Abstract
Background
Safety and efficacy of botulinum toxin A for glabellar line (GL) treatment are well established. Currently approved formulations require reconstitution before injection.
Objectives
The authors sought to assess 6-month efficacy, safety, and patient satisfaction of new ready-to-use abobotulinumtoxinA solution for injection (ASI) in patients with moderate-to-severe GL at maximum frown.
Methods
The authors conducted a phase 3, double-blind, randomized, placebo-controlled trial (NCT02353871). Patients (N = 185) were randomized (2:1) to receive ASI 50 U or placebo. GL severity was evaluated at days 8, 15, 29, 57, 85, 113, 148, and 183 employing a 4-point scale for investigator’s live assessment (ILA) and subject's self-assessment (SSA). Primary endpoint was ILA of GL at maximum frown at day 29, and secondary endpoints were ILA and SSA of GL at maximum frown (all time points), patient satisfaction with GL appearance, time to onset, and duration of action.
Results
Responder rates were significantly higher for ASI vs placebo (88.3% vs 1.4%; P < 0.0001) at day 29 by ILA and all time points by ILA (P < 0.0001-0.0441) and SSA (P < 0.0001-0.0036). Sixty percent of patients reported onset of treatment response on or before day 3 (P < 0.0001 vs placebo), and in 5% of patients, efficacy by ILA lasted 6 months (day 183; P = 0.0441 vs placebo). Patient satisfaction rates were significantly higher for ASI vs placebo at all visits (P < 0.0001). Safety was comparable with the known abobotulinumtoxinA profile.
Conclusions
ASI was significantly efficacious for improving moderate or severe GL vs placebo by investigator and patient assessment. ASI was associated with high patient satisfaction, a long duration of action, and comparable safety profile to abobotulinumtoxinA.
Level of Evidence: 1

Of the 5 factors known to contribute to “the aging face,”1 the skin and underlying muscles play a significant role in the emergence of noticeable lines and folds, including glabellar lines (GL).2 Patients and clinicians recognize the importance of GL in self-perception, perception by others, and emotional and psychological well-being.2-4
Clostridium botulinum toxin type A (BoNT-A) is a potent neurotoxin with 7 serotypes (A-G).5 All serotypes of BoNT-A act selectively on peripheral cholinergic nerve endings, blocking/inhibiting release of acetylcholine, and reducing muscle contraction.5 BoNT-A smooths facial lines, including GL, due to its efficacy in temporarily relaxing the procerus and corrugator muscle complex.5 The efficacy and safety of the 3 most commonly used BoNT-As (abobotulinumtoxinA: Dysport, Ipsen Ltd, Slough, UK/Azzalure, Galderma SA, Lausanne, Switzerland; incobotulinumtoxinA: Bocouture/Xeomin, Merz Pharmaceuticals, Inc., Frankfurt, Germany; onabotulinumtoxinA: Vistabel/Botox, Allergan, Inc., Irvine, CA) in improving the appearance of moderate-to-severe GL have been evaluated in several double-blind, randomized, placebo-controlled, phase 3 clinical trials over the last 15 years.6-13 In these trials, all BoNT-A preparations were well tolerated and reduced the severity of GL for up to 4 months.13,14
All currently approved formulations of BoNT-A are lyophilized preparations that require reconstitution with sodium chloride,15 a process potentially leading to dosing errors and inconsistency among injectors.16-18 To ensure patient safety for aesthetic procedures and avoid any potential practitioner errors, research into the safest and most practical injection procedures is ongoing.
AbobotulinumtoxinA solution for injection (ASI; Dysport, Ipsen Ltd, Slough, UK; Azzalure, Galderma Ltd, Lausanne, Switzerland) is an injectable, liquid form of BoNT-A. By virtue of its liquid formulation, ASI has the potential to improve safety and dosing accuracy compared with lyophilized BoNT-A preparations. The aim of the present phase 3 trial was to assess the efficacy, safety, and patient satisfaction of a single injection of liquid formulation abobotulinumtoxinA, ASI, for improving the appearance of moderate-to-severe GL over 6 months. Based on phase 2 study data indicating that a good benefit-to-risk profile was achieved at an ASI dose of 50 U (NCT01333397),19 this dose was selected for comparison with placebo in the present phase 3 double-blind controlled trial.
METHODS
Objectives
This study assessed the efficacy, safety, and patient satisfaction of a single injection of a liquid form of abobotulinumtoxinA, ASI 50 U, for improving the appearance of moderate-to-severe GL compared with placebo over a 6-month period.
Ethics
This study was conducted in accordance with the Declaration of Helsinki, with approval from independent ethics committees or institutional review boards (Committee for the Protection of People Île-de-France II, Ethik-Kommission Landesamt für Gesundheit und Soziales Berlin, and ethics committees of the medical associations of Bavaria, Nordrhein, Hamburg and Hessen), and in accordance with informed consent regulations and the International Conference on Harmonisation Consolidated Guideline on Good Clinical Practice. This study adhered to all local regulatory requirements. All patients provided written informed consent before initiation of any study-related procedure or administration of study treatment.
Study Design
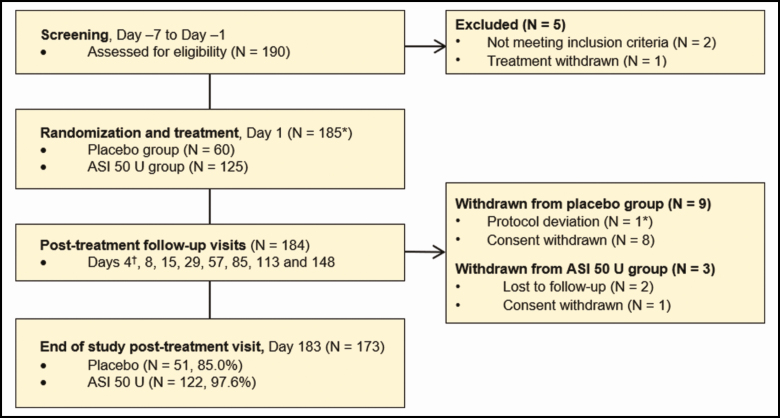
This was a phase 3, randomized, double-blind, placebo-controlled trial (NCT02353871) performed at 9 study centers across France and Germany between January 2015 and August 2015. An overview of the study design is shown in Figure 1.
Figure 1.
Study design and patient disposition. *One patient in the placebo group was randomized but did not receive study treatment. †Day 4 follow-up monitoring of adverse events and concomitant medications via telephone contact. ASI, abobotulinumtoxinA solution for injection.
Inclusion and Exclusion Criteria
All patients were botulinum toxin-naïve adults (aged between 18 and 65 years) with moderate-to-severe (Grade 2 or 3) GL at maximum frown, as assessed by both the investigator (investigator’s live assessment [ILA]; employing a validated 4-point photographic scale)20 and the patient (subject's self-assessment [SSA]; utilizing a 4-point categorical scale to assess the appearance of their GL)21 at baseline. Patients were dissatisfied or very dissatisfied (Grade 2 or 3) with their GL at baseline according to patient’s self-assessed level of satisfaction (using a validated 4-point categorical scale). Female patients were of non-childbearing potential (defined as postmenopausal for at least 1 year) or had a negative pregnancy test.
Patients were excluded from entering the study if they had undergone facial surgery or invasive procedures, including permanent injections, dermal short- and long-duration fillers, skin abrasion, laser procedures, or photorejuvenation before or during the study. Other reasons for exclusion were as follows: presence of marked facial asymmetry, ptosis, excessive dermatochalasis, deep dermal scarring, or thick sebaceous skin; a history of upper eyelid blepharoplasty or brow lift within the previous 5 years; inability to substantially reduce GL by physically spreading them apart; active infection or other skin problem in the glabellar area (eg, acute acne lesions or ulcers); concomitant therapy which, in the investigator’s opinion, would interfere with the evaluation of safety or efficacy of the study treatment; anxiety disorder, drug/alcohol misuse, or other psychiatric disorders; a history of facial nerve palsy; treatment with an experimental drug or device within 30 days before, or during, the study; known allergy or hypersensitivity to any BoNT-A serotype; use of medications that affect neuromuscular transmission; and presence of any other condition (eg, neuromuscular disorder or other disorder that could interfere with neuromuscular function), which, in the investigator’s opinion, might increase risk to the patient or influence the results of the study.
Randomization
Eligible patients (N = 185) were randomized 2:1 to receive either ASI 50 U or placebo utilizing computer-generated randomization lists that were created by a sponsor statistician independent from the study using a validated in-house system developed with SAS procedure PLAN (SAS Institute, Inc., Cary, NC). Randomization was stratified by gender and investigator-assessed severity of GL at maximum frown (moderate vs severe) at baseline.
Treatment
ASI was provided in a vial containing 125 U of abobotulinumtoxinA at a concentration of 200 U/mL. Placebo was provided as a liquid in a vial containing only the excipients of ASI. Each vial contained 0.625 mL of deliverable volume of solution. A volume of 0.25 mL was to be withdrawn from the vial into a syringe for patient administration. In both treatment groups, total treatment volume (0.25 mL) was divided into 5 injections (0.05 mL/injection; corresponding to 50 U of ASI divided into 10 U/injection), each of which was injected into 1 of 5 sites across the glabellar region. One injection was to be administered into the procerus muscle and 2 injections into each corrugator muscle: one directly above the inner canthus and above the bony orbital rim, and the other at 1 cm medially from the vertical pupillary axis and approximately 1 cm superior to the bony orbital rim.
All patients remained at the study center for 30 minutes after treatment for observation. Following telephone contact on day 4 postinjection to evaluate treatment-emergent adverse events (TEAEs) and use of concomitant medications and treatments, patients attended follow-up visits on days 8, 15, 29, 57, 85, 113, 148, and 183 (final study visit). All patients who attended the day 183 visit were considered to have completed the study. Any concomitant medications taken during the study, including prescription and over-the-counter drugs, herbal supplements, and treatments other than study treatment, were recorded.
Endpoints
The primary efficacy endpoint was the proportion of responders (defined under Assessments, below) on day 29, as measured by the investigator (ILA of the appearance of GL at maximum frown).
Secondary efficacy endpoints included:
Proportion of responders at each posttreatment visit (except day 29) as measured by ILA at maximum frown.
Proportion of responders at each posttreatment visit, as measured by SSA at maximum frown.
Proportion of patients with reduction of ≥2 grades in severity of GL at each posttreatment visit, as measured by ILA at maximum frown.
Proportion of responders at each posttreatment visit, as measured by ILA at rest.
Proportion of responders on day 29 who remained responders at each subsequent posttreatment visit, as measured by ILA at maximum frown.
Proportion of responders at each posttreatment visit, as measured by patient’s level of satisfaction with the appearance of their GL.
Time to onset of treatment response based on patient’s diary card.
ASI duration of action based on the ILA and SSA at maximum frown.
Tertiary efficacy endpoints included change from baseline to each posttreatment visit in the following FACE-Q patient-reported outcome parameters:22,23 Rasch transformed score of the satisfaction with facial appearance; Rasch transformed score of the psychological well-being; aging appearance score.
Patients were not anonymized to study investigators and coordinators with access to the electronic case report form (eCRF). Safety endpoints included the recording and monitoring of TEAEs and serious adverse events (AEs), facial physical examination, and vital signs (sitting blood pressure and heart rate).
Assessments
Investigators employed a validated 4-point photographic scale20 to assess GL severity at maximum frown and at rest (Grade 0, none; Grade 1, mild; Grade 2, moderate; Grade 3, severe), at baseline (day 1), and at each scheduled posttreatment office visit. SSA of GL at maximum frown was also recorded at baseline and at each scheduled posttreatment office visit, utilizing a 4-point categorical scale21 (Grade 0, no wrinkles; Grade 1, mild wrinkles; Grade 2, moderate wrinkles; Grade 3, severe wrinkles). A responder was defined as a patient with GL severity Grade 0 or 1 at maximum frown at a given visit, when Grade 2 or 3 at baseline. The duration of action for treatment was assessed utilizing the number of days taken for a responder to reexhibit Grade 2 or 3 severity GL, employing ILA and SSA evaluations.
Patients’ assessment of treatment response was recorded at baseline and each day until day 7 utilizing a diary card in which they responded “yes” or “no” to the question “Since being injected have you noticed an improvement in the appearance of your glabellar lines (lines between your eyebrows)?” Patients completed the diary card at approximately the same time every day. Patients graded their level of satisfaction with the appearance of their GL by means of a 4-point categorical scale (Grade 0, very satisfied; Grade 1, satisfied; Grade 2, dissatisfied; Grade 3, very dissatisfied) at baseline and each scheduled posttreatment office visit. A responder was defined as a patient with satisfaction Grade 0 or 1 at a given visit, when Grade 2 or 3 at baseline.
FACE-Q patient-reported outcomes were assessed employing a subset of 3 scales:22,23 satisfaction with facial appearance, including 10 items (each rated from 1, very dissatisfied to 4, very satisfied); psychological well-being, including 10 items (each rated from 1, definitely disagree to 4, definitely agree); and aging appraisal, utilizing a visual analog scale (−15, I look 15 years younger to +15, I look 15 years older).
TEAEs were monitored from the time the patient gave informed consent to the time when the patient’s participation in the study was considered to have ended. TEAEs, including information on seriousness, intensity, drug relationship, and AEs leading to withdrawal, were monitored at the study center for 30 minutes posttreatment, then on day 4 (telephone follow-up), and at each office visit following treatment. A facial physical examination, recording abnormalities of the skin and musculature, was performed by the investigator at baseline and each subsequent office visit and clinically significant findings recorded as TEAEs. Blood pressure and heart rate while sitting were measured and recorded at baseline (pretreatment and 30 minutes after treatment) and each subsequent office visit.
Statistical Analyses
Statistical analyses were performed employing SAS version 9.3 in accordance with International Conference on Harmonisation E9 guidelines and based on pooled data from individual study sites. A sample size of 24 randomized patients was required according to sample size calculations, and a 5% drop-out rate was assumed so that 27 randomized patients (ASI: n = 18; placebo: n = 9) was estimated to be sufficient to demonstrate superior efficacy of ASI 50 U. Safety assessment required 60 placebo patients; thus, 180 randomized patients were considered sufficient. This sample size resulted in 99% power for testing the primary efficacy endpoint. Efficacy analyses were based on the modified intent-to-treat population (randomized patients with baseline and ≥1 postbaseline value for ILA of GL at maximum frown).
The primary efficacy endpoint was analyzed utilizing a multivariate logistic regression model, including stratification factors (gender and GL baseline severity) and center as fixed effects. Adjusted proportion of responders and 95% confidence intervals (CI) were provided for each treatment group. Analyses of the secondary efficacy endpoint of ILA at maximum frown and at rest, SSA at maximum frown, and patient’s level of satisfaction at each visit were performed as described for the primary efficacy endpoint.
Time to onset of treatment response and duration of action of treatment were analyzed employing a stratified log-rank test and a Cox proportional hazard model, including treatment group, center, and stratification factors as fixed effect. Rasch transformed score for the FACE-Q satisfaction with facial appearance and psychological well-being scales were calculated by converting the total scores to a scale from 0 to 100. Rasch transformed scores and visual analog scale score were analyzed employing a general linear model, with stratification factor and center as fixed effects. The safety population included all randomized patients who received study treatment. AEs were coded using the Medical Dictionary for Regulatory Activities version 18.1.
RESULTS
Study Population
Overall, 185 patients were randomized into the ASI 50-U and placebo groups (n = 125 and 60, respectively). Of those patients, the study was completed by 97.6% of patients in the ASI 50-U group and 85.0% of patients in the placebo group. Patient disposition is shown in Figure 1. The modified intent-to-treat population consisted of 184 patients.
Demographic data and baseline characteristics are shown in Table 1. Overall, demographic characteristics were similar between treatment groups. The proportion of patients with severe GL (Grade 4 on photographic or categorical scales) at baseline was slightly higher when assessed by ILA compared with SSA (ILA: 58.4% and 57.6% for ASI 50 U and placebo, respectively; and SSA: 45.6% and 50.8%, respectively). The mean ± SD duration of follow-up was 179.0 ± 17.8 days in the ASI 50-U group and 171.1 ± 31.4 days in the placebo group.
Table 1.
Baseline Demographics and Patient Characteristics
| Demographic | ASI 50 U (N = 125) | Placebo (N = 60a) |
|---|---|---|
| Mean age (years ± SD) | 47.7 ± 9.75 | 48.0 ± 9.09 |
| Range (min, max) | 24-65 | 27-63 |
| Gender, n (%) | ||
| Female | 108 (86.4) | 52 (86.7) |
| Male | 17 (13.6) | 8 (13.3) |
| Race | ||
| Caucasian, n (%) | 124 (99.2) | 59 (98.3) |
| Black/African American | 0 | 1 (1.7) |
| Other | 1 (0.8) | 0 |
| ILA of GL at maximum frown, n (%) | ||
| Severe | 73 (58.4) | 34 (57.6) |
| Moderate | 52 (41.6) | 25 (42.4) |
| ILA of GL at rest, n (%) | ||
| Severe | 14 (11.2) | 6 (10.2) |
| Moderate | 39 (31.2) | 26 (44.1) |
| Mild | 62 (49.6) | 24 (40.7) |
| None | 10 (8.0) | 3 (5.1) |
| SSA of GL at maximum frown, n (%) | ||
| Severe | 57 (45.6) | 30 (50.8) |
| Moderate | 68 (54.4) | 29 (49.2) |
| Patients’ satisfaction with appearance of GL, n (%) | ||
| Very dissatisfied | 56 (44.8) | 26 (44.1) |
| Dissatisfied | 69 (55.2) | 33 (55.9) |
Ethnicity for all patients was not Hispanic/Latino. ASI, abobotulinumtoxinA solution for injection; GL, glabellar lines; ILA, investigator’s live assessment; SSA, subject's self-assessment; SD, standard deviation. aOne patient was randomized but did not receive study treatment.
Efficacy
Primary Efficacy Endpoint: Proportion of Responders at Day 29 as Measured by the Investigator (ILA of GL at Maximum Frown)
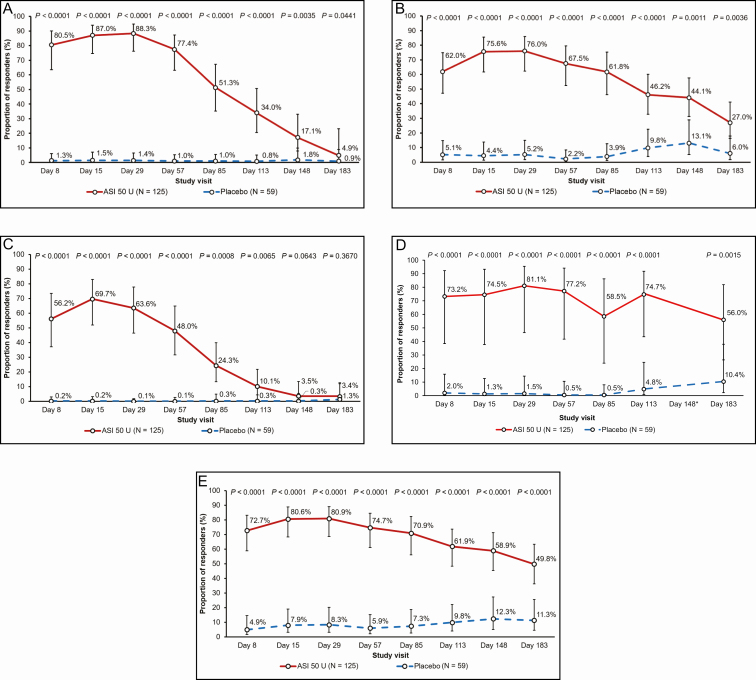
As shown in Figure 2A, the proportion of responders in the ASI 50-U group (88.3%) on day 29 by ILA of GL at maximum frown was significantly (P < 0.0001) greater than with placebo (1.4%).
Figure 2.
Proportion of responders (95% confidence interval) at each time point for (A) investigator’s live assessment of glabellar lines at maximum frown, (B) subject's self-assessment of glabellar lines at maximum frown, (C) reduction of ≥2 grades in severity of glabellar lines, by investigator’s live assessment at maximum frown, (D) investigator’s live assessment of glabellar lines at rest, and (E) patient satisfaction with appearance of glabellar lines. Data are presented as the adjusted proportion (95% confidence interval) at each visit. Responders were defined as patients with a severity grade of none or mild at a given visit, when severity was moderate or severe at baseline. *Proportion of responders was not calculable due to quasi-complete separation of data point. ASI, abobotulinumtoxinA solution for injection; Day, day postinjection.
Proportion of Responders at Each Posttreatment Visit According to the Investigator or Patient
The proportion of responders in the ASI 50-U group was consistently statistically significantly higher compared with placebo at each posttreatment visit according to both the ILA (P < 0.0001-0.0441) and SSA (P < 0.0001-0.0036) of GL at maximum frown (Figure 2A and 2B).
Peak efficacy of ASI 50 U, as measured by ILA, was reported at day 29 (88.3%, P < 0.0001 vs placebo) and lasted up to day 183 in 4.9% (P = 0.0441) of patients (Figure 2A). Notably, 80.5% (P < 0.0001) of patients in the ASI 50-U group were responders as early as day 8 by ILA. By SSA, peak efficacy in the ASI 50-U group was observed at day 29 (76.0%, P < 0.0001), lasting up to day 183 in 27.0% (P = 0.0036) of patients (Figure 2B). In the placebo group, the proportion of responders at maximum frown was between 0.8% and 1.8% by ILA and between 2.2% and 13.1% by SSA across all visits.
The proportion of responders with a reduction in GL severity grade of ≥2 was significantly higher in patients treated with ASI 50 U compared with placebo from day 8 (56.2% vs 0.2%; P < 0.0001) until day 113 (10.1% vs. 0.3%; P = 0.0065), as assessed by ILA at maximum frown (Figure 2C). The proportion of responders in the ILA of GL at rest was relatively stable up to Day 57; between Day 85 and Day 183, the responder rate was more variable over time. The proportion of responders treated with ASI was consistently statistically significant compared with placebo (P < 0.0001-0.0015) across study visits (Figure 2D).
Proportion of Responders on Day 29 With Continued Response Throughout the Study
The proportion of responders in the ASI 50-U group at day 29 (88.3%) who had a continued response at subsequent visits was 87.3% at day 57, gradually decreasing to 5.3% by day 183, according to ILA.
Proportion of Patients Satisfied With the Appearance of Their GL
Significantly larger proportions of patients satisfied with the appearance of their GL were observed in the ASI 50-U group at all study visits up to day 183 compared with placebo (P < 0.0001 at each visit; Figure 2E). Patient satisfaction in the ASI 50-U group peaked at day 29 (80.9%) and gradually decreased over subsequent study visits, with almost half of patients (49.8%) remaining satisfied with the appearance of their GL at day 183. In the placebo group, patient satisfaction with GL appearance ranged between 4.9% and 12.3% across all study visits.
Time to Onset of Treatment Response and Duration of Action
The 95% CI for time to onset of treatment response, based on patient’s diary card, in the ASI 50-U group was 2.0 to 3.0 days, with a median of 3.0 days. This was not calculable for placebo due to the very low numbers of responders. Treatment difference for ASI 50 U compared with placebo was significant (P < 0.0001; both log-rank test and Cox proportional hazard model). In the ASI 50-U group, 60.0% of patients reported onset of treatment response on or before day 3 postinjection (Table 2).
Table 2.
Time to Onset of Treatment Response by Treatment Group
| Days postinjection | ASI 50 U (N = 125) | Placebo (N = 58) | ||
|---|---|---|---|---|
| Onset of response, n (%) | Cumulative nonresponse ratea, % (95% CI) | Onset of response, n (%) | Cumulative nonresponse ratea, % (95% CI) | |
| 1 | 26 (20.8) | 79 (71, 85) | 6 (10.3) | 90 (78, 95) |
| 2 | 32 (25.6) | 54 (44, 62) | 0 | — |
| 3 | 17 (13.6) | 40 (31, 48) | 3 (5.2) | 84 (72, 92) |
| 4 | 12 (9.6) | 30 (23, 39) | 2 (3.4) | 81 (68, 89) |
| 5 | 6 (4.8) | 26 (18, 33) | 0 | — |
| 6 | 5 (4.0) | 21 (15, 29) | 0 | — |
| 7 | 1 (0.8) | 20 (14, 28) | 0 | — |
| >7 or no response | 25 (20.0) | — | 47 (81.0) | — |
Onset of response is defined as the first day patient responds “yes” to the question “Since being injected have you noticed an improvement in the appearance of your glabellar lines (lines between your eyebrows)?” ASI, abobotulinumtoxinA solution for injection; CI, confidence interval. aPercent of patients who had not responded by postinjection day, determined by Kaplan-Meier estimates.
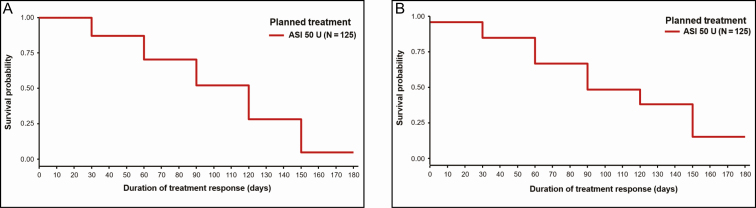
According to ILA at maximum frown, the median (95% CI) duration of action was significantly longer in the ASI 50-U group at 4.5 months (137.0 days; 95% CI: 106.0, 141.0) compared with placebo at 1.6 months (50.0 days; 95% CI: 29.0, 79.0; P < 0.0001, log-rank test; P = 0.0001 Cox proportional hazards model). Similarly, when measured by SSA, the ASI 50-U group had a significantly longer duration of action of 3.6 months (108.0 days; 95% CI: 105.0, 142.0) when compared with placebo at 1.2 months (36.0 days; 95% CI: 29.0, 50.0; P = 0.0037, log-rank test; P = 0.0186, Cox proportional hazards model). Overall, 71% and 67% of responders in the ASI 50-U group by ILA and SSA, respectively, had a duration of action greater than 3.0 months (90 days; Figure 3; Supplemental Table 1, available online at www.aestheticsurgeryjournal.com).
Figure 3.
Kaplan-Meier curves for duration of treatment response based on (A) investigator’s live assessment and (B) subject's self-assessment at maximum frown. ASI, abobotulinumtoxinA solution for injection.
FACE-Q Patient-Reported Outcomes
Least square (LS) mean change from baseline in satisfaction with facial appearance was significantly increased in the ASI 50-U group compared with placebo at each posttreatment visit, except day 183 (LS mean treatment difference in change from baseline ranged from 10.0 points on day 57, P < 0.0001 to 2.7 points on day 183, P = 0.2268). Similarly, the treatment difference for psychological well-being was significantly higher in the ASI 50-U group at all posttreatment visits in favor of abobotulinumtoxinA solution 50 U compared with placebo at all visits (LS mean treatment difference in change from baseline ranged from 11.6 points on day 57, P < 0.0001 to 5.4 points on day 183, P = 0.0279). For aging appearance appraisal, the treatment difference was statistically significant in favor of ASI 50 U at all visits, except day 148 (change from baseline ranged from 1.3 years at day 15, P < 0.0001 to 0.7 years at day 148, P = 0.0809).
Safety
Details of TEAEs are summarized in Table 3. The proportion of patients with ≥1 TEAE was higher in the ASI 50-U group (40.0%) compared with placebo (30.5%; Table 3). There were no deaths or withdrawals due to TEAEs during this study. Of the TEAEs considered treatment related in the ASI 50-U group, the most frequently reported were headache and injection-site pain (Table 3). The only treatment-related TEAE in the placebo group was injection-site pain, occurring in 3 patients (Table 3). None of the treatment-related TEAEs reported in this study were considered severe.
Table 3.
Overall Summary of TEAEs Occurring (Safety Population)
| Number of patients reporting at least one event, n (%) [number of events] | ASI 50 U (N = 125) | Placebo (N = 59) |
|---|---|---|
| At least one TEAE | 50 (40.0) [98] | 18 (30.5) [29] |
| TEAEs occurring in ≥2% patients | ||
| Infections and infestations | 25 (20.0) | 11 (18.6) |
| Nasopharyngitis | 10 (8.0) | 5 (8.5) |
| Influenza | 3 (2.4) | 3 (5.1) |
| Tonsillitis | 3 (2.4) | 0 |
| Nervous system disorders | 18 (14.4) | 3 (5.1) |
| Headache | 17 (13.6) | 2 (3.4) |
| General disorders and administration site conditions | 13 (10.4) | 3 (5.1) |
| Injection site pain | 10 (8.0) | 3 (5.1) |
| Eye disorders | 7 (5.6) | 0 |
| Skin and subcutaneous tissue disorders | 6 (4.8) | 2 (3.4) |
| Brow ptosis | 3 (2.4) | 0 |
| Gastrointestinal disorders | 3 (2.4) | 1 (1.7) |
| Musculoskeletal and connective tissue disorders | 2 (1.6) | 2 (3.4) |
| Injury, poisoning and procedural complications | 2 (1.6) | 2 (3.4) |
| Severe TEAEs | 3 (2.4) [3] | 4 (6.8) [4] |
| At least one related TEAE | 28 (22.4) [32] | 3 (5.1) [3] |
| Nervous system disorders | 11 (8.8) | 0 |
| Headache | 11 (8.8) | 0 |
| General disorders and administration site conditions | 11 (8.8) | 3 (5.1) |
| Injection-site pain | 10 (8.0) | 3 (5.1) |
| Injection-site hypoesthesia | 1 (0.8) | 0 |
| Eye disorders | 4 (3.2) | 0 |
| Eyelid edema | 2 (1.6) | 0 |
| Blepharochalasis | 1 (0.8) | 0 |
| Eyelid ptosis | 1 (0.8) | 0 |
| Skin and subcutaneous tissue disorders | 3 (2.4) | 0 |
| Brow ptosis | 3 (2.4) | 0 |
| Musculoskeletal and connective tissue disorders | 1 (0.8) | 0 |
| Muscle hemorrhage | 1 (0.8) | 0 |
| Injury, poisoning, and procedural complications | 1 (0.8) | 0 |
| Postprocedural contusion | 1 (0.8) | 0 |
Safety population defined as all randomized patients who received at least one injection of study treatment into at least one injection site. ASI, abobotulinumtoxinA solution for injection; TEAE, treatment-emergent adverse event.
Serious TEAEs were reported for 2 patients (1.6%) in the ASI 50-U group; these were severe mydriasis and moderate foot deformity due to hallux rigidus. A serious TEAE was reported for one patient (1.7%) in the placebo group (severe aphthous ulcer). None of these events were considered treatment related by the investigator. The majority of TEAEs were of mild or moderate intensity. Three patients had severe TEAEs in the ASI 50-U group (Table 3): mydriasis, nasopharyngitis, and sinusitis. In the placebo group, severe TEAEs were reported by 4 patients and included aphthous ulcer, influenza, tendon calcification, and ovarian cyst. None of the severe TEAEs reported in either treatment group were considered treatment related by the investigator.
No clinically meaningful changes in vital signs were observed at any study visit in either treatment group. Abnormal findings from the facial physical examination after treatment were reported slightly more frequently in the ASI 50-U group (up to 4 patients with abnormal findings in any facial area at any given visit) than in the placebo group (not more than one patient with abnormal findings in any facial area at any given visit). Similar numbers of abnormal findings were reported for all facial areas and at all visits. In most patients, the abnormality was present already before treatment (at baseline).
DISCUSSION
The present study investigated the efficacy and safety of a new ready-to-use liquid formulation of abobotulinumtoxinA (ASI) compared with placebo for improvement in the appearance of GL in botulinum toxin-naïve patients (N = 185). The dose of ASI administered in this study (50 U) was determined by previous efficacy and clinical safety data.19 The injection sites, doses per muscle, and route of administration were consistent with previous studies,8,24 international consensus recommendations,14 and the currently approved prescribing information for the treatment of GL with abobotulinumtoxinA (Dysport/Azzalure).25 Demographic and baseline characteristics were similar in both treatment groups, and, in agreement with the inclusion criteria, all patients had moderate or severe GL at maximum frown at baseline.
The results of the present study demonstrate not only the efficacy of ASI 50 U compared with placebo, but also support the comparable efficacy of ASI with the approved reconstituted formulation, abobotulinumtoxinA, reported by Ascher et al in the phase 2 comparator-controlled study.19 Ascher et al demonstrated a tendency to an increased responder rate in the ASI 50 U group for both ILA and SSA at day 29 compared with the aboBoNT-A 50 U group (ILA: 91.4% vs 77.1%, respectively, P = 0.1006; SSA: 85.7% vs 82.9%, P = 0.7426).19 In the same study, the responder rate on day 29 for ILA and SSA was significantly higher in the ASI 50 U dose groups compared with placebo. In this present study, a single injection of ASI 50 U was demonstrated to be efficacious for the reduction of moderate or severe GL according to the primary endpoint, with a significantly higher proportion of responders on day 29 with ASI 50 U compared with placebo (88.3% compared with 1.4%, respectively; P < 0.0001). These results are in line with the consistently high proportions of responders observed in previous studies evaluating the efficacy of 50 U of the reconstituted formulation abobotulinumtoxinA after a single treatment cycle by the investigator’s assessment at maximum frown. Brandt et al reported responder rates of 89.5% and 7.5% in patients treated with abobotulinumtoxinA 50 U and placebo, respectively, on day 30 postinjection (P < 0.001),26 and Ascher et al reported responder rates at day 30 of 75.9% and 6.7%, respectively (P < 0.001).24 Comparable results were observed at day 30 after each injection cycle of abobotulinumtoxinA 50 U during long-term open-label studies (Moy et al, 80%-91% at different injection cycles; and Rubin et al, 82%-88%).8,14,27 Although response rates in the present and previous studies are high, they do not reach 100%, even when defined as an improvement of ≥1 severity grade. The reasons for this can be easily explained because a standard dosage will always give standard results. Because patients’ muscles vary in size and activity, some patients may benefit from higher doses. As demonstrated by Kane et al,7 efficacy could be increased by adjusting the dosage of injections to the muscle mass of the patient.
In the present study, significantly higher responder rates were also observed by SSA of GL, at maximum frown on day 29, in the ASI 50-U group compared with placebo (76.0% vs 5.2%, respectively; P < 0.0001). Similarly to the primary endpoint, these responder rates were comparable to those observed by patient’s assessment at day 30 postinjection in previous studies for 50 U of the reconstituted formulation after a single treatment cycle (Brandt et al; 75.7% compared with 9.8% for abobotulinumtoxinA and placebo, respectively; P < 0.001)26 and the first injection of long-term open-label studies (Moy et al, 75%; and Rubin et al, 71%).8,27 The magnitude of the superiority of ASI 50 U compared with placebo observed in this study is greater by ILA than by SSA; this difference was expected because it is commonly reported in previous studies of reconstituted abobotulinumtoxinA.8,26,27 All patients were BoNT-A naïve, which ensures that the efficacy and placebo rates are not biased by previous experience with BoNT-A. Indeed, it could be speculated that non BoNT-A-naïve patients might have lowered the placebo rate because patients would know what to expect.
ASI 50 U had a fast onset of action, with 20.8% of patients reporting a response to treatment on day 1 postinjection, 25.6% on day 2, and 13.6% on day 3. The median time to onset of treatment response was 3 days, and a significant proportion of patients were considered responders (severity grade of 0 or 1; ILA of GL at maximum frown) as early as day 8 (80.5%). These results are considered to be within the expected range of onset of treatment response for reconstituted abobotulinumtoxinA.21
Results from all secondary endpoints were consistently in favor of ASI 50 U compared with placebo during the entire observation period of 183 days. Based on ILA at maximum frown, the median duration of action of ASI 50 U was 4.5 months (137 days; 95% CI: 106.0, 141.0) and treatment response lasted for up to 5 months (17.1% responders on day 148). Indeed, of the patients who were responders on day 29 based on the ILA at maximum frown, 20.1% were still identified as responders on day 148 and 5.3% even at day 183. The median duration of action observed here was longer that the median duration of between 2.8 and 3.8 months (85-117 days) observed in other abobotulinumtoxinA studies in GL.14 Notably, in the present study there was a large proportion of responders as early as day 8 both for ILA and SSA assessments and a relatively high proportion of patients still responding to ASI 50 U at days 113 and 148.
The proportion of patients who were “satisfied” or “very satisfied” with the appearance of their GL was significantly higher with ASI 50 U compared with placebo at all time points during the present study (P < 0.0001). Patient satisfaction peaked at day 29 postinjection (80.9%), with similar responder rates to those previously reported at 1 month postinjection with abobotulinumtoxinA 50 U (Ascher et al 2005: 78.0%).14,28 Interestingly, at day 15, responder rates in the present study were comparable to day 29 (80.6%), whereas a previous study by Ascher et al (2004) with abobotulinumtoxinA reported an increase in patient satisfaction between day 14 (65.5%) and day 29 (86.2%).14,24 One explanation might be a faster onset of effect in the prediluted solution for injection compared with the reconstituted toxin, as previously suggested in a phase 2 study by Ascher et al.19
As assessed using the FACE-Q, a validated and novel patient-reported outcome measure for aesthetic facial procedures,22,23 the present study reported statistically significant treatment differences in favor of the ASI 50-U group compared with placebo for patient satisfaction with facial appearance, psychological well-being, and aging appearance. The FACE-Q patient-reported outcomes recorded in the present study will be the focus of a future publication.
Although a higher proportion of patients in the ASI 50-U group had treatment-related TEAEs compared with placebo, TEAEs were transient and most were of mild or moderate intensity. Additionally, there were no deaths or TEAEs leading to withdrawal in this study. This safety profile was consistent with the well-established safety profile of abobotulinumtoxinA for treatment of GL, and the study did not identify any new or unexpected safety issues.
A recognized limitation of this study is the enrollment of few male patients, which reflects the current situation that male patients are less inclined to request BoNT-A therapy; however, this is comparable to other studies.14,29,30 One might hypothesize that with more male patients, with larger muscle mass, the response rate might have been lower; a previous study by Kane et al showed that response rates may be increased by adjusting the dosage by muscle mass.7 The dose of 50 U in this present study was selected based on a favorable benefit-to-risk profile from a phase 2 study, which examined the clinical effects of ASI at doses of 20, 50, and 75 U in female patients.19 This is the same dose as recommended in the prescribing information for reconstituted formulation abobotulinumtoxinA,25 the efficacy of which has been established.14 The advantages of utilizing a liquid formulation are to circumvent reconstitution errors and injection inconsistencies. However, it is also important to note that injections were not individualized but standardized to a given treatment protocol, and thus response rates may have been lower than is commonly seen in daily practice. It should also be noted that an active comparator, for example, the reconstituted formulation, was not used in this study; however, a comparator-controlled phase 2 study had already been conducted.19 Future studies could address whether gender/muscle mass influences the clinical effects of the liquid formulation.
CONCLUSIONS
This double-blind, randomized, placebo-controlled, phase 3 study demonstrated the efficacy of a novel, ready-to-use liquid formulation of abobotulinumtoxinA (ASI; 50 U; Dysport/Azzalure) for improving moderate or severe GL at maximum frown by day 29 after first injection compared with placebo, as assessed both by investigators (88%, P < 0.0001) and study patients (76%, P < 0.0001). Injection with ASI 50 U was also associated with statistically significantly higher proportions of responders by ILA of GL at rest. Patients’ level of satisfaction with the appearance of GL was consistently significantly higher with ASI 50 U compared with placebo from first injection until the end of the study.
Treatment onset with ASI 50 U was fast, with 60.0% of patients reporting onset of treatment response on or before day 3 postinjection (median of 3 days [95% CI: 2, 3]). A long treatment response was also observed (median 4.5 months [137 days; 95% CI: 106, 141]), with 66% of patients having a response >3 months, 19% having a response >5 months, and some patients (5%) remaining responders at 6 months (day 183, study end). Overall, ASI 50 U was well tolerated, with a safety profile comparable to that of abobotulinumtoxinA.
Supplementary Material
Acknowledgments
Medical writing and submission support, under the direction of the authors, were provided by Jacqueline Harte, Louise Prince, and Germanicus Hansa-Wilkinson of Watermeadow Medical, an Ashfield Company, funded by Ipsen.
Disclosures
Dr Ascher served as a consultant for and has received research grant support from Allergan (Irvine, CA), Ipsen (Paris, France), and Merz (Frankfurt, Germany). He is also an instructor and investigator for Ipsen. Dr Rzany is an advisor and/or speaker for Ipsen and Merz. Dr Kestemont received honoraria from Galderma (Lausanne, Switzerland) for participating in courses and workshops. Dr Hilton has received fees for participation as an investigator in clinical trials from Allergan, Ipsen, and Evolus. Dr Heckmann received honoraria from Allergan, Ipsen, and Evolus (Pune, India) for conducting clinical trials in the field of botulinum toxin research. Dr Noah is a speaker and advisor for Polytech Germany and has received honoraria from Urgo, Allergan, Ipsen, Johnson & Johnson, and Orthogen for conducting clinical trials. Dr Boineau has served as a consultant and speaker for Galderma. Dr Kerscher has received research support and has conducted clinical trials for Merz Pharmaceuticals GmbH (as Head of the Division of Cosmetic Sciences, University of Hamburg, Germany) and has acted as a speaker and/or investigator for Merz, Kythera, Q-Med/Galderma, and Pierre Fabre. Magali Volteau, Philippe Le Berre, and Philippe Picaut are employees of Ipsen. Dr Bodokh declared no potential conflicts of interest with respect to the research, authorship, and publication of this article. All non-Ipsen authors also received compensation from Ipsen for conducting this clinical trial.
Funding
This study was sponsored by Ipsen (Paris, France), who provided funding to the investigational centers involved. Medical writing support was provided by Watermeadow Medical, funded by Ipsen.
REFERENCES
- 1. Elson ML. Evaluation and treatment of the aging face. In: Elson ML, ed. Evaluation and Treatment of the Aging Face. New York, NY: Springer US; 1995:1-8. [Google Scholar]
- 2. Dessy LA, Fallico N, Mazzocchi M, Scuderi N. Botulinum toxin for glabellar lines: a review of the efficacy and safety of currently available products. Am J Clin Dermatol. 2011;12(6):377-388. [DOI] [PubMed] [Google Scholar]
- 3. De Boulle K, Fagien S, Sommer B, Glogau R. Treating glabellar lines with botulinum toxin type A-hemagglutinin complex: a review of the science, the clinical data, and patient satisfaction. Clin Interv Aging. 2010;5:101-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cox SE, Finn JC. Social implications of hyperdynamic facial lines and patient satisfaction outcomes. Int Ophthalmol Clin. 2005;45(3):13-24. [DOI] [PubMed] [Google Scholar]
- 5. Carruthers A, Carruthers J.. Botulinum Toxin. 4th ed. Elsevier Saunders; 2017. [Google Scholar]
- 6. Carruthers JA, Lowe NJ, Menter MA, et al. ; BOTOX Glabellar Lines I Study Group . A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849. [DOI] [PubMed] [Google Scholar]
- 7. Kane MA, Brandt F, Rohrich RJ, Narins RS, Monheit GD, Huber MB; Reloxin Investigational Group . Evaluation of variable-dose treatment with a new U.S. Botulinum Toxin Type A (Dysport) for correction of moderate to severe glabellar lines: results from a phase III, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619-1629. [DOI] [PubMed] [Google Scholar]
- 8. Moy R, Maas C, Monheit G, Huber MB; Reloxin Investigational Group . Long-term safety and efficacy of a new botulinum toxin type A in treating glabellar lines. Arch Facial Plast Surg. 2009;11(2):77-83. [DOI] [PubMed] [Google Scholar]
- 9. Rzany B, Flynn TC, Schlöbe A, Heinz M, Harrington L. Long-term results for incobotulinumtoxinA in the treatment of glabellar frown lines. Dermatol Surg. 2013;39(1 Pt 1):95-103. [DOI] [PubMed] [Google Scholar]
- 10. Carruthers A, Carruthers J, Coleman WP 3rd, et al. . Multicenter, randomized, phase III study of a single dose of incobotulinumtoxinA, free from complexing proteins, in the treatment of glabellar frown lines. Dermatol Surg. 2013;39(4):551-558. [DOI] [PubMed] [Google Scholar]
- 11. Hanke CW, Narins RS, Brandt F, et al. . A randomized, placebo-controlled, double-blind phase III trial investigating the efficacy and safety of incobotulinumtoxinA in the treatment of glabellar frown lines using a stringent composite endpoint. Dermatol Surg. 2013;39(6):891-899. [DOI] [PubMed] [Google Scholar]
- 12. Schlessinger J, Dover JS, Joseph J, et al. ; Dysport Study Group . Long-term safety of abobotulinumtoxinA for the treatment of glabellar lines: results from a 36-month, multicenter, open-label extension study. Dermatol Surg. 2014;40(2):176-183. [DOI] [PubMed] [Google Scholar]
- 13. Jones D, Carruthers J, Narins RS, et al. . Efficacy of incobotulinumtoxinA for treatment of glabellar frown lines: a post hoc pooled analysis of 2 randomized, placebo-controlled, phase 3 trials. Dermatol Surg. 2014;40(7):776-785. [DOI] [PubMed] [Google Scholar]
- 14. Rzany B, Ascher B, Monheit G. Treatment of glabellar lines with botulinum toxin type A (Speywood Unit): a clinical overview. J Eur Acad Dermatol Venereol. 2010;24(Suppl 1):1-14. [DOI] [PubMed] [Google Scholar]
- 15. Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7(2):31-39. [PMC free article] [PubMed] [Google Scholar]
- 16. Carey WD. Incorrect reconstitution of incobotulinumtoxinA leads to loss of neurotoxin. J Drugs Dermatol. 2014;13(6):735-738. [PubMed] [Google Scholar]
- 17. Berdot S, Sabatier B, Gillaizeau F, Caruba T, Prognon P, Durieux P. Evaluation of drug administration errors in a teaching hospital. BMC Health Serv Res. 2012;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bass Kaplan J. The dilution confusion: easy dosing for botulinum toxins. Plast Surg Nurs. 2016;36(1):24-27. [DOI] [PubMed] [Google Scholar]
- 19. Ascher B, Kestemont P, Boineau D, et al. . Liquid formulation of abobotulinumtoxinA exhibits a favorable efficacy and safety profile in moderate to severe glabellar lines: a randomized, double-blind, placebo- and active comparator-controlled trial. Aesthet Surg J. 2018;38(2):183-191. [DOI] [PubMed] [Google Scholar]
- 20. Honeck P, Weiss C, Sterry W, Rzany B; Gladys study group . Reproducibility of a four-point clinical severity score for glabellar frown lines. Br J Dermatol. 2003;149(2):306-310. [DOI] [PubMed] [Google Scholar]
- 21. Baumann L, Brandt FS, Kane MA, Donofrio LM. An analysis of efficacy data from four phase III studies of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6 Suppl):S57-S65. [DOI] [PubMed] [Google Scholar]
- 22. Pusic AL, Klassen AF, Scott AM, Cano SJ. Development and psychometric evaluation of the FACE-Q satisfaction with appearance scale: a new patient-reported outcome instrument for facial aesthetics patients. Clin Plast Surg. 2013;40(2):249-260. [DOI] [PubMed] [Google Scholar]
- 23. Klassen AF, Cano SJ, Schwitzer JA, Scott AM, Pusic AL. FACE-Q scales for health-related quality of life, early life impact, satisfaction with outcomes, and decision to have treatment: development and validation. Plast Reconstr Surg. 2015;135(2):375-386. [DOI] [PubMed] [Google Scholar]
- 24. Ascher B, Zakine B, Kestemont P, Baspeyras M, Bougara A, Santini J. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol. 2004;51(2):223-233. [DOI] [PubMed] [Google Scholar]
- 25. Ipsen Biopharm Ltd. Dysport Full Prescribing Information. 2017. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2019/01/21084019/Dysport_Full_Prescribing_Information.pdf. Accessed August 31, 2018. [Google Scholar]
- 26. Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893-1901. [DOI] [PubMed] [Google Scholar]
- 27. Rubin MG, Dover J, Glogau RG, Goldberg DJ, Goldman MP, Schlessinger J. The efficacy and safety of a new U.S. botulinum toxin type A in the retreatment of glabellar lines following open-label treatment. J Drugs Dermatol. 2009;8(5):439-444. [PubMed] [Google Scholar]
- 28. Ascher B, Zakine B, Kestemont P, et al. . Botulinum toxin A in the treatment of glabellar lines: scheduling the next injection. Aesthet Surg J. 2005;25(4):365-375. [DOI] [PubMed] [Google Scholar]
- 29. Nestor M, Ablon G, Pickett A. Key parameters for the use of abobotulinumtoxinA in aesthetics: onset and duration. Aesthet Surg J. 2017;37(Suppl 1):S20-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen JL, Schlessinger J, Cox SE, Lin X; Reloxin Investigational Group . An analysis of the long-term safety data of repeat administrations of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6 Suppl):S43-S49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.