Abstract
One of the hallmarks of hormone receptor (HR)-positive breast cancer is its dependence on the phosphatidylinositol-3-kinase (PI3K) pathway. Here, we review the epidemiologic, functional, and pharmacologic interactions between oncogenic PI3K and the estrogen receptor (ER). We discuss the epidemiology of PI3K pathway alterations, mechanisms of resistance to PI3K inhibitors, and the current mechanistic landscape of crosstalk between PI3K and ER, which provide the rationale for dual ER and PI3K inhibition and is now a standard of care in the treatment of ER+ PIK3CA-mutant metastatic breast cancer. We outline newer studies in this field that delineate the clinically relevant overlaps between PI3K and parallel signaling pathways, insulin signaling, and ER epigenetic modifiers. We also identify several caveats with the current data and propose new strategies to overcome these bottlenecks.
Keywords: PI3K pathway, estrogen receptor, breast cancer, PI3K inhibitors, PIK3CA, AKT
Key Message
In this review, we discuss the interplay between estrogen receptor (ER), PIK3CA, and the PI3K pathway that has culminated in the combination of fulvestrant with alpelisib as a new standard of care in the treatment of ER+ PIK3CA-mutant metastatic breast cancer.
Epidemiology of PI3K pathway alterations in HR-positive breast cancer
The PI3K pathway in cancer
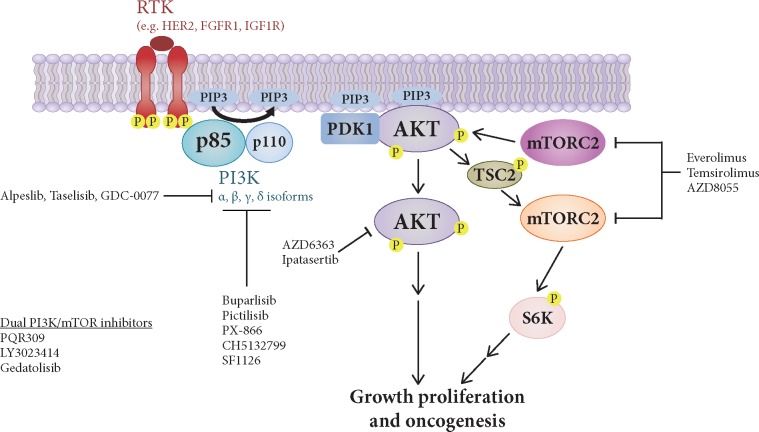
Activation of the phosphoinositide-3-kinase (PI3K) pathway occurs in a variety of cancers. (Figure 1). The PI3K pathway integrates extracellular signals that activate receptor tyrosine kinases (RTKs) and G-protein-coupled receptors (GPCRs) and is necessary for normal growth and proliferation [1]. PI3K-alpha (PI3Kα) is a heterodimeric protein complex composed of the catalytic subunit p110α (coded by the PIK3CA gene) and the regulatory subunit p85α (coded by the PIK3R1 gene) [2]. p110α binds to and is inhibited by the regulatory subunit p85α and catalyzes the phosphorylation of the lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3) [2]. Other p110 isoforms including p110β can signal through this pathway to produce PIP3. Accumulation of PIP3 at the plasma membrane functions as second messenger to initiate a downstream signaling cascade involving the activation of AKT by the PIP3-binding protein phosphoinositide-dependent kinase 1 (PDK1) and by mammalian target of rapamycin complex 2 (mTORC2). Activated AKT phosphorylates and disinhibits tuberous sclerosis complex 2 (TSC2), which is a negative regulator of mTOR and leads to downstream mitogenic signaling. PI3K requires multiple inputs for full activation, including binding by membrane-bound RTKs and Ras. Conversely, PIP3 can be dephosphorylated to PIP2 by the lipid phosphatase and tensin homolog (PTEN). Cellular and tumor dependence on the PI3K pathway occurs in several manners, including PIK3CA and AKT oncogene mutation, RTKs overexpression, and PTEN tumor suppressor loss of function. In this review, we discuss the molecular epidemiology of PI3K pathway alterations in estrogen receptor (ER)-positive (ER+) breast cancer (BC).
Figure 1.
Signaling by the phosphatidylinositol-3-kinase (PI3K)-AKT- mammalian target of rapamycin (mTOR) pathway. The PI3K-AKT-mTOR pathway is activated by various growth factor receptors such as HER2, fibroblast growth factor receptor 1 (FGFR1), insulin-like growth factor 1 R (IGF1R) among others. PI3K proteins are recruited to the plasma membrane, leading to phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 recruits to the membrane the AKT kinases that, once activated by PDK1 and mTORC2 phosphorylation, are able to phosphorylate TSC2 and negatively regulate the activity of the kinase mTOR. mTORC1 also phosphorylates and activates S6K. Also shown are few examples of PI3K-AKT-mTOR inhibitors in clinical trials.
PIK3CA mutations in ER+ BC
The most common PI3K pathway alteration in ER+ BC is PIK3CA oncogenic mutations, while PIK3CA gene amplification in the absence of mutation is relatively rare [3]. PIK3CA mutations are present in up to 40% of ER+ human epidermal growth factor receptor 2 (HER2)-negative primary and metastatic tumors and result in constitutive enzymatic activity [4–6]. Many retrospective studies, including a large meta-analysis of >10 000 patients with early-stage BC [7], have proposed that PIK3CA mutations are prognostic markers for improved relapse-free survival on univariate analysis [8, 9]. However, these studies have also confirmed that, when controlling for additional favorable-risk clinical variables such as ER positivity and receipt of hormonal therapy alone without chemotherapy, PIK3CA mutations are not prognostic for improved relapse-free survival or overall survival [7, 8]. A study in HER2-positive (HER2+) BC has found that PIK3CA mutations predict for decreased overall survival [7], suggesting additivity between upstream RTK signaling and PIK3CA mutation, and this has provided the preclinical rationale for investigating combination anti-HER2 therapy with PI3K pathway inhibition [10].
Many distinct cancer-associated PIK3CA mutations have been identified. The most frequent mutations are hotspot single amino acid substitutions in the helical (E545K and E542K in exon 9) or kinase (H1047R in exon 20) domains [11]. The mechanisms of activation of E545K and H1047R are distinct, where E545K mimics activation by RTK phosphopeptides and is dependent on Ras, and H1047R increases lipid membrane binding promoting access to PIP2 substrate and is Ras-independent. Some small studies analyzing mostly ER+ tumors have demonstrated decreased survival for patients with E545K- as compared with H1047R-containing tumors [12, 13], but these differences have not proven significant in a larger meta-analysis [7]. There is also a long tail of less frequent mutations scattered across the PIK3CA gene, the majority of which lead to partial activation in biochemical and cellular models [14]. The functional and clinical consequences of these mutations are less clear since most clinical studies focus on exon 9 and exon 20 mutations only. While these additional mutational mechanisms may be distinct, they likely converge on a model where PIK3CA mutations structurally open the PI3K complex, increasing membrane binding and kinase activity owing to less inhibition of p110α by p85α [15]. It should be noted that these mutations were probably underestimated in earlier studies where principally the E545K and H1047R hotspots (or some but not all exons) were sequenced. Using a targeted sequencing approach analyzing all exons of 417 genes including PIK3CA, our group has demonstrated that some novel rare PIK3CA mutations are associated with endocrine resistance in metastatic ER+ BC [6]. Despite the large number of PIK3CA mutations, inactivating PIK3R1 mutations that activate PI3K by disinhibiting p110α [15] are rarely observed in ER+ BC (3%).
Other PI3K pathway alterations in ER+ BC
Upstream RTKs overexpression or mutations can hyperactivate their downstream signaling pathways including PI3K. ERBB2 amplification/HER2 overexpression is found in 10% of ER+ BCs [16], and rare HER2 mutations associated with endocrine resistance have also been characterized [17, 18]. Fibroblast growth factor receptor 1 (FGFR1) amplification is found in up to 12% of ER+ BCs [19]. There are multiple direct and indirect mechanisms of interactions between upstream RTKs and the PI3K pathway, rationalizing combination therapies of PI3K inhibitors with RTK inhibitors. Mutations in Ras, which is also upstream of PI3K, are infrequent in BC (<1%, TCGA), though loss of negative regulators of Ras have been reported in luminal B tumors [20].
De novo PTEN mutations are rare in ER+ BC and are mutually exclusive with PIK3CA mutations in treatment naive breast tumors [21, 22]; however, proteomic approaches have shown decreased PTEN protein in breast tumors irrespective of PIK3CA mutations, suggesting additional regulatory mechanisms of PTEN. In a meta-analysis, PTEN protein loss by immunohistochemistry is prognostic for decreased survival across all receptor subtypes of BC [23], but given that is highly associated with ER- BC, its prognostic significance has not been elucidated in ER+ BC. However, as discussed below, acquired PTEN mutation is a mechanism of resistance to PI3Kα inhibitors in ER+ PIK3CA mutant BC [24].
AKT mutant isoforms are considered weak oncogenes, where AKT1 E17K hotspot mutations are found in 3% of ER+ BC [22] and are mutually exclusive with PIK3CA mutations in early-stage [22] and metastatic ER+ BC [6]. On the other hand, AKT2 and AKT3 can be amplified but are rarely mutated in ER+ BC [22].
Toward a higher order understanding of the role of the PI3K pathway in ER+ BC
No single alteration of the PI3K pathway is predictive of survival in early-stage or metastatic ER+ BC. While single PIK3CA mutants are oncogenic in multiple cellular models, these mutations are weak oncogenes in vivo, with variable penetrance resulting in pleiotropic histologies (not only adenocarcinomas but also sarcomas and squamous cell cancers) with partial levels of estrogen dependence [25]. The discordance in growth and proliferation between single PIK3CA mutations in in vitro and in vivo models implicates additional mechanisms (either intrinsic or extrinsic to the PI3K pathway) for the full PI3K oncogenic phenotype. The clinical corollary to this observation is the lack of benefit of PI3K inhibitor monotherapy in ER+ BC.
One obvious solution for tumor cells to augment PI3K pathway oncogenesis would be additional oncogenic or tumor suppressor mutations in the PI3K pathway; however, PIK3CA, AKT, and PTEN mutations are largely mutually exclusive in treatment-naive ER+ BC, suggesting that nongenomic mechanisms (e.g. gene expression or protein-based) may play an important role in oncogenesis. Indeed, a number of crosstalk epigenetic and posttranslational modification mechanisms exist between ER and PI3K (discussed below).
Moreover, many of the older BC sequencing studies have focused on localized primary tumors rather than on metastatic tumors, contain cohorts that eventually result in small numbers when stratified by receptor subtype, include tumors containing only the most frequent PIK3CA mutations, and do not stratify based on specific treatment outcomes [e.g. selective estrogen receptor degrader versus aromatase inhibitors (AIs) versus tamoxifen]. These important issues have begun to be addressed by large-scale sequencing efforts coupled with careful clinicogenomic annotation [6] to deconvolute PI3K pathway activating genotypes/phenotypes from clinical prognosis among untreated primary and endocrine-sensitive and endocrine-resistant metastatic ER+ breast tumors.
Preclinical mechanisms of resistance to PI3K inhibitors
Initial studies on PI3K inhibitor resistance focused on RTK pathway upregulation [26] through expression and activation of HER2, HER3, and other RTKs in ER+ BC [27–29]. These RTKs re-stimulate PI3K by binding to the nSH2 and cSH2 (Src homology 2) domains of p85α, relieving its inhibition of p110α. Multiple adaptive and acquired resistance mechanisms to PI3K inhibitors have now been described. These can occur directly in the canonical effectors of the PI3K-AKT-mTOR cascade or involve parallel pathways that feed into the PI3K effectors at proximal or distal points.
PI3K inhibitors potently decrease AKT signaling but AKT inhibitors can paradoxically re-sensitize some alpelisib-resistant ER+ BC cell lines to alpelisib, as ascertained through a combinatorial drug screen [30]. In this report, residual levels of AKT signaling were still present in T47D but not in MCF7 ER+ BC cell line, and this correlated with response to combined alpelisib and AKT inhibitor therapy, re-sensitizing alpelisib-resistant ER+ T47D BC cells but not alpelisib-resistant ER+ MCF7 BC cells. Thus, AKT inhibition by PI3K inhibitors is context-dependent even in ER+ BC. These findings are in agreement with BC sequencing studies that have demonstrated a lack of correlation between PIK3CA mutation and AKT phosphorylation, raising the possibility of autonomous activation mechanisms.
Consistently, a number of non-AKT-dependent mechanisms that drive resistance to PI3Kα inhibitors have been elucidated. These mechanisms involve pathways parallel to all branches of the PI3K pathway such as other p110 isoforms, other AGC family kinases (of which AKT is one member), and downstream signaling nodes with varied inputs and outputs.
While p110α is frequently mutated in ER+ BC, p110β, which can also drive PI3K pathway signaling, is rarely mutated in cancer. By measuring PIP3 restoration after alpelisib treatment of ER+ BC cells, Costa et al. demonstrated that p110β activation by RTKs can lead to non-AKT-dependent downstream PI3K pathway signaling [31]. Despite consistent PIP3 suppression, combining p110α with p110β inhibitors induced tumor regression in an ER+/HER2+ model but not in ER+/HER2- xenografts, suggesting a hierarchy for non-AKT-dependent mechanisms of resistance among different BC receptor subtypes.
In ER+ BC, PTEN mutations are rarely found de novo as described above. Our group has discovered that convergent loss of function PTEN mutations can drive resistance to alpelisib by analyzing an exceptional responder patient [24]. This heavily pre-treated patient had ER+ metastatic BC and initially responded to alpelisib monotherapy for 9.5 months. After therapy progression and expiration, we accessed 14 different metastases during a rapid autopsy of the patient. Ten of these lesions carried different genetic alterations converging to homozygous loss of function of PTEN. In ER+ PIK3CA-mutant BC cells, PTEN loss caused resistance to alpelisib but not to the pan-PI3K inhibitor BKM120. Speculating that loss of function PTEN mutations can drive resistance to PI3Kα inhibitors by decreasing amounts of PIP3, which indirectly increases the PIP2 substrate for the p110β isoform and downstream signaling, we tested the efficacy of combining alpelisib with a p110β inhibitor in a patient-derived model established from the same patient. Accordingly, this strategy caused tumor regression, suggesting that the combination of p110α and p110β inhibition may be a general strategy to overcome PI3K inhibitor resistance in ER+ BC when p110β becomes active as a consequence of pharmacological pressure.
Our group and others have demonstrated that persistent activity of either mTORC1 [30, 32] or PDK1 [33, 34] can drive intrinsic resistance to alpelisib in ER+ BC. These are AKT-independent mechanisms, as AKT signaling is profoundly inhibited by alpelisib in both sensitive and resistant BC cell lines in vitro and in vivo. Resistance to alpelisib can also be mediated by reactivation of mTOR by the secreted ligands insulin-like growth factor 1 (IGF1) and neuregulin [32]. The causative role of mTOR activity in this setting is underscored by the fact that suboptimal doses of everolimus can re-sensitize these tumors to alpelisib. PDK1 activates not only AKT but also other AGC family kinases including those of the serum/glucocorticoid regulated kinase (SGK) family, and SGK is upregulated in alpelisib-resistant BC cells and tumors [33]. Combination of PI3K and PDK1 inhibitors also reverts resistance to alpelisib in an mTORC1-dependent manner. Inhibition of other targets such as cyclin-dependent kinases 4 and 6 (CDK4/6) can re-sensitize ER+ BC cells that exhibit primary or acquired resistance to alpelisib [30]. Combination of the CDK4/6 inhibitor ribociclib with alpelisib delayed the development of resistance both in vitro and in vivo.
Cancer cell-extrinsic mechanisms of resistance to PI3K inhibitors have also been investigated. In a recent report, mouse models of multiple cancers including ER+ BC showed increased blood glucose and insulin on treatment with a wide range of small molecule inhibitors of the PI3K pathway, including PI3Kα-specific inhibitors [35]. This hyperinsulinemia resulted in increased PI3K pathway signaling and cell proliferation. In vivo, targeting this relief of negative feedback on insulin signaling through sodium/glucose cotransporter 2 (SGLT2) inhibition or ketogenic diet led to profound re-sensitization of PI3K inhibitors in many cancer histologies.
These recent studies on mechanisms of resistance raise several important points. First, unlike protein kinases where ‘on-target’ mutations are a common mechanism of acquired resistance, mutations in the ATP-binding site of PI3Kα (which may sterically hinder the binding of small molecule ATP competitors) have never been reported, to our knowledge. This lack of pressure on the target may be because PI3Kα-specific inhibitors in their current incarnation are not mutant selective and inhibit also the wild-type enzyme in both cancer cells and normal cells. Thus, selective pressures to alter other pathways may outweigh intrinsic pressure to mutate PIK3CA to cause resistance. Moreover, concomitant inhibition of wild-type PI3K may prevent dosing within an adequate therapeutic window.
Second, the large number of AKT-independent mechanisms for resistance to PI3K inhibitors highlights the problem with using phosphorylated AKT as a surrogate for direct readout of PI3K activation, given that in many cases, even with complete suppression of AKT, parallel mechanisms of resistance can simultaneously activate more distal components of the PI3K pathway. Additional biomarkers such as direct PIP3 measurement [31] (which is also feasible in tissue [36]) or immunohistochemistry for pS6 may be of utility in assessing the response to PI3K inhibitors in patients. Finally, it should be noted that many preclinical studies analyzed the effects of PI3K inhibitor monotherapy only, and there may be higher order interactions when studying the resistance to combined PI3K inhibition and anti-endocrine therapy.
Crosstalk between ER and PI3K pathway
Many years of work, from classical investigations of transcription factor (TF) function and growth factor signaling regulation to modern genome-wide epigenomic assays, have demonstrated that there is important crosstalk between ER and PI3K pathway. Even more importantly, these findings have shown to have significant implications for the biology of BC and response to both PI3K inhibitors and endocrine agents. In over 70% of patients with ER+ BC, ER serves as a master cellular regulator that controls transcriptional repertoires favoring uncontrolled cell proliferation and tumor growth [37]. The binding of estrogen to ER triggers receptor dimerization and recruitment of ER to the cis regulatory elements of its target genes to regulate transcription. ER binding to its target genes leads to recruitment of additional cooperating TFs and chromatin regulators which are critical for the function of this intricate network (referred to as the classical genomic signaling pathway) [38]. Estrogen genomic signaling induces the expression/activation of proteins important for tumor growth such as the insulin-like growth factor I receptor (IGF-IR), insulin growth factor II (IGFII) and downregulation of genes such as epidermal growth factor receptor (EGFR) and HER2. As such, ER is inhibited clinically using the ER antagonist tamoxifen, the AIs letrozole and exemestane, or the ER degrader fulvestrant.
The importance of PI3K pathway in ER+ BC is highlighted by the mentioned high frequency of activating somatic mutations of PIK3CA (∼40%) in this subset of BC. In addition, other genomic alterations resulting in hyperactivation of the PI3K pathway such as ERBB2 and AKT1 are frequent in ER+ BC [39, 40]. As the vast majority of PIK3CA-mutant tumors are ER+, studies have indicated that both pathways can coordinately support survival.
One of the first clinical evidences that supported the close interaction between the PI3K pathway and ER signaling came from the Breast Cancer Trials of Oral Everolimus-2 (BOLERO-2) phase III clinical trial that showed improvement in progression-free survival (PFS) in ER+ BC patients treated with the mTOR inhibitor everolimus in combination with the AI exemestane [41]. Despite the minimal activity of single-agent mTOR inhibitor (in part due to the activation of compensatory pathways), and that these patients were refractory to endocrine therapies, these encouraging results strongly suggested a synergistic activity in the dual targeting of PI3K and ER pathways. As specific PI3Kα inhibitors were emerging in the clinic, we and others studied the effects of these agents on ER signaling and its role on limiting their efficacy. In this regard, we have detected a highly uniform adaptive tumor response to PI3Kα inhibitors that is characterized by an increased dependency on the ER response, which mediates resistance to these agents and can be reversed by the addition of anti-ER therapies [42]. Thus, inhibition of PI3K paradoxically activates ER-mediated transcription in BC cells as a survival feedforward mechanism [42]. Of note, also in prostate cancer, which is characterized by its dependence on the nuclear hormone androgen receptor (AR) and presence of activation of PI3K signaling, it has been shown that PI3K pathway activates AR signaling to support survival [43]. Thus, inhibition of PI3K oncogenic pathway increases cell survival dependency on ER and AR in breast and prostate cancer, respectively.
The preclinical findings on ER upregulation upon PI3K inhibition paved the way for phase III registration clinical studies testing PI3Kα inhibitors in combination with the anti-ER agent fulvestrant in patients with advanced PIK3CA-mutant ER+ BC [44]. These efforts culminated with the registrational phase III clinical trial SOLAR 1, demonstrating that the addition of the PI3Kα inhibitor alpelisib to anti-endocrine therapy improved PFS in HR+ PIK3CA mutant metastatic BC from 5.7 to 11.0 months leading to Food and Drug Administration (FDA) approval of alpelisib [45]. Since most of the patients were selected to present a resistance to ET, PFS longer than 11 months could theoretically be achieved and should be explored in earlier disease settings.
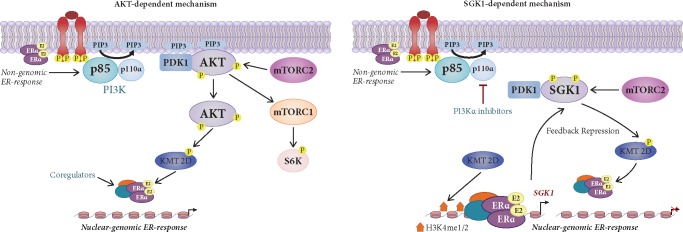
The mechanism of the interplay between the ER and PI3K pathways remained unclear until recently. In studying this intricate crosstalk, we demonstrated that PI3Kα inhibition remodels the chromatin landscape of BC on a genome-wide scale to enable a permissive gene regulatory state at ER loci in both cells and patient samples. By establishing a direct association between PI3K/AKT signaling and chromatin regulators, we identified the histone methyltransferase KMT2D as a key determinant of ER activation by the PI3K/AKT signaling pathway. We found that in scenarios of activated PI3K signaling, such as in PIK3CA mutant cells, KMT2D is directly phosphorylated and inactivated by AKT, resulting in the attenuation of histone methylation, loss of binding of the ER and consequent transcriptional suppression. Conversely, KMT2D activity and histone methylation is enhanced when PI3Kα is inhibited, facilitating the recruitment of ER transcriptional network and unleashing a dominant impact on transcription and promoting tumor growth. Consistently, KMT2D silencing in xenograft models substantially enhances the antitumor activity of PI3Kα inhibitors by suppressing ER transcriptional output (Figure 2). These results provide a better understanding of how ER-dependent transcription may be modulated by oncogenic pathways as feedforward survival mechanism and provide a rationale for epigenetic therapy for this disease [46]. In addition to the AKT-dependent mechanism of ER regulation via the phosphorylation of KMT2D, we recently discovered that the PI3K pathway propagates its effects to control KMT2D and ER function via the activity of SGK1, another downstream PI3K effector. We show that, upon PI3Kα inhibition, ER activates SGK1 transcription and elevates SGK1 protein levels. This in turn resulted in phosphorylation of KMT2D, suppressing its function, and attenuating ER-dependent expression. Thus, a negative feedback loop activates SGK1 to promote chromatin-based regulation of ER-dependent gene expression (Figure 2). Given that high levels of active SGK1 would inhibit ER activity making cells less dependent on the ER response [47], we speculate that SGK1 could be another important mediator of resistance not only to PI3K inhibitors, as we have previously reported [33], but also to endocrine therapies. Other hormone nuclear receptors such as PR or retinoic acid receptor [48, 49] have been previously linked to affect ER transcriptional activity, and their gene signatures are elevated by PI3K kinase inhibition [46]. Thus, it is plausible to surmise that their function could be also regulated by protein kinase inhibition and KMT2D. Moreover, it also remains to be investigated whether PI3K signaling can regulate transcription to promote growth by modulating the function of additional chromatin regulators.
Figure 2.
Proposed model of the phosphatidylinositol-3-kinase (PI3K)-estrogen receptor (ER) crosstalk. Estrogen activates nuclear ER known as the genomic pathway and membrane ER known as the non-genomic pathway. Membrane ER can associate with growth factor signaling components including PI3K. Activation of PI3K pathway activates its main effector AKT. After activation, AKT goes to the nucleus where it encounters nuclear substrate such as the H3K4 mono-and di-menthyltransferase KMT2D, an important regulator of nuclear ER. AKT phosphorylates KMT2D at S1331 to attenuate KMT2D function, inducing a loss of binding of KMT2D to ER target genes and a suppression of ER-dependent transcription (genomic pathway). Upon PI3Kα inhibition, AKT is no longer active and cannot phosphorylate KMT2D. KMT2D induces mono- and dimethylation of H3K4, is recruited to ER target loci and activates ER-dependent transcription (AKT-dependent mechanism). A target gene of ER is the PI3K effector SGK1. Upon PI3Kα inhibition, ER upregulates SGK1 transcription. Elevated SGK1, in turn, directly phosphorylates KMT2D at S1331, attenuating its function and subsequent ER activity as a negative repression feedback loop (SGK-dependent mechanism). ER-dependent increase of SGK1 upon PI3Kα inhibition in transient and may only be active few hours after PI3K pathway inhibition, while the overall ER-response can be sustained.
The regulatory crosstalk between PI3K and ER is bidirectional as not only upregulation of ER by PI3K inhibition mediates resistance to PI3K inhibitors but also PI3K pathway activation is associated with de novo and acquired resistance to endocrine therapies. In this regard, knockdown of PTEN or INPP4B phosphatases or upregulation of oncogenes that activate the PI3K pathway such as HER2 and IGF1R and activating AKT1 mutations have been shown to mediate resistance to anti-estrogen therapy in patients with ER+ BC [50]. Moreover, it has been reported that tamoxifen- or fulvestrant-resistant ER+ BC cells/xenografts, as well as long-term estrogen deprived ER+ BC cells, exhibit higher levels of activation of PI3K/AKT/mTOR signaling and hyperactivation of IGF-IR and or InsR [51]. In most of these models, endocrine therapy resistance was reversed with the inhibition of PI3K.
Given the complexity of the ER and PI3K pathways, their interplay occurs at numerous levels. Findings demonstrate that ER and its coregulators can be phosphorylated by oncogene-mediated signaling. A variety of kinases including MAPKs and downstream effectors of PI3K such as AKT and S6K directly phosphorylate ER leading to ligand-independent ER-dependent transcription [50, 52, 53]. However, treatment of cells with the pan PI3K inhibitor BKM120 did not decrease the phosphorylation of ER or ER-dependent transcription [54]. Another layer of interaction and regulation of ER signaling is the phosphorylation of ER coregulatory proteins such as KMT2D by growth factor kinases. Examples include the PI3K pathway mediated c-Jun phosphorylation [55]. c-Jun complexes with c-Fos to form the AP1 complex, which binds specific non-estrogen response element promoters of AP1-responsive genes to regulate transcription (referred to as the nonclassical genomic signaling pathway [56]). Other examples include phosphorylation of AIB1 coactivator by MAPK, which increases ER-dependent transcription [57]. Overexpression of AIB1 in MCF7 BC cell lines transfected with HER2 (MCF7/HER2+) converts tamoxifen-bound ER into an estrogen agonist rather than an antagonist, suggesting that ER-HER2 crosstalk has an important role in tamoxifen resistance.
Evidence from preclinical work has also implicated ER to regulate cellular functions independent of its classical transcriptional activity via nongenomic estrogen signaling mechanisms. A small pool of ER that is located at the plasma membrane resembles growth factor ligands and this is where part of the crosstalk between ER and the PI3K pathway occurs. Plasma membrane-bound ER increases the levels of second messengers such as cyclic AMP and rapidly initiates activation of various signaling molecules such as IGF-1R/InsR, EGFR, HER2, PI3K, MEK, and Src [58]. Several membrane ER isoforms have been identified, including ERα, splice variants of ERα (ER-36, ER-46), ERβ, and GPR30 [59]. Membrane ER may activate oncogenic kinases to promote endocrine resistance; however, the clinical validation of this phenomenon is still pending.
Thus, in ER+ breast tumors a complex cycle with cross-regulatory interactions and negative and positive feedbacks is established between ER and growth factor receptor network, leading to enhanced cell growth and proliferation [60]. These interactions are likely to differ in normal versus disease states as the activated PI3K pathway in cancer strongly affects the ER response and vice versa. The multidirectional interplay between the two most critical survival pathways in BC provides an explanation for the poor efficacy of single pathway therapy in ER+/PIK3CA mutant BCs. Most importantly, it has provided the molecular rationale for simultaneously targeting both pathways to achieve superior disease control culminating with the encouraging recent results of the phase III clinical trial targeting both ER and PI3Kα in BC patients with HR+/PIK3CA mutations and FDA approval of the first PI3K inhibitor, alpelisib [45].
Dual blockade of ER and PI3K: preclinical and clinical data
The alterations in key nodes of the PI3K pathway render effectors of this signaling cascade as attractive targets for anticancer drug development. Despite the high frequency of PIK3CA mutations in BC and in other cancers, the development of isotype specific PI3Kα inhibitors did not occur until recently [61]. Nevertheless, drug development of agents inhibiting components of the PI3K pathway blossomed, while at the same time evidence began to indicate the complexity of PI3K signaling, crosstalks with other pathways, and positive and negative regulatory feedbacks which in concert challenged the success of these agents.
The first inhibitors of the PI3K pathway that advanced in the clinic are the mTOR inhibitors everolimus and temsirolimus, both derivatives of rapamycin. While these inhibitors have been approved, they have limited activity as monotherapy [62, 63], likely due to narrow therapeutic windows and activation of compensatory mechanisms involved in intrinsic and adaptive resistance. With this in mind, competitive inhibitors of mTOR kinase (e.g. AZD8055), which are effective against both mTORC1 and mTORC2 and inhibit the feedback-loop-based activation of AKT caused by inhibition of mTORC1 alone, have been developed and are being clinically tested [64]. However, they too have only modest clinical activity when used as clinically achievable single agents.
Multiple inhibitors can also selectively inhibit AKT proteins, preventing the activation of mTORC1 and the downstream effects of the pathway. Encouraging levels of activity have been reported in patients with cancers harboring the AKT1 E17K oncogenic mutation in a multi-histology basket study testing the efficacy of AZD6363, a pan-AKT kinase inhibitor. This study provided the first evidence that AKT E17K can be a therapeutic target in human cancer [65].
Furthermore, early examples of PI3K inhibitors which were tested in the laboratory and in the clinic include the pan-PI3K inhibitors buparlisib, pictilisib, PX-866, CH5132799 and SF1126 among others, which inhibit to some degree the catalytic activity of all four PI3K class I isoforms PI3Kα (encoded by PIK3CA), PI3Kβ (encoded by PIK3CB), PI3Kγ (encoded by PIK3CG) and PI3Kδ (encoded by PIK3CD) [66]. In the case of buparlisib (BKM120) [67], in preclinical models, the combination of this agent and fulvesrant reduced tumor growth in an everolimus-resistant BC model [54, 68]. In the clinic, the phase III study of BKM120 plus fulvestrant versus placebo + fulvestrant did meet its primary end point of improved PFS but the real clinical benefit was minimal [69]. Overall, the median exposure to BKM120 was limited to 2 months of therapy due to high toxicity. While pan-PI3K inhibitors may lead to toxicity given their relatively narrow therapeutic window, there may also be specific features of this class of agents such as increased blood-brain-barrier penetration that may potentiate these toxicities, given that in the BKM120 group adverse effects such as mood disorders (i.e. depression and anxiety) were also observed [69]. Overall, while these agents work well in preclinical models, the clinical development of most pan-PI3K inhibitors has stopped due to insufficient efficacy, toxicity and the absence of biomarkers as predictors of sensitivity or resistance.
Dual inhibitors of PI3K and mTOR kinase, such as PQR309, LY3023414 and PF-04691502 among others, have been also developed and they have shown activities in preclinical models and continue to be actively investigated in the clinic as single agents or in combination with other targeted therapies [70]. Recent summaries of the armamentarium of PI3K-AKT-mTOR inhibitors have been reviewed in depth elsewhere [66, 71].
Thus, despite the significant amount of effort invested in the preclinical and clinical development of the PI3K pathway inhibitors, few of these agents have made or are making their way to the clinic. One reason includes their modest activity as monotherapy, which in case of everolimus is often explained by the disruption of the mTORC1-S6K1-mediated negative feedback loop. Thus, the identification of rational combinations therapies to increase survival for patients with all stages of ER+ disease is required. Examples include everolimus and exemestane, which have been approved for treatment of ER+ BC after failure of NSAI and have demonstrated the greatest translation into standard of care. And as mentioned, the identification of biomarkers of sensitivity to the PI3K pathway inhibitors has been lacking.
In order to ultimately deliver the full potential of PI3K inhibitors, some solutions may be (i) testing therapeutic schedules that may be less toxic, (ii) developing isotype-specific PI3Kα inhibitors expected to expand the therapeutic window and increase efficacy and (iii) combining therapies based on robust preclinical evidence. These recent isoform inhibitors seem to be achieving effects as monotherapy in PIK3CA-mutant tumors, and even so in combination with endocrine therapy. In this regard, β-sparing PI3K inhibitors such as taselisib in combination with the ER degrader fulvestrant had prolongation of PFS that was significant but unfortunately not clinically meaningful, possibly due to the narrow therapeutic index, which resulted in frequent treatment discontinuation [44]. Instead, combinatorial therapy of the anti-ER therapy fulvestrant together with specific PI3Kα inhibitors alpelisib has shown a clear clinical meaningful benefit in patients with ER+, HER2-negative, PIK3CA-mutant advanced metastatic BC [45], which has led to its recent FDA approval. The strong preclinical data in both mouse models and cultured BC cells showing that the combination of PI3K inhibitors with fulvestrant induces tumor regression have set the stage for these clinical trials to take place [42]. Therefore, a notable advancement in BC has been the increased knowledge of the interdependence between the ER and PI3K pathways and the necessity for dual inhibition. The understanding of the mechanisms of resistance to PI3K inhibitors by additional pre-clinical studies is necessary for the design of promising combinatorial treatments that will translate into practice-changing clinical trials.
In summary, after more than a decade of research PI3Kα inhibitors have become a standard of care in the treatment of ER+ BC in combination with fulvestrant based on the data of SOLAR-1 study [45]. Considering this new and energizing body of evidence, a coming wave of clinical and preclinical research and drug development of PI3Kα inhibitors will likely emerge. Research on the identification of the BC patients with increased sensitivity to PI3Kα inhibitors is also to be expected.
Acknowledgements
We would like to thank the members of Scaltriti laboratory for helpful comments and discussions.
Funding
This work was supported by grants from the NIH, including awards P30 CA008748, R01 CA190642 and R21 CA223789 and from the Breast Cancer Research Foundation. NV was supported by the Conquer Cancer Foundation of ASCO/Breast Cancer Research Foundation YIA, the Fund for Innovation in Cancer Informatics, and a grant from the Society of MSK. NV, ET and MS were supported by a kind gift from Mrs. Barbara Smith and her husband. This paper was published as part of a supplement funded by an educational grant from Novartis.
Disclosure
NV reports advisory board activities for Novartis. MS is in the advisory board of The Bioscience Institute and Menarini Ricerche, is a cofounder of Medendi Medical Travel and has received research funds from Puma Biotechnology, Daiichi-Sankio, Immunomedics, Targimmune and Menarini Ricerche. All remaining authors have declared no conflicts of interest.
References
- 1. Fruman DA, Chiu H, Hopkins BD. et al. The PI3K pathway in human disease. Cell 2017; 170(4): 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitman M, Downes CP, Keeler M. et al. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 1988; 332(6165): 644–646. [DOI] [PubMed] [Google Scholar]
- 3. Wu G, Xing M, Mambo E. et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res 2005; 7(5): R609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Isakoff SJ, Engelman JA, Irie HY. et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res 2005; 65(23): 10992–11000. [DOI] [PubMed] [Google Scholar]
- 5. Zhao JJ, Gjoerup OV, Subramanian RR. et al. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell 2003; 3(5): 483–495. [DOI] [PubMed] [Google Scholar]
- 6. Razavi P, Chang MT, Xu G. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 2018; 34: 427–438.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zardavas D, Te Marvelde L, Milne RL. et al. Tumor PIK3CA genotype and prognosis in early-stage breast cancer: a pooled analysis of individual patient data. J Clin Oncol 2018; 36(10): 981–990. [DOI] [PubMed] [Google Scholar]
- 8. Pang B, Cheng S, Sun SP. et al. Prognostic role of PIK3CA mutations and their association with hormone receptor expression in breast cancer: a meta-analysis. Sci Rep 2015; 4(1): 6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu YR, Jiang YZ, Zuo WJ. et al. PIK3CA mutations define favorable prognostic biomarkers in operable breast cancer: a systematic review and meta-analysis. Onco Targets Ther 2014; 7: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurvitz SA, Andre F, Jiang Z. et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol 2015; 16(7): 816–829. [DOI] [PubMed] [Google Scholar]
- 11. Samuels Y, Wang Z, Bardelli A. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004; 304(5670): 554. [DOI] [PubMed] [Google Scholar]
- 12. Barbareschi M, Buttitta F, Felicioni L. et al. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res 2007; 13(20): 6064–6069. [DOI] [PubMed] [Google Scholar]
- 13. Abramson VG, Cooper Lloyd M, Ballinger T. et al. Characterization of breast cancers with PI3K mutations in an academic practice setting using SNaPshot profiling. Breast Cancer Res Treat 2014; 145(2): 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Kwok-Shing Ng P, Kucherlapati M. et al. A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell 2017; 31: 820–832.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheung LW, Yu S, Zhang D. et al. Naturally occurring neomorphic PIK3R1 mutations activate the MAPK pathway, dictating therapeutic response to MAPK pathway inhibitors. Cancer Cell 2014; 26(4): 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis MJ, Tao Y, Young O. et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol 2006; 24(19): 3019–3025. [DOI] [PubMed] [Google Scholar]
- 17. Bose R, Kavuri SM, Searleman AC. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013; 3(2): 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nayar U, Cohen O, Kapstad C. et al. Acquired HER2 mutations in ER(+) metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat Genet 2019; 51(2): 207–216. [DOI] [PubMed] [Google Scholar]
- 19. Turner N, Pearson A, Sharpe R. et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 2010; 70(5): 2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olsen SN, Wronski A, Castano Z. et al. Loss of RasGAP tumor suppressors underlies the aggressive nature of luminal B breast cancers. Cancer Discov 2017; 7(2): 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saal LH, Holm K, Maurer M. et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 2005; 65(7): 2554–2559. [DOI] [PubMed] [Google Scholar]
- 22. Stemke-Hale K, Gonzalez-Angulo AM, Lluch A. et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 2008; 68(15): 6084–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li S, Shen Y, Wang M. et al. Loss of PTEN expression in breast cancer: association with clinicopathological characteristics and prognosis. Oncotarget 2017; 8: 32043–32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juric D, Castel P, Griffith M. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature 2015; 518(7538): 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koren S, Bentires-Alj M.. Mouse models of PIK3CA mutations: one mutation initiates heterogeneous mammary tumors. FEBS J 2013; 280(12): 2758–2765. [DOI] [PubMed] [Google Scholar]
- 26. O'Reilly KE, Rojo F, She QB. et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006; 66: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chandarlapaty S, Sawai A, Scaltriti M. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 2011; 19(1): 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serra V, Scaltriti M, Prudkin L. et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 2011; 30(22): 2547–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chakrabarty A, Sanchez V, Kuba MG. et al. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A 2012; 109(8): 2718–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vora SR, Juric D, Kim N. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014; 26(1): 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Costa C, Ebi H, Martini M. et al. Measurement of PIP3 levels reveals an unexpected role for p110beta in early adaptive responses to p110alpha-specific inhibitors in luminal breast cancer. Cancer Cell 2015; 27(1): 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elkabets M, Vora S, Juric D. et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med 2013; 5: 196ra199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castel P, Ellis H, Bago R. et al. PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kalpha inhibition. Cancer Cell 2016; 30(2): 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vasudevan KM, Barbie DA, Davies MA. et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell 2009; 16(1): 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hopkins BD, Pauli C, Du X. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 2018; 560(7719): 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clark J, Anderson KE, Juvin V. et al. Quantification of PtdInsP3 molecular species in cells and tissues by mass spectrometry. Nat Methods 2011; 8(3): 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Green KA, Carroll JS.. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer 2007; 7(9): 713–722. [DOI] [PubMed] [Google Scholar]
- 38. Lupien M, Eeckhoute J, Meyer CA. et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 2008; 132(6): 958–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ciriello G, Gatza ML, Beck AH. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015; 163(2): 506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baselga J, Campone M, Piccart M. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366(6): 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bosch A, Li Z, Bergamaschi A. et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med 2015; 7: 283ra251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carver BS, Chapinski C, Wongvipat J. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011; 19(5): 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baselga J, Cortés J, DeLaurentiis M. et al. SANDPIPER: phase III study of the PI3-kinase (PI3K) inhibitor taselisib (GDC-0032) plus fulvestrant in patients (pts) with estrogen receptor (ER)-positive, HER2-negative locally advanced or metastatic breast cancer (BC) enriched for pts with PIK3CA-mutant tumors. Cancer Res 2017; 35: TPS1119-TPS1119. [Google Scholar]
- 45. Andre F, Ciruelos E, Rubovszky G. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 2019; 380: 1929–1940. [DOI] [PubMed] [Google Scholar]
- 46. Toska E, Osmanbeyoglu HU, Castel P. et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 2017; 355(6331): 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toska E, Castel P, Chhangawala S. et al. PI3K inhibition activates SGK1 via a feedback loop to promote chromatin-based regulation of ER-dependent gene expression. Cell Rep 2019; 27(1): 294. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ross-Innes CS, Stark R, Holmes KA. et al. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev 2010; 24(2): 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mohammed H, Russell IA, Stark R. et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature 2015; 523(7560): 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Campbell RA, Bhat-Nakshatri P, Patel NM. et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 2001; 276(13): 9817–9824. [DOI] [PubMed] [Google Scholar]
- 51. Miller TW, Hennessy BT, Gonzalez-Angulo AM. et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest 2010; 120(7): 2406–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yamnik RL, Digilova A, Davis DC. et al. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem 2009; 284(10): 6361–6369. [DOI] [PubMed] [Google Scholar]
- 53. Kato S, Endoh H, Masuhiro Y. et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995; 270(5241): 1491–1494. [DOI] [PubMed] [Google Scholar]
- 54. Miller TW, Balko JM, Fox EM. et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov 2011; 1(4): 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Minden A, Lin A, Claret FX. et al. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 1995; 81(7): 1147–1157. [DOI] [PubMed] [Google Scholar]
- 56. Osborne CK, Schiff R, Fuqua SA, Shou J.. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res 2001; 7(Suppl 12): 4338s–4342s. discussion 4411s–4412s. [PubMed] [Google Scholar]
- 57. Font de Mora J, Brown M.. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 2000; 20(14): 5041–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aronica SM, Kraus WL, Katzenellenbogen BS.. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A 1994; 91(18): 8517–8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Z, Zhang X, Shen P. et al. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci U S A 2006; 103(24): 9063–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prat A, Baselga J.. The role of hormonal therapy in the management of hormonal-receptor-positive breast cancer with co-expression of HER2. Nat Rev Clin Oncol 2008; 5(9): 531–542. [DOI] [PubMed] [Google Scholar]
- 61. Fritsch C, Huang A, Chatenay-Rivauday C. et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 2014; 13(5): 1117–1129. [DOI] [PubMed] [Google Scholar]
- 62. Hudes G, Carducci M, Tomczak P. et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356(22): 2271–2281. [DOI] [PubMed] [Google Scholar]
- 63. Motzer RJ, Escudier B, Oudard S. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008; 372(9637): 449–456. [DOI] [PubMed] [Google Scholar]
- 64. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM.. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307(5712): 1098–1101. [DOI] [PubMed] [Google Scholar]
- 65. Hyman DM, Smyth LM, Donoghue MTA. et al. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol 2017; 35(20): 2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Janku F, Yap TA, Meric-Bernstam F.. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 2018; 15(5): 273–291. [DOI] [PubMed] [Google Scholar]
- 67. Maira S-M, Pecchi S, Huang A. et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther 2012; 11(2): 317–328. [DOI] [PubMed] [Google Scholar]
- 68. Liu P, Cheng H, Roberts TM, Zhao JJ.. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009; 8(8): 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Baselga J, Im SA, Iwata H. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18(7): 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shapiro GI, Bell-McGuinn KM, Molina JR. et al. First-in-human study of PF-05212384 (PKI-587), a small-molecule, intravenous, dual inhibitor of PI3K and mTOR in patients with advanced cancer. Clin Cancer Res 2015; 21(8): 1888–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rodon J, Tabernero J.. Improving the armamentarium of PI3K inhibitors with isoform-selective agents: a new light in the darkness. Cancer Discov 2017; 7(7): 666–669. [DOI] [PubMed] [Google Scholar]