Abstract
For many decades, T helper 2 (TH2) cells have been considered to predominantly regulate the pathogenic manifestations of allergic asthma, such as IgE-mediated sensitization, airway hyperresponsiveness, and eosinophil infiltration. However, recent discoveries have significantly shifted our understanding of asthma from a simple TH2 cell-dependent disease to a heterogeneous disease regulated by multiple T cell subsets, including T follicular helper (TFH) cells. TFH cells, which are a specialized cell population that provides help to B cells, have attracted intensive attention in the past decade because of their crucial role in regulating antibody response in a broad range of diseases. In particular, TFH cells are essential for IgE antibody class-switching. In this review, we summarize the recent progress regarding the role of TFH cells and their signature cytokine interleukin (IL)-21 in asthma from mouse studies and clinical reports. We further discuss future therapeutic strategies to treat asthma by targeting TFH cells and IL-21.
Keywords: T follicular helper (TFH) cell, interleukin-21 (IL-21), T follicular regulatory (TFR) cell, asthma, immunotherapy
Introduction
Asthma, one of the most common chronic and non-infectious diseases, affects around 334 million people worldwide (1). Although the mortality rate associated with asthma has declined remarkably with the regular use of inhaled corticosteroids or oral systemic corticosteroids (2, 3), the overall effectiveness of this therapeutic approach has remained debatable, since 5–25% of asthmatic patients are refractory and show resistance to current corticosteroid-based treatments (4). Concurrently, side effects such as poor immune response to infection and increased risk of osteoporosis are associated with long-term corticosteroid treatment in patients with asthma (5, 6). Therefore, novel treatments that can replace the current steroid-based therapies in a larger cohort of asthma patients and reduce the risk of side effects are urgently needed to not only improve patient outcomes but also reduce the economic burden associated with the management of severe asthma.
Because of their myriad effects on inflammatory responses in the respiratory tract, CD4+ T cells have been identified as potent regulators of asthma pathogenesis (7). In this regard, T helper 2 (TH2) cells have gradually gained recognition in studies on asthma biology (8, 9). Interleukin (IL)-4, IL-5, and IL-13, which are canonical type 2 cytokines produced by TH2 cells, prominently mediate the development of asthma and airway inflammation, manifesting as enhanced IgE-mediated sensitization, airway hyperreactivity (AHR), as well as eosinophil infiltration (1, 10). However, emerging evidence suggests that T follicular helper (TFH) cells, rather than TH2 cells, predominantly produce IL-4 and IL-21 in B cell follicles and closely regulate IgE class-switching during severe asthma development in both mice and humans (11–15). Therefore, a thorough understanding of TFH cells and their signature cytokine IL-21 is important to fully elucidate the pathogenesis of asthma. In this review, we have summarized recent discoveries related to the role of TFH cells and IL-21 in mouse models and patients with asthma. In addition, we have discussed the therapeutic strategies for asthma that are based on modulation of TFH cells and IL-21, which may potentially be translated into clinical use in the near future.
Biology of TFH Cells
Generation and Development of TFH Cells
T cell and B cell interactions, particularly the help provided by T cells to B cells, have been demonstrated for decades (16–19). However, the cellular processes underlying this “help” provided to B cell follicles were not fully understood until a specialized CXCR5-expressing CD4+ T cell population, which is uniquely regulated by Bcl-6, was identified (20–22). These cells, termed as TFH cells, can access B cell follicles and regulate the germinal center response (23). Over the past decade, significant progress has been achieved in studying TFH cells. These “help”-providing T follicular cells have been revealed to markedly express inducible co-stimulator molecules (ICOS), programmed cell death 1 (PD-1), and CD40-ligand (CD40L), which are essential for interacting with B cells (24). Moreover, TFH cells produce high amounts of the cytokine IL-21 in the B cell follicles (25, 26).
These molecules are not only determinative of the commitment of TFH cells but are also pivotal for the migration and full functionality of these cells in follicles. After activation by dendritic cells in T cell zone, primed T cells become precursor TFH (pre-TFH) cells and downregulate CCR7 and PSGL1 while upregulate CXCR5 for their migration into B cell follicles, where CXCL13, the ligand for CXCR5 is plentifully accumulated (24, 27) (Figure 1). Additionally, EBI2 and PD-1 are critical for the positioning of pre-TFH cells near the T-B border (28, 29). With sustained ICOS stimulation by B cells as well as downregulation of the adhesion molecules EBI2 and S1PR1, TFH cells are allowed to further develop into B cell follicles and are retained in the germinal centers to become germinal center TFH (GC-TFH) cells (23, 30, 31) (Figure 1). Bcl-6 is essential for this complex cellular process, since it promotes CXCR5 expression while repressing the expression of the transcription factors T-bet, GATA-3, and RORγt, molecules that are essential for the induction of other subsets of effector CD4+ T cells such as TH1, TH2, and TH17 cells (32, 33). Moreover, Bcl-6 inhibits CCR6, PSGL1, CCR7, and S1PR1, the cell surface molecules that regulate non-follicular localization of effector CD4+ T cells (23). Antagonistically, the transcriptional repressor B lymphocyte-induced maturation protein 1 (Blimp-1) negatively acts on Bcl-6 to inhibit TFH development. Transcription factors such as c-Maf, FOXO1, Id3, TCF-1, IRF4, and ASCL2 are also known to play important roles in fine-tuning the sophisticated cellular regulatory network during TFH development and function (23).
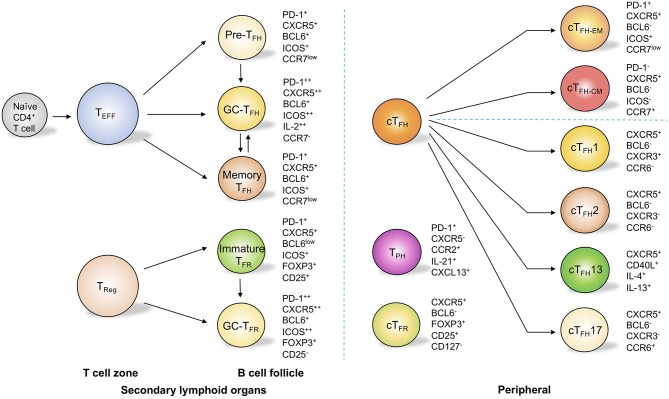
Figure 1.
TFH cell and related subsets in secondary lymph organs and in peripheral circulation. In secondary lymphoid organs, T follicular helper (TFH) cells are composed of subsets with distinct phenotypes. These subsets include pre-TFH cells, germinal center (GC) TFH cells, and memory TFH cells. Through the upregulation of programmed cell death protein 1 (PD-1) and CXC-chemokine receptor 5 (CXCR5), regulatory T (Treg) cells migrate into B cell follicles and become immature T follicular regulatory (TFR) cells and germinal center TFR cells. In the peripheral circulation, circulating TFH (cTFH) cells can be categorized into effector memory (cTFH−EM) cells and central memory (cTFH−CM) cells on the basis of the expression of PD-1 and CC-chemokine receptor 7 (CCR7). cTFH cells can also be sub-grouped into cTFH1, cTFH2, cTFH13, and cTFH17 cells on the basis of the differential expression of CXCR3 and CCR6 as well as the secretion of interleukin-4 (IL-4) and IL-13. Circulating TFR cells are similar to Treg cells but express CXCR5. Notably, T peripheral helper (TPH) cells do not express CXCR5 but can produce IL-21 and CXCL13, which allows them to provide help to B cells.
Cytokine Milieu Regulates TFH Cell Development and Differentiation
Development of TFH cells is also dependent on the cytokine milieu. Mouse studies have revealed that IL-6, IL-12, and IL-27 induce the expression of Bcl-6 and promote TFH lineage differentiation through the activation of the transcription factors STAT3 and/or STAT4 (34–38). In humans, TGF-β together with IL-12 and IL-23 may contribute to the generation of human TFH cells (39). In contrast, the TGF-β signal exerts suppressive effects in regulating the production of IL-21 and expression of ICOS and Bcl-6 in mouse TFH cells (39). Nevertheless, IL-2 is a suppressive molecule that inhibits the generation of both human and mouse TFH cells in a STAT5- and Blimp1-dependent manner (40, 41).
Circulating TFH Cells and Subsets of TFH Cells
Although TFH cells possesses distinctive characteristics in comparison with other subsets of CD4+ T cells, they can produce TH1, TH2, and TH17-type cytokines. Indeed, Reinhardt et al. (42), Zaretsky and Hirota etc. have shown that TFH cells, especially circulating or tissue-resident TFH cells, produce IL-4 or IL-17 to modulate antibody responses (43, 44). Bona fide germinal center TFH cells can also produce IL-4, IFN-γ, or IL-17 to regulate antibody outcomes (42–44).
After the contraction phase of the immune response, a small proportion of CD4+ T cells give rise to memory T cells, which confer long-lasting immunity to the host to defend it against recurrent invasions of pathogens. Indeed, MacLeod et al. (45) have shown that CXCR5+ memory CD4+ T (memory TFH) cells (Figure 1) accelerate the generation of functional TFH cells and promote OVA-specific IgG1 titers in OVA immunization. Moreover, influenza vaccination promotes the levels of circulating TFH cells (cTFH) cells in human blood, and these cTFH cells correlate with a boosting of antigen-specific B cell response (46). These data strongly suggest that memory TFH cells exist in circulating blood and that these cells can foster rapid and high-quality antibody response.
Interestingly, memory TFH cells in circulation are not only able to promote recall response, but are with plasticity to give rise to other functional effector T cells in different contexts (47, 48). It is also noticed in germinal center that GC-TFH cells switch to produce IL-4 from IL-21 as the germinal center reaction evolved (49). These evidences suggest that TFH cells are not terminally differentiated cells and maintain flexibility to convert into other functional CD4+ T cell subsets.
On the basis of the differential expressions of the chemokine receptors CXCR3 and CCR6, peripheral circulating TFH (cTFH) cells can be divided into three major subsets: cTFH1 cells (BCL6−CXCR3+CCR6−), cTFH2 cells (BCL6−CXCR3−CCR6−), and cTFH17 (BCL6−CXCR3−CCR6+) cells (50) (Figure 1). These subsets are transcriptionally different and produce distinct cytokines to regulate humoral response (50). Of note, cTFH2 and cTFH17 cells, but not the cTFH1 population, are characterized as efficient helper TFH cells to promote the class-switching of immunoglobulin (50). cTFH2 cells promote IgG and IgE secretion, whereas blood cTFH17 cells induce IgG and IgA secretion (50). Interestingly, a group of peripheral T cells defined as T peripheral helper cells (TPH) do not express CXCR5 but can produce IL-21 and CXCL13 (Figure 1), which allows them to provide help to B cells (51, 52). Meanwhile, a group of CD4+ T cells expressing CXCR3 and PD-1 but not CXCR5 have been found in both blood and tubulointerstitial areas in lupus patients (53). These cells provide the help to B cells through the production of IL-10 and succinate instead of IL-21 (53). It is with interest to know in the future how these non-classic “B cell help” CD4+ T cells correlate with each other and with classic TFH cells. Notably, classic human circulating TFH cells can also be categorized into distinct effector stages by evaluating the expression levels of ICOS, PD-1, and CCR7 (54, 55). On the basis of this strategy, activated-stage (effector memory) cTFH (cTFH−EM) cells are defined as PD-1+CXCR5+BCL6−ICOS+CCR7low cells, which are similar to pre-TFH cells, while PD-1−CXCR5+BCL6−ICOS−CCR7+ cells are characterized as central memory cTFH cells (cTFH−CM) and can persist for weeks after antigen stimulation (54, 55) (Figure 1). Interestingly, within blood cTFH1 cells, the helper ability is restricted mostly to the activated ICOS+PD-1+CCR7low subset, while within cTFH2 and cTFH17 cells, both activated and central memory subsets are capable of providing help signals to the B cells (56, 57). In fact, the activated ICOS+PD-1+CCR7low subset represents the most efficient helper cells among cTFH cells (56, 57). Beyond this classification, a study using a murine model with dedicator of cytokinesis 8 (Dock8) deficiency revealed a subset of IL-13-producing TFH cells associated with high-affinity IgE production (58) (Figure 1). These “TFH13” cells, which are present in both mice and humans, have a unique cytokine profile (IL-13+IL-4+) and co-express Bcl-6 and GATA-3 (58). These cells were further demonstrated to be responsible for the production of high-affinity anaphylactic IgE but not low-affinity IgE (58).
Role of TFH Cells in Asthma Pathogenesis
Since TFH cells are indispensable for antibody maturation, investigators have studied the role of these cells in many disease contexts, including asthma (23). Emerging evidence from both mouse and human studies has elucidated that subsets of TFH cells differentially contribute to the development of asthma (Table 1). These observations have broadened our understanding of asthma and provided novel options to treat asthma by targeting TFH cells from different angles.
Table 1.
T follicular cells in mouse/human asthma and related allergic diseases.
| Species | Model/Patients | Location | Dysfunction | Effect | References |
|---|---|---|---|---|---|
| Mouse | Peanut | mLN | TFH1↑ | TFH cells promote peanut-specific IgE production. | (59) |
| HDM | mLN | TFH2↑ | TFH cells are precursors of HDM-specific TH2 cells. | (11) | |
| HDM | mLN | TFH2↑ | TFH cells amplify TH2 cell function in allergic airway inflammation; TFH cells support the sustained production of IgE antibody in vivo. | (12–14) | |
| HDM | Lung | CD4+IL-21+↑ | Promotes local inflammation in the airway | (12, 60, 61) | |
| HDM and Peanut | mLN | TFH13↑ | TFH cells are required for production of high-affinity, but not low-affinity, IgE and subsequent allergen-induced anaphylaxis. | (58) | |
| HDM | mLN | TFH13↑, TFR ↓ | TFR cells can limit TFH13 cell-promoted IgE production. | (62) | |
| Transplantation (not clear in Asthma) | mLN, Spleen | TFH17↑, TFR ↓ | IL-10-producing marginal zone precursor B cells control the differentiation of TFH cells and are necessary for immune tolerance. | (63) | |
| OVA Immunization | mLN, Spleen | TFR ↓ | Deficiency of TFR cells leads to excessive humoral immune responses. | (64, 65) | |
| Human | Juvenile Dermatomyositis (not clear in Asthma) | Blood | cTFH1↓, cTFH2↑, cTFH17↑ | cTFH2 and cTFH17 cells, but not cTFH1 population, are characterized as efficient helper TFH cells to promote the class-switching of immunoglobulins. | (50) |
| Allergic Asthma | Blood | cTFH2↑ | TFH cells positively correlate with the total IgE level. | (66–68) | |
| Peanut-Allergen | Blood | cTFH13↑ | / | (58) | |
| HDM-Allergen | Blood | cTFH↑, cTFR ↓ | AIT efficiently modulates the balance of circulating TFH and TFR. | (69) | |
| Allergic Rhinitis | Blood | cTFR ↓ | AIT efficiently reinvigorates TFR cells to control IgE production. | (70) | |
| Asthma | Blood | cTFR ↓ | TFR cells produce high amounts of IL-10, which may inhibit the generation of pathogenic TFH cells. | (71–73) | |
| Rheumatoid Arthritis (not clear in Asthma) | Blood | TPH↑ | TPH cells promote B cell responses and antibody production. | (51) | |
| Lupus (not clear in Asthma) | Blood | TPH↑ | TPH cells stimulate B cell responses via IL-21. | (52) |
HDM, house dust mite; mLN, mediastinal lymph node; AIT, allergen-specific immunotherapy.
TFH Cells in Murine Asthma Models
Like in the case of other immune diseases, animal models serve as a feasible approach to investigate the pathogenesis of asthma. To fully understand how TFH cells participate in asthma development, multiple allergens such as house dust mite (HDM), ovalbumin (OVA), molds, and cockroach antigens have been utilized to induce asthma symptoms in mice (74).
Using an HDM-induced asthma mouse model, Ballesteros-Tato et al. (11) showed that the initial intranasal sensitization with HDM directly induces IL-4-producing TFH cells, and these cells then become IL-4+IL-13+ TH2 cells after the HDM challenge. Interestingly, depletion of TFH cells after HDM sensitization successfully prevents TH2 cell-mediated immunity after secondary exposure (11). These results are supported by recent studies showing that TFH cells can further differentiate into functional subsets to regulate antibody response (11, 47, 75, 76). Meanwhile, studies have also shown that the airborne allergen HDM independently induces TH2 or TFH cells to regulate eosinophilic airway inflammation and IgE production, which raises more questions related to the clear definition of the different roles of TH2 and TFH cells in HDM-induced asthma (12, 13). More importantly, these studies have revealed a rare but important IL-21 producing CD4+ T cells that are highly pathogenic and can synergize airway inflammation in the lung tissue (12, 60). These cells are different from classical TFH cells as they lack expression of Bcl-6 and CXCR5 and don't require ICOS signaling (12, 60, 61). In another peanut-induced asthma mouse model, TFH cells robustly promoted peanut-specific IgE production (59). In this model, depletion of TFH cells decreased IgE production and protected mice from anaphylaxis without affecting TH2 cells (59). Thus, TFH cells are necessary and sufficient for the B cell class-switching and sustained IgE production in the absence of TH2 cells (13, 59). In line with this result, mice with T cell specific IL-6R deficiency exhibit limited TFH expansion after HDM sensitization and significantly impaired IgE response (14). Moreover, a rare population defined as IL-13 producing TFH (TFH13) cells is reported to be essential for the production of high-affinity IgE antibody and the subsequent allergen-induced anaphylaxis (58). Eliminating TFH13 cells or TFH cell-derived IL-13 during allergen immunization results in the abrogation of high-affinity anaphylactic IgE (58).
TFH Cells in Human Asthma
In human studies, our group and other groups have found significantly higher circulating TFH cell (CXCR5+CD4+) levels in both child and adult asthma patients in comparison with healthy cohorts (66, 67). Additionally, a skewed peripheral TFH cell phenotype toward the TFH2 phenotype has been identified in asthma patients, where the frequency of TFH2 cells positively correlated with total IgE levels in the blood (66). We have further observed that circulating TFH cells enhance IgE production, which can be reduced by blocking IL-4 or IL-21 (77). Moreover, the levels of IL-4+IL-21+CXCR5+CD4+ T cells have been shown to positively correlate with the total IgE level in vivo (77). These results indicate that circulating CXCR5+CD4+ TFH cells support the germinal center production of IgE in asthma patients. Interestingly, studies using microRNA have revealed that miR-192 is a promising therapeutic target in asthma patients as it inhibits TFH cell differentiation (67, 78). Of note, allergen-specific immunotherapy (AIT), which leads to improved prognosis in allergic patients, efficiently reduces circulating TFH cell levels (68, 69). AIT treatment also markedly increases the frequency of T follicular regulatory (TFR) cells, which are known to suppress the germinal center reaction (69, 70).
Biology of IL-21
IL-21 and IL-21R were discovered in 2000 (79, 80). As a pleiotropic type I four-α-helical bundle cytokine, IL-21 is predominantly produced by NKT cells and activated CD4+ T cells such as TH9 cells, TH17 cells, and TFH cells (81, 82). IL-21 exerts its biological function via binding to its heterodimeric receptor. This receptor is composed of the common γ-chain subunit shared with IL-2 family cytokines, including IL-4, IL-7, IL-9, and IL-15, and its own unique receptor (designated IL-21R), a member of the class I cytokine receptor family (83). Although the production of IL-21 is restricted to lymphocytes, IL-21R is universally expressed on a large range of immune and non-immune cells, indicating its broad physiological effects (79, 80). Recent advances have revealed that IL-21 promotes the activation and cytotoxic function of NK and NKT cells (84, 85). IL-21 also enhances the anti-viral and tumor function of CD8+ T cells (82, 86, 87). In particular, IL-21 regulates the formation and function of CD4+ T cell subsets, including the promotion of IL-17-producing T cells (TH17) (88, 89), efficient development of TFH cells (90), and limitation of TFR cells (64). IL-21 is essential for B cell differentiation and activation. In this context, IL-21 induces B cell proliferation and differentiation to either memory B cells or terminally differentiated plasma cells egressing from the germinal center (82, 91). In addition, IL-21 plays fundamental roles in regulating Ig class-switching and maintaining germinal center reaction (82, 92, 93).
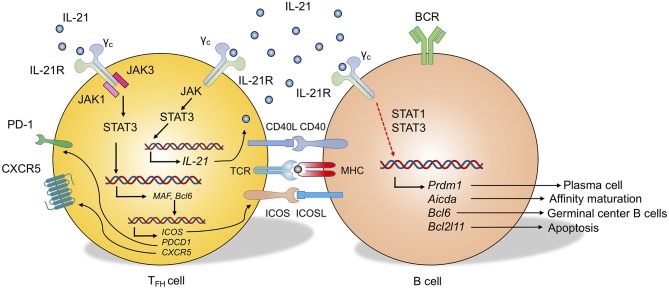
As a potent cellular modulator, IL-21 binds to the IL-21R and stabilizes the IL-21R-γc (common cytokine receptor γ chain) complex, which leads to the activation of downstream signaling cascades (94). Which signaling pathways are particularly important to regulate the formation, function, and fate of T and B cells? Janus kinase 1 (JAK1) and JAK3 have been shown to be largely activated by the IL-21R-γc complex. This activation leads to strong phosphorylation of signal transducer and activator of transcription protein 3 (STAT3), which will further dimerize and translocate into the nucleus for target genes (94). In T cells, activated STAT3 signaling results in increased expression of retinoic acid receptor-related orphan receptor-γt (RORC) and enhanced production of IL-17 and IL-21 (88, 89, 95, 96). This IL-21-STAT3 axis can also directly promote IL-6 mediated Bcl-6 expression, which induces the upregulation of CXCR5, ICOS, and PD-1 during TFH cell development (23, 25, 97) (Figure 2). Although future studies are required to determine whether IL-21 is superior to other STAT3 inducing cytokines such as IL-6 on the regulation of TFH cells in vivo (98), IL-21 is at least partially required for the potentiation of TFH-like cells in vitro (90, 99). Additionally, IL-21 also regulates the target genes in T cells through BATF, JUN, and IRF4 (100). In B cells, IL-21 maintains Bcl-6 expression in germinal center B cells (101, 102) while it increases the expression of Blimp1 (Prdm1), which promotes plasma cell differentiation (91). IL-21 also regulates the apoptosis of B cells through the modulation of BIM (Bcl-2 interacting mediator of cell death) (103, 104) (Figure 2).
Figure 2.
Role of IL-21 in TFH cell and B cell differentiation. IL-21 binds to the IL-21 receptor (IL-21R), which dimerizes with the common cytokine receptor-γ chain (γc) to form the IL-21R complex. In TFH cells, IL-21 signaling activates Janus kinase 1 (JAK1) and JAK3 to induce phosphorylation of signal transducer and activator of transcription 3 (STAT3, also STAT1 and STAT5 to some extent). STAT3 protein translocates into the cell nucleus and regulates the transcription of target genes, including IL-21, Maf, and Bcl6. This regulation leads to the autocrine IL-21 by TFH cells and the transcriptional program that upregulates genes encoding CXCR5, ICOS, and PD-1. In B cells, at least partially through STAT1 and STAT3, IL-21 signaling can regulate the gene expression of Prdm1 (Plasma cell differentiation), Acida (Affinity maturation), Bcl6 (Germinal center B cells), and Bcl2l11 (B cell apoptosis) which leads to the differentiation of B cells to multiple directions.
These IL-21-initiated pivotal signaling pathways can be targeted through agonists or antagonists (inhibitors) to modulate T and B cell development and function, and more importantly, intervene and treat multiple immune related diseases, including asthma.
Role of IL-21 in Asthma Pathogenesis
IL-21 in the Pathogenesis of Murine Asthma
The IL-21 transcript is upregulated in the lung and lung-draining lymph nodes during allergic airway response (12, 105). The protein levels of IL-21 and IL-21R are also increased in the pulmonary tissues of asthmatic rats (106). Additionally, IL-21 level is elevated in the serum and bronchoalveolar lavage fluid (BALF) of asthmatic mice (107, 108).
IL-21 has anti-IgE and anti-inflammatory effects (93, 109–113). Indeed, Il21r-deficient mice exhibit high levels of IgE, and IL-21 inhibits IL-4-induced IgE secretion by B cells (105, 114). In the OVA-induced asthma model, the administration of exogenous IL-21 reduced IgE production and decreased eosinophil recruitment into the airway (109). Consistent with this data, Lin et al. have confirmed in vivo that intranasal administration of IL-21-expressing adenovirus suppresses allergic responses (115). Additionally, in this model, administration of IL-21 not only reduces the frequency of TH2 cells but suppresses the secretion of TH2-associated cytokines such as IL-4, IL-5, and IL-13 (115). In line with this observation, Wu et al. have shown that nasal administration of rmIL-21 significantly reduced the AHR, inflammatory cell infiltration, IgE-producing B cell level, and total serum IgE level (116). As mentioned above, serum total and HDM-specific IgE antibody titers are markedly higher in Il21r-deficient mice (105). However, Il21r-deficient mice develop unexpectedly less AHR in an HDM model of asthma (105). Similar results showing a decline in AHR are also observed in an OVA-induced experimental asthma model using Il21r-deficient mice (114). These findings suggest that IL-21 is importantly involved in the development of asthma. While the mechanisms underlying the dichotomy in the role of IL-21 in regulating IgE and AHR remain poorly understood, they are presumably related to the location and timing of the differential accumulation of IL-21 during disease development.
IL-21 in Human Asthma
The main obstacle in studying the immunopathology of asthma in human subjects is the relative inaccessibility of inflamed tissues. Nonetheless, using bronchial biopsies, IL-21 expression has been shown to be elevated in both moderate and severely asthmatic individuals (105). Additionally, an increased IL-21 level appears to be associated with increased infiltration of inflammatory cells in the submucosa and is correlated with asthma severity (105). In addition, the plasma level of IL-21 is significantly elevated in asthma patients in comparison with healthy controls (117). Consistently, an increased frequency of IL-21-expressing CD4+ T cells is also observed in asthma patients. This increased frequency positively correlates with total IgE levels in the blood (77). Moreover, in vitro experiments have demonstrated that blocking IL-21 in the coculture assay of B cells with CXCR5+CD4+ T cells results in decreased IgE antibody production by B cells (77). Besides, Chatterjee et al. have reported that the exon-3 polymorphism C5250T of the IL-21 gene was significantly associated with atopic asthma and total IgE level (118).
Clinical Implications
The increasing number of studies on TFH cells and IL-21 have inspired numerous possibilities for the development novel immunotherapies to treat asthma. As mentioned above, modulating IL-21 and TFH cell-regulated IgE production may effectively control asthma development and alleviate inflammatory and hyperresponsiveness symptoms in patients.
TFH Cells and Serum IL-21 as Biomarkers in Asthma
Precise and early diagnosis of asthma and related syndromes is critical for the prompt control of disease development in patients. Lung function tests for timely and accurate diagnosis of asthma are not as feasible in children as they are in adults. As evidenced in recent clinical studies, the frequency of cTFH cells and/or the IL-21 level in peripheral blood mononuclear cells (PBMCs) appear to be the promising diagnostic biomarkers for IgE production and asthma symptoms (66, 67, 77). cTFH cells and IL-21 levels can be potentially included in future diagnostic criteria for asthma. Moreover, future portable devices equipped with a method to analyze cTFH cells and IL-21 may allow efficient and precise diagnosis of asthma in those who have a family history of the disease or are highly susceptible to severe asthma due to genetic defects and environmental factors.
Limiting Pathogenic TFH Cells in Asthma
Many approaches can be utilized to target pathogenic TFH cells. For example, Treg cells are known to reinstate immune tolerance and prevent exaggerated immune response through their immune-suppressive function (119). Deficiency of Treg cells in both mice and humans leads to the excessive humoral immune responses characterized by spontaneous germinal center formation and increased frequency of pathogenic TFH cells (65, 120, 121). Indeed, temporary depletion of Treg cells leads to enhanced secondary immune response upon antigen re-challenge (65). This enhanced memory immune response occurs partially through the reduction of CTLA-4-directed inhibition of CD80/CD86 on B cells, which results in an increased frequency of TFH cells (65).
Furthermore, by upregulating CXCR5, a significant proportion of Treg cells migrate into B cell follicles and exert suppressive functions on TFH cells and GC B cells (122, 123). These cells, which are termed as TFR cells, are considered to control autoimmunity and germinal center reaction (124) (Figure 1) as well as autoreactive B cell clones in infection (125). Human clinical studies have shown that allergen immunotherapy reinvigorates the TFR cells in patients with allergic rhinitis, and the addition of human TFR cells in the TFH and B cell coculture system remarkably reduced TFH cell-promoted IgE production (70). It is thus of interest to see how TFR cells respond in asthma patients in future studies. Moreover, specialized human IL-10-producing CD25+Foxp3− TFR cells effectively control IgE production (126). In the most recent study, Clement et al. revealed that TFR cells can limit TFH13 cell-promoted IgE in mouse, and depletion of TFR cells enhances antigen-specific IgE antibody and exacerbates lung inflammation (62). These studies suggest promising paths to inhibit pathogenic TFH cells and IgE production in asthma, and also shed light on the development of novel immunotherapies in asthma patients by promoting Treg/TFR cell-mediated suppression of TFH cells.
Administration of cytokine and/or antibodies has been considered to be an effective method to reinstate the balance of immune response in many types of diseases including asthma (127). IL-2 has been shown to vigorously suppress TFH cells (40). Indeed, clinical studies have proven that low-dose IL-2 treatment in systemic lupus erythematosus (SLE) patients safely and effectively limits autoimmunity partially through direct inhibition of self-reactive TFH cells (41). Besides, other cytokines may also potentiate the repression of pathogenic TFH cells in asthma. For example, IL-7 is reported to repress Bcl-6 and the gene profile of TFH cells in chronic viral infection, which leads to the generation of a memory pool of effector T cells (128). Although lack of CXCR5 and Bcl-6 expression, a specialized IL-21 producing CD4+ T cell population is reported to provide help to B cells and synergize airway inflammation in lung tissue (12, 60). The role of these cells in human asthma is still unknown, nevertheless, it is of great interest to understand these non-classic TFH cells in the future as targeting on their IL-21 production may ameliorate lung inflammation in asthma. Moreover, IL-10 resolves the inflammation in asthma (71–73). Studies have shown that IL-10-producing marginal zone precursor B cells control the differentiation of TFH cells and are necessary for immune tolerance (63). Treg cells and TFR cells produce high amounts of IL-10, which may be the underlying mechanism of the Treg/TFR cell-mediated inhibition of pathogenic TFH cells and allergen-specific IgE antibody production. Type I interferon counteracts with STAT3 to restrain TFH cells (129). Interestingly, type I interferon has been also shown to suppress infection-induced asthma (130, 131). In particular, future studies should aim to determine whether targeting of type I interferons will eliminate pathogenic TFH cells and resolve asthma in patients. Of note, combination therapy with mixed cytokines, cytokine-cells, and cytokine-chemical may provide even better suppression of pathogenic TFH cells. For example, the combination of IL-10 or IL-2 with Treg or TFR cells may synergize the immuno-suppressive function of pathogenic TFH cells and confer improved control of asthma symptoms in patients.
Modulating IL-21 Signaling in Asthma
IL-21 and IL-21R are emerging as promising targets for novel cytokine-based immunotherapies in many diseases, including SLE, primary immunodeficiency (PID), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and lymphoma (132–134). Phase I and phase II clinical trials have tested the efficacy and safety of IL-21 administration in limiting malignant melanoma (135–138). These studies provide evidence for the use of IL-21 as a safe and effective immunotherapeutic agent in a broad range of diseases. Because of IL-21's profound effects in controlling IgE production, supplementation of IL-21 may be useful to rebalance the elevated IgE level in asthma (93). It is possible that IL-21 may have multiple roles in asthma, wherein it may sustain germinal center reaction while limiting Ig class-switching toward IgE. This dichotomy in the effects of IL-21 in asthma may be due to the timing and location at which IL-21 is preferentially accumulated. Nevertheless, it is worthwhile to point out that IL-21 administration in other allergic mouse models, including skin allergy, allergic rhinitis, and anaphylaxis, impressively reduces allergen-specific IgE production (111–113). Again, these points of evidence provide confidence for the development and assessment of IL-21-based immunotherapy in allergic asthma.
On the other hand, amelioration of disease symptoms and improved health were observed after delivery of IL-21 neutralizing antibodies or IL-21R blockade in mice in multiple autoimmune and inflammatory disease models, including SLE (139), arthritis (140), graft-vs.-host disease (GVHD) (141, 142), and Crohn's Disease (143). Although it is still not very clear why IL-21 and IL-21R signaling play different roles in asthma, it will be very exciting to see more studies provide definitive evidence on IL-21's immunomodulating functions in regulating TFH cells, IgE production, and germinal center response in asthma.
Conclusion
In our review of the research using animal models and human patient samples, TFH cell and its signature cytokine IL-21 were evidenced to be largely involved in asthma. In particular, specialized subsets of TFH cells, such as TFH2 cells, TFH13 cells, and TFR cells closely regulate IgE production in asthma. Future studies using single-cell technology can help us better understand this heterogeneity of the TFH cell population in asthma patients and healthy cohorts. Future studies are also required to elucidate the connection between IL-21 and different subsets of TFH cells as well as TFR cells, and to determine how can we use this follicular regulatory network to control asthma disease. It also remains to be seen how TFH, TFR cells and IL-21 are used to better classify the asthma patients, which may help clinicians design personalized and precise medicine for different individuals with asthma.
Author Contributions
PZ, FG, and TZ wrote the manuscript. PZ revised the manuscript and led the submission.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Di Yu for reading the manuscript and providing constructive suggestions. The author apologizes to investigators whose contributions were not cited more extensively because of space limitations.
Footnotes
Funding. This work was supported by Six Talent Peaks Project in Jiangsu Province (2017-WSN-186) and Jiangsu Province 333 Project (BRA2019152) to FG, National Natural Science Foundation of China (81600692) to TZ and (81970759) to TZ and PZ, and Youth Science Funds of Shandong Academy of Sciences (2018QN003) to TZ.
References
- 1.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. (2018) 391:783–800. 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 2.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. (2000) 343:332–6. 10.1056/NEJM200008033430504 [DOI] [PubMed] [Google Scholar]
- 3.Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. (2001) 1:CD002178 10.1002/14651858.CD002178 [DOI] [PubMed] [Google Scholar]
- 4.Hansbro PM, Kim RY, Starkey MR, Donovan C, Dua K, Mayall JR, et al. Mechanisms and treatments for severe, steroid-resistant allergic airway disease and asthma. Immunol Rev. (2017) 278:41–62. 10.1111/imr.12543 [DOI] [PubMed] [Google Scholar]
- 5.Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. (2018) 52:1800703. 10.1183/13993003.00703-2018 [DOI] [PubMed] [Google Scholar]
- 6.Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet. (2018) 391:350–400. 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 7.Muehling LM, Lawrence MG, Woodfolk JA. Pathogenic CD4(+) T cells in patients with asthma. J Allergy Clin Immunol. (2017) 140:1523–40. 10.1016/j.jaci.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. (1992) 326:298–304. 10.1056/NEJM199201303260504 [DOI] [PubMed] [Google Scholar]
- 9.Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. (2014) 16:45–56. 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 10.Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol Rev. (2017) 278:162–72. 10.1111/imr.12557 [DOI] [PubMed] [Google Scholar]
- 11.Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, Leon B. T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity. (2016) 44:259–73. 10.1016/j.immuni.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coquet JM, Schuijs MJ, Smyth MJ, Deswarte K, Beyaert R, Braun H, et al. Interleukin-21-producing CD4(+) T cells promote type 2 immunity to house dust mites. Immunity. (2015) 43:318–30. 10.1016/j.immuni.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. (2017) 139:300–13.e7. 10.1016/j.jaci.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble A, Zhao J. Follicular helper T cells are responsible for IgE responses to Der p 1 following house dust mite sensitization in mice. Clin Exp Allergy. (2016) 46:1075–82. 10.1111/cea.12750 [DOI] [PubMed] [Google Scholar]
- 15.Kubo M. T follicular helper and TH2 cells in allergic responses. Allergol Int. (2017) 66:377–81. 10.1016/j.alit.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 16.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. (2000) 192:1545–52. 10.1084/jem.192.11.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nat Immunol. (2001) 2:876–81. 10.1038/ni0901-876 [DOI] [PubMed] [Google Scholar]
- 18.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. (2001) 193:1373–81. 10.1084/jem.193.12.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. (2000) 192:1553–62. 10.1084/jem.192.11.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. (2009) 325:1006–10. 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. (2009) 325:1001–5. 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. (2009) 31:457–68. 10.1016/j.immuni.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Vinuesa CG, Linterman MA, Yu D, MacLennan IC. Follicular Helper T Cells. Annu Rev Immunol. (2016) 34:335–68. 10.1146/annurev-immunol-041015-055605 [DOI] [PubMed] [Google Scholar]
- 24.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. (2019) 50:1132–48. 10.1016/j.immuni.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. (2008) 29:127–37. 10.1016/j.immuni.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 26.Chtanova T, Newton R, Liu SM, Weininger L, Young TR, Silva DG, et al. Identification of T cell-restricted genes, and signatures for different T cell responses, using a comprehensive collection of microarray datasets. J Immunol. (2005) 175:7837–47. 10.4049/jimmunol.175.12.7837 [DOI] [PubMed] [Google Scholar]
- 27.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. (2011) 34:932–46. 10.1016/j.immuni.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature. (2016) 533:110–4. 10.1038/nature17947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J, Hou S, Fang Q, Liu X, Liu X, Qi H. PD-1 controls follicular T helper cell positioning and function. Immunity. (2018) 49:264–74.e4. 10.1016/j.immuni.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. (2013) 496:523–7. 10.1038/nature12058 [DOI] [PubMed] [Google Scholar]
- 31.Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz LJ, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Kruppel-like factor 2. J Exp Med. (2015) 212:217–33. 10.1084/jem.20141432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, et al. BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J Exp Med. (2015) 212:539–53. 10.1084/jem.20141380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. (2014) 41:529–42. 10.1016/j.immuni.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweet RA, Lee SK, Vinuesa CG. Developing connections amongst key cytokines and dysregulated germinal centers in autoimmunity. Curr Opin Immunol. (2012) 24:658–64. 10.1016/j.coi.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 35.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. (2009) 31:158–69. 10.1016/j.immuni.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. (2009) 31:491–501. 10.1016/j.immuni.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 37.Papillion A, Powell MD, Chisolm DA, Bachus H, Fuller MJ, Weinmann AS, et al. Inhibition of IL-2 responsiveness by IL-6 is required for the generation of GC-TFH cells. Sci Immunol. (2019) 4:eaaw7636. 10.1126/sciimmunol.aaw7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou P, Liang K, Yu D. Germinal center TFH cells: T(w)o be or not t(w)o be, IL-6 is the answer. Sci Immunol. (2019) 4:aay7668. 10.1126/sciimmunol.aay7668 [DOI] [PubMed] [Google Scholar]
- 39.Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, et al. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol. (2014) 15:856–65. 10.1038/ni.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. (2012) 36:847–56. 10.1016/j.immuni.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. (2016) 22:991–3. 10.1038/nm.4148 [DOI] [PubMed] [Google Scholar]
- 42.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. (2009) 10:385–93. 10.1038/ni.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaretsky I, Atrakchi O, Mazor RD, Stoler-Barak L, Biram A, Feigelson SW, et al. ICAMs support B cell interactions with T follicular helper cells and promote clonal selection. J Exp Med. (2017) 214:3435–48. 10.1084/jem.20171129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, et al. Plasticity of Th17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. (2013) 14:372–9. 10.1038/ni.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacLeod MK, David A, McKee AS, Crawford F, Kappler JW, Marrack P. Memory CD4 T cells that express CXCR5 provide accelerated help to B cells. J Immunol. (2011) 186:2889–96. 10.4049/jimmunol.1002955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koutsakos M, Wheatley AK, Loh L, Clemens EB, Sant S, Nussing S, et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med. (2018) 10:eaan8405. 10.1126/scitranslmed.aan8405 [DOI] [PubMed] [Google Scholar]
- 47.Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. (2012) 13:491–8. 10.1038/ni.2261 [DOI] [PubMed] [Google Scholar]
- 48.Barr T, Gray D. TFH memory: more or less TFH? Euro J Immunol. (2012) 42:1977–80. 10.1002/eji.201242755 [DOI] [PubMed] [Google Scholar]
- 49.Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, et al. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. (2016) 17:1197–205. 10.1038/ni.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. (2011) 34:108–21. 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao DA, Gurish MF, Marshall JL, Slowikowski K, Fonseka CY, Liu Y, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature. (2017) 542:110–4. 10.1038/nature20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bocharnikov AV, Keegan J, Wacleche VS, Cao Y, Fonseka CY, Wang G, et al. PD-1hi CXCR5- T peripheral helper cells promote B cells responses in lupus via MAF and IL-21. JCI Insight. (2019). 10.1172/jci.insight.130062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caielli S, Veiga DT, Balasubramanian P, Athale S, Domic B, Murat E, et al. A CD4(+) T cell population expanded in lupus blood provides B cell help through interleukin-10 and succinate. Nat Med. (2019) 25:75–81. 10.1038/s41591-018-0254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. (2013) 39:770–81. 10.1016/j.immuni.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 55.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. (2013) 39:758–69. 10.1016/j.immuni.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. (2014) 35:436–42. 10.1016/j.it.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol. (2015) 16:142–52. 10.1038/ni.3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. (2019) 365:eaaw6433. 10.1126/science.aaw6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolence JJ, Kobayashi T, Iijima K, Krempski J, Drake LY, Dent AL, et al. Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol. (2018) 142:1144–58.e8. 10.1016/j.jaci.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van V, Beier KC, Pietzke LJ, Al Baz MS, Feist RK, Gurka S, et al. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat Commun. (2016) 7:10875 10.1038/ncomms10875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van DV, Bauer L, Kroczek RA, Hutloff A. ICOS costimulation differentially affects T cells in secondary lymphoid organs and inflamed tissues. Am J Resp Cell Mol. (2018) 59:437–47. 10.1165/rcmb.2017-0309OC [DOI] [PubMed] [Google Scholar]
- 62.Clement RL, Daccache J, Mohammed MT, Diallo A, Blazar BR, Kuchroo VK, et al. Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol. (2019) 20:1360–71. 10.1038/s41590-019-0472-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lal G, Kulkarni N, Nakayama Y, Singh AK, Sethi A, Burrell BE, et al. IL-10 from marginal zone precursor B cells controls the differentiation of Th17, Tfh and Tfr cells in transplantation tolerance. Immunol Lett. (2016) 170:52–63. 10.1016/j.imlet.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jandl C, Liu SM, Canete PF, Warren J, Hughes WE, Vogelzang A, et al. IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nat Commun. (2017) 8:14647. 10.1038/ncomms14647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. (2014) 41:1013–25. 10.1016/j.immuni.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 66.Gong F, Qian C, Zhu H, Zhu J, Pan Y, Dong Q, et al. Circulating follicular T-helper cell subset distribution in patients with asthma. Allergy Asthma Proc. (2016) 37:154–61. 10.2500/aap.2016.37.3982 [DOI] [PubMed] [Google Scholar]
- 67.Zhang D, Wu Y, Sun G. miR-192 suppresses T follicular helper cell differentiation by targeting CXCR5 in childhood asthma. Scand J Clin Lab Invest. (2018) 78:236–42. 10.1080/00365513.2018.1440628 [DOI] [PubMed] [Google Scholar]
- 68.Yao Y, Chen CL, Wang N, Wang ZC, Ma J, Zhu RF, et al. Correlation of allergen-specific T follicular helper cell counts with specific IgE levels and efficacy of allergen immunotherapy. J Allergy Clin Immunol. (2018) 142:321–4.e10. 10.1016/j.jaci.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 69.Schulten V, Tripple V, Seumois G, Qian Y, Scheuermann RH, Fu Z, et al. Allergen-specific immunotherapy modulates the balance of circulating Tfh and Tfr cells. J Allergy Clin Immunol. (2018) 141:775–7.e6. 10.1016/j.jaci.2017.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao Y, Wang ZC, Wang N, Zhou PC, Chen CL, Song J, et al. Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J Allergy Clin Immunol. (2019) 144:118–28. 10.1016/j.jaci.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 71.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med. (2005) 202:1459–63. 10.1084/jem.20052211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. (2005) 5:271–83. 10.1038/nri1589 [DOI] [PubMed] [Google Scholar]
- 73.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. (2005) 202:1539–47. 10.1084/jem.20051166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. (2008) 1:213–20. 10.1242/dmm.000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. (2011) 186:5556–68. 10.4049/jimmunol.1002828 [DOI] [PubMed] [Google Scholar]
- 76.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. (2011) 35:583–95. 10.1016/j.immuni.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong F, Zhu HY, Zhu J, Dong QJ, Huang X, Jiang DJ. Circulating CXCR5(+)CD4(+) T cells participate in the IgE accumulation in allergic asthma. Immunol Lett. (2018) 197:9–14. 10.1016/j.imlet.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 78.Yamamoto M, Singh A, Ruan J, Gauvreau GM, O'Byrne PM, Carlsten CR, et al. Decreased miR-192 expression in peripheral blood of asthmatic individuals undergoing an allergen inhalation challenge. BMC Genomics. (2012) 13:655. 10.1186/1471-2164-13-655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. (2000) 97:11439–44. 10.1073/pnas.200360997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. (2000) 408:57–63. 10.1038/35040504 [DOI] [PubMed] [Google Scholar]
- 81.Tangye SG. Advances in IL-21 biology - enhancing our understanding of human disease. Curr Opin Immunol. (2015) 34:107–15. 10.1016/j.coi.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 82.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Disc. (2014) 13:379–95. 10.1038/nrd4296 [DOI] [PubMed] [Google Scholar]
- 83.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Ann Rev Immunol. (2008) 26:57–79. 10.1146/annurev.immunol.26.021607.090316 [DOI] [PubMed] [Google Scholar]
- 84.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. (2006) 177:120–9. 10.4049/jimmunol.177.1.120 [DOI] [PubMed] [Google Scholar]
- 85.Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer (vol. 201, pg 1973, 2005). J Exp Med. (2005) 202:569 10.1084/jem.20042280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. (2009) 324:1576–80. 10.1126/science.1172815 [DOI] [PubMed] [Google Scholar]
- 87.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. (2009) 324:1569–72. 10.1126/science.1174182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. (2007) 282:34605–10. 10.1074/jbc.M705100200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. (2007) 448:484–7. 10.1038/nature05970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bauquet AT, Jin HL, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and T-H-17 cells. Nat Immunol. (2009) 10:167–75. 10.1038/ni.1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of blimp-1 and bcl-61. J Immunol. (2004) 173:5361–71. 10.4049/jimmunol.173.9.5361 [DOI] [PubMed] [Google Scholar]
- 92.Gharibi T, Majidi J, Kazemi T, Dehghanzadeh R, Motallebnezhad M, Babaloo Z. Biological effects of IL-21 on different immune cells and its role in autoimmune diseases. Immunobiology. (2016) 221:357–67. 10.1016/j.imbio.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 93.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. (2002) 298:1630–4. 10.1126/science.1077002 [DOI] [PubMed] [Google Scholar]
- 94.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. (2001) 167:1–5. 10.4049/jimmunol.167.1.1 [DOI] [PubMed] [Google Scholar]
- 95.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. (2007) 8:967–74. 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- 96.Ivanov, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. (2007) 19:409–17. 10.1016/j.smim.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. (2008) 29:138–49. 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. (2011) 6:e17739. 10.1371/journal.pone.0017739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao X, Wang H, Chen Z, Zhou P, Yu D. An optimised method to differentiate mouse follicular helper T cells in vitro. Cell Mol Immunol. (2019). 10.1038/s41423-019-0329-7. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. (2009) 31:941–52. 10.1016/j.immuni.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. (2010) 207:365–78. 10.1084/jem.20091777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. (2010) 207:353–63. 10.1084/jem.20091738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin HL, Carrio R, Yu AX, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or bim-dependent apoptosis. J Immunol. (2004) 173:657–65. 10.4049/jimmunol.173.1.657 [DOI] [PubMed] [Google Scholar]
- 104.Gowda A, Roda J, Hussain SRA, Ramanunni A, Joshi T, Schmidt S, et al. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro. Blood. (2008) 111:4723–30. 10.1182/blood-2007-07-099531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lajoie S, Lewkowich I, Herman NS, Sproles A, Pesce JT, Wynn TA, et al. IL-21 receptor signalling partially mediates Th2-mediated allergic airway responses. Clin Exp Allergy. (2014) 44:976–85. 10.1111/cea.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He Y, Lou X, Jin Z, Yu L, Deng L, Wan H. Mahuang decoction mitigates airway inflammation and regulates IL-21/STAT3 signaling pathway in rat asthma model. J Ethnopharmacol. (2018) 224:373–80. 10.1016/j.jep.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 107.Cheng S, Chen H, Wang A, Bunjhoo H, Cao Y, Xie J, et al. Exclusion of IL-21 in the pathogenesis of OVA-induced asthma in mice. Int J Clin Exp Med. (2014) 7:3202–8. [PMC free article] [PubMed] [Google Scholar]
- 108.Chen H, Cheng S, Wang A, Bunjhoo H, Cao Y, Xie J, et al. IL-21 does not involve in OVA-induced airway remodeling and chronic airway inflammation. Int J Clin Exp Med. (2015) 8:10640–5. [PMC free article] [PubMed] [Google Scholar]
- 109.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood. (2002) 100:4565–73. 10.1182/blood-2002-04-1115 [DOI] [PubMed] [Google Scholar]
- 110.Harada M, Magara-Koyanagi K, Watarai H, Nagata Y, Ishii Y, Kojo S, et al. IL-21-induced Bepsilon cell apoptosis mediated by natural killer T cells suppresses IgE responses. J Exp Med. (2006) 203:2929–37. 10.1084/jem.20062206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, et al. IL-21 administration into the nostril alleviates murine allergic rhinitis. J Immunol. (2007) 179:7157–65. 10.4049/jimmunol.179.10.7157 [DOI] [PubMed] [Google Scholar]
- 112.Kishida T, Hiromura Y, Shin-Ya M, Asada H, Kuriyama H, Sugai M, et al. IL-21 induces inhibitor of differentiation 2 and leads to complete abrogation of anaphylaxis in mice. J Immunol. (2007) 179:8554–61. 10.4049/jimmunol.179.12.8554 [DOI] [PubMed] [Google Scholar]
- 113.Tamagawa-Mineoka R, Kishida T, Mazda O, Katoh N. IL-21 reduces immediate hypersensitivity reactions in mouse skin by suppressing mast cell activation or IgE production. J Invest Dermatol. (2011) 131:1513–20. 10.1038/jid.2011.73 [DOI] [PubMed] [Google Scholar]
- 114.Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL, et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. (2007) 109:2023–31. 10.1182/blood-2006-05-021600 [DOI] [PubMed] [Google Scholar]
- 115.Lin PY, Jen HY, Chiang BL, Sheu F, Chuang YH. Interleukin-21 suppresses the differentiation and functions of T helper 2 cells. Immunology. (2015) 144:668–76. 10.1111/imm.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu J, Zhang S, Qin T, Jiang J, Liu Q, Zhang L, et al. IL-21 alleviates allergic asthma in DOCK8-knockout mice. Biochem Biophys Res Commun. (2018) 501:92–9. 10.1016/j.bbrc.2018.04.179 [DOI] [PubMed] [Google Scholar]
- 117.Gong F, Su Q, Jiang D, Chen J, Pan Y, Huang X. High frequency of circulating follicular helper T cells in patients with bronchial asthma. Clin Lab. (2014) 60:963–8. 10.7754/Clin.Lab.2013.130427 [DOI] [PubMed] [Google Scholar]
- 118.Chatterjee R, Batra J, Ghosh B. A common exonic variant of interleukin21 confers susceptibility to atopic asthma. Int Arch Allergy Immunol. (2009) 148:137–46. 10.1159/000155744 [DOI] [PubMed] [Google Scholar]
- 119.Wing JB, Sakaguchi S. Foxp3(+) T(reg) cells in humoral immunity. Int Immunol. (2014) 26:61–9. 10.1093/intimm/dxt060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tai Y, Sakamoto K, Takano A, Haga K, Harada Y. Dysregulation of humoral immunity in Foxp3 conditional-knockout mice. Biochem Biophys Res Commun. (2019) 513:787–93. 10.1016/j.bbrc.2019.04.090 [DOI] [PubMed] [Google Scholar]
- 121.Leonardo SM, De Santis JL, Gehrand A, Malherbe LP, Gauld SB. Expansion of follicular helper T cells in the absence of Treg cells: implications for loss of B-cell anergy. Eur J Immunol. (2012) 42:2597–607. 10.1002/eji.201242616 [DOI] [PubMed] [Google Scholar]
- 122.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. (2011) 17:983–8. 10.1038/nm.2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. (2011) 17:975–82. 10.1038/nm.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Deng J, Wei Y, Fonseca VR, Graca L, Yu D. T follicular helper cells and T follicular regulatory cells in rheumatic diseases. Nat Rev Rheumatol. (2019) 15:475–90. 10.1038/s41584-019-0254-2 [DOI] [PubMed] [Google Scholar]
- 125.Botta D, Fuller MJ, Marquez-Lago TT, Bachus H, Bradley JE, Weinmann AS, et al. Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat Immunol. (2017) 18:1249–60. 10.1038/ni.3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Canete PF, Sweet RA, Gonzalez-Figueroa P, Papa I, Ohkura N, Bolton H, et al. Regulatory roles of IL-10-producing human follicular T cells. J Exp Med. (2019) 216:1843–56. 10.1084/jem.20190493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. (2008) 8:218–30. 10.1038/nri2262 [DOI] [PubMed] [Google Scholar]
- 128.McDonald PW, Read KA, Baker CE, Anderson AE, Powell MD, Ballesteros-Tato A, et al. IL-7 signalling represses Bcl-6 and the TFH gene program. Nat Commun. (2016) 7:10285. 10.1038/ncomms10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. (2014) 40:367–77. 10.1016/j.immuni.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huber JP, Gonzales-van Horn SR, Roybal KT, Gill MA, Farrar JD. IFN-alpha suppresses GATA3 transcription from a distal exon and promotes H3K27 trimethylation of the CNS-1 enhancer in human Th2 cells. J Immunol. (2014) 192:5687–94. 10.4049/jimmunol.1301908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol. (2016) 17:65–75. 10.1038/ni.3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA. (2009) 106:1518–23. 10.1073/pnas.0807309106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kotlarz D, Zietara N, Uzel G, Weidemann T, Braun CJ, Diestelhorst J, et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. (2013) 210:433–43. 10.1084/jem.20111229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma J, Ma D, Ji C. The role of IL-21 in hematological malignancies. Cytokine. (2011) 56:133–9. 10.1016/j.cyto.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 135.Rasmussen MA, Colding-Jorgensen M, Hansen LT, Bro R. Multivariate evaluation of pharmacological responses in early clinical trials - a study of rIL-21 in the treatment of patients with metastatic melanoma. Br J Clin Pharmacol. (2010) 69:379–90. 10.1111/j.1365-2125.2009.03600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. (2008) 26:2034–9. 10.1200/JCO.2007.14.5193 [DOI] [PubMed] [Google Scholar]
- 137.Hashmi MH, Van Veldhuizen PJ. Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin's lymphoma. Expert Opin Biol Ther. (2010) 10:807–17. 10.1517/14712598.2010.480971 [DOI] [PubMed] [Google Scholar]
- 138.Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. (2009) 15:2123–9. 10.1158/1078-0432.CCR-08-2663 [DOI] [PubMed] [Google Scholar]
- 139.Zhang M, Yu G, Chan B, Pearson JT, Rathanaswami P, Delaney J, et al. Interleukin-21 receptor blockade inhibits secondary humoral responses and halts the progression of preestablished disease in the (NZB x NZW)F1 systemic lupus erythematosus model. Arthritis Rheumatol. (2015) 67:2723–31. 10.1002/art.39233 [DOI] [PubMed] [Google Scholar]
- 140.Roeleveld DM, Marijnissen RJ, Walgreen B, Helsen MM, van den Bersselaar L, van de Loo FA, et al. Higher efficacy of anti-IL-6/IL-21 combination therapy compared to monotherapy in the induction phase of Th17-driven experimental arthritis. PLoS ONE. (2017) 12:e0171757. 10.1371/journal.pone.0171757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hippen KL, Bucher C, Schirm DK, Bearl AM, Brender T, Mink KA, et al. Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes. Blood. (2012) 119:619–28. 10.1182/blood-2011-07-368027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bucher C, Koch L, Vogtenhuber C, Goren E, Munger M, Panoskaltsis-Mortari A, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. (2009) 114:5375–84. 10.1182/blood-2009-05-221135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Holm TL, Tornehave D, Sondergaard H, Kvist PH, Sondergaard BC, Hansen L, et al. Evaluating IL-21 as a potential therapeutic target in Crohn's disease. Gastroenterol Res Pract. (2018) 2018:5962624. 10.1155/2018/5962624 [DOI] [PMC free article] [PubMed] [Google Scholar]