Abstract
Aims
The aim of this study was to investigate the occurrence of myocardial injury and cardiac dysfunction after an endurance race by biomarkers and cardiac magnetic resonance in triathletes with and without myocardial fibrosis.
Methods and results
Thirty asymptomatic male triathletes (45 ± 10 years) with over 10 training hours per week and 55 ± 8 ml/kg per minute maximal oxygen uptake during exercise testing were studied before (baseline) and 2.4 ± 1.1 hours post-race. Baseline cardiac magnetic resonance included cine, T1/T2, late gadolinium enhancement (LGE) and extracellular volume imaging. Post-race non-contrast cardiac magnetic resonance included cine and T1/T2 mapping. Non-ischaemic myocardial fibrosis was present in 10 triathletes (LGE+) whereas 20 had no fibrosis (LGE–). At baseline, LGE + triathletes had higher peak exercise systolic blood pressure with 222 ± 21 mmHg compared to LGE– triathletes (192 ± 30 mmHg, P < 0.01). Post-race troponin T and creatine kinase MB were similarly increased in both groups, but there was no change in T2 and T1 from baseline to post-race with 54 ± 3 ms versus 53 ± 3 ms (P = 0.797) and 989 ± 21 ms versus 989 ± 28 ms (P = 0.926), respectively. However, post-race left atrial ejection fraction was significantly lower in LGE + triathletes compared to LGE– triathletes (53 ± 6% vs. 59 ± 6%, P < 0.05). Furthermore, baseline atrial peak filling rates were lower in LGE – triathletes (121 ± 30 ml/s/m2) compared to LGE + triathletes (161 ± 34 ml/s/m2, P < 0.01). Post-race atrial peak filling rates increased in LGE– triathletes to 163 ± 46 ml/s/m2, P < 0.001), but not in LGE + triathletes (169 ± 50ml/s/m2, P = 0.747).
Conclusion
Despite post-race troponin T release, we did not find detectable myocardial oedema by cardiac magnetic resonance. However, the unfavourable blood pressure response during exercise testing seemed to be associated with post-race cardiac dysfunction, which could explain the occurrence of myocardial fibrosis in triathletes.
Keywords: Athlete's heart, cardiac magnetic resonance, myocardial fibrosis, late gadolinium enhancement, T2 and T1 mapping, post-race myocardial oedema, post-race cardiac function
Introduction
Strenuous exercise results in mainly reversible changes of cardiac morphology and function and a transient increase of serum markers of myocardial injury.1–3 However, a portion of athletes reveal myocardial fibrosis on contrast-enhanced cardiac magnetic resonance (CMR), which represents an irreversible myocardial injury.4–7 One explanation for myocardial fibrosis in athletes could be repetitive exercise-related myocardial injuries triggered by hypoxia, inflammation or hypertensive overload.7 Accordingly, a recent study reported the occurrence of myocardial oedema using T2w CMR after a marathon race, which was associated with a decrease in cardiac function.8 However, accurate detection of myocardial oedema by T2w CMR is limited due to multiple factors.9 These limitations have been addressed by mapping techniques, which enable reliable detection of myocardial oedema by quantification of native T2 and T1 relaxation times.10,11
Another focus of the current research is the presence of exercise-induced cardiac dysfunction and its association with biomarkers of cardiac injury. Neilan et al. observed transient altered diastolic left ventricular (LV) filling, increased pulmonary pressures and right ventricular (RV) dimensions, and decreased RV systolic function after a marathon race.1 The post-race reduced diastolic LV filling was associated with an increase in N-terminal pro-brain natriuretic peptide.1 However, a more recent study reported that biomarkers of myocardial injury were not associated with systolic or diastolic cardiac dysfunction following an endurance exercise.12
We hypothesised that triathletes potentially develop exercise-induced myocardial injury, which is mirrored by increased cardiac biomarkers, myocardial oedema and altered systolic and diastolic cardiac function. The purpose of the study was therefore to analyse the occurrence of myocardial injury and cardiac dysfunction after an endurance race by biomarkers and CMR in triathletes with and without myocardial fibrosis.
Methods
Triathletes and controls
The local ethics committee approved the study and all participants gave written consent. Triathletes were contacted through advertisements at triathlon clubs and were included if they trained for a minimum of 10 hours per week and participated regularly in competitions in the past 3 years. Control subjects were eligible if they exercised less than 3 hours per week. Thirty consecutive male triathletes (aged 45 ± 10 years, range 18–61 years, 73% were 35–55 years of age) underwent baseline and post-race CMR. Baseline CMR was performed at least 30 days after the last race. Subjects were instructed to refrain from any exercise and alcohol consumption in the preceding 72 hours, whereas any food and caffeine intake was restricted 3 hours before baseline CMR. The interval between baseline and post-race CMR was more than one month. Blood samples were drawn immediately before each CMR from an antecubital vein in the supine position for 5 minutes to obtain haematocrit, creatine kinase, highly sensitive troponin T and N-terminal pro-brain natriuretic peptide (NT-proBNP). Cardiopulmonary exercise testing was performed 3 hours after the baseline CMR to objectify the exercise capacity of the triathletes as previously described.13 Study exclusion criteria were contraindications for CMR or any systemic disease. None of the triathletes had cardiovascular diseases and all reported no intake of any cardiac or illicit medication.
Post-race CMR and blood tests
All triathletes successfully finished their races with a total distance of 68 ± 77 km. Distances for each leg were: swimming 1.3 ± 0.5 km;, cycling 72 ± 89 km and running 16 ± 12 km. The total race time was 3.3 ± 2.7 hours. The participants were transported immediately post-race to our institution to obtain blood samples and CMR, which was performed at 2.4 ± 1.1 (range 1–5) hours post-race. There was no difference in the time to blood test between triathletes with non-ischaemic myocardial fibrosis (late gadolinium enhancement (LGE)+) with 2.4 ± 1.2 hours compared to triathletes with no fibrosis (LGE–) with 2.4 ± 1.1 hours (P = 0.999).
CMR protocol
CMR was performed using a 1.5 T Achieva scanner equipped with a five-channel cardiac phased array receiver coil (Philips Healthcare, Best, The Netherlands). The CMR protocol included standard steady-state free-precession cine CMR in short axis with retrospective ECG triggering and acquisition of 25 phases for LV and RV volumetry and LV mass assessment. Native T1 mapping was performed using a 5s (3s) 3s modified look-locker inversion recovery (MOLLI) sequence three short-axes slices (apical, mid and basal) before and 15 minutes after contrast media administration.13 For T2 mapping we used a gradient (echo planar imaging) and spin-echo multi-echo sequence in three short-axis sections corresponding to the MOLLI sections.14 Ten minutes after a bolus injection of 0.2 mmol/kg gadoter acid (Dotarem; Guerbet, Sulzbach, Germany) at a rate of 2.5 ml/s end-diastolic LGE images were acquired using end-diastolic phase-sensitive inversion recovery sequences in short-axis orientation covering the entire heart and in two, three and four-chamber views. More details on the magnetic resonance imaging sequences, acquisition parameters and extracellular volume (ECV) calculation are provided in Supplementary Appendix E1.
CMR data analysis
Two investigators independently and blindly analysed each CMR using cvi42 software (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada). CMR parameters were indexed to the calculated body surface area (BSA) and are given as the mean of the two observers. Evaluation of LV and RV volumes and LV mass was performed in standard fashion on short-axis cine images.9 Left atrial (LA) and right atrial (RA) volumes and ejection fraction were quantified using the biplane area-length method, excluding pulmonary veins and atrial appendage.15 Short axis cine CMR images at the tips of the mitral valve leaflets were used to measure inter-ventricular septum thickness in diastole.16 This parameter was not indexed to BSA to enable comparison with echocardiographic data from previous studies. Focal myocardial fibrosis was quantified on short axis LGE images using a threshold method with a cutoff greater than 5 standard deviations (SDs) above remote normal myocardium.9 Native T1, post-contrast T1 and ECV were measured on maps generated by a plug-in written for OsiriX software.17 Areas of focal LGE were excluded from T1 and ECV measurements to evaluate these parameters unbiased from the presence of LGE. Apical slices were not included in the global measurements of native T2 and T1 times due to concerns about error related to partial volume averaging.18 Endo and epicardial contours were manually drawn and propagated through the image stack. Meticulous care was taken not to include the blood volume into the measurements to avoid partial volume effects.19
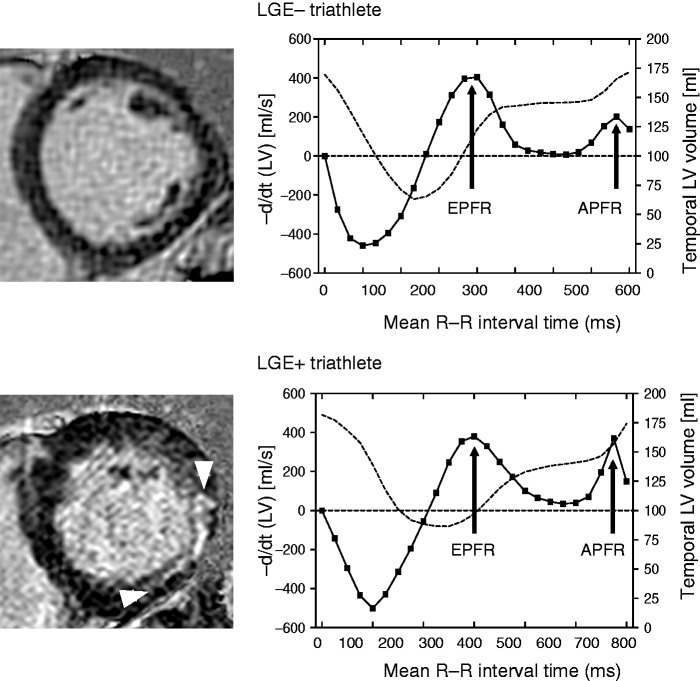
LV filling patterns were analysed with dedicated software (CMRtools, Cambs, UK) as previously reported.20 Briefly, LV three-dimensional (3D) volumetry was performed by manual delineation of the LV endocardial borders in end-diastolic, end-systolic and mid-diastolic short-axis views. Trabeculae and papillary muscles were excluded from the LV cavity, which enabled construction of time-volume curves from all time frames of the cardiac cycle. The differentiated time-volume curve is characterised by two diastolic peaks including the early peak filling rate and the atrial peak filling rate (Figure 1), which represent the maximum speed of passive LV filling and the maximum speed of LV filling secondary to atrial contraction.21
Figure 1.
Left ventricular (LV) volume (dashed line) and differentiated LV time-volume (solid line) curves analysed using cine cardiac magnetic resonance in a LGE– and LGE + triathlete. The differentiated time-volume curve is characterised by two diastolic peaks including the early peak filling rate (EPFR) and the atrial peak filling rate (APFR). The LGE– triathlete had normal peak filling rates with high EPFR and low APFR, whereas the LGE + triathlete had an increased APFR. LGE: late gadolinium enhancement.
Statistical analysis
Statistical analyses were performed using SPSS for Windows, version 21.0 (IBM SPSS Statistics, Armonk, NY, USA). Continuous data are presented as mean±SD and categorical data are presented as absolute numbers and percentages. Unpaired and paired two-sided Student's t-tests were used to compare continuous data. Categorical variables were compared using the chi2 test or Fisher's exact test as appropriate. Statistical significance was defined as P < 0.05.
Results
Baseline findings
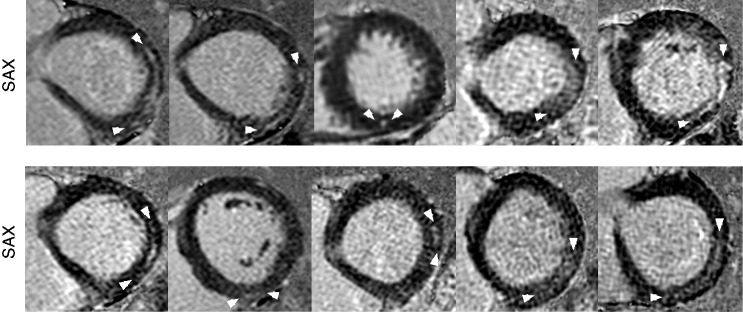
CMR revealed LGE in 10 of 30 (33%) male triathletes, but in none of the male controls (P < 0.01). All LGE + triathletes had non-ischaemic myocardial fibrosis, with a LGE pattern typical for myocarditis in eight triathletes including four triathletes with subepicardial LGE location and a thin epicardial gap (Figure 2), whereas four triathletes had mid-myocardial LGE. Two athletes had LGE located at the posterior right ventricular insertion point. LGE was predominantly located in the inferolateral segments (Figure 2). LGE + triathletes were smaller and had higher BMI compared to LGE– triathletes (Table 1). LGE + triathletes had higher peak exercise systolic blood pressure with 222 ± 21 mmHg compared to LGE– triathletes with 192 ± 30 mmHg (P < 0.01) and controls with 173 ± 24 mmHg (P < 0.0001). No difference in maximal oxygen uptake (VO2max) was observed between LGE + and LGE– triathletes. LGE– triathletes had higher creatine kinase compared to LGE + triathletes; however, highly sensitive troponin T was not different between the groups. LGE + triathletes had higher LV mass indices with 88 ± 7 g/m2 and larger LA end-systolic volume indices with 53 ± 14 ml/m2 compared to LGE– triathletes with 78 ± 10 g/m2 and 44 ± 10 ml/m2, respectively (P < 0.01, P < 0.05). Left and right heart ejection fractions were normal in both groups. Baseline T1 relaxation times were lower in LGE– triathletes, with 982 ± 22 ms compared to controls with 1021 ± 29 ms (P < 0.0001) (Table 1). There was a trend towards lower baseline T1 in LGE– triathletes (982 ± 22 ms) compared to LGE + triathletes (1002 ± 36 ms, P = 0.069). LGE + triathletes had higher ECV of 26.2 ± 1.4% compared to LGE – triathletes, with 24.5 ± 1.3% (P < 0.01), which was similar to controls (24.2 ± 2.9%). The early peak filling rate index was normal in both triathlete groups. LGE– triathletes had lower atrial peak filling rate indices compared to LGE + triathletes (P < 0.01) and compared to controls (P < 0.05, Table 1). LGE– triathletes had an increased peak filling rate ratio, with 2.1 ± 0.8 compared to controls with 1.6 ± 0.4 (P < 0.01, Table 1), whereas LGE + triathletes had an apparently normal peak filling rate ratio, with 1.6 ± 0.7 compared to controls (P = 0.956).
Figure 2.
Short axis late gadolinium enhancement (LGE) images depicting a non-ischaemic fibrosis pattern in all 10 LGE + triathletes. A LGE pattern typical for myocarditis was seen in eight triathletes including four triathletes with subepicardial LGE location and a thin epicardial gap, whereas four triathletes had mid-myocardial LGE. Two athletes had LGE located at the posterior right ventricular insertion point.
Table 1.
Baseline findings.
| Controls (n = 20) | LGE– triathletes (n = 20) | LGE + triathletes (n = 10) | P value LGE+ vs. LGE– | |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, years | 42 ± 12 | 42 ± 10 | 49 ± 8 | 0.074 |
| Weight, kg | 79 ± 9 | 79 ± 8 | 79 ± 11 | 0.922 |
| Height, m | 1.80 ± 0.09 | 1.84 ± 0.07 | 1.77 ± 0.08# | <0.05 |
| Body mass index, kg/m2 | 24.4 ± 2.4 | 23.3 ± 1.7 | 25.0 ± 2.7# | <0.05 |
| Body surface area, m2 | 1.98 ± 0.14 | 2.02 ± 0.14 | 1.96 ± 0.16 | 0.294 |
| Exercise testing | ||||
| Systolic BP at rest, mmHg | 121 ± 15 | 124 ± 10 | 130 ± 16 | 0.236 |
| Diastolic BP at rest, mmHg | 84 ± 11 | 85 ± 8 | 83 ± 9 | 0.559 |
| Peak systolic BP, mmHg | 173 ± 24 | 192 ± 30§ | 222 ± 21ll | <0.01 |
| Peak diastolic BP, mmHg | 81 ± 20 | 88 ± 23 | 88 ± 12 | 0.980 |
| Heart rate at rest, bpm | 73 ± 13 | 66 ± 14* | 66 ± 10 | 0.719 |
| Peak heart rate, bpm | 171 ± 15 | 171 ± 8 | 165 ± 10 | 0.091 |
| Δ heart rate rest/peak, bpm | 104 ± 16 | 117 ± 12† | 110 ± 15 | 0.182 |
| VO2max, ml/kg per min | 40 ± 6 | 57 ± 9§ | 53 ± 8** | 0.290 |
| Ventilatory threshold, % of VO2max | 68 ± 12 | 82 ± 7§ | 82 ± 8¶ | 0.828 |
| Maximal power, W | 240 ± 48 | 416 ± 103§ | 348 ± 83# | 0.081 |
| Laboratory | ||||
| Troponin T, pg/ml | 5 ± 3 | 6 ± 3 | 8 ± 6 | 0.427 |
| Creatine kinase, U/I | 210 ± 191 | 267 ± 195 | 128 ± 50 | <0.05 |
| Creatine kinase-MB, U/I | 5 ± 8 | 14 ± 16 | 4 ± 9 | 0.081 |
| NT-proBNP, pg/ml | 39 ± 25 | 34 ± 23 | 88 ± 150 | 0.119 |
| Haematocrit, % | 44 ± 3 | 44 ± 2 | 43 ± 6 | 0.658 |
| Haemoglobin, mg/dl | 14.9 ± 1.1 | 14.8 ± 0.8 | 14.7 ± 0.9 | 0.699 |
| Creatinine, mg/dl | 0.96 ± 0.19 | 1.04 ± 0.15 | 0.96 ± 0.11 | 0.183 |
| CMR – left heart | ||||
| LVEDVi, ml/m2 | 82 ± 12 | 102 ± 14§ | 100 ± 16¶ | 0.765 |
| LVESVi, ml/m2 | 32 ± 10 | 40 ± 8* | 36 ± 9 | 0.253 |
| LV ejection fraction, % | 62 ± 9 | 61 ± 5 | 64 ± 8 | 0.343 |
| Heart rate, beats/min | 66 ± 13 | 54 ± 8‡ | 56 ± 10ll | 0.537 |
| LV cardiac index, l/min/m2 | 3.3 ± 0.9 | 3.3 ± 0.5 | 3.5 ± 0.8 | 0.594 |
| LV mass index, g/m2 | 69 ± 8 | 78 ± 10† | 88 ± 7** | <0.01 |
| LV septum, mm | 9 ± 1 | 11 ± 1§ | 12 ± 2** | <0.05 |
| LAEDVi, ml/m2 | 13 ± 4 | 16 ± 6* | 22 ± 7# | <0.05 |
| LAESVi, ml/m2 | 41 ± 10 | 44 ± 10 | 53 ± 14ll | <0.05 |
| LA ejection fraction, % | 59 ± 9 | 64 ± 9 | 58 ± 12 | 0.148 |
| LGE size, %LV | 2.8 ± 1.7 | |||
| LGE mass index, g/m2 | 2.2 ± 1.6 | |||
| CMR – right heart | ||||
| RVEDVi, ml/m2 | 84 ± 13 | 103 ± 19 | 104 ± 21 | 0.952 |
| RVESVi, ml/m2 | 34 ± 10 | 44 ± 13 | 40 ± 14 | 0.389 |
| RV ejection fraction, % | 59 ± 7 | 58 ± 6 | 62 ± 10 | 0.201 |
| RV cardiac index, l/min/m2 | 3.3 ± 0.7 | 3.5 ± 0.8 | 3.2 ± 0.5 | 0.138 |
| RAEDVi, ml/m2 | 20 ± 5 | 28 ± 7 | 30 ± 10 | 0.801 |
| RAESVi, ml/ m2 | 38 ± 7 | 50 ± 14 | 52 ± 16 | 0.835 |
| RA ejection fraction, % | 48 ± 13 | 45 ± 9 | 42 ± 12 | 0.526 |
| Mapping parameters | ||||
| Global T2, ms | 55 ± 3 | 54 ± 2 | 53 ± 4 | 0.353 |
| Global T1, ms | 1021 ± 29 | 982 ± 22§ | 1002 ± 36 | 0.069 |
| Extracellular volume (%) | 24.2 ± 2.9 | 24.5 ± 1.3 | 26.2 ± 1.4ll | <0.01 |
| LV filling | ||||
| Early peak filling rate index, ml/s/m2 | 234 ± 68 | 242 ± 58 | 239 ± 87 | 0.889 |
| Atrial peak filling rate index, ml/s/m2 | 160 ± 57 | 121 ± 30* | 161 ± 34 | <0.01 |
| Peak filling rate ratio | 1.6 ± 0.4 | 2.1 ± 0.8† | 1.6 ± 0.7 | <0.05 |
Numbers are mean±SD for continuous and n (%) for categorical data.
*P < 0.05, †P < 0.01, ‡P < 0.001 or §P < 0.0001 for LGE– versus controls.
llP < 0.05, ¶P < 0.01, #P < 0.001 or **P < 0.0001 for LGE + versus controls.
LA: left atrial; LAEDVi: left atrial end-diastolic volume index; LAESVi: left atrial end-systolic volume index; LGE: late gadolinium enhancement; LV: left ventricular; LVEDVi: left ventricular end-diastolic volume index; LVESVi: left ventricular end-systolic volume index; NT-proBNP: N-terminal pro-brain natriuretic peptide; RA: right atrial; RAEDVi: right atrial end-diastolic volume index; RAESVi: right atrial end-systolic volume index; RV: right ventricular; RVEDVi: right ventricular end-diastolic volume index; RVESVi: right ventricular end-systolic volume index; VO2max: maximal oxygen uptake.
Post-race changes in all triathletes
Biomarkers of myocardial injury increased post-race in all triathletes including troponin T and creatine kinase MB (Table 2). Furthermore, NT-proBNP increased post-race, potentially related to increased LV pressure during competition. There was a weak positive correlation between troponin T and the time that had elapsed after the race until the blood samples were taken (r = 0.39, P < 0.05; Figure 3(a)). No association was found between NT-proBNP and the time to blood test (r = −0.14, P = 0.463; Figure 3(b)). A decrease of haematocrit and haemoglobin was observed, which is compatible with exercise-induced anaemia and plasma expansion. CMR revealed post-race decreased diastolic volumes of all four chambers, but the heart rate was increased, resulting in increased post-race LV and RV cardiac output (Table 2). LA ejection fraction decreased post-race, whereas LV, RV and RA ejection fractions remained constant. Global and regional T2 and T1 relaxation times were not increased post-race (Table 2 and Supplementary Figure 1). The atrial peak filling rate indices were increased and the peak filling rate ratio was decreased post-race, indicating a race-induced altered LV filling pattern.
Table 2.
Post-race changes in all triathletes.
| Parameter | Baseline All triathletes (n = 30) | Post-race All triathletes (n = 30) | P value pre vs. post-race |
|---|---|---|---|
| Laboratory | |||
| Troponin T, pg/ml | 7 ± 4 | 57 ± 86 | <0.01 |
| Creatine kinase, U/I | 220 ± 173 | 466 ± 246 | <0.0001 |
| Creatine kinase-MB, U/I | 10 ± 14 | 35 ± 15 | <0.0001 |
| NT-proBNP, pg/ml | 52 ± 90 | 127 ± 121 | <0.0001 |
| Haematocrit, % | 44 ± 3 | 42 ± 3 | <0.01 |
| Haemoglobin, mg/dl | 14.7 ± 0.8 | 14.4 ± 0.9 | <0.01 |
| Creatinine, mg/dl | 1.01 ± 0.14 | 1.12 ± 0.18 | <0.0001 |
| CMR – left heart | |||
| LVEDVi, ml/m2 | 102 ± 15§ | 96 ± 14 | <0.001 |
| LVESVi, ml/m2 | 38 ± 8* | 37 ± 10 | 0.512 |
| LV ejection fraction, % | 62 ± 6 | 62 ± 6 | 0.630 |
| Heart rate, beats/min | 54 ± 9‡ | 67 ± 10 | <0.0001 |
| LV cardiac index, l/min/m2 | 3.4 ± 0.6 | 3.9 ± 0.6 | <0.0001 |
| LV mass index, g/m2 | 82 ± 10§ | 82 ± 10 | 0.911 |
| LAEDVi, ml/m2 | 18 ± 7† | 16 ± 5 | 0.070 |
| LAESVi, ml/m2 | 47 ± 12§ | 37 ± 9 | <0.0001 |
| LA ejection fraction, % | 62 ± 10 | 57 ± 7 | <0.01 |
| CMR – right heart | |||
| RVEDVi, ml/m2 | 104 ± 19‡ | 98 ± 16 | <0.05 |
| RVESVi, ml/m2 | 43 ± 13* | 41 ± 12 | 0.136 |
| RV ejection fraction, % | 59 ± 8 | 59 ± 7 | 0.865 |
| RAEDVi, ml/m2 | 28 ± 8† | 28 ± 9 | 0.612 |
| RAESVi, ml/m2 | 51 ± 14† | 46 ± 11 | <0.05 |
| RA ejection fraction, % | 44 ± 10 | 41 ± 8 | 0.162 |
| RV cardiac index, l/min/m2 | 3.3 ± 0.6 | 3.8 ± 0.7 | <0.001 |
| Mapping parameters | |||
| Global T2, ms | 54 ± 3 | 53 ± 3 | 0.797 |
| Global T1, ms | 989 ± 28‡ | 989 ± 21 | 0.926 |
| Extracellular volume (%) | 25.1 ± 1.6 | ||
| Diastolic function | |||
| Early peak filling rate index, ml/s/m2 | 241 ± 67 | 239 ± 60 | 0.855 |
| Atrial peak filling rate index, ml/s/m2 | 134 ± 37 | 165 ± 54 | <0.01 |
| Peak filling rate ratio | 1.9 ± 0.8* | 1.6 ± 0.7 | <0.05 |
Numbers are mean ± SD for continuous and n (%) for categorical data.
*P < 0.05, †P < 0.01, ‡P < 0.001 and §P < 0.0001 for triathletes at baseline compared to controls.
LA: left atrial; LAEDVi: left atrial end-diastolic volume index; LAESVi: left atrial end-systolic volume index; LGE: late gadolinium enhancement; LV: left ventricular; LVEDVi: left ventricular end-diastolic volume index; LVESVi: left ventricular end-systolic volume index; NT-proBNP: N-terminal pro-brain natriuretic peptide; RA: right atrial; RAEDVi: right atrial end-diastolic volume index; RAESVi: right atrial end-systolic volume index; RV: right ventricular; RVEDVi: right ventricular end-diastolic volume index; RVESVi: right ventricular end-systolic volume index; VO2max: maximal oxygen uptake.
Figure 3.
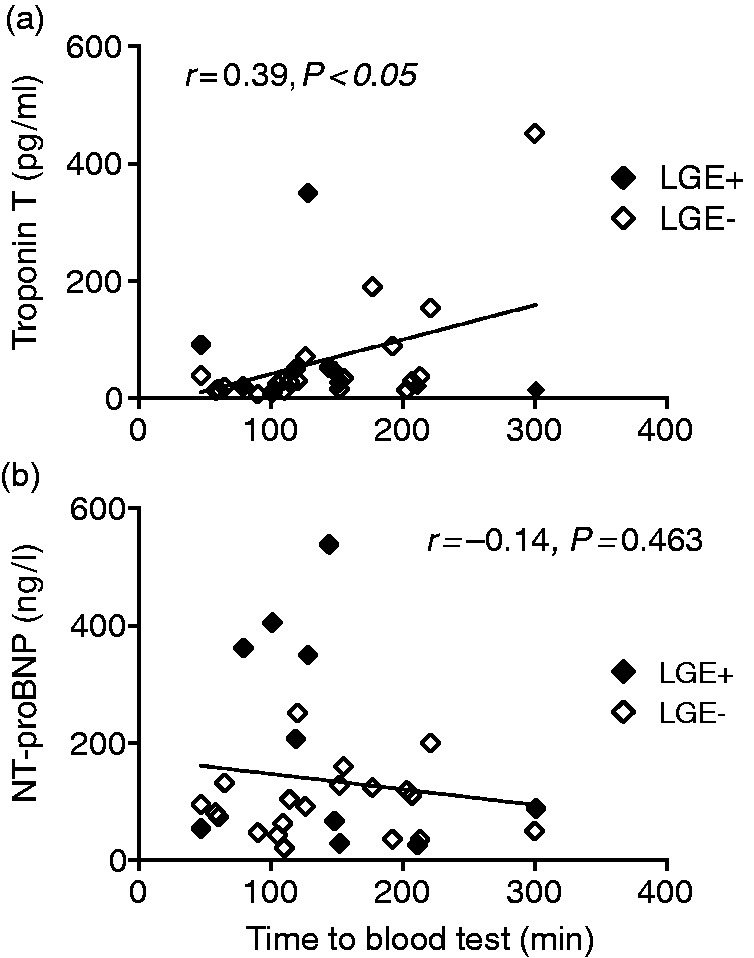
Correlation between troponin T (a) and NT-proBNP (b) and the time elapsed after the race until blood samples were taken.
Post-race changes in LGE + and LGE– triathletes
Troponin T and creatine kinase MB increased post-race similarly in both triathlete groups (Table 3 and Figure 4). Baseline NT-proBNP was not different between both groups (P = 0.119), but there was a trend towards higher post-race NT-proBNP in LGE + triathletes, with 185 ± 185 pg/ml compared to LGE– triathletes, with 98 ± 58 pg/ml (P = 0.061, Table 3). LGE + triathletes had lower post-race haematocrit levels compared to LGE– triathletes (P < 0.05). LGE + triathletes had lower mean post-race LA ejection fraction, with 53 ± 6% compared to LGE– triathletes, with 59 ± 6% (P < 0.05). Post-race global and regional T2 and T1 relaxation times were not different between LGE– and LGE + triathletes (Table 3, Supplementary Figures 2 and 3). The atrial peak filling rate index increased in LGE– triathletes from 121 ± 30 ml/s/m2 at baseline to 163 ± 57 ml/s/m2 post-race (P < 0.001), whereas this parameter remained unchanged post-race in LGE + triathletes. The peak filling rate ratio decreased in both triathlete groups; however, LGE– triathletes had normal post-race values, whereas LGE + triathletes had subnormal post-race values.
Table 3.
Post-race changes in LGE + and LGE– triathletes.
| LGE– triathletes
(n = 20) |
P value | LGE + triathletes
(n = 10) |
P value | |||
|---|---|---|---|---|---|---|
| Baseline | Post-race | Baseline | Post-race | |||
| Laboratory | ||||||
| Troponin T, pg/ml | 6 ± 3 | 65 ± 103 | <0.05 | 8 ± 6 | 40 ± 26 | <0.01 |
| CK, U/I | 267 ± 195* | 510 ± 252 | <0.001 | 128 ± 50 | 377 ± 218 | <0.01 |
| CK-MB, U/I | 14 ± 16 | 35 ± 15 | <0.001 | 4 ± 9 | 36 ± 18 | <0.01 |
| NT-proBNP, pg/ml | 34 ± 23 | 98 ± 58 | <0.0001 | 88 ± 150 | 185 ± 185 | <0.05 |
| Haematocrit, % | 44 ± 2 | 43 ± 2* | <0.05 | 43 ± 6 | 41 ± 4 | 0.056 |
| Haemoglobin, mg/dl | 14.8 ± 0.8 | 14.5 ± 0.9 | 0.109 | 14.7 ± 0.9 | 14.1 ± 1.0 | <0.05 |
| Creatinine, mg/dl | 1.04 ± 0.15 | 1.15 ± 0.17 | <0.001 | 0.96 ± 0.11 | 1.07 ± 0.20 | <0.05 |
| CMR – left heart | ||||||
| LVEDVi, ml/m2 | 102 ± 14 | 96 ± 13 | <0.01 | 100 ± 16 | 96 ± 17 | 0.162 |
| LVESVi, ml/m2 | 40 ± 8 | 37 ± 9 | 0.281 | 36 ± 9 | 37 ± 11 | 0.417 |
| LV ejection fraction, % | 61 ± 5 | 61 ± 6 | 0.872 | 64 ± 8 | 62 ± 7 | 0.432 |
| Heart rate, beats/min | 54 ± 8 | 67 ± 11 | <0.0001 | 56 ± 10 | 66 ± 10 | <0.01 |
| LV cardiac index, l/min/m2 | 3.3 ± 0.5 | 3.9 ± 0.6 | <0.001 | 3.5 ± 0.8 | 3.9 ± 0.5 | 0.089 |
| LV mass index, g/m2 | 78 ± 10† | 79 ± 10* | 0.341 | 88 ± 7 | 87 ± 9 | 0.557 |
| LAEDVi, ml/m2 | 16 ± 6* | 14 ± 5† | <0.001 | 22 ± 7 | 20 ± 5 | <0.05 |
| LAESVi, ml/m2 | 44 ± 10 | 34 ± 7† | 0.114 | 53 ± 14 | 43 ± 9 | 0.399 |
| LA ejection fraction, % | 64 ± 9 | 59 ± 6* | <0.05 | 58 ± 12 | 53 ± 6 | 0.151 |
| CMR – right heart | ||||||
| RVEDVi, ml/m2 | 103 ± 19 | 100 ± 16 | 0.145 | 104 ± 21 | 95 ± 17 | 0.080 |
| RVESVi, ml/m2 | 44 ± 13 | 42 ± 12 | 0.174 | 40 ± 14 | 37 ± 12 | 0.453 |
| RV ejection fraction, % | 58 ± 6 | 58 ± 7 | 0.999 | 62 ± 10 | 61 ± 8 | 0.839 |
| RAEDVi, ml/m2 | 28 ± 7 | 27 ± 8 | 0.861 | 30 ± 10 | 29 ± 10 | 0.736 |
| RAESVi, ml/m2 | 50 ± 14 | 45 ± 10 | <0.01 | 52 ± 16 | 49 ± 13 | 0.442 |
| RA ejection fraction, % | 45 ± 9 | 40 ± 8 | 0.085 | 42 ± 12 | 42 ± 8 | 0.942 |
| RV cardiac index, l/min/m2 | 3.2 ± 0.5 | 3.9 ± 0.8 | <0.001 | 3.5 ± 0.8 | 3.7 ± 0.6 | 0.461 |
| Mapping parameters | ||||||
| Global T2, ms | 54 ± 2 | 53 ± 4 | 0.353 | 53 ± 4 | 54 ± 2 | 0.387 |
| Global T1, ms | 982 ± 22 | 989 ± 21 | 0.605 | 1002 ± 36 | 995 ± 22 | 0.697 |
| Diastolic function | ||||||
| Early peak filling rate index, ml/s/m2 | 242 ± 58 | 248 ± 45 | 0.639 | 239 ± 87 | 221 ± 82 | 0.433 |
| Atrial peak filling rate index, ml/s/m2 | 121 ± 30† | 163 ± 57 | <0.001 | 161 ± 34 | 169 ± 50 | 0.747 |
| Peak filling rate ratio | 2.1 ± 0.8* | 1.7 ± 0.7 | <0.05 | 1.6 ± 0.7 | 1.3 ± 0.5 | 0.214 |
Numbers are mean±SD for continuous and n (%) for categorical data.
*P < 0.05 and †P < 0.01 for LGE + versus LGE–.
LA: left atrial; LAEDVi: left atrial end-diastolic volume index; LAESVi: left atrial end-systolic volume index; LGE: late gadolinium enhancement; LV: left ventricular; LVEDVi: left ventricular end-diastolic volume index; LVESVi: left ventricular end-systolic volume index; NT-proBNP: N-terminal pro-brain natriuretic peptide; RA: right atrial; RAEDVi: right atrial end-diastolic volume index; RAESVi: right atrial end-systolic volume index; RV: right ventricular; RVEDVi: right ventricular end-diastolic volume index; RVESVi: right ventricular end-systolic volume index; VO2max: maximal oxygen uptake.
Figure 4.
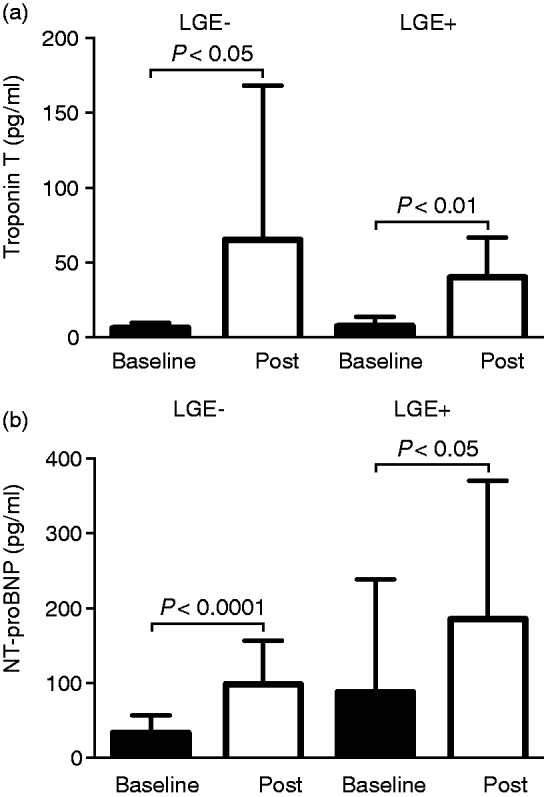
Troponin T (a) increased similarly in LGE– and LGE + triathletes after the race. N-terminal pro-brain natriuretic peptide (NT-proBNP) (b) also increased in both triathlete groups; however, there was a trend towards higher post-race values in LGE + triathletes compared to LGE– triathletes (P = 0.061). LGE: late gadolinium enhancement.
Discussion
This study analysed the occurrence of myocardial oedema and cardiac dysfunction including LV filling by CMR after an endurance race in triathletes with and without myocardial fibrosis. The major findings were: (a) We did not observe myocardial oedema post-race by T2 and T1 mapping despite increased cardiac biomarkers; (b) LGE + triathletes had a reduced mean post-race LA ejection fraction compared to LGE– triathletes indicating exercise-induced LA dysfunction; and (c) The atrial peak filling rate index increased post-race in LGE– triathletes, but not in LGE + triathletes, potentially indicating an exercise-induced impairment of the LV filling pattern.
Myocardial oedema after competition
Gaudreault et al. reported the presence of transient myocardial oedema in runners detected by standard T2w CMR within the first 6–48 hours after a marathon.8 In contrast, we did not find elevated myocardial T2 and T1 relaxation times following an endurance race. Although we cannot rule out mild oedema below the detectable limit, the presence of myocardial oedema in our cohort is very unlikely considering the superior accuracy of T2 and T1 mapping to detect myocardial oedema compared to standard T2w short tau inversion recovery (STIR) imaging.22,23 However, some differences in the study design could also explain these contradicting oedema findings. Firstly, the interval between race and CMR scan was different. Gaudreault et al. performed CMR within 6 to 48 hours post-race, whereas we obtained the CMR within 5 hours after the race. Although experimental studies showed that myocardial oedema occurs within 15 minutes after acute myocardial injury,24 we cannot exclude that the myocardial oedema was missed in our study by performing CMR ‘too early’. Secondly, it is possible that our triathletes were better trained compared to the athletes of Gaudreault et al., who found myocardial oedema more frequently in less fit marathon runners.8 In conclusion, our data at least show that there is no detectable myocardial oedema by mapping techniques early after an endurance race in trained triathletes.
Change of cardiac volumes and function in triathletes following a competitive race
Unlike previous studies, which either showed unchanged12 or increased right heart volumes2 or decreased left heart volumes2,12,25 we observed decreased post-race diastolic volumes for all four heart chambers. It has been suggested that a reduced diastolic LV volume is related to a lower volume status of the athlete.26 However, our data showed that the decrease in the heart volumes was accompanied by an increase in heart rate, which resulted in increased LV and RV cardiac output post-race. The increased heart rate and cardiac output are most likely related to normal thermoregulation of the body caused by hyperthermia, as 70–80% of the produced energy is released as heat.27
Differences in clinical and CMR parameters between LGE + and LGE– triathletes at baseline
Our LGE + triathletes were characterised by a larger LV mass index, higher peak exercise systolic blood pressure and larger LA volume at baseline. It is well known for patients with hypertension and for the general population that an increased LV mass is associated with an increased LA volume.28 Furthermore, it has been shown for the general population that LA ejection fraction is an independent predictor of mortality independent of traditional risk factors and LV ejection fraction.29 Currently, it is questionable whether data from the general population can be applied to healthy triathletes. Nevertheless, the increase of exercise systolic blood pressure, LV mass index and LA volume in LGE + triathletes suggests that the higher LV mass in this group does not represent normal LV hypertrophy in terms of an athlete's heart. Our data suggest a pathological response to exercise with consequential structural changes including excessive LV hypertrophy and LA dilatation. Analysis of the LV filling by CMR revealed that LGE– triathletes had ‘supranormal’ diastolic LV filling, whereas LGE + triathletes had ‘pseudonormal’ diastolic LV filling, which was similar to that of controls. It was reported that endurance athletes are characterised by a ‘supranormal’ LV filling pattern with an increased E/A ratio.30 The authors suggested that the ‘supranormal’ LV filling is attributed to an increased LV relaxation and reduced myocardial stiffness in athletes.30 Conversely, the ‘pseudonormal’ diastolic LV filling in our LGE + triathletes is most likely related to increased myocardial stiffness either caused by abnormal LV hypertrophy or by the observed increased ECV indicating diffuse myocardial fibrosis.
Differences in race-induced changes between LGE + and LGE– triathletes
LGE + triathletes had a lower post-race LA ejection fraction compared to LGE– triathletes, indicating a race-induced LA dysfunction in LGE + triathletes. Furthermore, we observed a trend for higher post-race NT-proBNP values in LGE + triathletes, potentially related to increased LV pressure during competition in these triathletes. Our data suggest that the reduced LA ejection fraction in LGE + triathletes could potentially be mediated by a higher blood pressure during competition, resulting in LA dysfunction. This assumption is further supported by the observed increased peak systolic blood pressure during exercise testing. In conclusion, our data support the hypothesis that an unfavourable blood pressure response to exercise may result in myocardial injury resulting in LA dysfunction.
Limitations
We have not performed blood pressure measurements during the race, which could have further supported the hypothesis that the systolic blood pressure is pathologically increased in LGE + triathletes during competition. However, we studied blood response to exercise testing at baseline revealing higher systolic blood pressure in LGE + triathletes compared to LGE– triathletes. Furthermore, previous studies revealed that a substantial number of athletes are characterised by an unfavourable blood pressure response to exercise, which can be seen as a precursor of hypertension at rest in athletes.31,32
Conclusions
This study about post-race myocardial injury and cardiac dysfunction revealed that the observed troponin T increase was not accompanied by myocardial oedema detectable by T2 or T1 relaxation times. However, we observed elevated post-race NT-proBNP values, possibly related to increased LV pressure during competition. Interestingly, this NT-proBNP release was more pronounced in LGE + triathletes, who were characterised by higher peak systolic blood pressure during exercise testing, indicating unfavourable blood pressure response during workout. In addition, LGE + triathletes had lower post-race LA ejection fraction compared to LGE– triathletes, which could also be a result of increased exercise blood pressure. Further studies are needed including blood pressure measurements during competition to understand better the mechanisms that are responsible for race-induced cardiac dysfunction and myocardial injury.
Author contribution
ET, KM, AP and GKL contributed to the conception or design of the work. ET, BS, JS, KM, RF, BjS, MW, CS, ES, SB, UKR, PS, AP, MP and GKL contributed to the acquisition, analysis, or interpretation of data for the work. ET, KM and GKL drafted the manuscript. All authors critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Stehning is an employee of Philips Healthcare, Hamburg, Germany. The remaining authors report no conflicts of interest.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Neilan TG, Januzzi JL, Lee-Lewandrowski E, et al. Myocardial injury and ventricular dysfunction related to training levels among non-elite participants in the Boston marathon. Circulation 2006; 114: 2325–2333. [DOI] [PubMed] [Google Scholar]
- 2.La Gerche A, Burns AT, Mooney DJ, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 2012; 33: 998–1006. [DOI] [PubMed] [Google Scholar]
- 3.Skadberg Ø, Kleiven Ø, Bjørkavoll-Bergseth M, et al. Highly increased troponin I levels following high-intensity endurance cycling may detect subclinical coronary artery disease in presumably healthy leisure sport cyclists: the North Sea Race Endurance Exercise Study (NEEDED) 2013. Eur J Prev Cardiol 2017; 24: 885–894. [DOI] [PubMed] [Google Scholar]
- 4.Breuckmann F, Möhlenkamp S, Nassenstein K, et al. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology 2009; 251: 50–57. [DOI] [PubMed] [Google Scholar]
- 5.La Gerche A, Baggish AL, Knuuti J, et al. Cardiac imaging and stress testing asymptomatic athletes to identify those at risk of sudden cardiac death. JACC: Cardiovasc Imaging 2013; 6: 993–1007. [DOI] [PubMed] [Google Scholar]
- 6.Zorzi A, Perazzolo Marra M, Rigato I, et al. Non-ischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol 2016; 9: e004229. DOI: 10.1161/CIRCEP.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Schoor FR, Aengevaeren VL, Hopman MTE, et al. Myocardial fibrosis in athletes. Mayo Clin Proc 2016; 91: 1617–1631. [DOI] [PubMed] [Google Scholar]
- 8.Gaudreault V, Tizon-Marcos H, Poirier P, et al. Transient myocardial tissue and function changes during a marathon in less fit marathon runners. Can J Cardiol 2013; 29: 1269–1276. [DOI] [PubMed] [Google Scholar]
- 9.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson 2013, pp. 15: 35. DOI: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giri S, Chung Y-C, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magnetic Reson 2009; 11: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ugander M, Bagi PS, Oki AJ, et al. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JCMG 2012; 5: 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson M, O'Hanlon R, Prasad S, et al. Biological markers of cardiac damage are not related to measures of cardiac systolic and diastolic function using cardiovascular magnetic resonance and echocardiography after an acute bout of prolonged endurance exercise. Br J Sports Med 2011; 45: 780–784. [DOI] [PubMed] [Google Scholar]
- 13.Tahir E, Starekova J, Muellerleile K, et al. Myocardial fibrosis in competitive triathletes detected by contrast-enhanced CMR correlates with exercise-induced hypertension and competition history. JACC: Cardiovasc Imaging 2018; 11: 1260–1270. [DOI] [PubMed] [Google Scholar]
- 14.Baessler B, Schaarschmidt F, Stehning C, et al. A systematic evaluation of three different cardiac T2-mapping sequences at 1.5 and 3T in healthy volunteers. Eur J Radiol 2015; 84: 2161–2170. [DOI] [PubMed] [Google Scholar]
- 15.Le T-T, Tan RS, De Deyn M, et al. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magnetic Reson 2016; 18: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 17.Radunski UK, Lund GK, Stehning C, et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC: Cardiovasc Imaging 2014; 7: 667–675. [DOI] [PubMed] [Google Scholar]
- 18.Moon JC, Messroghli DR, Kellman P, et al. Society for Cardiovascular Magnetic Resonance Imaging, Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magnetic Reson 2013; 15: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schelbert EB, Piehler KM, Zareba KM, et al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc 2015; 4: e002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoennagel BP, Fischer R, Grosse R, et al. Peak filling rates assessed by CMR imaging indicate diastolic dysfunction from myocardial iron toxicity. JACC: Cardiovasc Imaging 2016; 9: 1353–1354. [DOI] [PubMed] [Google Scholar]
- 21.Aquaro GD, Pizzino F, Terrizzi A, et al. Diastolic dysfunction evaluated by cardiac magnetic resonance: the value of the combined assessment of atrial and ventricular function. Eur Radiol 2019; 29: 1555–1564. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira VM, Piechnik SK, Dall'Armellina E, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC: Cardiovasc Imaging 2013; 6: 1048–1058. [DOI] [PubMed] [Google Scholar]
- 23.Tahir E, Sinn M, Bohnen S, et al. Acute versus chronic myocardial infarction: diagnostic accuracy of quantitative native T1 and T2 mapping versus assessment of edema on standard T2-weighted cardiovascular MR images for differentiation. Radiology 2017; 285: 83–91. [DOI] [PubMed] [Google Scholar]
- 24.Jennings RB, Schaper J, Hill ML, et al. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res 1985; 56: 262–278. [DOI] [PubMed] [Google Scholar]
- 25.O'Hanlon R, Wilson M, Wage R, et al. Troponin release following endurance exercise: is inflammation the cause? a cardiovascular magnetic resonance study. J Cardiovasc Magnetic Reson 2010; 12: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kean AJ, McCloskey VR, Seghatol FF, et al. Preservation of ventricular function in amateur athletes after completion of a marathon. J Am Soc Echocardiogr 2006; 19: 202–205. [DOI] [PubMed] [Google Scholar]
- 27.di Prampero PE. Energetics of muscular exercise. Rev Physiol Biochem Pharmacol 1981; 89: 143–222. [DOI] [PubMed] [Google Scholar]
- 28.Oliver W, Matthews G, Ayers CR, et al. Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging 2017; 10: pii: e005047. DOI: 10.1161/CIRCIMAGING.116.005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Matulevicius SA, Ayers CR, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J 2013; 34: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claessens PJ, Claessens CW, Claessens MM, et al. Supernormal left ventricular diastolic function in triathletes. Tex Heart Inst J 2001; 28: 102–110. [PMC free article] [PubMed] [Google Scholar]
- 31.Pressler A, Jähnig A, Halle M, et al. Blood pressure response to maximal dynamic exercise testing in an athletic population. J Hypertens 2018; 36: 1803–1809. [DOI] [PubMed] [Google Scholar]
- 32.Caselli S, Serdoz A, Mango F, et al. High blood pressure response to exercise predicts future development of hypertension in young athletes. Eur Heart J 2019; 40: 62–68. [DOI] [PubMed] [Google Scholar]