Abstract
In this review, we discuss biomarkers of response and resistance to PI3K inhibitors (PI3Ki) in estrogen receptor-positive breast cancer, both in the early and advanced settings. We analyse data regarding PIK3CA mutations, PI3K pathway activation, PTEN expression loss, Akt signalling, insulin levels, 18FFDG-PET/CT imaging, FGFR1/2 amplification, KRAS and TP53 mutations. Most of the discussed data comprise retrospective and exploratory studies, hence many results are not conclusive. Therefore, among all of these biomarkers, only PIK3CA mutations have proved to have a predictive value for treatment with the α-selective PI3Ki alpelisib (SOLAR-1 trial) and the β-sparing PI3Ki taselisib (SANDPIPER trial) in the advanced setting. Since the accuracy of current individual biomarkers is not optimal, a composite biomarker, including DNA, RNA and protein expression data, to more precisely assess the PI3K/AKT/mTOR pathway activation status, may arise as a promising approach. Finally, we describe the rational for new combination therapies involving PI3Ki and anti-HER2 agents, chemotherapy, CDK4/6 inhibitors, mTOR inhibitors or new endocrine treatments and discuss the ongoing trials in this field.
Keywords: breast neoplasms, predictive biomarkers, PI3K inhibitors, PIK3CA, gene sequencing
Key Message
So far, only PIK3CA mutations have proved to have a predictive value for treatment with α-selective and β-sparing PI3K inhibitors in the advanced breast cancer setting, and its use has recently entered clinical practice. As new drug combinations with PI3K inhibitors are being developed, there is an unmet need to find biomarkers for adequate treatment tailoring.
Introduction
Since the landmark BOLERO-2 trial demonstrated the benefit of targeting the PI3K/AKT/mTOR pathway in breast cancer (BC) [1], there has been an enormous effort to find new agents and innovative combinations targeting this pathway. Yet, given the toxicities and costs associated with these agents, research has focussed on ways to better identify which patients would benefit the most from these treatments. In this review, we will discuss biomarkers of response and resistance specifically to PI3K inhibitors (PI3Ki) in estrogen receptor (ER)-positive BC. Furthermore, we will describe the rationale for new combination therapies involving PI3Ki and the ongoing trials evaluating these strategies.
Biomarkers of response and resistance
PI3K/AKT/mTOR pathway can be activated by multiple factors, such as oncogenic genomic alterations in PIK3CA, PIK3R1, PTEN, AKT, among others [2]. Some of these alterations have been extensively studied as potential biomarkers of response and/or resistance to PI3Ki. Although there is a large amount of preclinical data on this topic, we will preferentially focus on biomarkers that were tested in human BC samples and were correlated with clinical end points, usually within trials.
Potential biomarkers to predict the benefit from PI3Ki
PIK3CA gene mutations and PI3K pathway activation status
Preclinical studies show that PIK3CA-mutated (PIK3CA-mut) BC cells are more sensitive to PI3Ki [3, 4], yet clinical data assessing its predictive value are contradictory (Table 1). In the neoadjuvant setting, a PIK3CA-mut has no predictive value for treatment with the pan-PI3Ki pictilisib (OPPORTUNE trial) or the α-selective PI3Ki alpelisib (NEO-ORB trial), but seems to predict benefit from the β-sparing PI3Ki taselisib (LORELEI trial) [5, 6, 12, 18].
Table 1.
Summary of studies assessing biomarkers of response and/or resistance to treatment with PI3K inhibitors
| Trial | Phase | Population | Treatment | Tested tissue | Mutated/altered population | WT/normal/ITT population | Comments/conclusion |
|---|---|---|---|---|---|---|---|
| Pan-PI3Ki | |||||||
| Neoadjuvant | |||||||
| OPPORTUNE [5, 6] | II | ER+/HER2−, operable BC (T≥1 cm), n=167 → 75 initial analysis; 136 evaluable final analysis | Anastrozole± pictilisib×2w | IHC (Ki67, PgR, PTEN)+targeted NGS of >400 genes+CNV analyses+reverse-phase protein arrays and RNA profiling; BC subtype was defined using the NanoString PAM50 algorithm |
|
PIK3CA WT: geometric mean Ki67 suppression ratio: 0.63 (95% CI 0.39–1.0) | No predictive value (overall); differences according to type of mutation? |
| PTEN negative (n=7): ratio 0.59 (95% CI 0.08–4.13) | PTEN positive (n=108): ratio 0.58 (95% CI 0.40–0.83) | No predictive value | |||||
| PI3K pathway alterationa: ratio 0.92 (95% CI 0.57–1.48) | No PI3K pathway alteration: ratio 0.52 (95% CI 0.33–0.83) | No predictive value | |||||
| Luminal B subtype (n=33): ratio 0.37 (95% CI 0.21–0.67) | Luminal A subtype (n=20): ratio 1.01 (95% CI 0.52–1.96) | Luminal B subtype: apparent predictive value | |||||
| PgR negative (n=21): ratio 0.35 (95% CI 0.14–0.87) | PgR positive (n=115): ratio 0.65 (95% CI 0.43–0.98) | PgR negative: apparent predictive value | |||||
| Metastatic | |||||||
| BELLE-2 [7] | III | ER+/HER2−, after AI, n=1147 | Fulvestrant±buparlisib | Archived primary tumour tissue—IHC (PTEN) and analysis of PIK3CA (exons 1, 7, 9, and 20) by Sanger sequencing, n=851 | PI3K pathway activatedb,c (44%): PFS HR 0.76 (95% CI 0.60–0.97); OS HR 0.81 (95% CI 0.61–1.08) | ITT (with known PI3K status): PFS HR 0.80 (95% CI 0.68–0.94); non-activated PI3K pathway: OS HR 0.98 (95% CI 0.77–1.24) |
No predictive value |
| Blood (ctDNA) at baseline—analysis of PIK3CA (exons 1, 7, 9, and 20) by PCR, n=587 | PIK3CA-mut: PFS HR 0.58 (95% CI 0.41–0.82); OS HR 0.81 (95% CI 0.56–1.17) | PIK3CA WT: PFS HR 1.02 (95% CI 0.79–1.30); OS HR 1.12 (95% CI 0.83–1.50) | Predictive value: benefit only in PIK3CA-mut | ||||
| BELLE-3 [8] | III | ER+/HER2−, after ET + everolimus, n=432 | Fulvestrant±buparlisib | New or archived (73%) tissue—analysis of PIK3CA (exons 7, 9 and 20) by PCR, n=320 | PIK3CA-mut: PFS HR 0.39 (95% CI 0.23–0.65) | PIK3CA WT: PFS HR 0.81 (95% CI 0.59–1.12) | Predictive value: benefit only in PIK3CA-mut |
| Blood (ctDNA) at baseline—analysis of PIK3CA (exons 9 and 20) by PCR, n=348 | PIK3CA-mut: PFS HR 0.46 (95% CI 0.29–0.73) | PIK3CA WT: PFS HR 0.73 (95% CI 0.53–1.00) | |||||
| BELLE-4 [9] | II/III | HER2−, no prior CT for ABC; prior ET allowed; n=416 (302 ER+ [73%]) | Paclitaxel±buparlisib | Archived (most) or fresh biopsy tissue—IHC (PTEN) and analysis of PIK3CA (exons 1, 7, 9, and 20) by Sanger sequencing | PI3K pathway activatedb,c: PFS HR 1.17 (95% CI 0.63–2.17) | PI3K pathway non-activated: PFS HR 1.18 (95% CI 0.76–1.83) | No predictive value |
| FERGI [10] | II |
Part 1: ER+/HER2−, after AI, n=168 |
Fulvestrant±pictilisib 340 mg | Tissue (not specified)—analysis of PIK3CA missense mutations (C420R; E542K; E545A, E545G, or E545K; and H1047L, H1047R, or H1047Y) by PCR | PIK3CA-mutb: PFS HR 0.73 (95% CI 0.42–1.28) | PIK3CA WT: PFS HR 0.72 (95% CI 0.42–1.23) | No predictive value |
| Part 2: ER+/HER2−, after AI, only PIK3CA-mut, n=61 | Fulvestrant ± pictilisib 260 mg | PIK3CA-mutb: PFS HR 1.07 (95% CI 0.53–2.18) | NA | No benefit from pictilisib in PIK3CA-mut patients | |||
| PEGGY [11] | ER+/HER2−, first-/second-line CT for ABC, n=183 | Paclitaxel±pictilisib 260 mg | Archived primary tumour or fresh biopsy metastatic tissue—analysis of PIK3CA (exons 7, 9, and 20) by PCR, n=168 | PIK3CA-mut: PFS HR 1.06 (95% CI 0.52–2.12) | ITT: PFS HR 0.95 (95% CI 0.62–1.46) | No predictive value | |
| β-Sparing PI3Ki | |||||||
| Neoadjuvant | |||||||
| LORELEI [12, 13] | II | ER+/HER2−, stage I–III operable (T≥2 cm), n=334 | Letrozole ± taselisib×16w | Primary BC, at baseline, week 3 and surgery—IHC (pAKT, pPRAS40 and pS6) and analysis of PIK3CA (exons 1, 4, 7, 9, and 20) by PCR | PIK3CA-mut: ORR: 56% (taselisib) versus 38% (P), OR 2.03 (95% CI 1.06–3.88); pCR: 1.4% (taselisib) versus 0% (P) | PIK3CA WT: ORR: 46% versus 40% (P), OR 1.22 (95% CI 0.68–2.21); pCR: 2.2% (taselisib) versus 1.1% (P) | Apparent predictive value |
| No association between baseline phosphoproteins levels and response (ORR or cell cycle arrest) | No predictive value | ||||||
| Metastatic | |||||||
| Saura [14] | Ib | ER+ ABC, after ≥1 ET line, n=28 | Letrozole+taselisib | Archived or fresh tissue—analysis of PIK3CA (exons 1, 4, 7, 9, and 20) by PCR | PIK3CA-mut: ORR 38% | PIK3CA WT: ORR 9% | Numerically higher ORR in the PIK3CA-mut group |
| Dickler [15] | II | ER+/HER2− ABC, after ≥1 ET line, n=47 | Fulvestrant+taselisib | Archived or fresh tissue—analysis of PIK3CA (exons 1, 4, 7, 9, and 20) by PCR | PIK3CA-mut: CBR 38.5% (95% CI 13.9–68.4) | PIK3CA WT: CBR 23.8% (95% CI 8.2–47.2) | Numerically higher CBR in the PIK3CA-mut group |
|
PIPA [16] |
Ib | ER+/HER2− PIK3CA-mutant ABC cohort, after ≥1 line of ET, n=24 | Fulvestrant + taselisib + palbociclib | Archive or fresh tumour biopsy and blood (ctDNA)—analysis of PIK3CA mutations (method?) | PIK3CA-mutb: ORR: 33%, CBR 58%, mPFS 7.9 months (95% CI 5.6–11.8) | NA | Clinical benefit in patients with a PIK3CA mutation |
| SANDPIPER [17] | III | Cohort PIK3CA-mut: ER+/HER2− ABC, after AI, n=516 | Fulvestrant±taselisib | Archived or fresh tissue—analysis of PIK3CA (exons 1, 4, 7, 9, and 20) by PCR | PIK3CA-mutb: PFS HR 0.70 (95% CI 0.56–0.89)d | NA | Predictive value: benefit only in PIK3CA-mut (but similar HR) |
| Cohort PIK3CA WT: ER+/HER2− ABC, after AI, n=115 | NA | PIK3CA WTb: PFS HR 0.69 (95% CI 0.44–1.08) | |||||
| α-Selective PI3Ki | |||||||
| Neoadjuvant | |||||||
| NEO-ORB [18] | II | ER+/HER2−, T1c-T3, n=257 | Letrozole ± alpelisib×24w | Primary BC—analysis of PIK3CA (exons 1, 4, 7, 9, and 20) by PCR |
PIK3CA-mutb: ORR: 43% (alpelisib) versus 45% (P), P=0.435; pCR: 1.7% (alpelisib) versus 3.0% (P), P=0.282 |
PIK3CA WT: ORR: 63% (alpelisib) versus 61% (P), P=0.611; pCR: 2.8% (alpelisib) versus 1.7% (P), P=0.697 | No predictive value |
| PgR negative: in PIK3CA-mut (n=19): ORR 33% (alpelisib) versus 43% (P); in PIK3CA WT (n=17): 67% (alpelisib) versus 64% (P) | PgR positive: in PIK3CA-mut (n=128): ORR 46% (alpelisib) versus 45% (P); in PIK3CA WT (n=113): 63% (alpelisib) versus 60% (P) | No predictive value | |||||
| Metastatic | |||||||
| Mayer et al. [19] | Ib | ER+/HER2− ABC, endocrine-resistant, n=26 | Letrozole + alpelisib | Archive or fresh tumour (74% primary tumour) biopsy—analysis of PIK3CA, PTEN, and AKT1 by multiplex PCR; NGS of 341 genes + FGFR1/2 amplification by FISH | PIK3CA-mut: CBR 44% | PIK3CA WT: CBR 20% | Numerically higher CBR in the PIK3CA-mut group |
| Juric [20] | Ib | ER+/HER2− ABC, endocrine-resistant (heavily pre-treated), n=81 | Fulvestrant + alpelisib | Archive or fresh tumour biopsy—analysis of PIK3CA by NGS | PIK3CA-mut: ORR 29% (95% CI 17% to 43%); mPFS 9.1 months (95% CI 6.6–14.6) | PIK3CA WT: ORR 0; mPFS 4.7 months (95% CI 1.9–5.6) | Numerically higher ORR in the PIK3CA-mut group |
| Sharma [21] | I/II | HER2− ABC, after ≥1 line of CT (any setting), n=43 (of which 70% were ER+) | Nab-paclitaxel + alpelisib | Tumour tissue and blood (ctDNA)—analysis of PIK3CA and PTEN mutations by NGS | PI3K pathway activatede (n=19; 44%): mPFS 13 months (95% CI 9–17) | Non-activated PI3K pathway (n=23; 53%): mPFS 7 months (95% CI 3–11) | Apparent prognostic value (PFS HR 0.40; 95% CI 0.18–0.90) |
| SOLAR-1 [22, 23] | III | Cohort PIK3CA-mut: ER+/HER2− ABC, after AI, n=341 | Fulvestrant ± alpelisib |
|
NA | Predictive value: benefit only in PIK3CA-mut, independent of exon or type of mutation | |
| Cohort PIK3CA WT: ER+/HER2− ABC, after AI, n=231 | NA |
|
|||||
Definition of activated PI3K pathway: OPPORTUNE: not defined.
Stratification factor and/or assignment criteria to a specific treatment cohort.
Definition of activated PI3K pathway: BELLE-2 and BELLE-4: PI3K pathway activated: PIK3CA-mutation and/or no PTEN expression (by immunohistochemistry).
Primary end point.
Definition of activated PI3K pathway: Sharma et al.: PI3K pathway activated: presence of PIK3CA-activating or PTEN-inactivating mutations in either tumour tissue or ctDNA.
ABC, advanced breast cancer; AI, aromatase inhibitor; BC, breast cancer; CBR, clinical benefit rate; CI, confidence interval; CNV, copy number variations; CT, chemotherapy; ctDNA, circulating tumour DNA; ER+, estrogen receptor positive; ET, endocrine therapy; FISH, fluorescent in situ hybridization; HER2+, HER2 positive; HER2−, HER2 negative; HR, hazard ratio; IHC, immunohistochemistry; ITT, intention-to-treat population; mPFS, median progression-free survival; mut: mutation; NA, not applicable; NGS, next-generation sequencing; OR, odds ratio; ORR, overall response rate; P, placebo; PCR, polymerase chain reaction; PFS, progression-free survival; PgR, progesterone receptor; PIK3CA-mut, mutation in the PIK3CA gene; Ph, phase of the clinical trial; T, tumour size; WT, wild-type.
In the advanced setting, results may be also difficult to interpret. Some studies on pan-PI3Ki report that PIK3CA-mut has predictive value when detected in the blood [7] or in the blood and tissue [8], while others report no predictive value [9–11]. Nevertheless, it seems that a PIK3CA-mut may identify patients who benefit the most from β-sparing PI3Ki (taselisib) and α-selective PI3Ki (alpelisib), as patients with PIK3CA-wild-type tumours did not benefit from these targeted drugs in clinical trials [14, 15, 17, 19, 20, 22]. Furthermore, the two most frequently mutated regions (exon 9—helical domain and exon 20—kinase domain) of PIK3CA have been explored as biomarkers of response. Analyses in the neoadjuvant setting suggested that mutations in exon 9 conferred a higher sensitivity to pictilisib when compared with mutations in exon 20 [5, 6]. Yet, the large SOLAR-1 trial showed a benefit from alpelisib in PIK3CA-mut patients, independently of the type of mutation found [22].
A potential reason for PIK3CA mutational status contradictory results may be due to its variable oncogenic potential, leading to different degrees of tumour cells’ addiction to PI3K/AKT/mTOR pathway activation [24]. Indeed, Loi et al. have developed a PI3KCA-mutant-related gene signature (PIK3CA-GS), which could predict the occurrence of mutations in PIK3CA and AKT1, and was correlated with a PTEN-loss gene signature [25]. Interestingly, the authors showed that higher PIK3CA-GS scores (i.e. corresponding to the mutant-like phenotype) were associated with low levels of pathway activation. On the other hand, patients with lower PIK3CA-GS scores (i.e. with higher pathway activation) had the greater benefit from treatment with letrozole/everolimus [25, 26]. Moreover, Mertins et al. showed that some PIK3CA-mut breast tumours do not present downstream pathway activation, further demonstrating the variable oncogenic potential of the PIK3CA-mut [27]. One possibility is that the tumour may require another hit for full activation of the pathway and, indeed, it has recently been demonstrated that the occurrence of double PIK3CA mutations in cis leads to an increased PI3K pathway activity and downstream signalling in breast tumours compared with single hotspot mutations [28]. In addition, these double mutations rendered tumours more sensitive to α-selective PI3Ki.
On the other hand, even in PIK3CA-mut tumours, resistance to PI3Ki can be mediated by activation of alternative pathways that drive cell proliferation (MAPK, ER, HER2, AXL, PIM-1, FOXO transcription factors); by signalling via other PI3K isoforms when a specific subunit is blocked; by activation of downstream effectors in the PI3K pathway such as AKT and mTOR; by loss of regulators of PI3K signalling such as PTEN; or by epigenomic crosstalk between PI3K and ER pathways, resulting in upregulation of ER-dependent transcription upon PI3K inhibition (Figure 1) [2, 29–32]. In order to overcome this classification issue, studies in metastatic BC have also analysed the benefit from PI3Ki according to a ‘PI3K pathway activation’ status biomarker. Its definition, however, varied between studies and usually combined DNA with protein expression assessment (Table 1). Even so, none of these PI3Ki trials indicated a predictive value of an ‘activated PI3K pathway’ biomarker [7, 9].
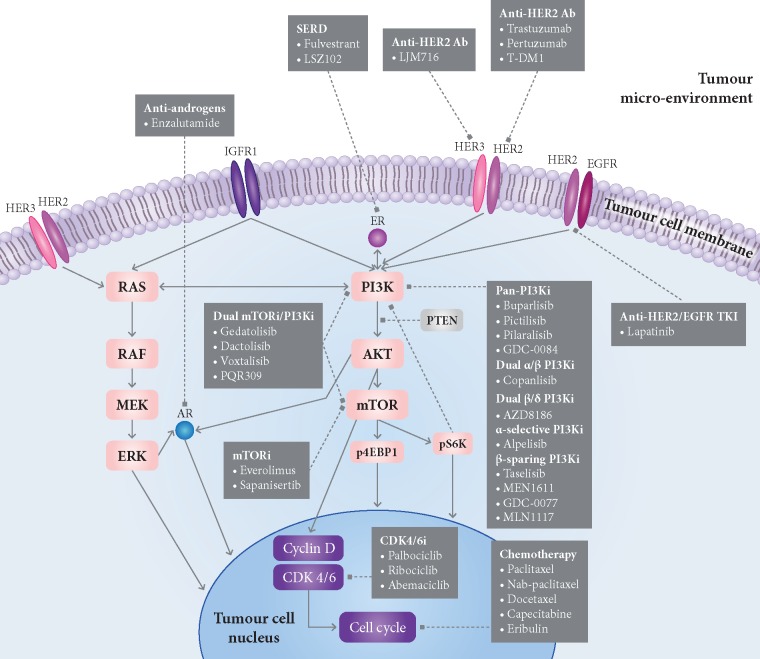
Figure 1.
Mechanisms of resistance to PI3K inhibitors in estrogen receptor (ER)-positive breast cancer and current and future drug combination strategies involving PI3K inhibitors. In PIK3CA-mutated breast tumours, resistance to PI3K inhibitors can be mediated by multiple mechanisms, including activation of alternative pathways that drive cell proliferation (e.g. RAS/MEK/ERK pathway, ER pathway, or HER2 pathway); by signalling via other PI3K isoforms when a specific subunit is blocked; by activation of downstream effectors in the PI3K pathway such as AKT and mTOR; by loss of regulators of PI3K signalling such as PTEN; or by epigenomic crosstalk between PI3K and ER pathways, resulting in upregulation of ER-dependent transcription. Ab, monoclonal antibody; AR, androgen receptor; CDK4/6i, CDK4/6 inhibitors; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HER3, human epidermal growth factor receptor 3; IGFR1, insulin growth factor receptor 1; mTOR, mTOR inhibitors; PI3Ki, PI3K inhibitors; SERD, selective estrogen receptor degraders; T-DM1, ado-trastuzumab emtansine; TKI, tyrosine kinase inhibitor. Dashed arrows, inhibitory function; bold arrows, activation function. Note: within each drug class, we have only included compounds that have been or that are currently being tested in combination with PI3K inhibitors in clinical trials (see Tables 2 and 3 for more details).
Other explanation to these contradictory results may relate to tumour heterogeneity—in some cases, PIK3CA mutations may not be early clonal events, but subclonal drivers present only in a part of the metastatic lesions and, thus, targeting this pathway may be less efficacious. Yet, results from the AURORA program show a high concordance rate of PIK3CA-mut between primary tumours and matched metastasis, suggesting that, in most cases, detected PIK3CA-mut are clonal [33]. On the other hand, its predictive value may change according to specific targeted agents—a hint to this differential effect is given by the β-sparing and α-selective PI3Ki studies [17, 22]: a predictive effect of PIK3CA-mut was demonstrated in both trials, but not in all trials testing pan-PI3Ki. PIK3CA mutations’ predictive value may also depend on disease setting: its oncogenic potential may be less important in the early when compared with the advanced setting [34], in which it has a role on the development of resistance to endocrine treatment [2]. Thus, its presence in endocrine treatment-resistant tumours in the advanced setting may predict benefit from targeted inhibition with PI3Ki, but not in ‘treatment-naïve’ tumours, like the ones in the neoadjuvant setting.
18FFDG-PET/CT imaging
In a phase Ib study testing the combination of buparlisib and letrozole, patients with no early metabolic response (at 2 weeks) by 18FFDG-PET/CT scan presented rapid disease progression [35]. Likewise, patients who presented an early metabolic response had a higher chance of staying longer on treatment, suggesting that a decrease in tumour metabolism predicts response to PI3Ki. While interesting, these data need validation in larger studies.
Other potential biomarkers of response
The OPPORTUNE trial suggested that patients with progesterone receptor-negative or luminal B tumours may benefit more from pictilisib due to the drug’s antiproliferative effect [5, 6], but this was not demonstrated in the NEO-ORB trial [18].
Potential biomarkers of resistance to PI3Ki
PTEN expression loss
Preclinical data suggest that cells with PTEN expression loss are more sensitive to AKT/PI3K inhibitors (PI3Ki) [4]. Juric et al. reported the case of a patient who progressed while being treated with alpelisib, in which all progressing metastatic lesions showed a de novo loss of PTEN expression, by different but convergent genetic alterations [36]. Then, the authors functionally analysed PTEN-null xenografts derived from this patient, which were also resistant to alpelisib. On the other hand, it is known that PTEN-deficient tumours are dependent on PI3Kβ signalling [37] and this may explain why patients included in the OPPORTUNE trial derived benefit from the pan-PI3Ki pictilisib (which also targets PI3Kβ), whether they had PTEN-positive or PTEN-negative tumours [5, 6]. Nonetheless, assessment of ‘PTEN status’ can be challenging, as its ‘loss’ has been determined by the allelic or complete loss of the PTEN gene [19, 21], but also by the immunohistochemical expression of the PTEN protein or of other downstream markers, like phosphorylated-Akt [5, 7, 9, 12, 13, 38]. Moreover, these studies have used different PTEN antibodies and variable definitions of PTEN status, making comparisons difficult between them.
High insulin levels
It is well known that PI3K mediates cellular responses to insulin and that its inhibition leads to hyperglycaemia [17, 22]. A recent report has shown that PI3Ki-induced hyperglycaemia leads to an increase in insulin release and that this is sufficient to re-activate PI3K signalling in tumour models in mice, even in the presence of PI3Ki, leading ultimately to treatment resistance [39]. The authors have also demonstrated that this insulin feedback can be prevented or attenuated using dietary (e.g. ketogenic diet) and pharmacological measures (e.g. sodium-glucose cotransporter inhibitors), which improve the efficacy of PI3Ki. On the other hand, administration of exogenous insulin to control hyperglycaemia could further activate PI3K signalling in tumour cells and impair the efficacy of PI3Ki.
This hypothesis could partly explain why in the SANDPIPER trial there were differences in taselisib efficacy according to the region of the world: the hazard ratio (HR) was 0.38 [95% confidence interval (CI) 0.19–0.75] in Asia, 0.57 (95% CI 0.41–0.79) in Western Europe/USA/Canada/Australia, and 1.18 (95% CI 0.78–1.77) in Latin America/Eastern Europe [20]. Thus, differences in patients’ degree of insulin resistance, diet, and in management of hyperglycaemia (e.g. insulin use) according to each region could justify these discrepancies.
Although there is not yet clinical data to support this hypothesis, this is being explored on the datasets from PI3Ki clinical trials. Some PI3Ki trials already recommended the preferential use of oral antidiabetic drugs for hyperglicaemia management [12, 22]. Yet, if this hypothesis is proved, this would lead to further adaptions on the design of clinical trials using PI3Ki and also on the selection and follow-up of patients in daily clinical practice.
Other biomarkers of resistance
Data suggest that in PI3Ki-resistant cell lines there is low-level Akt signalling, and these can be resensitized by using the AKTi MK-2206 [32]. Nonetheless, a phase I trial combining neoadjuvant MK-2206 with anastrozole in patients with PIK3CA-mut tumours showed incomplete target inhibition and lack of further Ki67 suppression [40].
A small number of patients with FGFR1/2 amplification, KRAS or TP53 mutations did not derive clinical benefit in a phase Ib trial of letrozole/alpelisib [19], but this needs confirmation in larger studies.
Limitations of biomarker research
Despite intense research efforts to find predictive biomarkers of response/resistance to PI3Ki, so far only PIK3CA mutations (detected either in tissue or blood) have been approved by the US Food and Drug Administration (FDA) as a predictive biomarker for the use of alpelisib [41]. Moreover, PIK3CA mutations have been recently classified in the tier of evidence IA of genomic alterations in breast cancer (BC) of the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT), as predictors of benefit from α-selective PI3Ki [42].
There are several reasons that may explain the lack of definitive findings regarding the predictive value of PIK3CA-mut with other PI3Ki or for the other biomarkers studied so far. The first is that many of these analyses are retrospective, exploratory and based on a small number of patients. As some of the tested genetic alterations (e.g. mutations in PTEN) have a low frequency, statistical challenges and the risk of overfitting exist. Only the more recent trials have prospectively assessed PIK3CA-mut status or PI3K pathway activation before patients inclusion [7, 9, 10, 16–18, 22], but still the predictive value of PIK3CA-mut was only proven in the β-sparing and α-selective PI3Ki trials. This may partly be explained by the low tolerability of pan-PI3Ki when compared with isoform-selective PI3Ki: exposure to pan-PI3Ki was often reduced due to their toxicity, and this could have led to inadequate pathway inhibition. Thus, it may have confounded the interpretation of biomarker data, leading to contradictory results in the pan-PI3Ki trials [5–11].
Furthermore, differences in methods (use of Sanger sequencing, PCR, next-generation sequencing, etc.) and types of mutations assessed may have also influenced results. Lastly, these biomarkers have been mostly evaluated at baseline only. Data from CDK4/6i trials show that tumour genome may change under selective therapeutic pressure [43], thus it would be interesting to assess genetic alterations over time in patients treated with PI3Ki as well. A convenient technique to perform this would be through circulating tumour (ct)DNA [44]. Another issue relates to timing of assessment: most trials tested biomarkers on archived tissue, usually the primary breast tumour. Some of them assessed the concordance of PIK3CA-mut status between (archived) tissue versus ctDNA and it varied between 70% and 83% [7, 8, 45]. Interestingly, in BELLE-2, 21% patients with PIK3CA-wild-type tumour tissue had PIK3CA-mut ctDNA, which suggests tumour evolution between initial diagnosis and the time at which patients started a PI3Ki [7]. Thus, biomarkers like PIK3CA-mut should be assessed (either in blood or in a recent tissue biopsy) at the time of PI3Ki treatment initiation and not in archived tissue. Of note, in SOLAR-1, the number of patients with a PIK3CA-mut in ctDNA was lower than in the archival tissue (186 versus 341 patients, respectively), thus suggesting that a proportion of patients with PIK3CA-mut tissue had no identifiable PIK3CA-mut in ctDNA [23]. This is in line with the preliminary findings from the AURORA program, in which more than half of patients with a PIK3CA-mut identified in metastatic tissue (taken just before inclusion) did not present an identifiable PIK3CA-mut on synchronous ctDNA [33], which may be explained by many factors (e.g. low tumour burden, among others). This is the reason why FDA recommends that patients who have a negative ctDNA PIK3CA-mut test should undergo tumour biopsy for PIK3CA-mut assessment [41]. Still, as in SOLAR-1, patients with PIK3CA-wild-type ctDNA did not benefit from the addition of alpelisib (HR 0.80; 95% CI 0.60–1.06), it would be important to analyse the benefit of alpelisib in the subgroup of ‘discordant’ patients, who have PIK3CA-mut tissue, but a PIK3CA-wild-type ctDNA.
Future research
Genomic, transcriptomic and proteomic information assessed in breast tumour samples from clinical trials testing PI3Ki should be publically available. This would allow the combination of all this information, in order to better understand the predictive and prognostic role of PIK3CA-mut and other genetic alterations in advanced BC. Furthermore, future trials should prospectively assess these biomarkers, not only at baseline but throughout treatment and at disease progression. As an example, there is an ongoing prospective trial (CICLADES, NCT03318263), longitudinally assessing ctDNA for ESR1, PIK3CA and AKT1 mutations during first-line endocrine treatment with/without targeted therapy, in order to assess their predictive value.
As immunotherapy is emerging as a possible treatment of BC patients, we should also assess the effects of the different PI3Ki on tumour microenvironment and how it can predict response to these treatments. Finally, as new combination therapies involving PI3Ki are being developed, biomarkers to predict which patients will benefit from them should also be sought.
Combination therapies involving PI3Ki
Antitumour activity of PI3Ki in preclinical studies is encouraging, and β-sparing and α-selective PI3Ki have demonstrated to be effective in metastatic BC patients with PIK3CA-mut tumours [17, 22]. Nonetheless, disease progression invariably occurs during PI3Ki treatment, and therefore strategies to overcome resistance and improve patients’ outcomes are necessary. Given the resistance mechanisms previously described, combination of PI3Ki with targeted therapies that suppress alternative pathways, or blockade of PI3K pathway at downstream levels are potential strategies to overcome resistance (Figure 1; Tables 2 and 3).
Table 2.
Phase I/II/III combination trials with PI3K inhibitors in the early and metastatic estrogen receptor (ER)-positive breast cancer setting, with published results
| Trial | Phase | Nb. of pts | Inclusion criteria | Treatment arms (control versus experimental) | Results (control versus experimental)a | Comments |
|---|---|---|---|---|---|---|
| Neoadjuvant setting | ||||||
| With anti-HER2 therapy (and chemotherapy) | ||||||
| NeoPHOEBE [63] | II | 50 | HER2+, tumour diameter >2 cm by clinical examination and/or >1.5 cm by ultrasound/MRI | Paclitaxel+trastuzumab versus paclitaxel+trastuzumab+buparlisib |
|
EFS: Not reported; trial stopped earlier due to an excess in liver toxicity in the experimental arm |
| Metastatic setting | ||||||
| With CDK4/6 inhibitors | ||||||
| Juric [47] | I | 36 | ER+/HER2− ABC | Cohort 3: alpelisib+ribociclib+letrozole | ORR: 7%, SD: 22% | All grade AE > 35% of patients: nausea, hyperglycaemia, neutropenia and fatigue |
| Forero-Torres [48] | I | 35 | ER+/HER2− ABC without previous mTORi or PI3Ki therapy |
|
|
RP2D=180 mg/week |
| PIPA [16] | I | 35 | PIK3CA-mut ABC |
|
|
– |
| With chemotherapy | ||||||
| PEGGY [11] | II | 183 | ER+/HER2− ABC; prior CT not allowed with the exception of capecitabine or mTORi | Placebo+paclitaxel versus pictilisib plus paclitaxel |
|
Pictilisib did not improve PFS also in the PIK3CA-mut subgroup |
| BELLE-4 [9] | II | 416 |
|
Placebo+paclitaxel versus buparlisib+paclitaxel |
|
Tendency for better mPFS for PI3K activated population with buparlisib. Trial halted before entering phase III |
| McRee [49] | I | 25 | ABC for which capecitabine was deemed a reasonable option | Escalating doses of buparlisib (three levels) and capecitabine (two levels) | NA | Buparlisib MTD: 100 mg daily; capecitabine MTD: 1000 mg/m2 twice daily |
| Sharma [21] | I/II | 43 | HER2− ABC, >6 months from prior solvent-based taxane | Alpelisib+nab-paclitaxel |
|
PI: Alpelisib RP2D: 350 mg/day |
| With anti-HER2 therapy (±chemotherapy) | ||||||
| Cruz Zambrano [50] | I | 64 | Refractory solid tumours, including 11 HER2− and 11 HER2+ ABC |
|
|
Buparlisib MTD arm 2 and 4: 100 mg/day |
| Rodon-Ahnertb [51] | I | 46 | Refractory solid tumours, including 11 HER2− and 11 HER2+ ABC |
|
|
Dactolisib MTD arm 1 and 3: 800 mg/m2/week |
| Saura [52] and Pistilli [53] | I/II | 68 | HER2+ ABC after failing trastuzumab | Phase I: escalating doses of buparlisib+trastuzumab; phase II: RP2D found in phase I for the combination | ORR: 10% |
|
| Tolaney [54] | I/II | 42 | HER2+ ABC after failing trastuzumab |
|
NA | Pilaralisib MTD: 400 mg; did not enter phase II |
| Shah [55] | I | 10 | PIK3CA-mut HER+ ABC after progression under pertuzumab and T-DM1 | Alpelisib+LJM716+trastuzumab | SD: 83% (5 in 6 patients) | Combination too toxic to warrant further testing |
| PIKHER2[56] | I | 25 | HER2+ ABC after progression under trastuzumab | Escalating doses of buparlisib+lapatinib | DCR: 79% | RP2D: buparlisib 80 mg/day+lapatinib 1000 mg/day |
| Jain [57] | I | 17 | HER2+ ABC after a taxane+trastuzumab-based therapy | De-escalating doses of alpelisib combined with T-DM1 | mPFS: 6 m |
|
| Metzger Filho [58] | I | 26 | HER+ ABC regardless of previous lines of anti-HER2 therapy | Cohort A: taselisib + T-DM1 |
|
No DLT in tested doses |
| Schöffski [59] | I | 69 |
|
|
|
Pictilisib 260 mg selected as RP2D but further development of the drug halted |
| PANTHERA [46] | I/II | 12 | HER2+ ABC progressing after ≥1 line of trastuzumab or T-DM1 | Copanlisib+trastuzumab | ORR: 0%; DCR: 75% |
|
| With mTOR inhibitors | ||||||
| Baselga [60] | I | 7 | ER+/HER2− ABC | Escalating doses of alpelisib+everolimus+exemestane | NA | MTD for alpelisib: 200 mg |
Wherever applicable.
Same study reported in 2 separated abstracts, one for each pair of arms.
ABC, advanced breast cancer; AE, adverse events; CI, confidence interval; CT, chemotherapy; DCR, disease control rate; EFS, event-free survival; ER, estrogen receptor; ET, endocrine therapy; HR, hazard ratio; m, months; mt, mutant; MTD, maximum tolerated dose; NA, not available; ORR, overall response ratio; pCR, pathological complete response; mPFS, median progression-free survival; RP2D, recommended phase II dose; SD, stable disease; T-DM1, ado-trastuzumab emtansine.
Table 3.
Ongoing phase I/II/III combination trials with PI3K inhibitors in the early and metastatic estrogen-receptor positive breast cancer settings
| ClinicalTrials.gov identifier (Trial name) | Phase | Design | Patient population | Number of patients | Treatment arms | Objectives |
|---|---|---|---|---|---|---|
| PI3K inhibitors + CDK4/6 inhibitors (±dual PI3K/mTOR inhibitors) | ||||||
| NCT03128619a | I/II | Randomized, open-label, three-arm trial | ER+/HER2−, stage II/III | 102 |
|
|
| NCT02626507 | I | Open-label, single arm trial | ER+/HER2−, stage I–IV, intended for surgery of the primary tumour | 18 | Gedatolisib + fulvestrant + palbociclib (+ goserelin if pre-menopausal) |
|
| Metastatic | ||||||
| PI3K inhibitors + CDK4/6 inhibitors | ||||||
| NCT03939897 | I/II | Open label, non-randomized, two-arm trial | ER+/HER2−, endocrine-resistant ABC | 194 | Copanlisib + abemaciclib + fulvestrant versus abemaciclib + fulvestrant |
|
| NCT03128619a | Ib | Single-arm, open-label trial | ER+/HER2− ABC, first-line treatment | 102 | Copanlisib + palbociclib + letrozole |
|
| NCT02088684 | Ib/II | Randomized, open-label, three-arm trial | ER+/HER2− ABC; ≤2 lines of chemotherapy in phase Ib and ≤1 line in phase II | 70 | Ribociclib+fulvestrant + buparlisib versus ribociclib + alpelisib + fulvestrant versus ribociclib + fulvestrant |
|
| NCT02154776 (LeeBLet) | I | Single arm, open-label trial | ER+/HER2− ABC; ≤2 lines of chemotherapy | 13 | Buparlisib + ribociclib + letrozole |
|
| PI3K inhibitors + chemotherapy | ||||||
| NCT03218826 | I | Single arm, open-label trial | PTEN or PIK3CB mutated, HER2− ABC, among other tumours | 58 | AZD8186 + docetaxel |
|
| PI3K inhibitors + anti-HER2 agents (±chemotherapy) | ||||||
| NCT01285466 | Ib | Open label, non-randomized, multi-arm trial | HER2+ ABC eligible for paclitaxel and trastuzumab (for the cohort of breast cancer patients) | 110 | Dactolisib + paclitaxel + trastuzumab or Buparlisib + paclitaxel + trastuzumab |
|
| NCT02390427 | Ib | Non-randomized, open label, four-arm trial | HER2+ ABC with previous anti-HER2 treatment | 76 |
|
|
| NCT00928330 | I | Non-randomized, open-label trial | HER2+ ABC, after progressing on trastuzumab-based treatment | 57 |
|
Primary: change in cardiac function, among others; Secondary: PK, PFS, among others |
| NCT03765983 | II | Single arm, open label trial | HER+ ABC with CNS involvement | 47 | GDC-0084 + trastuzumab |
|
|
Ib | Single arm, open label trial | HER2+ ABC after trastuzumab and paclitaxel | 24 | Copanlisib + T-DM1 |
|
|
I | Single arm, open label trial | PIK3CA mutated HER2+ ABC, after > 2 lines of treatment, including trastuzumab | 48 | MEN1611 + trastuzumab ± fulvestrant |
|
| Multiple targeting of the PI3K/AKT/mTOR pathway | ||||||
| NCT03006172 | I | Open label, non-randomized, multi-arm trial | For breast cancer cohorts: ER+/HER2− PIK3CA-mutant, progressing on previous therapy | 196 | GDC-0077 + palbociclib + letrozole or fulvestrant, among others |
|
| NCT02684032 | I | Open label, non-randomized, multi-arm trial | ER+/HER2− ABC in various settings | 148 | Gedatolisib + palbociclib + fulvestrant or letrozole |
|
| NCT02077933 | Ib | Open label, non-randomized, crossover assignment trial |
|
79 |
|
|
| NCT01899053 | I | Open label, non-randomized, parallel assignment trial | All solid tumours except brain primary with no standard therapy available | 101 | Sapanisertib + MLN1117 |
|
| NCT01248494 | Ib | Open label, randomized, multi-arm trial | ER+ ABC, no limit on prior number of therapies; HR+/HER2+ patients must have failed trastuzumab | 72 |
|
|
| NCT01082068 | I/II | Open label, non-randomized, parallel assignment trial | HR+/HER2− ABC refractory to a non-steroidal aromatase inhibitor | 72 |
|
|
| NCT02723877 (PIQHASSO) | I/II | Single arm, open label trial | HER2− ABC previously treated with an anthracycline and a taxane | 41 | PQR309 + eribulin |
|
| PI3K inhibitors + selective estrogen receptor degraders (SERD) | ||||||
| NCT02734615 | Ib | Open label, randomized, parallel assignment trial | ER+/HER2− ABC | 312 |
|
|
| PI3K inhibitors + androgen receptor inhibitor | ||||||
| NCT03207529 | I | Single arm, open-label trial | ER+ or −, HER2−, AR+, PTEN+ ABC | 28 | Alpelisib + enzalutamide |
|
Duplicated study as it comprises two phases in different settings.
ABC, advanced breast cancer; AE, adverse events; AR, androgen receptor; CBR, clinical benefit rate; CNS, central nervous system; DCR, disease control rate; DLT, dose limiting toxicities; DoR, duration of response; ER+, estrogen receptor-positive; ER-, estrogen receptor-negative; MTD, maximum tolerated dose; OS, overall survival; ORR, objective response rate; pCR, pathologic complete response; PFS, progression-free survival; PK, pharmacokinetic parameters; RP2D, recommended phase II dose.
With anti-HER2 agents
In preclinical models of HER2-positive BC cells, PI3K pathway activation induces resistance, while treatment with PI3Ki restores sensitivity to anti-HER2 therapies, and the combination of anti-HER2 with PI3Ki has synergic antitumour activity [61]. Likewise, in HER2-positive BC patients, presence of a PIK3CA-mut is associated with worse response rates to neoadjuvant treatment [62]. Phase I/II studies demonstrated the overall feasibility of combining anti-HER2 treatments with PI3Ki (Table 2) [46, 50–59, 63]. A phase II study including HER2-positive, trastuzumab-resistant metastatic BC patients treated with buparlisib and trastuzumab showed an overall response rate of 10%, but grade ≥ 3 toxicities were observed in 70% of patients [52]. The NeoPHOEBE trial randomized HER2-positive BC patients to neoadjuvant trastuzumab/paclitaxel with/without buparlisib. This trial was interrupted after enrolment of only 50 patients due to an increased incidence of severe liver toxicity. Pathological complete response rates did not differ between buparlisib and placebo, yet a significant decrease in Ki67 was observed with buparlisib (75%) versus placebo (26.7%), suggesting that PI3Ki may be active in HER2-positive BC [63]. Despite this promising activity, the high frequency of severe toxicities was concerning. As isoform-selective PI3Ki might have a more favourable toxicity profile, ongoing studies are evaluating their combination with anti-HER2 treatments in HER2-positive BC patients (Table 3) [46].
With chemotherapy
PI3K pathway activation induces resistance to chemotherapy in BC cells [64]. In most clinical studies evaluating PIK3CA-mut as a predictor of chemotherapy response in BC, inferior response rates were observed in patients with PIK3CA-mut tumours when compared with patients with PIK3CA-wild-type tumours [62, 65]. Therefore, the combination of PI3Ki and chemotherapy is being investigated as an attempt to overcome treatment resistance, but no promising results have been observed so far (Table 2) [9, 11, 21, 49, 59]. Ongoing trials are evaluating the association of PI3Ki with chemotherapy in HER2-negative BC (Table 3).
With CDK4/6 inhibitors
Cyclin-dependent kinases (CDK) are involved in cell cycle regulation, and the dysregulated activation of these proteins is a mechanism of resistance to endocrine treatment [66]. Preclinical studies demonstrated an interaction between the CDK4/6 and PI3K pathways in ER-positive BC cells: the antitumour effect of CDK4/6 inhibitors (CDK4/6i) was impaired with PI3K/AKT/mTOR pathway activation, while in BC cells harbouring a PI3KCA-mut, co-treatment with CDK4/6i and PI3Ki was more effective than a PI3Ki alone. This suggested that PI3K activation is a potential mechanism of resistance to CDK4/6i [32]. Early phase trials have already shown the combination of CDK4/6i and PI3Ki may be active in BC (Table 2) [16, 47, 48]. To further explore their potential synergistic effect, ongoing studies are currently evaluating their combination (Table 3).
With new endocrine agents
Survival and proliferation of ER-positive BC cells is highly dependent on ER signalling [67]. ER pathway can remain active, even in the presence of endocrine treatment, through mutations in ESR1 gene, or via the activation of downstream effectors by alternative kinases such as PI3K, HER2 and MAPK [68]. The selective ER modulators/degraders (SERMs/SERDs) are agents designed to bind to the ER, block its signalling and/or increase its degradation. There are new SERDs/SERMs with the potential to bind to the mutated ER and thereby restore the effective blockade of the ER pathway in ESR1-mutated BC cells. Since ESR1-mutation and PIK3CA-mut are both involved in endocrine resistance in ER-positive BC, an ongoing study is evaluating the combination of a new SERD (LSZ102) with alpelisib in endocrine-resistant BC patients (Table 3).
Multiple targeting of the PI3K/AKT/mTOR pathway
Although PI3Ki effectively block PI3K and down-regulate its stimuli to cell proliferation, BC cells are able to reactivate PI3K/AKT/mTOR pathway signalling through the activation of downstream effectors such as AKT and mTOR, and thereby develop resistance to PI3Ki [29]. A potential strategy to overcome this resistance mechanism is the concomitant inhibition of multiple targets on the PI3K/AKT/mTOR pathway, which can be achieved by the combination of different inhibitors, or by agents that block multiple kinases [2, 60]. Thus, ongoing studies are evaluating the blockade of PI3K/AKT/mTOR signalling at multiple sites as a way to overcome treatment resistance in BC (Table 3).
With antiandrogens
PIK3CA-mut can be found in up to 40% of BC patients whose tumours express androgen receptors (AR), and the expression of AR is higher in BC that harbour mutations in the PI3K kinase domain than in PIK3CA wild-type BC [69, 70]. In preclinical models of luminal and triple-negative BC cells, there is a significant cross-talk between the PI3K and the AR pathways, with the activation of the AR inducing PTEN expression and rendering BC cells more sensitive to PI3K inhibition [71]. In cell lines and xenograft models of triple-negative BC cells that express AR, a synergy between the combination of PI3Ki and AR inhibitors has been demonstrated, with the combination exerting a more robust antitumoural effect than each agent alone [69]. Based on this preclinical data, the combination of the α-selective PI3Ki alpelisib with enzalutamide (an AR antagonist) is currently being evaluated in a phase I study in HER2-negative metastatic BC patients whose tumours express both AR and PTEN by immunohistochemistry (Table 3).
Conclusion
Despite intense research efforts, so far, only PIK3CA mutations have proved to have a predictive value for treatment with α-selective and β-sparing PI3Ki in the advanced setting. Thus, its assessment has recently entered clinical practice. Even so, a composite biomarker, which could more accurately assess PI3K/AKT/mTOR pathway activation, would be the preferred approach. This question is even more pressing as new drug combinations with PI3Ki are being developed and may enter clinical practice in the future, making better treatment tailoring an urgent need.
Acknowledgements
DE acknowledges the support of the European Society for Medical Oncology (ESMO) for a Medical Research Fellowship at Institut Jules Bordet (Brussels, Belgium). All the authors thank the very helpful input provided by the reviewers of this manuscript.
Funding
This paper was published as part of a supplement funded by an educational grant from Novartis.
Disclosure
MB: travel grants: Roche/GNE. RC: speaking honoraria: Boehringer-Ingelheim, Janssen; travel grant: Astra-Zeneca. DE: fellowship funding: Novartis. EdA: honoraria and advisory board: Roche/GNE; travel grants: Roche/GNE, GSK/Novartis; he is the co-principal investigator of the LORELEI trial (NCT02273973). MB, RC, DE, EdA: research grants for their Institute: Roche/GNE, Radius, Astra-Zeneca, Lilly, MSD, Novartis, Synthon, Servier, and Pfizer.
References
- 1. Baselga J, Campone M, Piccart M. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366(6): 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janku F, Yap TA, Meric-Bernstam F, Meric-Bernstam F.. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 2018; 15(5): 273.. [DOI] [PubMed] [Google Scholar]
- 3. Beaver JA, Gustin JP, Yi KH. et al. PIK3CA and AKT1 mutations have distinct effects on sensitivity to targeted pathway inhibitors in an isogenic luminal breast cancer model system. Clin Cancer Res 2013; 19(19): 5413–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Brien C, Wallin JJ, Sampath D. et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3′ kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res 2010; 16(14): 3670–3683. [DOI] [PubMed] [Google Scholar]
- 5. Schmid P, Pinder SE, Wheatley D. et al. Phase II randomized preoperative window-of-opportunity study of the PI3K inhibitor pictilisib plus anastrozole compared with anastrozole alone in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2016; 34(17): 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmid P, Pinder S, Wheatley D. et al. Abstract P2-08-02: interaction of PIK3CA mutation subclasses with response to preoperative treatment with the PI3K inhibitor pictilisib in patients with estrogen receptor-positive breast cancer. Cancer Res 2019; 79(Suppl 4): P2-08-02. [Google Scholar]
- 7. Baselga J, Im S-A, Iwata H. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18(7): 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Leo A, Johnston S, Lee KS. et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2018; 19(1): 87–100. [DOI] [PubMed] [Google Scholar]
- 9. Martín M, Chan A, Dirix L. et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol 2017; 28(2): 313–320. [DOI] [PubMed] [Google Scholar]
- 10. Krop IE, Mayer IA, Ganju V. et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016; 17(6): 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vuylsteke P, Huizing M, Petrakova K. et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann Oncol 2016; 27(11): 2059–2066. [DOI] [PubMed] [Google Scholar]
- 12. Saura C, de Azambuja E, Hlauschek D. et al. LBA10_PRPrimary results of LORELEI: a phase II randomized, double-blind study of neoadjuvant letrozole (LET) plus taselisib versus LET plus placebo (PLA) in postmenopausal patients (pts) with ER+/HER2-negative early breast cancer (EBC). Ann Oncol 2017; doi:10.1093/annonc/mdx440.001 [Google Scholar]
- 13. Nuciforo P, Hlauschek D, Saura C. et al. Exploratory analysis of the effect of taselisib on downstream pathway modulation and correlation with tumor response in ER-positive/HER2-negative early-stage breast cancer from the LORELEI trial. J Clin Oncol 2019; 37(Suppl 15): 1050. [Google Scholar]
- 14. Saura C, Sachdev J, Patel MR. et al. Abstract PD5-2: ph1b study of the PI3K inhibitor taselisib (GDC-0032) in combination with letrozole in patients with hormone receptor-positive advanced breast cancer. Cancer Res 2015; 75(Suppl 9): PD5-2. [Google Scholar]
- 15. Dickler MN, Saura C, Richards DA. et al. Phase II study of taselisib (GDC-0032) in combination with fulvestrant in patients with HER2-negative, hormone receptor-positive advanced breast cancer. Clin Cancer Res 2018; 24(18): 4380–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pascual J, MacPherson IR, Armstrong AC. et al. PIPA: a phase Ib study of β-isoform sparing phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib (T) plus palbociclib (P) and fulvestrant (FUL) in PIK3CA-mutant (mt) ER-positive and taselisib (T) plus palbociclib (P) in PIK3CA-mutant (mt) ER-negative advanced breast cancer. J Clin Oncol 2019; 37(Suppl 15): 1051.30817251 [Google Scholar]
- 17. Baselga J, Dent SF, Cortés J. et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): primary analysis from SANDPIPER. J Clin Oncol 2018; 36(Suppl 18): LBA1006. [Google Scholar]
- 18. Mayer IA, Prat A, Egle D. et al. A phase II randomized study of neoadjuvant letrozole plus alpelisib for hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (NEO-ORB). Clin Cancer Res 2019; 25(10): 2975–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayer IA, Abramson VG, Formisano L. et al. A phase Ib study of alpelisib (BYL719), a PI3Kα-specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res 2017; 23(1): 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juric D, Janku F, Rodón J. et al. Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA-wild-type estrogen receptor-positive advanced breast cancer: a phase 1b clinical trial. JAMA Oncol 2018; e184475.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma P, Abramson VG, O’Dea A. et al. Clinical and biomarker results from phase I/II study of PI3K inhibitor BYL 719 (alpelisib) plus nab-paclitaxel in HER2-negative metastatic breast cancer. J Clin Oncol 2018; 36(Suppl 15): 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. André F, Ciruelos E, Rubovszky G. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019; 380(20): 1929–1940. [DOI] [PubMed] [Google Scholar]
- 23. Juric D, Ciruelos E, Rubovszky G. et al. Abstract GS3-08: alpelisib + fulvestrant for advanced breast cancer: subgroup analyses from the phase III SOLAR-1 trial. Cancer Res 2019; 79(Suppl 4): GS3-08. [Google Scholar]
- 24. Zardavas D, Phillips WA, Loi S.. PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res 2014; 16(1): 201.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loi S, Haibe-Kains B, Majjaj S. et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci USA 2010; 107(22): 10208–10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loi S, Michiels S, Baselga J. et al. PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS One 2013; 8(1): e53292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mertins P, Mani DR, Ruggles KV. et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 2016; 534(7605): 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vasan N, Razavi P, Johnson JL. et al. 1ODouble PIK3CA mutations in cis enhance PI3Kα oncogene activation and sensitivity to PI3Kα inhibitors in breast cancer. Ann Oncol 2019; doi:10.1093/annonc/mdz095. [Google Scholar]
- 29. Castel P, Scaltriti M, Mechanisms of Resistance to PI3K and AKT Inhibitors In Yarden Y, Elkabets M (eds), Resistance to Anti-cancer Therapeutics Targeting Receptor Tyrosine Kinases and Downstream Pathways. Cham: Springer International Publishing; 2018; 117–146. [Google Scholar]
- 30. Bosch A, Li Z, Bergamaschi A. et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 2015; 7(283): 283ra51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toska E, Osmanbeyoglu HU, Castel P. et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 2017; 355(6331): 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vora SR, Juric D, Kim N. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014; 26(1): 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aftimos PG, Antunes De Melo e Oliveira AM, Hilbers F. et al. 152OFirst report of AURORA, the breast international group (BIG) molecular screening initiative for metastatic breast cancer (MBC) patients (pts). Ann Oncol 2019; doi:10.1093/annonc/mdz100.003 [Google Scholar]
- 34. Mayer IA, Arteaga CL.. PIK3CA activating mutations: a discordant role in early versus advanced hormone-dependent estrogen receptor-positive breast cancer? J Clin Oncol 2014; 32(27): 2932–2934. [DOI] [PubMed] [Google Scholar]
- 35. Mayer IA, Abramson VG, Isakoff SJ. et al. Stand up to cancer phase Ib study of Pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2014; 32(12): 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juric D, Castel P, Griffith M. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 2015; 518(7538): 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wee S, Wiederschain D, Maira S-M. et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA 2008; 105(35): 13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. André F, Hurvitz S, Fasolo A. et al. Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2—overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J Clin Oncol 2016; 34(18): 2115–2124. [DOI] [PubMed] [Google Scholar]
- 39. Hopkins BD, Pauli C, Du X. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018; 560(7719): 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma CX, Suman V, Goetz MP. et al. A phase II trial of neoadjuvant MK-2206, an AKT inhibitor, with anastrozole in clinical stage II or III PIK3CA-mutant ER-positive and HER2-negative breast cancer. Clin Cancer Res 2017; 23(22): 6823–6832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FDA approves first PI3K inhibitor for breast cancer. http://www.fda.gov/news-events/press-announcements/fda-approves-first-pi3k-inhibitor-breast-cancer (7 August 2019, date last accessed).
- 42. Condorelli R, Mosele F, Verret B. et al. Genomic alterations in breast cancer: level of evidence for actionability according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol 2019; 30(3): 365–373. [DOI] [PubMed] [Google Scholar]
- 43. O’Leary B, Hrebien S, Morden JP. et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. Nat Commun 2018; 9(1): 896.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arnedos M, Vicier C, Loi S. et al. Precision medicine for metastatic breast cancer—limitations and solutions. Nat Rev Clin Oncol 2015; 12(12): 693–704. [DOI] [PubMed] [Google Scholar]
- 45. Moynahan ME, Chen D, He W. et al. Correlation between PIK3CA mutations in cell-free DNA and everolimus efficacy in HR+, HER2− advanced breast cancer: results from BOLERO-2. Br J Cancer 2017; 116(6): 726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hassan A, Gullo G, O’Reilly S. et al. Abstract OT3-06-01: phase Ib clinical trial of coPANlisib in combination with Trastuzumab emtansine (T-DM1) in pre-treated unresectable locally advanced or metastatic HER2-positive breAst cancer (BC) “PANTHERA”-CTRIAL-IE 17-13. Cancer Res 2019; 79(Suppl 4): OT3-06-01. [Google Scholar]
- 47. Juric D, Ismail-Khan R, Campone M. et al. Abstract P3-14-01: phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2– breast cancer: safety, preliminary efficacy and molecular analysis. Cancer Res 2016; 76(Suppl 4): P3-14-01. [Google Scholar]
- 48. Forero-Torres A, Wesolowski R, Bardia A. et al. Phase Ib study to assess the safety, tolerability, and clinical activity of gedatolisib in combination with palbociclib and either letrozole or fulvestrant in women with metastatic or locally advanced/recurrent breast cancer (B2151009; NCT02684032). J Clin Oncol 2017; 35(Suppl 15): TPS1113. [Google Scholar]
- 49. McRee AJ, Marcom PK, Moore DT. et al. A phase I trial of the PI3K inhibitor buparlisib combined with capecitabine in patients with metastatic breast cancer. Clin Breast Cancer 2018; 18(4): 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cruz Zambrano C, Schuler MH, Machiels J-P. et al. Phase lb study of buparlisib (BKM120) plus either paclitaxel (PTX) in advanced solid tumors (aST) or PTX plus trastuzumab (TZ) in HER2+ breast cancer (BC). J Clin Oncol 2014; 32(Suppl 15): 627.24470012 [Google Scholar]
- 51. Rodon Ahnert J, Schuler MH, Machiels J-P. et al. Phase lb study of BEZ235 plus either paclitaxel (PTX) in advanced solid tumors (aST) or PTX plus trastuzumab (TZ) in HER2+ breast cancer (BC). J Clin Oncol 2014; 32(Suppl 15): 621. [Google Scholar]
- 52. Pistilli B, Pluard T, Urruticoechea A. et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res Treat 2018; 168(2): 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saura C, Bendell J, Jerusalem G. et al. Phase Ib study of Buparlisib plus Trastuzumab in patients with HER2-positive advanced or metastatic breast cancer that has progressed on Trastuzumab-based therapy. Clin Cancer Res 2014; 20(7): 1935–1945. [DOI] [PubMed] [Google Scholar]
- 54. Tolaney S, Burris H, Gartner E. et al. Phase I/II study of pilaralisib (SAR245408) in combination with trastuzumab or trastuzumab plus paclitaxel in trastuzumab-refractory HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2015; 149(1): 151–161. [DOI] [PubMed] [Google Scholar]
- 55. Shah PD, Chandarlapaty S, Dickler MN. et al. Phase I study of LJM716, BYL719, and trastuzumab in patients (pts) with HER2-amplified (HER2+) metastatic breast cancer (MBC). J Clin Oncol 2015; 33(Suppl 15): 590. [Google Scholar]
- 56. Guerin M, Rezai K, Isambert N. et al. PIKHER2: a phase IB study evaluating buparlisib in combination with lapatinib in trastuzumab-resistant HER2-positive advanced breast cancer. Eur. J. Cancer 2017; 86: 28–36. [DOI] [PubMed] [Google Scholar]
- 57. Jain S, Santa-Maria CA, Rademaker A. et al. Phase I study of alpelisib (BYL-719) and T-DM1 in HER2-positive metastatic breast cancer after trastuzumab and taxane therapy. J Clin Oncol 2017; 35(Suppl 15): 1026. [DOI] [PubMed] [Google Scholar]
- 58. Metzger Filho O, Goel S, Barry WT. et al. A mouse-human phase I co-clinical trial of taselisib in combination with TDM1 in advanced HER2-positive breast cancer (MBC). J Clin Oncol 2017; 35(Suppl 15): 1030. [Google Scholar]
- 59. Schöffski P, Cresta S, Mayer IA. et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res 2018; 20(1): 109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baselga J, Curigliano G, Martín M. et al. Abstract CT061: a phase Ib study of alpelisib (BYL719) + everolimus ± exemestane in patients with advanced solid tumors or HR+/HER2-breast cancer. Cancer Res 2016; 76(Suppl 14): CT061. [Google Scholar]
- 61. Rexer BN, Chanthaphaychith S, Dahlman KB, Arteaga CL.. Direct inhibition of PI3K in combination with dual HER2 inhibitors is required for optimal antitumor activity in HER2+ breast cancer cells. Breast Cancer Res 2014; 16(1): R9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Loibl S, von Minckwitz G, Schneeweiss A. et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol 2014; 32(29): 3212–3220. [DOI] [PubMed] [Google Scholar]
- 63. Loibl S, de la Pena L, Nekljudova V. et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur J Cancer 2017; 85: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jin W, Wu L, Liang K. et al. Roles of the PI-3K and MEK pathways in Ras-mediated chemoresistance in breast cancer cells. Br J Cancer 2003; 89(1): 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Majewski IJ, Nuciforo P, Mittempergher L. et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol 2015; 33(12): 1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Musgrove EA, Sutherland RL.. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 2009; 9(9): 631.. [DOI] [PubMed] [Google Scholar]
- 67. Moy B. Estrogen receptor pathway: resistance to endocrine therapy and new therapeutic approaches. Clin Cancer Res 2006; 12(16): 4790–4793. [DOI] [PubMed] [Google Scholar]
- 68. García-Becerra R, Santos N, Díaz L, Camacho J.. Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance. IJMS 2012; 14(1): 108–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lehmann BD, Bauer JA, Schafer JM. et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 2014; 16(4): 406.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gonzalez-Angulo AM, Stemke-Hale K, Palla SL. et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res 2009; 15(7): 2472–2478. [DOI] [PubMed] [Google Scholar]
- 71. Wang Y, Yu Q, He X. et al. Activation of AR sensitizes breast carcinomas to NVP-BEZ235’s therapeutic effect mediated by PTEN and KLLN upregulation. Mol Cancer Ther 2014; 13(2): 517–527. [DOI] [PubMed] [Google Scholar]