Abstract
Aim:
To describe treatment patterns among patients with stage III melanoma who underwent surgical excision in years 2011–2016, and assess outcomes among patients who subsequently received systemic adjuvant therapy versus watch-and-wait.
Methods:
Chart review of 380 patients from 17 melanoma centers in North America, South America and Europe.
Results:
Of 129 (34%) patients treated with adjuvant therapy, 85% received interferon α-2b and 56% discontinued treatment (mostly due to adverse events). Relapse-free survival was significantly longer for patients treated with adjuvant therapy versus watch-and-wait (hazard ratio = 0.63; p < 0.05). There was considerable heterogeneity in adjuvant treatment schedules and doses. Similar results were found in patients who received interferon-based adjuvant therapy.
Conclusion:
Adjuvant therapies with better safety/efficacy profiles will improve clinical outcomes in patients with stage III melanoma.
Keywords: : adjuvant therapy, interferon, melanoma, metastatic melanoma, nodal disease, stage III melanoma
Practice points.
As the treatment landscape for stage III melanoma evolves, it is important to have an understanding of the real-world patterns of use and impact of adjuvant therapy in the interferon (IFN) era.
This analysis included 380 patients with stage III melanoma (as recorded in the patients’ charts as part of the routine care) who underwent surgical resection in years 2011–2016 in three global regions, North America, South America, and Europe: 251 (66%) were managed under a watch-and-wait approach and 129 (34%) received adjuvant therapy. In the latter group, 121 patients received IFN-based adjuvant therapy (IFNα2b or pegylated IFNα2b), and 8 received other systemic adjuvant therapies.
The most widely cited reasons for using the watch-and-wait approach were patients’ decision and physician’s perception of poor tolerability of approved adjuvant therapies.
Among patients who received adjuvant therapy, IFNα2b was the most common treatment (85%). However, substantial variation was observed in IFNα2b starting dose/schedule and 56% discontinued therapy.
Despite the heterogeneity in treatment, patients who received adjuvant therapy had a significantly longer relapse-free survival (RFS) compared with patients managed with watch-and-wait (adjusted hazard ratio = 0.63; p < 0.05). Similar results were observed in a subset of patients only treated with IFN-based adjuvant therapy (RFS: adjusted hazard ratio = 0.57; p < 0.01).
The results of this study offer valuable real-world evidence from melanoma centers regarding clinical challenges in the adjuvant setting; there is a need for improved adjuvant therapies to address the unmet need for more tolerable therapies with clearer efficacy benefits in both RFS and overall survival.
Although cutaneous melanoma represents approximately 5% of skin cancers, it accounts for a considerable portion of skin cancer-related deaths [1,2]. In 2018, an estimated 287,723 individuals were diagnosed with cutaneous melanoma worldwide [3]. Over the last several decades, the global incidence of melanoma has increased 3–6% annually and is projected to continue to increase in coming years for both early and advanced stages [2,4,5,6,7].
Surgical excision remains the initial standard of care for patients with primary, node-negative melanoma [8,9,10]. Although surgical excision can greatly benefit patients, some patients experience relapse during their lifetime or face a higher risk of relapse that substantially increases with T substage following surgery [11]. A recent systematic review reported that patients with stage III disease had a 5-year relapse-free survival (RFS) <50% [12,13]. Among patients with stage III disease, there is considerable variability among different stage III substages, regardless of the American Joint Committee on Cancer (AJCC) classification system used. A long-term follow-up of the international Phase III COMBI-AD trial reported 4-year RFS rates of 62%, 37% and 30% in the placebo arm for patients with stage IIIA, IIIB and IIIC melanoma, respectively, when using the 7th edition of the AJCC classification [14]; these rates became 71%, 40%, 33% and 18% for patients with stage IIIA, IIIB, IIIC and IIID, respectively, after the authors performed a post hoc analysis in which tumor stages were determined according to the 8th edition of the AJCC classification [15,16]. Adjuvant therapy has been shown to reduce the risk of relapse in many patients with locally and regionally advanced disease.
Historically, the main adjuvant treatment for resected high-risk melanoma has been high-dose interferon α-2b (IFNα2b) [8]. Results from the pivotal trial that led to its approval in 1995 showed a 5-year RFS rate of 37% among treated patients versus 26% among those untreated after surgical resection [17]. In the same study, the overall survival (OS) was 46% among treated patients versus 37% for untreated [17]. However, conclusive evidence of the benefit of IFNα2b on RFS and OS has been lacking since the publication of that study. For example, high-dose IFNα2b did not consistently or significantly improve OS across studies [17,18,19]. Similarly, the subsequently developed pegylated derivative (i.e., pegylated IFNα2b [pegIFNα2b]; approved in 2011 in the USA [20]) significantly extended RFS, but not OS [21,22]. Moreover, concerns regarding the toxicity associated with these therapies, particularly IFNα2b, led to a high variability in its practical use [17,23]. These inconsistent clinical trial results may limit the use of IFN (IFNα2b or pegIFNα2b) among patients at highest risk of relapse [24]. Furthermore, there is a scarcity of real-world studies regarding the use of adjuvant therapies such as IFN and associated patient outcomes, which could provide a benchmark for the more recently approved adjuvant therapies. These new therapies hold the prospect of improving treatment efficacy, including both RFS and OS, and also of reducing toxicity compared with IFN-based therapies [25].
Examples of recent therapies include immune checkpoint inhibitors nivolumab (approved in the USA [26] in 2017 and Europe [27] in 2018) and pembrolizumab (approved in the USA [28] in 2019 and Europe [29] in 2018) as well as ipilimumab (approved in the USA [30] in 2015) in addition to the small molecule inhibitors dabrafenib plus trametinib (targeted therapies; approved in 2018 the USA [31,32] and Europe [33,34]) for BRAFV600-mutated tumors, which represent the most common type of mutation. Despite these changes, assessing the impact of adjuvant therapy during the IFN era may still provide meaningful insights about the potential benefits and challenges associated with recently approved adjuvant treatments.
The present study was conducted to describe real-world treatment patterns among patients with melanoma who underwent surgical excision in years 2011–2016, and assess clinical outcomes among those who subsequently received systemic adjuvant therapy versus watch-and-wait. The analysis aimed to provide a global perspective of these measurements across eight countries in North America, South America and Europe.
Materials & methods
Data source & study design
In this multicountry, retrospective, center-based chart review study, participating physicians were recruited from 17 melanoma treatment centers or hospitals in North America (Canada and the USA), South America (Argentina and Brazil) and Europe (France, Germany, Spain and the UK). Using a standardized case report form, data abstractors (e.g., physicians, nurse practitioners) extracted de-identified patient-level information (i.e., patient characteristics, treatments and outcomes) for patients who satisfied inclusion criteria from July 2017 to July 2018. Each data abstractor contributed information for 2–50 patient charts.
The date of the first surgical resection for stage III cutaneous melanoma was defined as the index date. By design, all patients had stage III surgery before 1 January 2011 as well as ≥24 months from the index date to the date of chart abstraction (i.e., 24 months of potential follow-up). Study outcomes (patterns of adjuvant therapy, relapses, deaths) were evaluated from the index date until the earliest among death and date of chart abstraction (follow-up period).
Study sample & study cohorts
To be included in this study, patients were required to meet the following criteria: diagnosis of resectable stage III cutaneous melanoma (as recorded in the patients’ charts as part of the routine care; patients presenting with initial resectable lymph node recurrence after a diagnosis of stage I or II melanoma were eligible); ≥18 years of age on the date of the diagnosis of stage III cutaneous melanoma; (3) evidence of complete surgical resection of stage III cutaneous melanoma after 1 January 2011; have ≥24 months of clinical follow-up history following surgical resection, with the exception of deceased patients for whom follow-up until death was required.
Patients were excluded if they had any evidence of incomplete surgical resection as initial surgery for stage III cutaneous melanoma; received a diagnosis of any other type of malignancy before being diagnosed with stage III cutaneous melanoma, unless the patient was disease free of other types of malignancy for ≥5 years prior to the diagnosis of stage III cutaneous melanoma; received adjuvant treatment for melanoma in a clinical trial setting.
Patients were classified into one of two cohorts based on the therapy received after complete surgical resection of stage III cutaneous melanoma and before a relapse. Those who initiated systemic adjuvant therapy in this latter period were included in the adjuvant therapy cohort, while remaining patients were included in the watch-and-wait cohort. Supplementary analyses were performed to assess study outcomes among patients who specifically received an IFN-based adjuvant therapy.
Study measures & outcomes
Patient characteristics that were assessed at the index date included demographics (e.g., age, sex), country of residence, insurance coverage (e.g., public vs private), employment status (e.g., full time, part time, voluntary), days from stage III melanoma diagnosis to surgical resection, and characteristics of stage III melanoma (i.e., substage, tumor thickness, ulceration/mitosis, lymph node involvement, primary location of melanoma; Table 1). Additional characteristics included BRAFV600 testing and BRAFV600 mutation status (e.g., type of mutation). Treatment patterns (e.g., type of adjuvant therapy, dose increases, dose decreases, dose interruptions, add-on therapy) and reasons for treatment discontinuation (e.g., adverse events) were also assessed after the index date and before the first relapse. Physician rationale (e.g., physician reasons for prescribing adjuvant therapy or preferring watchful waiting or other therapies) was described. RFS and OS post-index dates were assessed in patients with adjuvant therapy versus watch-and-wait. All study measures and outcomes were evaluated for the overall study population (all patients with stage III tumor) and in subgroups of patients with stage IIIA, IIIB and IIIC melanoma, based on the AJCC 7th edition [14].
Table 1. . Patient baseline characteristics.
| Baseline characteristics | All patients | Adjuvant therapy‡ | Watch-and-wait§ | p-value¶ |
|---|---|---|---|---|
| N = 380 | N = 129 | N = 251 | ||
| Demographics | ||||
| Country of residence N, (%) | < 0.001† | |||
| – Argentina | 20 (5.3) | 0 (0.0) | 20 (8.0) | |
| – Brazil | 8 (2.1) | 7 (5.4) | 1 (0.4) | |
| – Canada | 15 (3.9) | 12 (9.3) | 3 (1.2) | |
| – France | 67 (17.6) | 17 (13.2) | 50 (19.9) | |
| – Germany | 120 (31.6) | 31 (24.0) | 89 (35.5) | |
| – Spain | 80 (21.1) | 46 (35.7) | 34 (13.5) | |
| – UK | 10 (2.6) | 1 (0.8) | 9 (3.6) | |
| – USA | 60 (15.8) | 15 (11.6) | 45 (17.9) | |
| Age at index date (years) | ||||
| – Mean ± SD [median] | 57.2 ± 14.2 [57] | 52.9 ± 11.6 [52] | 59.3 ± 14.9 [61] | < 0.001† |
| – 65 years or older, N (%) | 127 (33.4) | 22 (17.1) | 105 (41.8) | < 0.001† |
| Males, N (%) | 211 (55.5) | 73 (56.6) | 138 (55.0) | 0.849 |
| Insurance coverage at the time of stage III melanoma, N (%)# | ||||
| Public insurance plan only | 265 (69.7) | 95 (73.6) | 170 (67.7) | 0.284 |
| Private insurance plan only | 42 (11.1) | 12 (9.3) | 30 (12.0) | 0.544 |
| Mix of public/private insurance plan | 9 (2.4) | 5 (3.9) | 4 (1.6) | 0.303 |
| Unknown | 64 (16.8) | 17 (13.2) | 47 (18.7) | 0.221 |
| Employment status at the time of stage III melanoma, N (%) | ||||
| Full time | 160 (42.1) | 67 (51.9) | 93 (37.1) | 0.008† |
| Part time | 17 (4.5) | 6 (4.7) | 11 (4.4) | 1 |
| Voluntary work | 2 (0.5) | 1 (0.8) | 1 (0.4) | 1 |
| Sick leave | 5 (1.3) | 3 (2.3) | 2 (0.8) | 0.445 |
| Unemployed | 23 (6.1) | 10 (7.8) | 13 (5.2) | 0.442 |
| Early retirement | 2 (0.5) | 1 (0.8) | 1 (0.4) | 1 |
| Normal retirement | 99 (26.1) | 16 (12.4) | 83 (33.1) | < 0.001† |
| Other (e.g., disabled, student) | 7 (1.8) | 2 (1.6) | 5 (2.0) | 1 |
| Unknown | 65 (17.1) | 23 (17.8) | 42 (16.7) | 0.901 |
| Comorbidity | ||||
| CCI | ||||
| – Mean ± SD [median] | 2.3 ± 0.8 [2] | 2.2 ± 0.6 [2] | 2.4 ± 0.9 [2] | 0.002† |
| – CCI ≤2, N (%) | 292 (76.8) | 112 (86.8) | 180 (71.7) | 0.001† |
| Tumor stage at initial diagnosis, N (%) | ||||
| Stage I | 64 (16.8) | 11 (8.5) | 53 (21.1) | < 0.001† |
| Stage II | 146 (38.4) | 54 (41.9) | 92 (36.7) | |
| Stage III | 145 (38.2) | 61 (47.3) | 84 (33.5) | |
| Unknown | 25 (6.6) | 3 (2.3) | 22 (8.8) | |
| Stage III characteristics | ||||
| Substage at stage III diagnosis, N (%) | 0.157 | |||
| – Stage IIIA | 146 (38.4) | 55 (42.6) | 91 (36.3) | |
| – Stage IIIB | 168 (44.2) | 58 (45.0) | 110 (43.8) | |
| – Stage IIIC | 66 (17.4) | 16 (12.4) | 50 (19.9) | |
| Lymph node involvement, N (%) | 0.070 | |||
| –Yes | 377 (99.2) | 126 (97.7) | 251 (100.0) | |
| – No | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| –Unknown | 3 (0.8) | 3 (2.3) | 0 (0.0) | |
| Tumor ulceration or mitoses ≥1/mm2, N (%) | 0.274 | |||
| – Absent | 207 (54.5) | 63 (48.8) | 144 (57.4) | |
| – Present | 145 (38.2) | 56 (43.4) | 89 (35.5) | |
| – Unknown | 28 (7.4) | 10 (7.8) | 18 (7.2) | |
| Tumor thickness, N (%) | 0.153 | |||
| – ≤1 mm | 39 (10.3) | 10 (7.8) | 29 (11.6) | |
| – 1.01–2.0 mm | 95 (25.0) | 39 (30.2) | 56 (22.3) | |
| – 2.01–4.0 mm | 106 (27.9) | 28 (21.7) | 78 (31.1) | |
| – >4.0 mm | 112 (29.5) | 42 (32.6) | 70 (27.9) | |
| – Unknown | 28 (7.4) | 10 (7.8) | 18 (7.2) | |
| Primary location of melanoma, N (%) | 0.154 | |||
| – Legs | 132 (34.7) | 45 (34.9) | 87 (34.7) | |
| – Back | 81 (21.3) | 37 (28.7) | 44 (17.5) | |
| – Trunk | 69 (18.2) | 22 (17.1) | 47 (18.7) | |
| – Arms | 54 (14.2) | 16 (12.4) | 38 (15.1) | |
| – Head | 28 (7.4) | 5 (3.9) | 23 (9.2) | |
| – Other | 12 (3.2) | 3 (2.3) | 9 (3.6) | |
| – Unknown | 4 (1.1) | 1 (0.8) | 3 (1.2) | |
| Stage III surgery characteristics | ||||
| Days from stage III diagnosis to surgery, mean ± SD [median] | 30.7 ± 89.7 [8] | 28.4 ± 56.2 [10] | 31.9 ± 102.8 [7] | 0.856 |
| Year of surgery, N (%) | < 0.001† | |||
| – 2011 | 73 (19.2) | 19 (14.7) | 54 (21.5) | |
| – 2012 | 65 (17.1) | 31 (24.0) | 34 (13.5) | |
| – 2013 | 72 (18.9) | 39 (30.2) | 33 (13.1) | |
| – 2014 | 101 (26.6) | 25 (19.4) | 76 (30.3) | |
| – 2015 | 64 (16.8) | 15 (11.6) | 49 (19.5) | |
| – 2016 | 5 (1.3) | 0 (0.0) | 5 (2.0) | |
| BRAF mutation test | ||||
| Tested for BRAF mutation, N (%) | 0.492 | |||
| – Yes | 245 (64.5) | 80 (62.0) | 165 (65.7) | |
| – No | 125 (32.9) | 44 (34.1) | 81 (32.3) | |
| – Unknown | 10 (2.6) | 5 (3.9) | 5 (2.0) | |
| Timing of first BRAF test, N (% tested) | ||||
| – Before relapse | 182 (74.3) | 67 (83.8) | 115 (69.7) | 0.028† |
| – At/after relapse†† | 63 (25.7) | 13 (16.3) | 50 (30.3) | |
| – Mutation identified, N (% tested) | 136 (55.5) | 47 (58.8) | 89 (53.9) | 0.566 |
| Type of mutation, N (% with BRAF) | 0.302 | |||
| – V600E only | 108 (79.4) | 34 (72.3) | 74 (83.1) | |
| – V600K only | 9 (6.6) | 3 (6.4) | 6 (6.7) | |
| – V600R only | 1 (0.7) | 0 (0.0) | 1 (1.1) | |
| – V600K and V600E | 7 (5.1) | 5 (10.6) | 2 (2.2) | |
| – V600, not specified | 3 (2.2) | 1 (2.1) | 2 (2.2) | |
| – Unknown | 8 (5.9) | 4 (8.5) | 4 (4.5) | |
Statistical significance at the 5% level.
The adjuvant therapy cohort was defined as patients who received systemic therapy following initial surgery for stage III (but prior to relapse).
The watch-and-wait cohort was defined as patients who did not receive any systemic therapy following initial surgery for stage III (i.e., watch-and-wait/observation) (prior to relapse).
Statistical comparisons between the adjuvant therapy and watch-and-wait cohorts were performed using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables.
Other type of plan and no insurance plan categories for insurance coverage at the time of stage III melanoma are not shown, as no patient in the study sample was included in either of these categories.
During the follow up period, a relapse (loco-regional, unresectable/metastatic, or secondary primary melanoma) was observed for 168 patients (43 in adjuvant therapy cohort and 125 in watch-and-wait cohort).
CCI: Charlson Comorbidity Index; N: Sample size; SD: Standard deviation.
Statistical analyses
Descriptive analyses were performed using means, medians and standard deviations (SD) to summarize continuous variables, and frequencies and percentages for categorical variables. Time-to-event (i.e., RFS and OS) outcomes were assessed using Kaplan–Meier analyses. RFS was measured from the date of initial surgery for stage III melanoma to the earliest among the date of first relapse (event), date of death (event), or end of follow up (i.e., end of care for the patient or date of data collection; censoring) among patients with known information on time of relapse/death. Similarly, OS was measured from the date of initial surgery for stage III melanoma to the earliest among date of death (event), or end of follow up (i.e., end of care for the patient or date of data collection; censoring) for patients with known information on time of death.
RFS and OS were also compared between cohorts using unadjusted and adjusted Cox proportional hazards models. Hazard ratios (HRs) and 95% CI were reported for each outcome evaluated. Adjusted Cox proportional hazard models were used to control for the following baseline covariates: age at index date, sex, insurance coverage, employment status, tumor substage and Charlson Comorbidity Index (CCI).
Analyses were conducted using SAS Enterprise Guide version 7.1 and R version 3.4.2.
Results
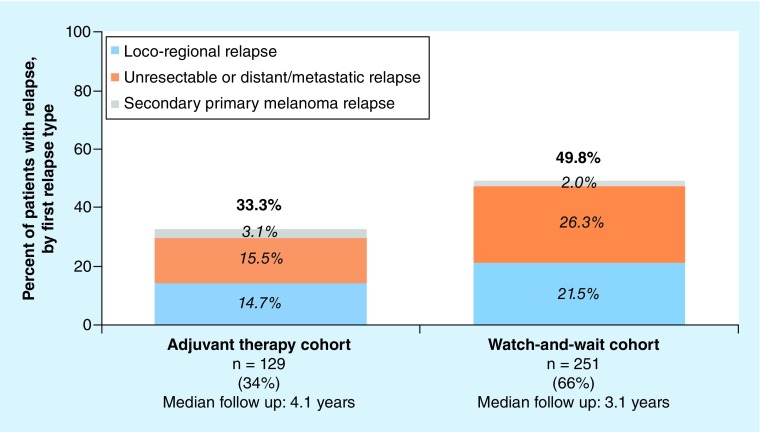
In this study, information was abstracted for 380 patients who met the inclusion criteria. The countries with the greatest geographical representation in the study sample were Germany (32%), Spain (21%), France (18%), the USA (16%) and Argentina (5%). Of the 380 patients, 129 (34%) were included in the adjuvant therapy cohort and 251 (66%) in the watch-and-wait cohort (Table 1; see Supplementary Table 1 for patients treated with IFN-based adjuvant therapy). Patients were followed, on average, for 3.5 years postindex date (median follow up: adjuvant therapy = 4.1 years; watch-and-wait = 3.1 years; Figure 1).
Figure 1. . First relapse postindex date in adjuvant therapy and watch-and-wait cohorts, by type of first relapse.
Baseline characteristics
Use of adjuvant therapy varied significantly across countries (p < 0.001; Table 1). For example, no patients were initiated on adjuvant therapy in Argentina as compared with >80% in Brazil (7/8 patients) and Canada (12/15 patients). Patients in the adjuvant therapy cohort were significantly younger than patients in the watch-and-wait cohort (median age [years]: adjuvant therapy = 52; watch-and-wait = 61; p < 0.001). Among these patients, 145 (38.2%) had primary stage III melanoma and 210 (55.3%) had recurrent stage III at the initial melanoma diagnosis (i.e., lymph node recurrence after resection of stage I or II melanoma); tumor stage at the initial diagnosis was unknown for 25 (6.6%) patients.
The proportion of patients working full time in the adjuvant therapy cohort was significantly greater than that in the watch-and-wait cohort (adjuvant therapy = 52%; watch-and-wait = 37%; p < 0.01). Patients in the adjuvant therapy cohort had a significantly lower CCI compared with those in the watch-and-wait cohort (CCI ≤2: adjuvant therapy = 87%; watch-and-wait = 72%; p < 0.01); however, melanoma clinical characteristics (e.g., stage, ulceration) were generally similar between cohorts.
Overall, 65% of the study samples were tested for BRAFV600 mutation at some time over their course of melanoma. Of the patients tested, the majority (74% overall) were tested before experiencing a relapse (Table 1; see Supplementary Table 1 for patients treated with IFN-based adjuvant therapy). Among the patients who were tested, 56% had a mutation identified, with V600E being the most common type of mutation (79% of those with mutation identified). Baseline characteristics of patients with stage IIIA–IIIC melanoma can be found in Table 2 (see Supplementary Table 2 for patients treated with IFN-based adjuvant therapy).
Table 2. . Patient baseline characteristics stratified by stage.
| Baseline characteristics | Stage IIIA | Stage IIIB | Stage IIIC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All patients | Adjuvant therapy‡ | Watch-and-wait§ | All patients | Adjuvant therapy‡ | Watch-and-wait§ | All patients | Adjuvant therapy‡ | Watch-and-wait§ | |
| N = 146 | N = 55 | N = 91 | N = 168 | N = 58 | N = 110 | N = 66 | N = 16 | N = 50 | |
| Demographics | |||||||||
| Country of residence (%) | |||||||||
| – Argentina | 10 (6.8)† | 0 (0.0)† | 10 (11.0)† | 6 (3.6)† | 0 (0.0)† | 6 (5.5)† | 4 (6.1) | 0 (0.0) | 4 (8.0) |
| – Brazil | 7 (4.8)† | 6 (10.9)† | 1 (1.1)† | 1 (0.6)† | 1 (1.7)† | 0 (0.0)† | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| – Canada | 7 (4.8)† | 5 (9.1)† | 2 (2.2)† | 5 (3.0)† | 5 (8.6)† | 0 (0.0)† | 3 (4.5) | 2 (12.5) | 1 (2.0) |
| – France | 14 (9.6)† | 8 (14.5) | 6 (6.6) | 40 (23.8)† | 7 (12.1)† | 33 (30.0)† | 13 (19.7) | 2 (12.5) | 11 (22.0) |
| – Germany | 51 (34.9)† | 15 (27.3)† | 36 (39.6)† | 49 (29.2)† | 13 (22.4)† | 36 (32.7)† | 20 (30.3) | 3 (18.8) | 17 (34.0) |
| – Spain | 31 (21.2)† | 14 (25.5)† | 17 (18.7)† | 37 (22.0)† | 26 (44.8)† | 11 (10.0)† | 12 (18.2) | 6 (37.5) | 6 (12.0) |
| – UK | 1 (0.7)† | 0 (0.0)† | 1 (1.1)† | 6 (3.6)† | 0 (0.0)† | 6 (5.5)† | 3 (4.5) | 1 (6.3) | 2 (4.0) |
| – USA | 25 (17.1)† | 7 (12.7)† | 18 (19.8)† | 24 (14.3)† | 6 (10.3)† | 18 (16.4)† | 11 (16.7) | 2 (12.5) | 9 (18.0) |
| Age at index date (years) | |||||||||
| Mean ± SD [median] | 56.2 ± 14.2† [55] | 52.1 ± 10.6† [50] | 58.7 ± 15.5† [60] | 56.2 ± 14.1† [56] | 52.3 ± 11.5† [55] | 58.3 ± 14.9† [59] | 61.6 ± 13.8 [65] | 58.2 ± 14.4 [61] | 62.7 ± 13.5 [66] |
| >65 years, N(%) | 44 (30.1)† | 8 (14.5)† | 36 (39.6)† | 49 (29.2)† | 7 (12.1)† | 42 (38.2)† | 34 (51.5) | 7 (43.8) | 27 (54.0) |
| Males, N (%) | 90 (61.6) | 36 (65.5) | 54 (59.3) | 87 (51.8) | 29 (50.0) | 58 (52.7) | 34 (51.5) | 8 (50.0) | 26 (52.0) |
| Insurance coverage at the time of stage III melanoma¶, N (%) | |||||||||
| Public insurance plan only | 93 (63.7) | 37 (67.3) | 56 (61.5) | 125 (74.4) | 45 (77.6) | 80 (72.7) | 47 (71.2) | 13 (81.3) | 34 (68.0) |
| Private insurance plan only | 22 (15.1) | 8 (14.5) | 14 (15.4) | 14 (8.3) | 3 (5.2) | 11 (10.0) | 6 (9.1) | 1 (6.3) | 5 (10.0) |
| Mix of public/private insurance plan | 3 (2.1) | 1 (1.8) | 2 (2.2) | 4 (2.4)† | 4 (6.9)† | 0 (0.0)† | 2 (3.0) | 0 (0.0) | 2 (4.0) |
| Unknown | 28 (19.2) | 9 (16.4) | 19 (20.9) | 25 (14.9) | 6 (10.3) | 19 (17.3) | 11 (16.7) | 2 (12.5) | 9 (18.0) |
| Employment status at the time of stage III melanoma, N (%) | |||||||||
| Full time | 64 (43.8) | 27 (49.1) | 37 (40.7) | 76 (45.2) | 32 (55.2) | 44 (40.0) | 20 (30.3) | 8 (50.0) | 12 (24.0) |
| Part time | 6 (4.1) | 1 (1.8) | 5 (5.5) | 9 (5.4) | 4 (6.9) | 5 (4.5) | 2 (3.0) | 1 (6.3) | 1 (2.0) |
| Voluntary work | 1 (0.7) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (2.0) |
| Sick leave | 2 (1.4) | 2 (3.6) | 0 (0.0) | 3 (1.8) | 1 (1.7) | 2 (1.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unemployed | 7 (4.8) | 4 (7.3) | 3 (3.3) | 13 (7.7) | 6 (10.3) | 7 (6.4) | 3 (4.5) | 0 (0.0) | 3 (6.0) |
| Early retirement | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 1 (1.7) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Normal retirement | 36 (24.7)† | 4 (7.3)† | 32 (35.2)† | 34 (20.2)† | 6 (10.3)† | 28 (25.5)† | 29 (43.9) | 6 (37.5) | 23 (46.0) |
| Other (e.g., military) | 2 (1.4) | 1 (1.8) | 1 (1.1) | 5 (3.0) | 1 (1.7) | 4 (3.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 28 (19.2) | 15 (27.3) | 13 (14.3) | 26 (15.5) | 7 (12.1) | 19 (17.3) | 11 (16.7) | 1 (6.3) | 10 (20.0) |
| Comorbidity | |||||||||
| CCI | |||||||||
| – Mean ± SD [median] | 2.3 ± 0.9 [2]† | 2.2 ± 0.6 [2]† | 2.4 ± 1.0 [2]† | 2.3 ± 0.7 [2] | 2.3 ± 0.7 [2] | 2.3 ± 0.7 [2] | 2.5 ± 0.7 [2] | 2.2 ± 0.4 [2] | 2.6 ± 0.8 [2] |
| – CCI ≤2, N (%) | 115 (78.8)† | 50 (90.9)† | 65 (71.4)† | 136 (81.0) | 49 (84.5) | 87 (79.1) | 41 (62.1) | 13 (81.3) | 28 (56.0) |
| Tumor stage at initial diagnosis, N (%) | |||||||||
| Stage I | 36 (24.7) | 9 (16.4) | 27 (29.7) | 21 (12.5)† | 2 (3.4)† | 19 (17.3)† | 7 (10.6) | 0 (0.0) | 7 (14.0) |
| Stage II | 41 (28.1) | 17 (30.9) | 24 (26.4) | 73 (43.5)† | 30 (51.7)† | 43 (39.1)† | 32 (48.5) | 7 (43.8) | 25 (50.0) |
| Stage III | 65 (44.5) | 28 (50.9) | 37 (40.7) | 58 (34.5)† | 26 (44.8)† | 32 (29.1)† | 22 (33.3) | 7 (43.8) | 15 (30.0) |
| Unknown | 4 (2.7) | 1 (1.8) | 3 (3.3) | 16 (9.5)† | 0 (0.0)† | 16 (14.5)† | 5 (7.6) | 2 (12.5) | 3 (6.0) |
| Stage III characteristics | |||||||||
| Lymph node involvement, N (%) | |||||||||
| – Yes | 145 (99.3) | 54 (98.2) | 91 (100.0) | 167 (99.4) | 57 (98.3) | 110 (100.0) | 65 (98.5) | 15 (93.8) | 50 (100.0) |
| – No | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| – Unknown | 1 (0.7) | 1 (1.8) | 0 (0.0) | 1 (0.6) | 1 (1.7) | 0 (0.0) | 1 (1.5) | 1 (6.3) | 0 (0.0) |
| Tumor ulceration or mitoses ≥1/mm2, N (%) | |||||||||
| – Absent | 126 (86.3) | 47 (85.5) | 79 (86.8) | 55 (32.7)† | 10 (17.2)† | 45 (40.9)† | 26 (39.4) | 6 (37.5) | 20 (40.0) |
| – Present | 14 (9.6) | 4 (7.3) | 10 (11.0) | 94 (56.0)† | 42 (72.4)† | 52 (47.3)† | 37 (56.1) | 10 (62.5) | 27 (54.0) |
| – Unknown | 6 (4.1) | 4 (7.3) | 2 (2.2) | 19 (11.3)† | 6 (10.3)† | 13 (11.8)† | 3 (4.5) | 0 (0.0) | 3 (6.0) |
| Tumor thickness, N (%) | |||||||||
| – ≤1.0 mm | 16 (11.0) | 5 (9.1) | 11 (12.1) | 16 (9.5) | 4 (6.9) | 12 (10.9) | 7 (10.6) | 1 (6.3) | 6 (12.0) |
| – 1.01–2.0 mm | 14 (9.6) | 6 (10.9) | 8 (8.8) | 50 (29.8) | 25 (43.1) | 25 (22.7) | 31 (47.0) | 8 (50.0) | 23 (46.0) |
| – 2.01–4.0 mm | 58 (39.7) | 17 (30.9) | 41 (45.1) | 38 (22.6) | 8 (13.8) | 30 (27.3) | 10 (15.2) | 3 (18.8) | 7 (14.0) |
| – >4.0 mm | 52 (35.6) | 23 (41.8) | 29 (31.9) | 45 (26.8) | 15 (25.9) | 30 (27.3) | 15 (22.7) | 4 (25.0) | 11 (22.0) |
| – Unknown | 6 (4.1) | 4 (7.3) | 2 (2.2) | 19 (11.3) | 6 (10.3) | 13 (11.8) | 3 (4.5) | 0 (0.0) | 3 (6.0) |
| Primary location of melanoma, N (%) | |||||||||
| – Legs | 45 (30.8) | 18 (32.7) | 27 (29.7) | 62 (36.9) | 21 (36.2) | 41 (37.3) | 25 (37.9) | 6 (37.5) | 19 (38.0) |
| – Back | 35 (24.0) | 19 (34.5) | 16 (17.6) | 30 (17.9) | 13 (22.4) | 17 (15.5) | 16 (24.2) | 5 (31.3) | 11 (22.0) |
| – Trunk | 35 (24.0) | 11 (20.0) | 24 (26.4) | 23 (13.7) | 9 (15.5) | 14 (12.7) | 11 (16.7) | 2 (12.5) | 9 (18.0) |
| – Arms | 19 (13.0) | 4 (7.3) | 15 (16.5) | 28 (16.7) | 11 (19.0) | 17 (15.5) | 7 (10.6) | 1 (6.3) | 6 (12.0) |
| – Head | 6 (4.1) | 1 (1.8) | 5 (5.5) | 18 (10.7) | 3 (5.2) | 15 (13.6) | 4 (6.1) | 1 (6.3) | 3 (6.0) |
| – Other | 5 (3.4) | 1 (1.8) | 4 (4.4) | 5 (3.0) | 1 (1.7) | 4 (3.6) | 2 (3.0) | 1 (6.3) | 1 (2.0) |
| – Unknown | 1 (0.7) | 1 (1.8) | 0 (0.0) | 2 (1.2) | 0 (0.0) | 2 (1.8) | 1 (1.5) | 0 (0.0) | 1 (2.0) |
| Stage III surgery characteristics | |||||||||
| Days from stage III diagnosis to surgery, mean ± SD [median] | 39.0 ± 126.8 [11.5] | 23.4 ± 41.1 [10] | 48.5 ± 157.0 [13] | 24.8 ± 49.6 [6.5] | 32.5 ± 66.9 [6.5] | 20.8 ± 37.2 [6.5] | 27.4 ± 66.8 [12.5] | 31.1 ± 61.0 [17.5] | 26.2 ± 69.1 [9] |
| Year of surgery, N (%) | |||||||||
| – 2011 | 28 (19.2)† | 7 (12.7)† | 21 (23.1)† | 28 (16.7)† | 8 (13.8)† | 20 (18.2)† | 17 (25.8) | 4 (25.0) | 13 (26.0) |
| – 2012 | 20 (13.7)† | 10 (18.2)† | 10 (11.0)† | 35 (20.8)† | 19 (32.8)† | 16 (14.5)† | 10 (15.2) | 2 (12.5) | 8 (16.0) |
| – 2013 | 26 (17.8)† | 17 (30.9)† | 9 (9.9)† | 35 (20.8)† | 17 (29.3)† | 18 (16.4)† | 11 (16.7) | 5 (31.3) | 6 (12.0) |
| – 2014 | 41 (28.1)† | 13 (23.6)† | 28 (30.8)† | 45 (26.8)† | 8 (13.8)† | 37 (33.6)† | 15 (22.7) | 4 (25.0) | 11 (22.0) |
| – 2015 | 29 (19.9)† | 8 (14.5)† | 21 (23.1)† | 24 (14.3)† | 6 (10.3)† | 18 (16.4)† | 11 (16.7) | 1 (6.3) | 10 (20.0) |
| – 2016 | 2 (1.4)† | 0 (0.0)† | 2 (2.2)† | 1 (0.6)† | 0 (0.0)† | 1 (0.9)† | 2 (3.0) | 0 (0.0) | 2 (4.0) |
| BRAF mutation test | |||||||||
| – Tested for BRAF mutation, N (%) | |||||||||
| – Yes | 75 (51.4) | 30 (54.5) | 45 (49.5) | 117 (69.6) | 39 (67.2) | 78 (70.9) | 53 (80.3) | 11 (68.8) | 42 (84.0) |
| – No | 64 (43.8) | 20 (36.4) | 44 (48.4) | 49 (29.2) | 19 (32.8) | 30 (27.3) | 12 (18.2) | 5 (31.3) | 7 (14.0) |
| – Unknown | 7 (4.8) | 5 (9.1) | 2 (2.2) | 2 (1.2) | 0 (0.0) | 2 (1.8) | 1 (1.5) | 0 (0.0) | 1 (2.0) |
| – Timing of first BRAF mutation test, N (%) | |||||||||
| – Before relapse | 50 (66.7) | 22 (73.3) | 28 (62.2) | 96 (82.1) | 34 (87.2) | 62 (79.5) | 36 (67.9)† | 11 (100.0)† | 25 (59.5)† |
| – At/after relapse# | 25 (33.3) | 8 (26.7) | 17 (37.8) | 21 (17.9) | 5 (12.8) | 16 (20.5) | 17 (32.1)† | 0 (0.0)† | 17 (40.5)† |
| Mutation identified, N (tested) | 43 (57.3) | 16 (53.3) | 27 (60.0) | 68 (58.1) | 27 (69.2) | 41 (52.6) | 25 (47.2) | 4 (36.4) | 21 (50.0) |
| Type of mutation, N (% with BRAF) | |||||||||
| – V600E only | 36 (83.7) | 13 (81.3) | 23 (85.2) | 50 (73.5) | 18 (66.7) | 32 (78.0) | 22 (88.0) | 3 (75.0) | 19 (90.5) |
| – V600K only | 1 (2.3) | 0 (0.0) | 1 (3.7) | 7 (10.3) | 3 (11.1) | 4 (9.8) | 1 (4.0) | 0 (0.0) | 1 (4.8) |
| – V600K and V600E | 2 (4.7) | 1 (6.3) | 1 (3.7) | 1 (1.5) | 0 (0.0) | 1 (2.4) | 2 (8.0) | 1 (25.0) | 1 (4.8) |
| – Unknown | 4 (9.3) | 2 (12.5) | 2 (7.4) | 3 (4.4) | 3 (11.1) | 0 (0.0) | – | – | – |
Statistical significance at the 5% level.
The adjuvant therapy cohort was defined as patients who received systemic therapy following initial surgery for stage III (but prior to relapse).
The watch-and-wait cohort was defined as patients who did not receive any systemic therapy following initial surgery for stage III (i.e., watch-and-wait/observation) (prior to relapse).
Other type of plan and no insurance plan categories for insurance coverage at the time of stage III melanoma are not shown, as no patient in the study sample was included in either of these categories.
During the follow up, a relapse (loco-regional, unresectable/metastatic, or secondary primary melanoma) was observed for 168 patients (43 in adjuvant therapy cohort and 125 in watch-and-wait cohort).
Statistical comparisons between the adjuvant therapy and watch-and-wait cohorts were performed using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables.
CCI: Charlson Comorbidity Index; N: Sample size; SD: Standard deviation.
Treatment patterns
Adjuvant therapy
Treatment patterns for patients who received adjuvant therapy following surgery for stage III melanoma are summarized in Table 3. In 88% of this patient cohort, the primary reason cited by physicians’ for initiating adjuvant therapy was it being an ‘approved adjuvant therapy’ (Table 3). Most patients in the adjuvant therapy cohort were treated with IFNα2b (85%) followed by pegIFNα2b (9%); the remaining patients received other systemic therapies (Table 3). Of the 110 patients treated with IFNα2b, 74 (67%) received high-dose IFNα2b, 15 (14%) received intermediate-dose IFNα2b and 21 (19%) received low-dose IFNα2b; the definition of high-, intermediate- and low-dose IFNα2b was based on the information available in the patients' chart. There was considerable variation in the treatment doses and schedules for induction and maintenance therapy. The median duration of therapy, among patients who had stopped therapy by the time of data collection with known treatment duration, was 9.4 months.
Table 3. . Treatment patterns of patients who received adjuvant therapy post surgery for stage III melanoma.
| Treatment patterns | All patients N = 129 | Interferon α-2b N = 110 | Peginterferon α-2b N = 11 | Other systemic therapy† N = 8 |
|---|---|---|---|---|
| Primary reason for choosing therapy‡, N (%) | ||||
| Approved adjuvant therapy | 114 (88.4) | 100 (90.9) | 11 (100.0) | 3 (37.5) |
| As per national treatment guidelines | 3 (2.3) | 2 (1.8) | 0 (0.0) | 1 (12.5) |
| As per institutional treatment guidelines | 5 (3.9) | 5 (4.5) | 0 (0.0) | 0 (0.0) |
| Potential improvement on health-related QOL | 1 (0.8) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Potential prolongation of survival | 2 (1.6) | 0 (0.0) | 0 (0.0) | 2 (25.0) |
| Unknown | 4 (3.1) | 2 (1.8) | 0 (0.0) | 2 (25.0) |
| Starting dose, N (%) | ||||
| Starting dose: MU/m2 | NA | NA | ||
| Lower dose than recommended | 5 (4.6) | 0 (0.0) | ||
| 20 MU/m2 (recommended) | 39 (35.5) | 0 (0.0) | ||
| Higher dose than recommended | 28 (25.5) | 0 (0.0) | ||
| Unknown | 38 (34.5) | 11 (100.0) | ||
| Schedule, N (%) | ||||
| Induction | NA | NA | ||
| Less frequent than recommended | 56 (50.9) | 9 (81.8) | ||
| Five-times a week for 4 weeks (recommended) | 46 (41.8) | 0 (0.0) | ||
| More frequent than recommended | 1 (0.9) | 0 (0.0) | ||
| Unknown | 7 (6.4) | 2 (18.2) | ||
| Maintenance | NA | NA | ||
| Less frequent than recommended | 35 (31.8) | 5 (45.5) | ||
| Three-times a week for 48 weeks (recommended) | 17 (15.5) | 0 (0.0) | ||
| More frequent than recommended | 13 (11.8) | 1 (9.1) | ||
| No maintenance | 43 (39.1) | 5 (45.5) | ||
| Unknown | 2 (1.8) | 0 (0.0) | ||
| Overall treatment duration | ||||
| Patients who stopped therapy with known treatment duration information | 110 (85.3) | 98 (89.1) | 8 (72.7) | 4 (50.0) |
| Months of adjuvant therapy (among patients who stopped therapy), mean ± SD [median] | 9.5 ± 6.5 [9.4] | 9.2 ± 6.1 [9.6] | 13.7 ± 10.6 [8.9] | 7.4 ± 6.6 [7.0] |
| Modification of adjuvant treatment, N (%) | ||||
| Any modification§ | 48 (37.2) | 40 (36.4) | 7 (63.6) | 1 (12.5) |
| Dose interruption | 22 (17.1) | 17 (15.5) | 4 (36.4) | 1 (12.5) |
| Dose decrease | 34 (26.4) | 28 (25.5) | 6 (54.5) | 0 (0.0) |
| Dose increase | 2 (1.6) | 2 (1.8) | 0 (0.0) | 0 (0.0) |
| Reason(s) for first modification§ | ||||
| Adverse events | 39 (81.3) | 32 (80.0) | 7 (100.0) | 0 (0.0) |
| Disease relapse | 2 (4.2) | 1 (2.5) | 0 (0.0) | 1 (100.0) |
| Decrease in QOL | 4 (8.3) | 4 (10.0) | 0 (0.0) | 0 (0.0) |
| Other | 6 (12.5) | 5 (12.5) | 1 (14.3) | 0 (0.0) |
| Discontinuation of adjuvant treatment, N (%) | ||||
| Yes | 72 (55.8) | 57 (51.8) | 10 (90.9) | 5 (62.5) |
| Discontinuation decision | ||||
| – Physician decision | 56 (77.8) | 48 (84.2) | 5 (50.0) | 3 (60.0) |
| – Patient decision | 15 (20.8) | 8 (14.0) | 5 (50.0) | 2 (40.0) |
| – Unknown | 1 (1.4) | 1 (1.8) | 0 (0.0) | 0 (0.0) |
| Reason(s)§,¶, N (%) | ||||
| – Severe adverse events# | 16 (22.2) | 15 (26.3) | 1 (10.0) | 0 (0.0) |
| – Nonsevere adverse events | 27 (37.5) | 23 (40.4) | 2 (20.0) | 2 (40.0) |
| – Disease relapse | 12 (16.7) | 8 (14.0) | 1 (10.0) | 3 (60.0) |
| – Decrease in QOL | 5 (6.9) | 2 (3.5) | 3 (30.0) | 0 (0.0) |
| – Completion of planned therapy | 8 (11.1) | 5 (8.8) | 3 (30.0) | 0 (0.0) |
| – Other | 4 (5.6) | 3 (5.3) | 1 (10.0) | 0 (0.0) |
| No | 50 (38.8) | 47 (42.7) | 0 (0.0) | 3 (37.5) |
| – Unknown | 7 (5.4) | 6 (5.5) | 1 (9.1) | 0 (0.0) |
Other systemic therapies include melphalan + TNFα, pembrolizumab, dabrafenib + trametinib, dabrafenib, dacarbazine, sargramostim.
Response options not selected are not shown (i.e., patient’s request; patient age or co-morbidities; intolerance to previous treatment regimen; other).
Multiple response options may be selected for one patient.
Response options not selected are not shown (i.e., resource/budget constraint; death).
A severe adverse event was defined as an event that led to a hospitalization or urgent intervention, limited the patient’s ability to care for him/herself, was life threatening or resulted in death.
MU: Million unit; QOL: Quality of life; N: Sample size; SD: Standard deviation.
Among patients whose IFNα2b starting dose was known, almost half did not receive the recommended dose (33/72; 46%), and more than half did not receive the recommended schedule (57/103; 55%). Deviations from the recommended induction IFNα2b dose/schedule among patients who did not receive the recommended dose included higher doses and/or less frequent induction schedules. A large proportion of IFNα2b users (39%) did not go on to receive the maintenance phase.
Among patients who were treated with adjuvant therapy (IFNα2b or other), 37% had a treatment modification during treatment (most commonly dose reduction), and 56% discontinued adjuvant treatment (as reported by the physician). Adverse events were the most common reason for both treatment modifications (81%) and treatment discontinuation (60%; Table 3). Ten (8%) patients received radiation therapy post surgery. Treatment patterns for patients with stage IIIA, IIIB and IIIC tumors are presented in Supplementary Tables 3–5.
Watch-and-wait approach
Treatment patterns for patients in the watch-and-wait cohort are summarized in Table 4. The primary reasons for choosing a watch-and-wait approach over adjuvant therapy were heterogeneous. Two of the most common reasons were ‘currently approved therapies have poor tolerability profile’ (44/251 patients; 18%) and ‘patient decision’ (48/251 patients; 19%). Nineteen (8%) patients received radiation therapy post surgery (Table 4). Treatment patterns for patients with different substages are presented separately for each tumor substage in Supplementary Table 6.
Table 4. . Treatment patterns of patients who received watch-and-wait post surgery for stage III melanoma.
| Treatment patterns | All patients |
|---|---|
| N = 251 | |
| Reason for selecting watch-and-wait, N (%) | |
| Clinical symptoms/co-morbidity making patient ineligible | 15 (6.0%) |
| –Atherosclerotic disease | 1 (0.4%) |
| – Autoimmune disease | 1 (0.4%) |
| – Bipolar disorder, schizophrenia | 1 (0.4%) |
| – COPD, chronic anemia | 1 (0.4%) |
| – Coronary artery disease | 1 (0.4%) |
| – Depressive syndrome | 1 (0.4%) |
| – Diabetes, heart valve | 1 (0.4%) |
| – Hypertrophic cardiomyopathy, cardiac arrhythmia | 1 (0.4%) |
| – Myocardial infarction | 2 (0.8%) |
| – Pregnancy | 1 (0.4%) |
| – Primary biliary cirrhosis | 1 (0.4%) |
| – Rheumatic arthritis | 1 (0.4%) |
| – Rhizomelic arthritis | 1 (0.4%) |
| – Stroke | 1 (0.4%) |
| Patient not eligible for clinical trial | 20 (8.0%) |
| No clinical trial available | 7 (2.8%) |
| Per treatment guidelines, adjuvant therapy is not recommended if disease-free post surgery | 11 (4.4%) |
| Uncertainty about impact of therapy on future treatment | 2 (0.8%) |
| Currently approved therapies have limited efficacy | 28 (11.2%) |
| Currently approved therapies have poor tolerability profile | 44 (17.5%) |
| Currently approved therapies have negative impact on health-related quality of life | 3 (1.2%) |
| Currently approved therapies are burdensome and inconvenient for patient’s daily life | 11 (4.4%) |
| Other reasons | 82 (32.7%) |
| –Considered radiotherapy | 3 (1.2%) |
| –Disease progression | 3 (1.2%) |
| – Disease surveillance and monitoring | 9 (3.6%) |
| – Inconvenient systemic therapies with limited efficacy | 1 (0.4%) |
| – Patient’s age | 9 (3.6%) |
| –Patient decision | 48 (19.1%) |
| –Patient did not return to center | 2 (0.8%) |
| –Physician decision | 1 (0.4%) |
| –Systemic therapies with limited efficacy and binding treatment | 1 (0.4%) |
| – Unknown | 5 (2.0%) |
| Unknown | 28 (11.2%) |
| Observed duration of ‘watch-and-wait’ period post stage III surgery | |
| Months to relapse/end of follow up (all patients), mean ± SD [median] | 28.0 ± 19.8 [27.5] |
| Months to relapse (126 patients with relapse), mean ± SD [median] | 14.7 ± 13.8 [11.7] |
| Radiation therapy post stage III surgery and prior to first relapse | |
| Patients with radiation therapy, N (%) | 19 (7.6%) |
| Months with radiation therapy, mean ± SD [median] | 0.9 ± 0.8 [0.9] |
| Primary reason for radiation therapy, N (%) | |
| –As per institutional guidelines | 3 (15.8%) |
| – As per national treatment guidelines in Argentina | 1 (5.3%) |
| – As per national treatment guidelines in Germany | 9 (47.4%) |
| – Benefits shown in delaying time to relapse | 5 (26.3%) |
| – Unknown | 1 (5.3%) |
COPD: Chronic obstructive pulmonary disease; N: Sample size; SD: Standard deviation.
Time-to-event analyses
Overall, 44% (168/380) of patients in the study sample experienced a relapse over a median follow up of 3.3 years post index (adjuvant therapy = 33% [43/129] over 4.1 years, watch-and-wait = 50% [125/251] over 3.1 years; Figure 1). The risk of relapse was highest for patients with stage IIIC melanoma (70%); patients with stage IIIA or IIIB had a similar risk of relapse (stage IIIA: 39%, stage IIIB: 39%; Supplementary Figure 1). The first relapse was characterized as unresectable or distant/metastatic for 47% (20/43) of patients with a relapse in the adjuvant therapy cohort and 53% (66/125) of patients with a relapse in the watch-and-wait cohort. Among patients who relapsed, 74% (32/43) and 74% (93/125) of those in the adjuvant therapy cohort and watch-and-wait cohort, respectively, experienced an unresectable or distant/metastatic relapse by the end of follow up.
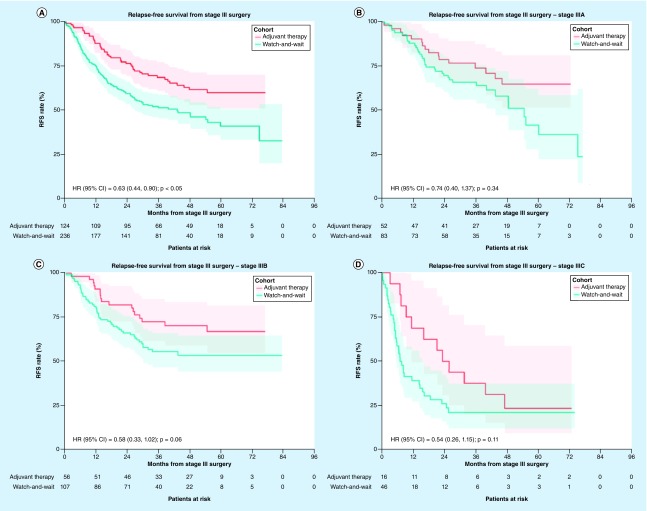
Patients who received adjuvant therapy had a significantly longer RFS compared with those in the watch-and-wait cohort, even after adjusting for differences in baseline characteristics between the cohorts (adjusted HR [95% CI] = 0.63 [0.44–0.90]; p < 0.05; Figure 2A). After 3 years of follow up, 68.7% of patients in the adjuvant therapy cohort were alive and free of relapse; this figure was 52.0% in the watch-and-wait cohort (Figure 2A). When evaluating RFS for patients with different melanoma substages, effect size numerically increased with tumor severity (i.e., stage IIIA: adjusted HR [95% CI] = 0.74 [0.40–1.37], stage IIIC: adjusted HR [95% CI] = 0.54 [0.26–1.15], all p > 0.05), but statistical significance was not reached for any of these comparisons between cohorts for each stage separately (Figure 2B–D). The results from the multivariate Cox model for RFS are summarized in Supplementary Tables 7–10. Results were largely similar when comparing RFS in a subset of patients treated with IFN-based adjuvant therapy and watch-and-wait (overall: HR [95% CI] = 0.57 [0.39–0.84]; p < 0.01; Supplementary Figure 2).
Figure 2. . Relapse-free survival following surgery for stage III melanoma among patients who received adjuvant therapy versus watch-and-wait.
(A) Overall, (B) among patients with stage IIIA, (C) stage IIIB, (D) and stage IIIC melanoma. The adjusted hazard ratio was estimated from a model controlling for baseline characteristics including age at surgery date for stage III, sex, insurance coverage, employment status, subclass of stage III and Charlson Comorbidity Index.
HR: Hazard ratio; RFS: Relapse-free survival.
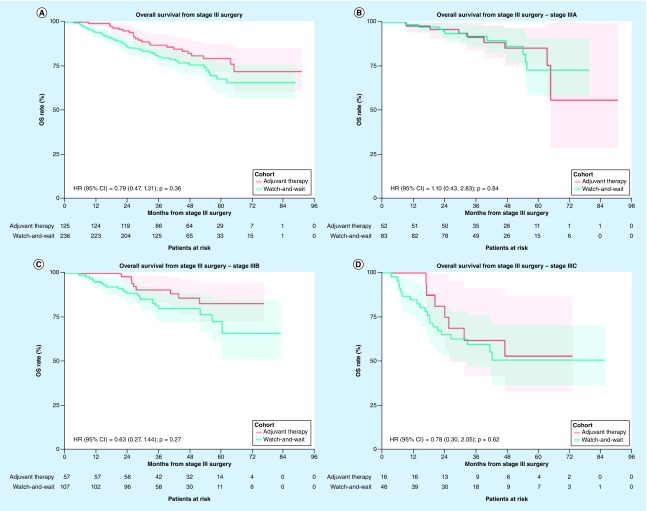
There was a trend toward worse OS in the watch-and-wait cohort, but the difference was not statistically significantly different between both cohorts (adjusted HR [95% CI] = 0.79 [0.47–1.31]; p = 0.36; Figure 3A). After 3 years of follow up, 87.5% of patients in the adjuvant therapy cohort were still alive; this figure was 81.2% among those in the watch-and-wait cohort (Figure 3A). Similar results were observed when assessing patient subgroups defined by melanoma substages, except in patients with stage IIIA melanoma for whom OS was numerically – albeit nonsignificantly – lower in the adjuvant therapy cohort relative to the watch-and-wait cohort (Figure 3B–D). The results from the multivariate Cox model for OS are summarized in Supplementary Tables 11–14. Results were largely similar when comparing OS in a subset of patients treated with IFN-based adjuvant therapy and watch-and-wait (overall: HR [95% CI] = 0.75 [0.43–1.31]; p = 0.31; Supplementary Figure 3).
Figure 3. . Overall survival following surgery for stage III melanoma among patients who received adjuvant therapy versus watch-and-wait.
(A) Overall, (B) among patients with stage IIIA, (C) stage IIIB and (D) stage IIIC melanoma. The adjusted hazard ratio was estimated from a model controlling for baseline characteristics including age at surgery date for stage III, sex, insurance coverage, employment status, subclass of stage III and Charlson Comorbidity Index.
HR: Hazard ratio; OS: Overall survival.
Discussion
In this multicountry, center-based chart review study, real-world treatment patterns and outcomes were evaluated for patients diagnosed with stage III melanoma who underwent complete resection in years 2011–2016 (IFN era) and were treated in a melanoma center. Results demonstrated that only a third of patients with stage III melanoma received adjuvant therapy following surgical resection and that the vast majority of patients were treated with high-dose IFNα2b. Overall, use of adjuvant therapies versus watch-and-wait varied considerably by country. While a lot of heterogeneity was observed in physicians’ reasons for selecting the watch-and-wait approach, the tolerability of approved adjuvant therapies available during the study period (i.e., mainly IFNα2b and pegIFNα2b) was one of the most commonly cited reasons. Approximately half of patients did not receive the recommended treatment schedule of IFN-based adjuvant therapy, and about a quarter were initiated on higher-than-recommended IFN doses. Among patients who received adjuvant therapy, most discontinued treatment prematurely due to unacceptable adverse events. The results of the time-to-event analyses support a clear benefit of adjuvant therapy use with respect to RFS, although the OS benefit was less clear.
The use of high-dose IFNα2b as an adjuvant therapy was a topic of intense debate prior to the advent of new therapies [35,36]. An earlier study from the initial Eastern Cooperative Oncology Group 1684 trial reported a significant, albeit relatively modest, improvement in RFS and OS among patients with melanoma stage IIB or III treated with high-dose IFNα2b versus observation only (median: 1.7 vs 1 year; 3.8 vs 2.8 years, respectively) [17]. These observations may not, however, be comparable to those of the present study due to the inclusion of stage IIB patients, the exclusion of patients with resectable lymph node recurrence and the many updates of the AJCC staging system since this trial was conducted. However, in a subsequent study, the improvement in OS vanished over a longer follow up period [19], and a second trial conducted by the same group of investigators failed to reproduce the OS benefit initially observed [18]. It is nonetheless important to acknowledge that the patient populations enrolled across these trials differed with respect to clinical characteristics due to the different time periods of enrollment [19]. In addition to inconsistencies in survival benefits, high-dose IFNα2b is associated with substantial toxicities [17]. Common adverse events include hepatotoxicity, chronic fatigue and neurological abnormalities [37]. Although toxicities associated with high-dose IFNα2b have been reported to be reversible or mitigated following dose reduction, it remains a common reason for premature treatment discontinuation [38,39].
In the present study, only a minority (∼a third) of the patients used adjuvant therapy. This finding is consistent with the results from two previously published retrospective cohort studies by Zhang et al. [40] and Hackshaw et al. [41], although the proportions of patients on adjuvant therapy were much lower in the two latter studies (Zhang et al.: 9%; Hackshaw et al.: 8.4%). This apparent discrepancy can be attributed to the fact that these studies included many patients with earlier stage disease who did not require adjuvant therapy based on guidelines [9,10] rather than focusing on patients with stage III melanoma. It is possible that the limited use of IFNα2b in the real-world practice of included melanoma centers reflects the inconclusive impact of high-dose IFNα2b on OS as well as its poor tolerability profile reported in trials and meta-analyses [17,18,19,24,42]. This possibility could also account for the variability in the dosing regimens at treatment initiation and during treatment observed in the present study. For example, the recommended induction and maintenance schedules were adhered to in 42 and 16% of patients, respectively. More specifically, a quarter received a higher starting dose than recommended, and less frequent induction and maintenance dosing schedules than recommended were used by 58 and 32% of patients, respectively. The high proportion of patients initiated on higher than recommended starting doses may be explained by the fact that previous studies have shown RFS benefits following increasing IFN dose [17]. Furthermore, more than a quarter had a dose reduction during follow up. This finding may be related to a previous study, which showed that reducing the dose of the conventional regimen by 25% did not hinder efficacy [43]. Due to lack of alternative treatment options at the time of data collection, physicians may also try to find ways to improve tolerability without compromising efficacy. This variation in treatment schedules aligns with prior studies, for example, a multicountry retrospective and prospective observational study found that 33% of patients with stage IIIB/C melanoma who were treated with high-dose IFN experienced a dose delay and/or reduction, and 29% discontinued therapy [44]. Similarly, results from another retrospective study reported modifications in both induction and maintenance schedules in 38 and 28% of patients, respectively [45]. The variability in treatment dose, schedule and duration, has resulted in a lack of clear insight regarding the risk benefit to patients [46,47]. Although it is known that adverse events can affect patient adherence to therapy, the threshold of toxicity that some patients can tolerate varies widely resulting in a dramatic impact on treatment patterns and patient outcomes [46,47,48,49].
The most common reasons for adopting a watch-and-wait approach were the poor tolerability profile of approved adjuvant therapies and patient decision, which align with the possibility that the poor tolerability of IFNα2b contributes to its low use in the real-world practice of included melanoma centers. In addition, patients initiated on adjuvant therapy were significantly younger and had less co-morbidities, suggesting physicians deemed them fitter to receive IFNα2b and tolerate its side effects. However, despite their seemingly superior health status, most of these patients did not remain on therapy for the full duration of the original Kirkwood 52 week, which is consistent with results from several previous studies [38,40,41]. Adverse events were cited as the main reason for treatment discontinuation. Notably, the observed proportion of patients who did not initiate any maintenance phase reached nearly 40%. Given that a one-month induction phase alone (i.e., without any maintenance phase) is not better than observation [50], this result suggests that a large fraction of patients may not experience any benefits from IFN-based adjuvant therapy due to early discontinuation before the initiation of the maintenance phase.
With respect to the effectiveness of adjuvant therapy, the findings of the current study align with previous trials. For example, results pertaining to RFS were consistent with the effectiveness of adjuvant therapy to reduce the risk of relapse in stage III melanoma, which has been reported in virtually all pivotal trials and meta-analyses [17,18,19,24,42]. The marginal and nonsignificant impact on OS also aligns with most, though not all, trials and meta-analyses on IFNα2b [18,19,42], which was, by far, the most common regimen in the current study.
However, the magnitude of the difference in RFS observed in the current study was higher than expected based on previous trials evaluating newer agents. Of note, all of these trials used a placebo control instead of an IFN-based adjuvant treatment, and regulatory bodies determined that this was a sufficient evidence to warrant drug approval. The HR in this study was seemingly better than that reported in the placebo-controlled trial of ipilimumab (HR = 0.76 vs placebo) [51] but worse than that from other trials that evaluated more recently approved therapies like dabrafenib plus trametinib (HR = 0.47 vs placebo) [52], nivolumab (HR = 0.65 vs ipilimumab) [53] and pembrolizumab (HR = 0.57 vs placebo) [54]. Two factors may explain this observation. First, the median RFS in the watch-and-wait cohort in the current study (43.1 months) appeared much longer than that observed in the placebo arms of previous randomized controlled trials (~15 months) [52,54]. This suggests that patients included in the watch-and-wait cohort in the current study had a more favorable prognosis than the patients enrolled in the clinical trials. The present study also included fewer patients with stage IIIC disease (17%) as compared with other randomized controlled trials (~40%) [51,52,53,54]. A recent Phase III placebo-controlled trial conducted by Maio et al. investigated the efficacy of the BRAF inhibitor vemurafenib as postsurgical adjuvant therapy for melanoma and enrolled patients with stage IIC, IIIA, or IIIB (cohort 1) and patients with stage IIIC melanoma (cohort 2) [55]. While the difference in RFS was significant in cohort 1 (HR = 0.54; p = 0.0010), a nonsignificant and substantially reduced impact was observed in cohort 2 (HR = 0.80; p = 0.26) [55]. Thus, it is conceivable that the inclusion of fewer patients with stage IIIC led to a more pronounced impact, although this possibility has not been formally tested.
Taken together, the results of this study highlight the lack of consensus regarding the use of adjuvant therapy for stage III melanoma following surgical resection, and the high rate of treatment discontinuation in the IFN era. Despite this, treated patients had significantly longer RFS than those in the watch-and-wait cohort. Nevertheless, there was no significant difference in OS. The benefits associated with adjuvant therapy are expected to improve with the recent introduction of novel therapies. Future research should aim to assess whether and how the benefits of these novel therapies translate into the real world.
Limitations
This study should be interpreted within the context of certain limitations. First, results pertaining to the overall study population may not apply to patients in each country assessed. For example, no patients were initiated on adjuvant therapy in Argentina as compared with >80% initiated on adjuvant therapy in Brazil and Canada. Furthermore, the melanoma centers included in this study may have heterogeneous practice patterns to treat melanoma such as different recommendations for initiating a patient on adjuvant therapy, different preferences for dosage and schedule of IFN therapy, or different recommendations for BRAFV600 testing. Second, approximately two-thirds of the patients in this study were covered by public insurance plans, which may limit generalizations of these results to patients insured under other types of plans. Third, most patients included in the present study were treated before the advent of new adjuvant therapies. The diagnostic workup, treatment patterns and outcomes of these patients may not reflect those of contemporary patients with stage III melanoma.
Conclusion
During the 2011–2016 study period, only about a third of patients were initiated on adjuvant therapy following surgical resection for stage III melanoma, with IFNα2b and pegIFNα2b being, by far, the most common adjuvant treatments. The majority of treated patients did not receive the recommended starting dosage of 20 million unit/m2, did not receive the treatment on the recommended schedule, and also discontinued treatment before the end of the standard 52-week schedule; similar results were found when separately assessing patients with different tumor substage. Despite this, RFS was significantly higher among patients who received adjuvant therapy versus those who underwent a watch-and-wait approach in the main analysis. In light of the recent approval of new treatments in the adjuvant setting, these data contribute to documenting the reasons for using or withdrawing adjuvant therapy as well as the potential challenges, which new agents may help overcome.
Future perspective
The treatment landscape for stage III melanoma has evolved considerably since the recent approval of several novel immunotherapies (i.e., nivolumab, pembrolizumab and ipilimumab) and small molecule inhibitors (i.e., dabrafenib plus trametinib). Treatment with these recently approved agents is expected to improve treatment efficacy by extending RFS and OS while also reducing the intolerable adverse events commonly associated with IFN-based therapies. As more data on these new therapies become available, real-world analyses like those performed in the present study will be important to help choose between BRAF/MEK inhibitors and immunotherapies in patients with BRAF-mutated tumors.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/full/10.2217/mmt-2019-0015
Author contributions
P Mohr, F Kiecker, V Soriano, O Dereure, K Mujika, P Saiag, J Utikal, R Koneru, C Robert, F Cuadros, M Chacon, RU Villarroel, YG Najjar, L Kottschade, EM Couselo, R Koruth, A Guérin, R Burne, R Ionescu-Ittu and JS Zager contributed to the study design, data collection, analysis, interpretation and writing of the manuscript. M Perrinjaquet contributed to the study design, data collection and writing of the manuscript.
Financial & competing interests disclosure
This study was funded by Novartis Pharmaceuticals Corporation. P Mohr: honoraria: Amgen, Bristol-Myers Squibb, Merck, Merck Sharp & Dohme, Pierre Fabre, Novartis, Sanofi and Roche. F Kiecker: advisory boards: Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi and Roche; honoraria: Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre and Roche; clinical trial participation (principal investigator): Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi and Roche. R Koneru: research funding from Novartis; advisory boards: Novartis, Bristol-Myers Squibb, Merck. O Dereure: advisory board or honoraria and travel support: Bristol-Myers Squibb, Merck Sharp and Dohme, Roche, Novartis, Pierre Fabre, Sanofi. P Saiag: personal fees: Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck-Serono, Pfizer, Roche-Genentech, Pierre Fabre and Novartis; nonfinancial support from Bristol-Myers Squibb, Merck Sharp & Dohme, Roche-Genentech and Novartis. J Utikal: advisory board or honoraria and travel support: Amgen, Bristol-Myers Squibb, GlaxoSmithKline, LeoPharma, Merck Sharp & Dohme, Novartis, Pierre Fabre and Roche. C Robert: honoraria: Amgen, Bristol-Myers Squibb, Merck, Merck Sharp & Dohme, Pierre Fabre, Novartis, Sanofi and Roche. F Cuadros received research funding from Novartis; advisory boards: Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme; speakers bureau: Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme. RU Villarroel: Advisory Board: Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb; honoraria: Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb; clinical trial participation principal investigator: Novartis, Merck Sharp & Dohme. YG Najjar: research funding: Merck; advisory board: Array Biopharma; clinical trial participation: Novartis, Genentech, Merck and Array Biopharma. L Kottschade: advisory board: Array BioPharma; research funding: Bristol-Myers Squibb. EM Couselo: advisory board: Amgen, Bristol-Myers Squibb, Merck, Sharp & Dohme, Novartis, Pierre Fabre, Sanofi and Roche; honoraria: Amgen, Bristol-Myers Squibb, Merck, Sharp & Dohme, Novartis, Pierre Fabre and Roche; clinical trial participation (principal investigator): Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Merck, Sharp & Dohme, Novartis, Pierre Fabre, Sanofi and Roche. R Koruth is an employee of Novartis Pharmaceutical Corporation. A Guérin, R Burne and R Ionescu-Ittu are employees of Analysis Group, Inc., which has received consulting fees from Novartis. M Perrinjaquet is an employee of Navigant Germany GmbH, whose parent company has received consulting fees from Novartis. JS Zager: research funding: Novartis, Amgen, Philogen, Provectus, Delcath Systems, Castle Biosciences; advisory boards: Merck and Array Biopharma; medical advisory board: Delcath Systems; consulting: Philogen; speakers bureau: Sun Pharma and Array Biopharma. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by S Rochette and G DeWalt, employees of Analysis Group, Inc.; support for this assistance was provided by Novartis Pharmaceuticals Corporation. Data collection was coordinated by M Perrinjaquet and E Chater, employees of Navigant, while support for this assistance was provided by Novartis Pharmaceuticals Corporation.
Ethical conduct of research
The study underwent ethics committee review and obtained institutional review board approval for all participating sites. Upon regulatory or institutional review board requirements where applicable, informed consent forms were signed by patients included in the study.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.American Cancer Society. Key statistics for melanoms skin cancer (2019). https://www.cancer.org/cancer/melanoma-skin-cancer/about/key-statistics.html
- 2.Matthews NH, Li WQ, Qureshi AA, Weinstock MA, Cho E. Epidemiology of melanoma. : Cutaneous Melanoma: Etiology and Therapy, Ward WH, Farma JM, Brisbane (). Codon Publications, Brisbane, QLD, Australia: (2017). [PubMed] [Google Scholar]
- 3.World Health Organization: International Agency for Research on Cancer. World – incidence, mortality and prevalence by cancer site (2019). http://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- 4.Clarke CA, Mckinley M, Hurley S. et al. Continued increase in melanoma incidence across all socioeconomic status groups in California, 1998–2012. J. Invest. Dermatol. 137(11), 2282–2290 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Saraiya M, Patel P. et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J. Am. Acad. Dermatol. 65(1 Suppl. 5), S17–S25. e11–13 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Guy GP, Jr, Thomas CC, Thompson T. et al. Vital signs: melanoma incidence and mortality trends and projections – United States, 1982–2030. MMWR Morb. Mortal. Wkly Rep. 64(21), 591–596 (2015). [PMC free article] [PubMed] [Google Scholar]
- 7.Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept 7(2), 1–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha A, Tarhini AA. Adjuvant therapy for melanoma. Curr. Oncol. Rep. 19(5), 36 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U, Committee EG. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26(Suppl. 5), v126–132 (2015). [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology – cutaneous melanoma V1.2019 (2018). https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf [DOI] [PubMed]
- 11.Rockberg J, Amelio JM, Taylor A, Jorgensen L, Ragnhammar P, Hansson J. Epidemiology of cutaneous melanoma in Sweden-stage-specific survival and rate of recurrence. Int. J. Cancer 139(12), 2722–2729 (2016). [DOI] [PubMed] [Google Scholar]; • Retrospective population-based study that links hospital electronic medical records to registry data to estimate stage-specific survival and relapse/progression among Swedish patients with cutaneous metastatic melanoma prior to the widespread use of targeted therapies.
- 12.Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. J. Clin. Oncol. 28(18), 3042–3047 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe – a systematic review of the literature. Clin. Epidemiol. 8, 109–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Systematic literature review of stage-specific rates of survival and/or recurrence among adults diagnosed with cutaneous metastatic melanoma throughout Europe.
- 14.Balch CM, Gershenwald JE, Soong SJ. et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 27(36), 6199–6206 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauschild A, Dummer R, Schadendorf D. et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-mutant stage III melanoma. J. Clin. Oncol. (2018) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A long-term follow up study of the Phase III COMBI-AD trial that provides updated analyses on the efficacy of adjuvant dabrafenib plus trametinib in metastatic melanoma. Post hoc analyses were conducted to assess the effect of tumor stage and pathologic factors on relapse-free survival.
- 16.Gershenwald JE, Scolyer RA, Hess KR. et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 67(6), 472–492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon α-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 14(1), 7–17 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Kirkwood JM, Ibrahim JG, Sondak VK. et al. High- and low-dose interferon α-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J. Clin. Oncol. 18(12), 2444–2458 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood JM, Manola J, Ibrahim J. et al. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin. Cancer Res. 10(5), 1670–1677 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Schering Corporation. Sylatron™, prescribing information. NJ, USA: (2011). https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103949s5153lbl.pdf [Google Scholar]
- 21.Eggermont AM, Suciu S, Santinami M. et al. Adjuvant therapy with pegylated interferon α-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised Phase III trial. Lancet 372(9633), 117–126 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Eggermont AM, Suciu S, Testori A. et al. Long-term results of the randomized Phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon α-2b versus observation in resected stage III melanoma. J. Clin. Oncol. 30(31), 3810–3818 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Kirkwood JM, Resnick GD, Cole BF. Efficacy, safety, and risk-benefit analysis of adjuvant interferon α-2b in melanoma. Semin. Oncol. 24(4 Suppl. 1), S16–S23 (1997). [PubMed] [Google Scholar]
- 24.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon α adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J. Natl Cancer Inst. 102(7), 493–501 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Dimitriou F, Braun RP, Mangana J. Update on adjuvant melanoma therapy. Curr. Opin. Oncol. 30(2), 118–124 (2018). [DOI] [PubMed] [Google Scholar]; • A review summarizing results from selected Phase III clinical trials and the evolution of adjuvant therapeutic strategies for high-risk melanoma.
- 26.Food and Drug Administration. OPDIVO (nivolumab), prescribing information. Silver Spring, Maryland, USA: (2014). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s069lbl.pdf [Google Scholar]
- 27.European Medicines Agency. Summary of product characteristics (Opdivo) (2015). https://www.ema.europa.eu/documents/product-information/opdivo-epar-product-information_en.pdf
- 28.Food and Drug Administration. KEYTRUDA®(pembrolizumab), prescribing information. Silver Spring, Maryland, USA: (2018). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125514s042lbl.pdf [Google Scholar]
- 29.European Medicines Agency. Keytruda (2015). https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda
- 30.Food and Drug Administration. YERVOY®(ipilimumab), prescribing information. Silver Spring, Maryland, USA: (2011). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125377s096lbl.pdf [Google Scholar]
- 31.Food and Drug Administration. TAFINLAR®(dabrafenib), prescribing information. Silver Spring, Maryland, USA: (2013). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202806s010lbl.pdf [Google Scholar]
- 32.Food and Drug Administration. MEKINIST®(trametinib), prescribing information. Silver Spring, Maryland, USA: (2013). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/204114Orig1s009lbl.pdf [Google Scholar]
- 33.European Medicines Agency. Mekinist (2014). https://www.ema.europa.eu/en/medicines/human/EPAR/mekinist
- 34.European Medicines Agency. Tafinlar (2013). https://www.ema.europa.eu/en/medicines/human/EPAR/tafinlar
- 35.Bajetta E. Adjuvant use of interferon α 2b is not justified in patients with stage IIb/III melanoma. Nat. Clin. Pract. Oncol. 5(1), 4–5 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Kirkwood JM, Tarhini AA, Moschos SJ, Panelli MC. Adjuvant therapy with high-dose interferon α 2b in patients with high-risk stage IIB/III melanoma. Nat. Clin. Pract. Oncol. 5(1), 2–3 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Kirkwood JM, Bender C, Agarwala S. et al. Mechanisms and management of toxicities associated with high-dose interferon α-2b therapy. J. Clin. Oncol. 20(17), 3703–3718 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Levesque N, Mitchinson K, Lawrie D. et al. Health management program: factors influencing completion of therapy with high-dose interferon α-2b for high-risk melanoma. Curr. Oncol. 15(1), 36–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kefford RF. Adjuvant therapy of cutaneous melanoma: the interferon debate. Ann. Oncol. 14(3), 358–365 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Le TK, Shaw JW, Kotapati S. Retrospective analysis of drug utilization, health care resource use, and costs associated with IFN therapy for adjuvant treatment of malignant melanoma. Clinicoecon Outcomes Res. 7, 397–407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hackshaw MD, Krishna A, Mauro DJ. Retrospective US database analysis of drug utilization patterns, health care resource use, and costs associated with adjuvant interferon α-2b therapy for treatment of malignant melanoma following surgery. Clinicoecon Outcomes Res. 4, 169–176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-α for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat. Rev. 29(4), 241–252 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Gogas H, Dafni U, Bafaloukos D. et al. A randomized Phase III trial of 1 month versus 1 year adjuvant high-dose interferon α-2b in patients with resected high risk melanoma. J. Clin. Oncol. 25(Suppl. 18), 8505–8505 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Harries M, Mohr P, Grange F. et al. Treatment patterns and outcomes of stage IIIB/IIIC melanoma in France, Germany and the UK: a retrospective and prospective observational study (MELABIS). Int. J. Clin. Pract. 71(5), e12946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A retrospective, international study, based on data from patient medical records and patient surveys, assesses the variation in treatment patterns associated with adjuvant interferon therapy for stage IIIB/IIIC melanoma.
- 45.Espinosa E, Soriano V, Malvehy J. et al. Treatment patterns of adjuvant interferon-α2b for high-risk melanoma: a retrospective study of the Grupo Espanol Multidisciplinar de Melanoma – Prima study. Melanoma Res. 26(3), 278–283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilbridge KL, Weeks JC, Sober AJ. et al. Patient preferences for adjuvant interferon α-2b treatment. J. Clin. Oncol. 19(3), 812–823 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Eggermont AM, Suciu S, Mackie R. et al. Post-surgery adjuvant therapy with intermediate doses of interferon α 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet 366(9492), 1189–1196 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Schuchter LM. Adjuvant interferon therapy for melanoma: high-dose, low-dose, no dose, which dose? J. Clin. Oncol. 22(1), 7–10 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Ziefle S, Egberts F, Heinze S. et al. Health-related quality of life before and during adjuvant interferon-α treatment for patients with malignant melanoma (DeCOG-trial). J. Immunother. 34(4), 403–408 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Agarwala SS, Lee SJ, Yip W. et al. Phase III randomized study of 4 weeks of high-dose interferon-α-2b in stage T2bNO, T3a-bNO, T4a-bNO, and T1-4N1a-2a (microscopic) melanoma: a trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E1697). J. Clin. Oncol. 35(8), 885–892 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eggermont AM, Chiarion-Sileni V, Grob JJ. et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375(19), 1845–1855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long GV, Hauschild A, Santinami M. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 377(19), 1813–1823 (2017). [DOI] [PubMed] [Google Scholar]; • A Phase III study that examines the efficacy of adjuvant dabrafenib plus trametinib in BRAF V600E/V600K stage III melanoma.
- 53.Weber J, Mandala M, Del Vecchio M. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377(19), 1824–1835 (2017). [DOI] [PubMed] [Google Scholar]; • A Phase III study that examines the efficacy of adjuvant nivolumab versus ipilimumab among patients with advanced melanoma.
- 54.Eggermont AMM, Blank CU, Mandala M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378(19), 1789–1801 (2018). [DOI] [PubMed] [Google Scholar]; • A Phase III study that examines the efficacy of adjuvant pembrolizumab among patients with resected high-risk stage III melanoma.
- 55.Maio M, Lewis K, Demidov L. et al. Adjuvant vemurafenib in resected, BRAF(V600) mutation-positive melanoma (BRIM8): a randomised, double-blind, placebo-controlled, multicentre, Phase III trial. Lancet Oncol. 19(4), 510–520 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.