sensitivity Analysis
In addition to the range of vaccination coverages, the impact of additional input parameters has been tested in a univariate sensitivity analysis. Productivity loss parameters and vaccine efficacy have been varied to assess the resulting variation of outcomes. The two most influential parameters are varied simultaneously in a bivariate analysis to assess larger variations.
Results
Table 2 shows the projected impact of increasing levels of malaria vaccine coverage on cumulative Ghanaian GDP over 30 years, relative to the baseline of no vaccination.
Table 2.
Projected Impact of Increasing Malaria Vaccine Coverage on GDP in Ghana Over 30 Years, Relative to Baseline With No Vaccination
| Vaccine Coverage in Children Under the Age of 5 | |||||
|---|---|---|---|---|---|
| 20% | 40% | 60% | 80% | 100% | |
| Cumulative GDP (2015 US$, billions) | 1.38 | 2.77 | 4.15 | 5.54 | 6.93 |
| % of total cumulative GDP | 0.13% | 0.26% | 0.39% | 0.52% | 0.65% |
| Annual mean GDP (2015 US$, millions) | 46.0 | 92.2 | 138.4 | 184.6 | 230.8 |
| Mean annual GDP growth (%) | 0.1% | 0.2% | 0.3% | 0.4% | 0.5% |
| Mean annual GDP per capita growth (%) | 0.05% | 0.10% | 0.15% | 0.20% | 0.25% |
| Mean annual household disposable income (2015 US$, millions) | 45.8 | 91.7 | 137.5 | 183.5 | 229.5 |
GDP, gross domestic product.
At 100% malaria vaccine coverage in children under the age of 5, the projected economic benefit over 30 years in Ghana would amount to an additional US$6.93 billion (in 2015 prices) more than the baseline with no vaccine program. Annual mean GDP would increase by US$46.0 million at 20% vaccine coverage, rising to US$230.8 million at 100% coverage, equivalent to an increase in annual GDP growth of 0.1% and 0.5%, respectively. Mean annual GDP per capita (i.e., allowing for increases in population size resulting from reduced malaria childhood mortality) would grow by 0.05% at 20% vaccine coverage and by 0.25% at 100% vaccine coverage. This economic gain would occur despite the fact that the vaccinated children are not economically active.
Figure 2 shows the projected evolution in GDP per capita over time. In the first year after beginning the vaccination program, projected GDP per capita would rise immediately compared with the baseline. This reflects the reduction in malaria episodes in children, which allows adults to spend more days at work instead of caring for sick children and thus produces an immediate increase in labor productivity.
Figure 2.
Projected mean annual percentage change in GDP per capita in Ghana over 30 years with increasing levels of malaria vaccination coverage, relative to baseline with no vaccination.
Over the subsequent years, up to Year 11, projected GDP per capita falls, as more children survive but they are not yet old enough to enter the labor force; so the dependency ratio increases. At Year 11, the first cohort of vaccinated children (those vaccinated at the age of 4 [i.e., just under the age of 5] in the first year of the vaccination program) move into the labor force. These vaccinated children would have experienced fewer malaria episodes in childhood, and consequently have improved labor productivity resulting from fewer missed schooldays and less malaria-related cognitive impairment. The projected GDP per capita thus begins to increase after Year 11, and progressively increases over the remaining timeframe of the model. The improved GDP per capita in this later phase of the model, after the children who benefited from vaccination enter the workforce, outweighs the temporary decrease in GDP per capita in the earlier phase when these children were still dependent. Therefore, the cumulative effect over the 30-year time horizon would be a net gain in GDP per capita (Table 2). The overall effect of malaria vaccination of children under the age of 5 behaves as an initial investment providing long-term economic benefits.
We furthermore test the model by varying key parameters, for the 100% coverage scenario. Results are summarized in Figure 3. As expected, the model is stable around the midpoint estimates, and varying parameter values raise (lower) the vaccine’s economic benefit.
Figure 3.
Tornado diagram: change in cumulative GDP (billion 2015 US$) with full coverage.
The two parameters that have the largest impact on our results are 1) days absent from work to provide care for children and 2) productivity loss with >3 episodes of childhood malaria (from Table 1). To provide the highest (lowest) boundary for the benefit of the malaria vaccine, we run the model with these parameters. The cumulative GDP for a 30-year time horizon is between US$5.90 and US$7.96 billion with the midpoint value US$6.93 (see results in Figure 3 and Table 2).
Our result and the sensitivity analysis show the importance of the labor efficiency to the health intervention. We are more comprehensive with how malaria episodes affect labor efficiency by including impacts on childhood human capital development, children’s health on adults’ work absenteeism, and adult absenteeism due to malaria illnesses. Demographic projections are based on fertility, mortality, and migration; however, only the direct effect of malaria reduction on mortality is included in our model because we do not have good evidence on the fertility and migration responses to malaria, which could have reversed these trends. Future avenues of research could introduce these elements as well.
Discussion
To our knowledge, this modeling study represents the first attempt to explore the potential macroeconomic impact of malaria vaccination in children in sub-Saharan Africa. Our approach uses the established economic technique of CGE modeling, combined with an explicit component to model the impact of malaria on the economy via the mechanisms of effects on childhood mortality and labor productivity.
Taking Ghana as an example, our results indicate that a vaccination program in children under the age of 5 using a vaccine with 50% efficacy against malaria episodes and 20% efficacy against malaria mortality would raise projected annual GDP growth by 0.1% to 0.5%, depending on the level of vaccine coverage. This projected economic benefit is remarkable given that our model only considered the impact of vaccination on malaria in children. It reflects the value of investing in improved childhood health to obtain long-term economic benefits resulting from higher productivity when these healthier children enter the workforce as adults.
Our results are likely to be conservative, because the model focuses on improved labor productivity resulting from improved childhood health, and does not include several other factors that could be affected by malaria vaccination. First, a reduction in childhood mortality could lead to a reduction in total fertility in the long-term (sometimes referred to as a “demographic dividend” or “demographic transition”), and our model does not take this into account as a specific effect of malaria vaccination. Second, the model does not include any potential increases in trade, tourism, or foreign investment that could result from a reduction in malaria. Third, fewer childhood malaria episodes would mean that households have to spend less on out-of-pocket expenses such as treatment and transport to a clinic. They would then be free to divert more of their income to consumption of other goods and services, which would tend to increase demand in the economy and further increase GDP. This is not considered in the model, which will therefore tend to underestimate vaccine benefit.
A previous study estimated the direct economic cost of malaria in children under the age of 5 in Ghana, Tanzania, and Kenya using a probabilistic accounting model.17 This study estimated a net income benefit of only US$27.8 million (in 2015 prices) per year for Ghana, which is much lower than our estimate of a net benefit of US$229.5 million per year in household disposable income with 100% vaccine coverage. This difference illustrates the potential importance of CGE modelling that combines both direct and indirect effects, which were absent from the previous study that only considered direct costs.
The results show the variation in the timing of different effects (see Figure 2). The vaccine intervention provides an immediate increase in GDP per capita resulting from fewer days lost to care for sick children, which thereafter declines in the medium-run as the number of dependent children in the population increases because of reduced childhood mortality. Finally, in the long term, there is a sustained increase in GDP per capita as cohorts of vaccinated children enter the workforce with improved labor productivity resulting from better health in childhood.
Only RTS,S was assessed in the current model to reduce malaria in order to capture its specific effect. However, several other preventive malaria interventions, mainly bednet distribution, have been implemented in Ghana and these were assumed to be maintained at their current coverage for all baseline and counterfactual scenarios. The overall impact of intensifying preventative interventions could bring an extra economic growth when reducing malaria further. However if RTS,S is introduced when malaria has already been reduced, the potential extra gain with RTS,S will also be reduced.
The model currently assumes that malaria vaccination would be costless to the Ghanaian economy, with the costs of the vaccine funded by programs such as GAVI. This is a simplified assumption, and is consistent with the funding of the pilot vaccination program. However, in the longer term, Ghana may be self-financing for vaccines. Future research could extend the model to introduce vaccine costs and sources of funding, which could explore issues such as the long-term financial sustainability and the return on investment of potential vaccine programs. In addition, future research could further explore the impact of malaria vaccination on households at different socioeconomic levels, which could provide valuable information on the equity of malaria prevention programs.
Conclusion
We have adapted a CGE model by adding a health component to simulate the impact of malaria and malaria vaccination on economic growth via effects on demography and labor productivity. Using Ghana as an example, our results indicate that vaccination of children under the age of 5 against malaria with 100% coverage could increase GDP by an average of 0.5% per year over a 30-year period. Investing in improving childhood health by vaccinating against malaria could result in substantial long-term macroeconomic benefits when these children enter the workforce as adults. These macroeconomic benefits are not captured by conventional cost-effectiveness analyses, which may therefore underestimate the economic benefits of vaccination.
Supplemental Material
Supplemental material, 20191118_MDMPP_Malaria_Tech_APPENDIX_FINAL.rjf_online_supp for Exploring the Use of a General Equilibrium Method to Assess the Value of a Malaria Vaccine: An Application to Ghana by Erez Yerushalmi, Priscillia Hunt, Stijn Hoorens, Christophe Sauboin and Richard Smith in MDM Policy & Practice
Supplemental Material
Supplemental material, Figures_EXCEL for Exploring the Use of a General Equilibrium Method to Assess the Value of a Malaria Vaccine: An Application to Ghana by Erez Yerushalmi, Priscillia Hunt, Stijn Hoorens, Christophe Sauboin and Richard Smith in MDM Policy & Practice
Supplemental Material
Supplemental material, Focus_On_Patient_REVISED.rjf_online_supp for Exploring the Use of a General Equilibrium Method to Assess the Value of a Malaria Vaccine: An Application to Ghana by Erez Yerushalmi, Priscillia Hunt, Stijn Hoorens, Christophe Sauboin and Richard Smith in MDM Policy & Practice
Acknowledgments
The authors are grateful to James Thurlow, Xinshen Diao, and their coauthors from the International Food Policy Research Institute (IFPRI) for providing access to the Ghana Social Accounting Matrix. The authors also thank Christopher Adam, Patricia Akweongo, Jonathan Cave, Simo Goshev, Clara Helene Rübner Jørgensen, Carlo Perroni, Jeffrey Round, Lei Zhang, Sani Ziv, and RAND Health researchers for helpful discussions, advice, and suggestions that improved earlier versions of this study. The authors thank Benoit Guerin, Eanna Kelly, Jirka Taylor, and Shelly Culbertson (RAND Europe) for research assistance and editorial support. The authors would also like to thank Carole Nadin (Fleetwith Ltd, on behalf of GSK) for medical writing assistance and Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Fabien Debailleul coordinated manuscript development and editorial support.
This study benefited from comments and suggestions from discussants and participants in the Sustainable Development, Energy & the Environment Meeting on Malaria at Wilton Park, 2012; Poverty Reduction, Education & Growth (PEGNet) Conference in Senegal, 2012; International Society For Pharmacoeconomics and Outcomes Research (ISPOR) Annual European Congress in Berlin, 2012; the Centre for the Study of African Economies Conference (CSAE) in Oxford, 2013; and the University of Warwick economic seminar series.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Erez Yerushalmi, Priscillia Hunt, and Stijn Hoorens report that this work was supported by an unrestricted research grant from the GSK group of companies. Christophe Sauboin is an employee of the GSK group of companies and reports ownership of stock options/restricted shares from the GSK group of companies. Richard Smith reports no conflict of interest.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support for this study was provided by GlaxoSmithKline Biologicals SA (TrackHO Number: HO-13-13516). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Author Contributions: All authors comply with the ICMJE criteria for authorship. S. Hoorens, P. Hunt, C. Sauboin, R. Smith, E. Yerushalmi were involved in the conception and/or the design of the study. S. Hoorens, P. Hunt, C. Sauboin, R. Smith, E. Yerushalmi participated in the collection or generation of the study data. S. Hoorens, P. Hunt, R. Smith, E. Yerushalmi performed the study. P. Hunt, C. Sauboin, E. Yerushalmi contributed to the analysis tools. S. Hoorens, P. Hunt, C. Sauboin, R. Smith, E. Yerushalmi were involved in the analyses and/or the interpretation of the data. All authors read and approved the present article.
ORCID iDs: Erez Yerushalmi  https://orcid.org/0000-0002-9421-9067
https://orcid.org/0000-0002-9421-9067
Christophe Sauboin  https://orcid.org/0000-0003-0913-039X
https://orcid.org/0000-0003-0913-039X
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at http://journals.sagepub.com/home/mpp.
Contributor Information
Erez Yerushalmi, Birmingham City Business School, Birmingham City University, Birmingham, UK; Institute for Employment Research (IER), University of Warwick, Coventry, UK.
Priscillia Hunt, RAND Corporation, Santa Monica, CA, USA; Institute for the Study of Labor (IZA), Bonn, Germany.
Stijn Hoorens, RAND Europe, Brussels, Belgium.
Christophe Sauboin, Department of Health Economics, GSK, Wavre, Belgium.
Richard Smith, College of Medicine and Health, University of Exeter, Exeter, UK.
References
- 1. World Health Organization. Malaria vaccine: WHO position paper—January 2016. Wkly Epidemiol Rec. 2016;91(4):33–51. [PubMed] [Google Scholar]
- 2. World Health Organization. World malaria report 2017 [cited January 11, 2018]. Available from: http://who.int/malaria/publications/world-malaria-report-2017/report/en/
- 3. Goodman CA, Coleman PG, Mills AJ. Cost-effectiveness of malaria control in sub-Saharan Africa. Lancet. 1999;354(9176):378–85. [DOI] [PubMed] [Google Scholar]
- 4. White MT, Conteh L, Cibulskis R, Ghani AC. Costs and cost-effectiveness of malaria control interventions—a systematic review. Malar J. 2011;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morel CM, Lauer JA, Evans DB. Cost effectiveness analysis of strategies to combat malaria in developing countries. BMJ. 2005;331(7528):1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lubell Y, Riewpaiboon A, Dondorp AM, et al. Cost-effectiveness of parenteral artesunate for treating children with severe malaria in sub-Saharan Africa. Bull World Health Organ. 2011;89(7):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mori AT, Norheim OF, Robberstad B. Budget impact analysis of using dihydroartemisinin-piperaquine to treat uncomplicated malaria in children in Tanzania. Pharmacoeconomics. 2016;34(3):303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mulligan JA, Mandike R, Palmer N, et al. The costs of changing national policy: lessons from malaria treatment policy guidelines in Tanzania. Trop Med Int Health. 2006;11(4):452–61. [DOI] [PubMed] [Google Scholar]
- 9. Demarteau N, Breuer T, Standaert B. Selecting a mix of prevention strategies against cervical cancer for maximum efficiency with an optimization program. Pharmacoeconomics. 2012;30(4):337–53. [DOI] [PubMed] [Google Scholar]
- 10. Jit M, Hutubessy R. Methodological challenges to economic evaluations of vaccines: is a common approach still possible? Appl Health Econ Health Policy. 2016;14(3):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sauboin C, Van Vlaenderen I, Van Bellinghen L-A, Standaert B. Reducing malaria mortality at the lowest budget: an optimization tool for selecting malaria preventative interventions applied to Ghana. MDM Policy Pract. 2019;4(2):1–11. doi: 10.1177/2381468319861346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sauboin C, Van Bellinghen L-A, Van de, Velde N, Van Vlaenderen I. Economic impact of introducing the RTS,S malaria vaccine: cost-effectiveness and budget impact analysis in 41 countries. MDM Policy Pract. 2019. doi: 10.1177/2381468319873324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Penny MA, Verity R, Bever CA, et al. Lancet. 2016;387(10016):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galactionova K, Tediosi F, Camponovo F, Smith TA, Gething PW, Penny MA. Vaccine. 2017;35(1):53–60. [DOI] [PubMed] [Google Scholar]
- 15. Winskill P, Walker PG, Griffin JT, Ghani AC. BMJ Glob Health. 2017;2(1):e000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winskill P, Walker PG, Cibulskis RE, Ghani AC. Malar J. 2019;18(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sicuri E, Vieta A, Lindner L, Constenla D, Sauboin C. The economic costs of malaria in children in three sub-Saharan countries: Ghana, Tanzania and Kenya. Malar J. 2013;12:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–5. [DOI] [PubMed] [Google Scholar]
- 19. Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64(1–2 Suppl):85–96. [DOI] [PubMed] [Google Scholar]
- 20. Bloom DE, Brenzel L, Cadarette D, Sullivan J. Moving beyond traditional valuation of vaccination: needs and opportunities. Vaccine. 2017;35(Suppl. 1):A29–A35. [DOI] [PubMed] [Google Scholar]
- 21. Standaert B, Rappuoli R. Towards a more comprehensive approach for a total economic assessment of vaccines? 1. The building blocks for a health economic assessment of vaccination. J Mark Access Health Policy. 2017;5(1):1335162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beutels P, Edmunds WJ, Smith RD. Partially wrong? Partial equilibrium and the economic analysis of public health emergencies of international concern. Health Econ. 2008;17(11):1317–22. [DOI] [PubMed] [Google Scholar]
- 23. Smith R. Why a macroeconomic perspective is critical to the prevention of noncommunicable disease. Science. 2012;337(6101):1501–3. [DOI] [PubMed] [Google Scholar]
- 24. Kotsopoulos N, Connolly MP. Is the gap between micro- and macroeconomic assessments in health care well understood? The case of vaccination and potential remedies. J Mark Access Health Policy. 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith RD, Keogh-Brown MR, Barnett T, Tait J. The economy-wide impact of pandemic influenza on the UK: a computable general equilibrium modelling experiment. BMJ. 2009;339:b4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith RD, Yago M, Millar M, Coast J. Assessing the macroeconomic impact of a healthcare problem: the application of computable general equilibrium analysis to antimicrobial resistance. J Health Econ. 2005;24(6):1055–75. [DOI] [PubMed] [Google Scholar]
- 27. Smith RD, Keogh-Brown MR, Barnett T. Estimating the economic impact of pandemic influenza: An application of the computable general equilibrium model to the UK. Soc Sci Med. 2011;73(2):235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith RD, Keogh-Brown MR. Macroeconomic impact of a mild influenza pandemic and associated policies in Thailand, South Africa and Uganda: a computable general equilibrium analysis. Influenza Other Respir Viruses. 2013;7(6):1400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yerushalmi E, Hunt PE, Hoorens S, Sauboin C, Smith RD. The macro-economic impact of reducing malaria: an application of a dynamic general equilibrium modelling to Ghana. Value Health. 2012;15(7):A400. [Google Scholar]
- 30. Rutherford TF. Extension of GAMS for complementarity problems arising in applied economic analysis. J Econ Dyn Control. 1995;19(8):1299–324. [Google Scholar]
- 31. Rutherford TF. Applied general equilibrium modeling with MPSGE as a GAMS subsystem: an overview of the modeling framework and syntax. Comput Econ. 1999;14(1-2):1–46. [Google Scholar]
- 32. Breisinger C, Diao X, Thurlow J. Modeling growth options and structural change to reach middle income country status: the case of Ghana. Econ Modelling. 2009;26(2):514–25. [Google Scholar]
- 33. Breisinger C, Diao X, Thurlow J, Al Hassan RM. Potential impacts of a green revolution in Africa—the case of Ghana. J Int Dev. 2011;23(1):82–102. [Google Scholar]
- 34. Round J. Constructing SAMs for development policy analysis: lessons learned and challenges ahead. Econ Syst Res. 2010;15(2):161–83. [Google Scholar]
- 35. Health Policy Project. Software and models: Spectrum Policy Modeling System [cited December 1, 2012]. Available from: http://www.healthpolicyproject.com/index.cfm?id=software&get=Spectrum
- 36. World Health Organization. WHO welcomes global health funding for malaria vaccine [cited January 8, 2019]. Available from: https://www.who.int/en/news-room/detail/17-11-2016-who-welcomes-global-health-funding-for-malaria-vaccine
- 37. Smith T, Maire N, Dietz K, Killeen GF, Vounatsou P, Molineaux L, et al. Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;75(2 Suppl):11–8. [DOI] [PubMed] [Google Scholar]
- 38. Asante KP, Abdulla S, Agnandji S, Lyimo J, Vekemans J, Soulanoudjingar S, et al. Safety and efficacy of the RTS,S/AS01E candidate malaria vaccine given with expanded-programme-on-immunisation vaccines: 19 month follow-up of a randomised, open-label, phase 2 trial. Lancet Infect Dis. 2011;11(10):741–9. [DOI] [PubMed] [Google Scholar]
- 39. Mapping Malaria Risk in Africa (MARA), 2002, MARALITe Version 3. Available from: https://www.mara-database.org/
- 40. Aikins M. Cost-Effectiveness Analysis of Insecticide Impregnated Mosquito Nets (bed nets) Used as a Malaria Control Measure: A study from The Gambia (Thesis). London: University of London; 1995. [Google Scholar]
- 41. Cropper M, Haile M, Lampieti J, Poulos C, Whittington D. The value of preventing malaria in Tembien, Ethiopia (Policy Research Working Paper Series 2273 No. Policy Research Working Paper 2273). Washington, DC: The World Bank; 2000. [Google Scholar]
- 42. Ettling MB, Shepard DS. Economic cost of malaria in Rwanda. Trop Med Parasitol. 1991;42(3):214–18. [PubMed] [Google Scholar]
- 43. Ettling M, McFarland DA, Schultz LJ, Chitsulo L. Economic impact of malaria in Malawian households. Trop Med Parasitol. 1994;45(1):74–9. [PubMed] [Google Scholar]
- 44. Guiguemde TR, Coulibaly N, Coulibaly SO, Ouedraogo JB, Gbary AR. [An outline of a method for estimating the calculated economic cost of malaria cases: its application to a rural area in Burkina Faso (Western Africa)]. Trop Med Int Health. 1997;2(7):646–53. [DOI] [PubMed] [Google Scholar]
- 45. Leighton C, Foster R. Economic Impacts of Malaria in Keyna and Nigeria (No. Major Applied Research Paper No. 6). Bethesda, MA: Abt Associates Inc; 1993. [Google Scholar]
- 46. Sauerborn R, Ibrango I, Nougtara A, et al. The economic costs of illness for rural households in Burkina Faso. Trop Med Parasitol. 1995;46(1):54–60. [PubMed] [Google Scholar]
- 47. Asante FA, Asenso-Okyere K, Kusi A. The Economic Impact of The Burden of Malaria in Ghana (No. Technical Publication 66). Legon: University of Ghana, Institute of Statistical Social and Economic Research; 2005. [Google Scholar]
- 48. Bleakley H. Disease and Development: Evidence from the American South. J Eur Econ Assoc. 2003;1(2-3):376–86. [Google Scholar]
- 49. Cutler D, Fung W, Kremer M, Singhal M, Vogl T. Early-life malaria exposure and adult outcomes: evidence from malaria eradication in India. Am Econ J Appl Econ. 2010;2:72–94. [Google Scholar]
- 50. Bleakley H. Malaria eradication in the Americas: a retrospective analysis of childhood exposure. Am Econ J Appl Econ. 2010;2(2):1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 20191118_MDMPP_Malaria_Tech_APPENDIX_FINAL.rjf_online_supp for Exploring the Use of a General Equilibrium Method to Assess the Value of a Malaria Vaccine: An Application to Ghana by Erez Yerushalmi, Priscillia Hunt, Stijn Hoorens, Christophe Sauboin and Richard Smith in MDM Policy & Practice
Supplemental material, Figures_EXCEL for Exploring the Use of a General Equilibrium Method to Assess the Value of a Malaria Vaccine: An Application to Ghana by Erez Yerushalmi, Priscillia Hunt, Stijn Hoorens, Christophe Sauboin and Richard Smith in MDM Policy & Practice
Supplemental material, Focus_On_Patient_REVISED.rjf_online_supp for Exploring the Use of a General Equilibrium Method to Assess the Value of a Malaria Vaccine: An Application to Ghana by Erez Yerushalmi, Priscillia Hunt, Stijn Hoorens, Christophe Sauboin and Richard Smith in MDM Policy & Practice