Abstract
Bimetallic CuZn catalysts have been recently proposed as alternatives in order to achieve selectivity control during the electrochemical reduction of CO2 (CO2RR). However, fundamental understanding of the underlying reaction mechanism and parameters determining the CO2RR performance is still missing. In this study, we have employed size-controlled (∼5 nm) Cu100–xZnx nanoparticles (NPs) supported on carbon to investigate the correlation between their structure and composition and catalytic performance. By tuning the concentration of Zn, a drastic increase in CH4 selectivity [∼70% Faradaic efficiency (F.E.)] could be achieved for Zn contents from 10 to 50, which was accompanied by a suppression of the H2 production. Samples containing a higher Zn concentration displayed significantly lower CH4 production and an abrupt switch in the selectivity to CO. Lack of metal leaching was observed based on quasi in situ X-ray photoelectron spectroscopy (XPS). Operando X-ray absorption fine structure (XAFS) spectroscopy measurements revealed that the alloying of Cu atoms with Zn atoms takes place under reaction conditions and plays a determining role in the product selectivity. Time-dependent XAFS analysis showed that the local structure and chemical environment around the Cu atoms continuously evolve during CO2RR for several hours. In particular, cationic Zn species initially present were found to get reduced as the reaction proceeded, leading to the formation of a CuZn alloy (brass). The evolution of the Cu–Zn interaction with time during CO2RR was found to be responsible for the change in the selectivity from CH4 over Cu-ZnO NPs to CO over CuZn alloy NPs. This study highlights the importance of having access to in depth information on the interplay between the different atomic species in bimetallic NP electrocatalysts under operando reaction conditions in order to understand and ultimately tune their reactivity.
1. Introduction
The electrochemical reduction of CO2 (CO2RR) into useful chemicals and fuels has received much attention as a means to build carbon recycling systems.1,2 However, efficient and inexpensive electrocatalysts are still required to reduce the thermodynamically stable CO2 molecule while suppressing the H2 evolution reaction (HER). While various metal surfaces (bulk foils) have been identified to efficiently reduce CO2 into value-added carbon-based products such as CO (Au, Ag, and Zn), formic acid (Sn, In, and Bi), and hydrocarbons (Cu),3 their catalytic activity, selectivity, and stability are still insufficient for industrial acceptance. In order to enhance the performance of metal catalysts, several strategies have been proposed, including nanostructuring the metals,4−11 engineering the metal/electrolyte interface,12−17 or introducing a secondary metal to create bimetallic structural motifs.18−27
The utilization of bimetallic catalysts has been considered a promising approach to obtain improved catalytic performance for CO2RR.28,29 CuZn is of particular interest due to its low cost and lack of toxicity. Recent studies have demonstrated enhanced reactivity of CuZn catalysts for CO2RR.30−36 For example, nanoporous CuZn catalysts prepared by annealing and subsequent reduction of commercial CuZn alloy foils showed four and six times higher Faradaic efficiency (F.E.) for CO and HCOOH than those of the untreated CuZn foils.32 Zn-coated Cu electrodes exhibited higher selectivity for CH4 (52% F.E.) than bare Cu (23% FE for CH4).33 Oxide-derived CuZn catalysts were favorable toward the formation of C2 products (i.e., C2H4 and C2H5OH), and it was possible to tune the ratio of these products by varying the amount of Zn.34 This trend in the C2 selectivity was postulated to be due to the spillover of CO from Zn to Cu sites, which was thought to facilitate the production of C2 products at the Cu site.34 Such CO spillover effects were found to be facilitated when a homogeneous distribution of Cu and Zn atoms was formed in the CuZn catalysts.35
However, despite the former encouraging empirical results, significant discrepancies in the product selectivity of seemingly similar CuZn systems have been reported, and fundamental understanding is still missing regarding the reaction pathways, catalyst structure, and composition leading to a given selectivity trend. Moreover, uncertainties remain concerning the relative importance of the different parameters that may be adjusted in order to tune the materials electrocatalytic performance. For the bulk CuZn catalysts previously investigated, grain boundaries,37−39 local pH effects,40−42 or the presence of cationic metal species6,43−46 have been suggested to contribute to their altered product selectivity. However, the complexity of these material systems hinders our ability to disentangle the specific role of different parameters, which is required in order to understand the intrinsic reactivity of the CuZn system for CO2RR.
In this work, morphologically and chemically well-defined ∼5 nm CuZn nanoparticles (NPs) were synthesized via inverse micelle encapsulation and used to investigate the correlation between their structure, composition, and electrocatalytic activity and selectivity. By means of quasi in situ X-ray photoelectron spectroscopy (XPS) and operando X-ray absorption fine-structure spectroscopy (XAFS), the evolution of the structure, chemical state, and composition of the CuZn NPs was investigated under working CO2RR conditions. The gradual formation of a Cu–Zn alloy could be observed in the course of the CO2RR and correlated to the switch in selectivity from CH4 to CO.
2. Experimental Section
2.1. Preparation of CuZn NPs
Bimetallic CuZn nanoparticles (NPs) with the same nominal NP size but variable composition were prepared by an inverse micelle encapsulation method.47 The elemental composition was modified by controlling the molar ratio of the Cu and Zn precursor salts. CuCl2 and Zn(CH3COO)2 were dissolved in a solution of toluene and tetrahydrofuran, followed by the addition of a second toluene solution with a poly(styrene)-block-poly(2-vinylpyridine) diblock copolymer (PS(48500)-P2VP(70000), Polymer Source, Inc.). The mixture was then stirred for 2 days. The encapsulated CuZn NPs were deposited on glassy carbon substrates. After deposition, an O2-plasma etch treatment (20 W for 10 min, ∼400 mTorr O2) was used to remove the polymeric ligands. The specific synthesis parameters of the seven samples used in this study can be found in Table S1.
2.2. Morphological Characterization
Atomic force microscopy (AFM, Bruker, Multimode 8) was used to determine the NP height (hp). The CuZn NPs were deposited on silicon wafers for more accurate measurement of hp, since the glassy carbon electrode used for the electrochemical measurements has a rougher surface that makes difficult the background subtraction needed for the NP height determination. The average NP height was used to calculate the average CuZn geometric surface area of the NPs, ACuZn = 4π(hp/2)2, assuming spherical micellar NPs. The metal NP surface area was then multiplied by the NP number density to estimate the total surface area of the CuZn NPs in each sample. This parameter was used for the normalization of the current data.
2.3. Electrochemical Characterization
Electrochemical CO2 reduction experiments were conducted using an Autolab potentiostat (Multi Autolab M204) in an H-type two-compartment electrochemical cell made of polyether ether ketone (PEEK) separated by an anion-exchange membrane (Selemion AMV). A platinum mesh counter electrode and a leak-free Ag/AgCl reference electrode (Innovative Instruments) were used in a three electrode configuration. Purified 0.1 M KHCO3 solutions were prepared by treating the electrolyte with Chelex 100 Resin (Bio-Rad). The electrolyte was saturated with CO2 until a pH of 6.8 was achieved. Each data point presented corresponds to an identical freshly prepared sample measured with the chronoamperometric technique at −1.35 V versus RHE.
The gas products were quantified by gas chromatography (GC, Agilent 7890B) equipped with thermal conductivity (TCD) and flame ionization (FID) detectors. The GC was directly connected to the electrochemical cell for online gas analysis. CO2 gas was bubbled through the electrolyte at an average rate of 20 mL min–1. The formic acid concentration was analyzed by high-performance liquid chromatography (HPLC, Shimadzu Prominence) equipped with a NUCLEOGEL SUGAR 810 column and a refractive index detector (RID).
2.4. Structural and Chemical Characterization
Quasi in situ X-ray photoelectron spectroscopy (XPS) measurements were performed in an ultrahigh-vacuum setup equipped with a nonmonochromatic Al X-ray source (hv = 1486.6 eV) and a hemispherical electron analyzer (Phoibos 100, SPECS GMbH). The XPS analysis chamber was connected to an electrochemical cell allowing sample transfer without exposure to air after electrochemistry. The measurements were conducted using an analyzer pass energy of Epass = 13 eV and a source power of P = 300 W. All spectra were aligned to the carbon peak (Ebin = 284.8 eV) of the glassy carbon substrate. The composition ratio of Cu to Zn was calculated taking into account the relative sensitivity factors (RSF) of the metals (Cu 2p3/2: 20.28; Zn 2p3/2: 23.93) for an angle of 54° between the X-ray source and the analyzer and the transmission function of the analyzer.
Operando X-ray absorption fine-structure spectroscopy (XAFS) measurements were performed at the SAMBA beamline at SOLEIL synchrotron (France). A homemade electrochemical cell was used to acquire the XAFS spectra. All samples were measured in air and under operando conditions, while the potential was kept constant at −1.35 V versus RHE in a CO2-saturated 0.1 M KHCO3 electrolyte. XAFS spectra for the Cu K-edge (E0 = 8979 eV) and Zn K-edge (E0 = 9659 eV) were collected separately. Identically prepared (same NP solution) but different (fresh) samples were used for measurements at either the Cu or the Zn absorption edges. Further details of the XAFS measurements are given in the Supporting Information. Under CO2RR conditions, multiple XAFS spectra were acquired, until there were no visible changes. This took several hours for these samples. Here we use time-dependent X-ray absorption near edge structure (XANES) data to follow the evolution of the sample structure and composition. We also analyzed extended X-ray absorption fine structure (EXAFS) data and compared the data obtained for samples in their as-prepared state in air and in their final state after several hours under applied potential. In addition to the evaluation of the XANES data, EXAFS spectra collected after 1 h CO2RR under potential were also analyzed. Note, however, that the acquisition of a single EXAFS spectrum took ca. 12 min, which limits our time resolution. Moreover, due to the low signal-to-noise ratio, analysis of individual EXAFS spectra is problematic, and several spectra needed to be merged. Therefore, the representative EXAFS spectrum for a sample after 1 h of CO2RR was obtained after merging 4 spectra collected during 40–80 min of CO2RR.
Alignment, background subtraction, and normalization of the XAFS spectra were performed using the conventional approach as implemented in the Athena software.48 Linear combination fitting was used to process the XANES data. To obtain quantitative information about the distributions of bond lengths, we performed Cu- and Zn K-edge EXAFS data fitting. Details of EXAFS data fitting are given in the Supporting Information.
3. Results and Discussion
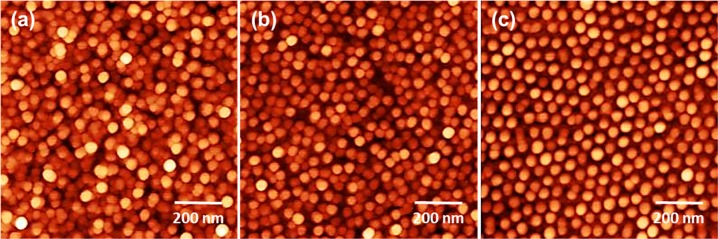
Figures 1 and S1 present AFM images of CuZn NPs with variable composition ratios. The AFM images show that all NPs exhibit spherical shape with uniform coverage across the substrate and narrow size distribution, with an average size of ca. 5 nm. Figure S2 displays the NP height histograms and the average values are included in Table S1. Following our previous work,46 the metal NP coverage was chosen to be large enough (Table S1) so that the interparticle reactant diffusion and probability of readsorption of intermediates is not hindered.
Figure 1.
AFM images of Cu100–xZnx NPs supported on SiO2/Si(111). (a) Cu100, (b) Cu50Zn50, and (c) Zn100.
In order to investigate the surface composition and chemical state of the CuZn NPs in their as-prepared state and after CO2RR, quasi in situ XPS measurements were carried out. Figures 2 and S3 show the XPS spectra of the CuZn NPs in their as-prepared state and after CO2RR. The surface composition ratio was obtained by integrating the areas of the Cu 2p3/2 and Zn 2p3/2 spectra. It was confirmed that the composition of the as-prepared samples was consistent with the starting molar ratio of the precursor salts, and that it did not change after the CO2RR (Figure S4).
Figure 2.
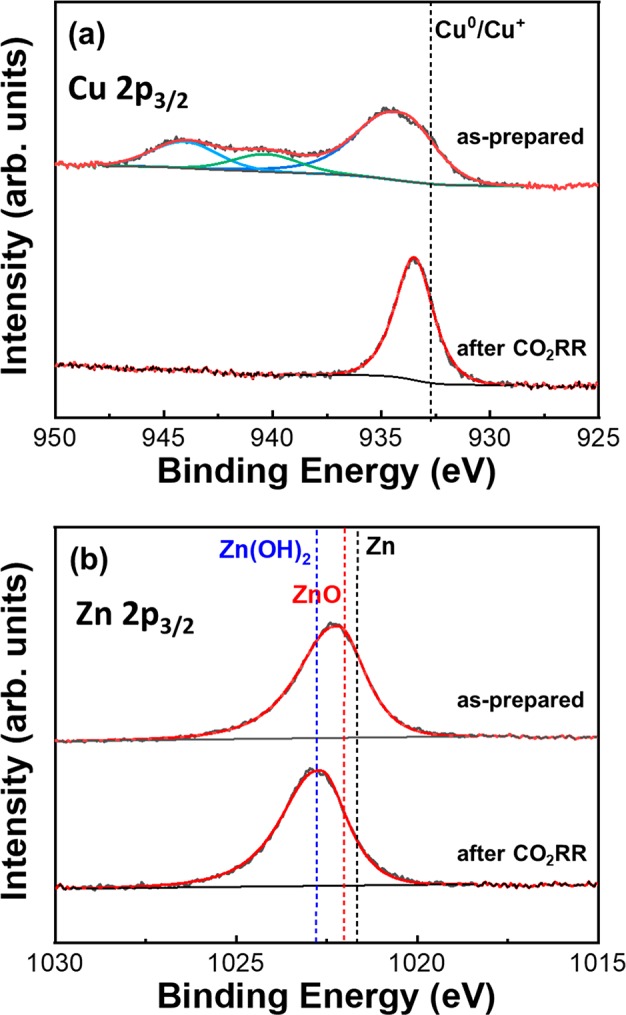
Quasi in situ XPS spectra of the (a) Cu 2p3/2 and (b) Zn 2p3/2 core level regions of Cu50Zn50 NPs deposited on glassy carbon acquired before and after (without air exposure) 1 h of CO2RR at −1.35 V vs RHE.
As shown in Figure 2a, Cu in the as-prepared samples after O2-plasma treatment was found to be in Cu2+ state, as evidenced by the shakeup satellites.47,49 After 1 h CO2RR, the shakeup features vanished. Simultaneously, a shift of the Cu 2p3/2 main peak toward lower binding energies was observed (ΔE = 1.0 eV). Nevertheless, a ∼0.8 eV higher binding energy than that of bulk metallic Cu was observed after CO2RR. This binding energy shift can be attributed to the initial and final state effects typically observed for small NPs.49 We also attempted to detect the presence of Cu+ species and distinguish them from Cu metal by using the Cu LMM Auger peak.50 Unfortunately, the former signal for our low-metal coverage NP samples was beyond the detection limit. The Zn 2p region (Figures 2b and S3) shows that before CO2RR the NPs are in the Zn2+ state (ZnO). The small change of 0.5 eV toward higher binding energies of the Zn 2p3/2 peak obtained after 1 h CO2RR is attributed to the presence of Zn(OH)2.51
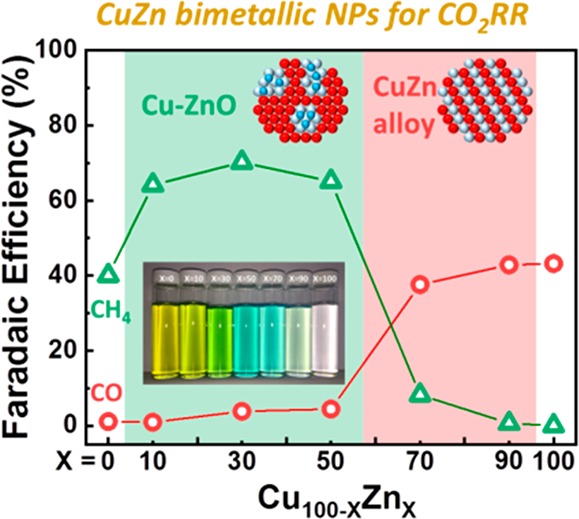
Figure 3 shows the current density and F.E. for the CuZn NP samples as a function of their composition. As shown in Figure 3a, the Cu100 NPs exhibit the highest activity among all investigated samples, and the activity was found to monotonically decrease with increasing Zn content in the CuZn NPs. Interestingly, the F.E. of the CuZn NPs showed different selectivity during CO2RR depending on the Cu to Zn ratio (Figure 3b). Cu100 and Zn100 NPs produced CH4 and CO as the main products, respectively. The three Cu100–xZnx NPs (x = 10, 30, and 50) exhibited enhanced CH4 F.E. compared to pure Cu NPs. Nonetheless, the increased CH4 selectivity of these three samples was abruptly suppressed when the Zn content was higher than 70%, whereas CO selectivity was found to increase.
Figure 3.
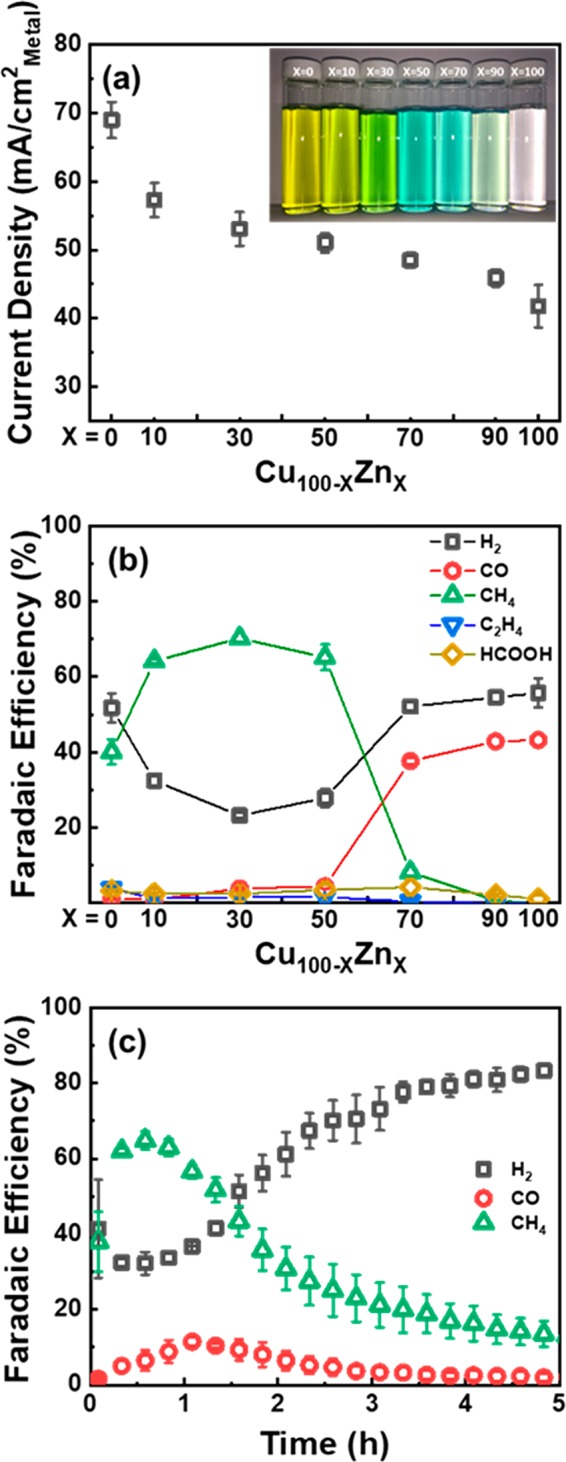
Activity and selectivity measurements of CO2RR over Cu100–xZnx NPs. (a) Geometric current density and (b) Faradaic efficiency toward H2, CO, CH4, C2H4, and HCOOH measured during 1 h of electrolysis at −1.35 V vs RHE in 0.1 M KHCO3 as a function of the NP composition. (c) Stability test of Cu50Zn50 NPs for the main products of CO2RR at −1.35 V vs RHE as a function of time. The insert in (a) shows a photograph of the vials with the different CuZn NP solutions.
In order to further understand the reactivity of the CuZn NPs for CO2RR, we also compared the partial current density of the major products as a function of the NP composition, as shown in Figure S5. Interestingly, we found that H2 production showed a different trend depending on the CH4 and CO production of the CuZn NPs. The H2 production was significantly suppressed upon increasing the Zn concentration for the Cu-rich CH4-producing CuZn NPs (i.e., Cu90Zn10, Cu70Zn30, and Cu50Zn50), while no changes in H2 with Zn concentration were observed when the Zn-rich CO-producing CuZn NPs (i.e., Cu30Zn70 and Cu10Zn90) were considered. This result suggests that the protons that should be used to generate H2 from water splitting have now a more favorable pathway toward CH4 formation in the Cu-rich NPs.
In general, the product selectivity of CO2 reduction is determined by the binding strength of adsorbed *CO and *H on the metal surface.52,53 According to previous studies, since a bulk polycrystalline Cu electrode has a moderate binding energy for adsorbed *CO, such *CO species can be further hydrogenated to produce CH4.54−56 On the other hand, a relatively weak binding strength of adsorbed *CO is known for polycrystalline Zn electrodes, leading to CO production without further reaction.34,35 These are also the main products detected for our monometallic Cu100 and Zn100 NP samples. However, it should be also considered that the enhanced fraction of low-coordinated atoms in NP samples such as those available here (5 nm) affect the relative binding of *H and *CO with respect to bulk systems of analogous composition, leading to distinct selectivity trends, in particular, enhanced H2 production, as previously reported.47,51 Interestingly, we found for our CuZn NPs increased CH4 production as compared to Cu100 NPs. However, when the Zn content was higher than 70% in the bimetallic NPs, only CO and H2 were produced. In the latter case, the proton and adsorbed *CO species that were not participating in the hydrogenation with adsorbed *CO on the Cu site may be released as H2 and CO. Furthermore, the stability tests of the Cu50Zn50 sample showed that the enhanced CH4 selectivity started to drop after 1 h, which is accompanied by a slight increase in CO selectivity (Figure 3c). This result indicates that the functionality of the Cu sites has changed over time.
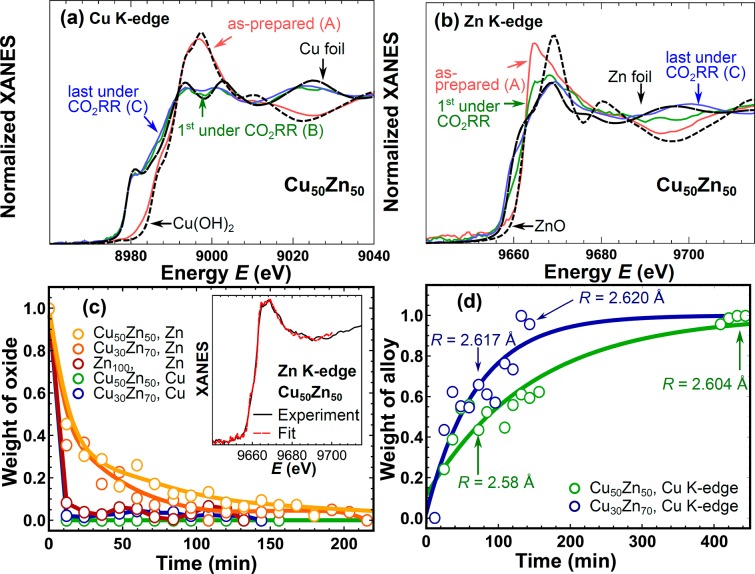
To gain more detailed information about the chemical state and structure of the electrocatalysts during CO2RR, XAFS measurements were conducted under operando conditions. Figure 4 shows selected Cu and Zn K-edge XANES spectra for Cu50Z50 NPs in their as-prepared state and under CO2RR conditions. Similar plots for Cu30Zn70 and Zn100 samples are given in Figure S6. Complete sets of time-dependent XANES spectra for these samples are shown in Figure S7. The position of the absorption edge in the XANES spectra of the as-prepared samples and the direct comparison with the reference materials (Figure 4 and S8) indicate that the Cu and Zn components are initially completely oxidized and predominantly in the 2+ oxidation state. The local structure around the Cu atoms is similar to that in Cu(OH)2, while from the Zn K-edge data we could not assign the observed XANES features to any particular reference material and can only conclude that some disordered oxide (or hydroxide) structure is formed.51
Figure 4.
Selected (a) Cu K-edge and (b) Zn K-edge XANES spectra of Cu50Zn50 NPs corresponding to as-prepared oxidized samples (spectrum A), samples immediately after the onset of CO2RR conditions (spectrum B) and the final spectrum collected after 7 h under CO2RR conditions (spectrum C). (c) Linear combination analysis results for the Zn K-edge and Cu K-edge of XANES data of Cu50Zn50 and Cu30Zn70 NPs obtained using spectrum A (oxidized sample) and spectrum C (completely reduced sample) as reference. The inset shows a representative linear combination fit (result for Zn K-edge spectrum of Cu50Zn50 NPs immediately after the onset of CO2RR conditions). (d) Linear combination analysis results of Cu K-edge XANES data using spectra B and C as reference, where the latter corresponds to the most alloyed state. Cu-M interatomic distances, as extracted from EXAFS data fitting, are also shown. Solid lines are guides for the eye.
Under CO2RR conditions, both Cu and Zn are reduced, as evidenced by the observed changes in the Cu and Zn K-edge XANES spectra, which become more similar to those of Cu and Zn foils (Figures 4 and S6–S7). Interestingly, the reduction rates appear to be very different for Cu and Zn. We observed that the Cu K-edge XANES spectra did not change much after the prompt initial reduction during the first scan under applied potential. However, at the Zn K-edge some variations in the XANES spectra for samples under potential were observed that had significantly longer characteristic times. To quantify these trends, we performed linear combination analysis using as reference the corresponding XANES spectra of the as-prepared sample (spectrum A in Figures 4a,b and S6), and the final spectrum (spectrum C in Figures 4a,b and S6) obtained after several hours under CO2RR conditions. The results for different samples and absorption edges are compared in Figure 4c. It is evident that the reduction of ZnO species takes several hours, while that of the Cu oxide species is completed during the first XAFS scan within several minutes. In agreement with the quasi in situ XPS results, neither the Zn on the NP surface nor that inside the NP core is completely reduced after 1 h of CO2RR. The reduction rate of Zn depends, however, on the Cu100–xZnx composition, with ZnO species in pure Zn NPs becoming reduced significantly faster than those in the bimetallic CuZn NPs. Since the Cu oxide species initially present in the small (∼5 nm) NPs were found by XAFS to become reduced very promptly (within several minutes), they can be ruled out as responsible for the much slower change in the selectivity observed.
Even though the chemical state of the Cu species was stable (reduced) during the reaction, small variations in the Cu K-edge XANES spectra can still be observed, which can be linked to gradual changes in the local structure around Cu. To track these changes, linear combination analysis is employed, using in this case as references Cu K-edge XANES spectra corresponding to the final state (spectrum C in Figures 4a and S6b) and the first Cu K-edge XANES spectrum for a reduced sample (obtained after several minutes under CO2RR conditions, spectrum B in Figures 4a and S6b). The obtained results, Figure 4d, demonstrate that the local structure around the Cu atoms continues evolving even after Cu is completely reduced. It should be noted that the characteristic times at which changes in the Cu K-edge XANES spectra are observed (Figure 4d) correlate well with the characteristic times of the reduction of the cationic Zn species (Figure 4c). Thus, from this observation, we can infer that the changes observed originate from the gradual enrichment of the samples with reduced Zn species and the interaction between the metallic Cu and metallic Zn species formed during CO2RR.
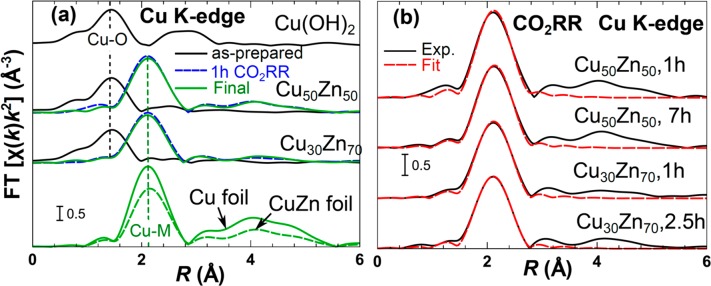
To better understand the nature of the Cu–Zn interactions, EXAFS spectra, which are more sensitive to the geometry of the environment around the absorbing atoms, were analyzed. Fourier transformed (FT) Cu K-edge EXAFS spectra of Cu50Zn50, Cu30Zn70, and Zn100 NPs measured for the as-prepared samples, for samples after 1 h of CO2RR and in the final state (after 7 h, 2.5 h, and 2 h of CO2RR for Cu50Zn50, Cu30Zn70, and Zn100 NPs, correspondingly) are shown in Figure 5a (Cu-K-edge) and Figure S8a (Zn-K-edge). In agreement with the XANES data analysis, the FT-EXAFS spectra of the as-prepared samples reveal the presence of M–O (M = Cu and Zn) structural motifs, as evidenced by a strong peak at ca. 1.5 Å (phase uncorrected). Overall, the FT-EXAFS spectra for the Cu K-edge in the as-prepared samples are close to those of Cu(OH)2.
Figure 5.
Fourier-transformed (FT) k2-weighted Cu K-edge EXAFS data for Cu50Zn50 and Cu30Zn70 NPs measured (a) as-prepared in air, after 1 h of CO2RR (dashed line) and in the final state (solid line). (b) EXAFS fitting results for Cu50Zn50 and Cu30Zn70 NPs under CO2RR for the different times indicated. Reference spectra from a Cu foil, CuZn foil, and Cu(OH)2 are also shown for comparison.
Under CO2RR conditions, the M–O peak disappears completely in the Cu K-edge data, while a strong peak at ca. 2.1–2.2 Å appears, which can be associated with the presence of M–M bonds. Thus, complete reduction of cationic Cu species in all CuZn samples is observed, in agreement with XANES data. Note here that since Cu and Zn are neighbors in the Periodic Table we cannot easily distinguish between Cu–Zn and Cu–Cu (or Zn–Zn and Zn–Cu) contributions. By comparing spectra obtained after 1 h of CO2RR with the final spectra obtained after several hours of CO2RR, small variations can be observed (more pronounced in the Zn K-edge data, Figure S9a), suggesting some gradual structural changes not only in the Zn but also in the Cu atomic environment.
For quantitative analysis, EXAFS data fitting was performed. For samples under CO2RR conditions, Cu K-edge EXAFS fitting results are summarized in Figure 5b and in Table S2. The fitting results of the Cu and Zn K-edge EXAFS data of the as-prepared samples are presented in the Figure S9b–d and Tables S3 and S4. The main finding from the Cu K-edge EXAFS data analysis is that the Cu–M interatomic distance is different in Cu50Zn50 (2.58 ± 0.01 Å after 1 h under CO2RR conditions) as compared to the Cu30Zn70 (2.617 ± 0.008 Å) NP sample (Table S2 and Figure 5b). Both distances are in between those of a Cu foil (2.56 Å) and a brass CuZn foil (2.62 Å), suggesting alloying of Cu with Zn. The increase of the interatomic distances in fcc-type Cu–Zn alloys with increasing Zn concentration is in agreement with X-ray diffraction data.57 Moreover, the interatomic distances change with time. Table S2 provides the values of the fitting variables obtained for the Cu30Zn70 NPs after 2.5 h CO2RR and for Cu50Zn50 NPS after 7 h. For both samples we observed an increase of the Cu–M distance with time, reaching 2.620 ± 0.008 Å for Cu30Zn70 NPs and 2.604 ± 0.008 Å for Cu50Zn50 NPs. In both cases the interatomic distances get closer to that in a CuZn brass foil. These time-dependent changes in interatomic distances can explain the observed time-dependent changes in the XANES spectra (Figure 4d) as well as the switch in the selectivity. Note that the sensitivity of the Cu K-edge XANES spectra to interatomic distance changes has been demonstrated in our recent work.58 This allows us now to track the changes in the catalyst structure with better time-resolution than what was possible based on the EXAFS analysis. By combining insights from EXAFS and XANES data analyses, we conclude that the interaction (alloying) between the Cu and Zn atoms gradually takes place under CO2RR conditions, and that it changes the local structure around the Cu atoms, making it more similar to that of bulk CuZn brass. Importantly, such changes took place in both samples (Cu50Zn50 and Cu30Zn70 NPs), but the local structure around Cu in the Zn-rich sample after 1 h of CO2RR was already closer to that in the brass CuZn foil, as suggested by the larger Cu–M distance for this sample. Therefore, this result implies that the ratio of the Cu and Zn elements and their oxidation state is critical in determining both, the alloying degree immediately after the onset of the CO2RR, as well as its evolution as a function of time.
Finally, based on the analysis of operando XAFS data we have observed a correlation between the product selectivity of the CuZn NPs and the reduction of the ZnO species and concomitant Cu–Zn alloy formation. It should be noted that there are a number of commonalities between the CO2 electrochemical reduction and the CO2 hydrogenation processes also taking place over Cu/ZnO catalysts, where the formation of brass might occur under certain reaction conditions and affect the catalytic selectivity. Since alloying in bimetallic systems induces the change of the geometric (or strain effect) structure, this transformation can lead to the tuning of the product selectivity in CuZn NPs. According to a recent experimental and theoretical study, an expansive strain in the Cu lattice should result in a more favorable stabilization of the reaction intermediates, leading to the formation of products beyond *CO.49 This expansive Cu strain could explain the enhanced CH4 selectivity in the Cu-rich CuZn NPs. Alloy formation, however, results also in a ligand effect and modification of the electronic structure, e.g., in a change of the d-band center of the metal atoms.28,29 When upon reduction of the ZnO species a CuZn alloy is formed, the d-band center of Cu is shifted away from the Fermi level.59 Such shift results in a weakening of the binding strength of *CO due to the occupancy of antibonding states.18,60 Therefore, product selectivity in the Zn-rich CuZn NPs seems to be controlled by the ligand effect rather than strain effects, leading to the loss of the functionality of the Cu site for further reduction of CO intermediates.
Importantly, our time-dependent XAFS analysis revealed that alloying between Cu and Zn atoms gradually takes place upon reduction of the cationic Zn species. This fact explains why the selectivity of CO2RR for CH4 deteriorates over time. In fact, our durability data acquired for the Cu50Zn50 NPs showed that the concentration of the initially produced CH4 gradually decreased after 1 h reaction (Figure 3c). In parallel, the concentration of CO slightly increased. This is assigned to the Cu–Zn alloy formation and the loss of the Cu site ability to hydrogenate adsorbed *CO. This result highlights the critical correlation of the structure and composition of the NP surface and the CO2RR selectivity. Furthermore, it also implies that maintaining the metallic Cu-ZnO interface is vital for the stable production of CO2 products, which can be affected by the reduction of the cationic Zn species in the CuZn alloyed catalysts. In conclusion, the product selectivity of CuZn NPs is determined by a combination of geometric and ligand effects, which become more or less prominent depending on the degree of alloying of the Cu–Zn species. In the first few hours, when the contribution of the Cu–Zn alloy is not significant, CH4 formation over Cu-ZnO NPs is observed. Selectivity, however, switches to the formation of a CO+H2 mix when brass is formed under CO2RR conditions.36
Considering that our CuZn NP system was prepared as a model catalyst system, CH4 and CO are the products to be expected when Cu is in contact with ZnO species within a nanoparticle. It is however known from previous studies that small Cu NPs favor CO production (and H2) over hydrocarbons and alcohols.61 Therefore, a comparison with other data in the literature on Cu–Zn systems must address two aspects: (i) whether a size effect should be considered and/or (ii) if the investigation has been conducted on a Cu-ZnO system or Cu–Zn alloy. Interestingly, several previous papers have reported the production of C2 products such as ethylene and ethanol in CuZn catalysts containing Cu/ZnO or CuOx/ZnO species.33,34 Others however reported only CO and H2 production when the samples investigated consisted of Cu–Zn alloys already in the as-prepared state.36 Our data serve to consolidate the different findings available in the Cu–Zn literature, in particular, to clarify that for bulk-like systems (films and large NPs), Cu–Zn alloys will lead to the exclusive production of CO and H2, while dealloyed Cu/ZnO systems might also result in other products such as CH4 for our NPs or even C2+ products for larger material systems.
4. Conclusion
In summary, we have demonstrated that the product selectivity of CuZn NPs can be correlated with the initial Cu/Zn ratio as well as with the degree of alloying between the Cu and Zn components, which was found to evolve under CO2RR conditions. In particular, time-dependent XAFS data revealed that metallic Cu in close proximity to ZnO leads to the production of CH4 at the initial stages of the reaction. The progressive reduction of the ZnO species taking place under CO2RR conditions and the concomitant enhanced Cu–Zn interaction and brass alloy formation lead to the switch in the selectivity to the exclusive generation of CO and H2. Our operando spectroscopy study provides crucial information on the nature of the active species and structure of CuZn NP catalysts. We believe that our findings can help to guide the rational design of bimetallic NP catalysts for CO2RR.
Acknowledgments
This work was funded by the German Federal Ministry of Education and Research (BMBF) under Grant 03SF0523C (CO2EKAT), the European Research Council under Grant ERC-OPERANDOCAT (ERC-725915), and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy, EXC 2008/1 (UniSysCat), 390540038.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b10709.
XAS data and fitting results, AFM images, quasi in situ XPS spectra, and electrochemical data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Whipple D. T.; Kenis P. J. A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. 10.1021/jz1012627. [DOI] [Google Scholar]
- Kondratenko E. V.; Mul G.; Baltrusaitis J.; Larrazabal G. O.; Perez-Ramirez J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. 10.1039/c3ee41272e. [DOI] [Google Scholar]
- Hori Y.Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry, Vayenas C. G., White R. E., Gamboa-Aldeco M. E., Eds.; Springer: New York, NY, 2008; pp 89–189. [Google Scholar]
- Kim C.; Jeon H. S.; Eom T.; Jee M. S.; Kim H.; Friend C. M.; Min B. K.; Hwang Y. J. Achieving Selective and Efficient Electrocatalytic Activity for CO2 Reduction Using Immobilized Silver Nanoparticles. J. Am. Chem. Soc. 2015, 137, 13844–13850. 10.1021/jacs.5b06568. [DOI] [PubMed] [Google Scholar]
- Mistry H.; Choi Y.-W.; Bagger A.; Scholten F.; Bonifacio C. S.; Sinev I.; Divins N. J.; Zegkinoglou I.; Jeon H. S.; Kisslinger K.; Stach E. A.; Yang J. C.; Rossmeisl J.; Roldan Cuenya B. Enhanced Carbon Dioxide Electroreduction to Carbon Monoxide over Defect-Rich Plasma-Activated Silver Catalysts. Angew. Chem., Int. Ed. 2017, 56, 11394–11398. 10.1002/anie.201704613. [DOI] [PubMed] [Google Scholar]
- Mistry H.; Varela A. S.; Bonifacio C. S.; Zegkinoglou I.; Sinev I.; Choi Y.-W.; Kisslinger K.; Stach E. A.; Yang J. C.; Strasser P.; Cuenya B. R. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 2016, 7, 12123. 10.1038/ncomms12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiudice A.; Lobaccaro P.; Kamali E. A.; Thao T.; Huang B. H.; Ager J. W.; Buonsanti R. Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction. Angew. Chem., Int. Ed. 2016, 55, 5789–5792. 10.1002/anie.201601582. [DOI] [PubMed] [Google Scholar]
- Jeon H. S.; Kunze S.; Scholten F.; Roldan Cuenya B. Prism-Shaped Cu Nanocatalysts for Electrochemical CO2 Reduction to Ethylene. ACS Catal. 2018, 8 (1), 531–535. 10.1021/acscatal.7b02959. [DOI] [Google Scholar]
- Li Y.; Cui F.; Ross M. B.; Kim D.; Sun Y.; Yang P. Structure-Sensitive CO2 Electroreduction to Hydrocarbons on Ultrathin 5-fold Twinned Copper Nanowires. Nano Lett. 2017, 17, 1312–1317. 10.1021/acs.nanolett.6b05287. [DOI] [PubMed] [Google Scholar]
- Raciti D.; Livi K. J.; Wang C. Highly Dense Cu Nanowires for Low-Overpotential CO2 Reduction. Nano Lett. 2015, 15, 6829–6835. 10.1021/acs.nanolett.5b03298. [DOI] [PubMed] [Google Scholar]
- Saberi Safaei T.; Mepham A.; Zheng X.; Pang Y.; Dinh C.-T.; Liu M.; Sinton D.; Kelley S. O.; Sargent E. H. High-Density Nanosharp Microstructures Enable Efficient CO2 Electroreduction. Nano Lett. 2016, 16, 7224–7228. 10.1021/acs.nanolett.6b03615. [DOI] [PubMed] [Google Scholar]
- Lum Y.; Yue B.; Lobaccaro P.; Bell A. T.; Ager J. W. Optimizing C–C Coupling on Oxide-Derived Copper Catalysts for Electrochemical CO2 Reduction. J. Phys. Chem. C 2017, 121, 14191–14203. 10.1021/acs.jpcc.7b03673. [DOI] [Google Scholar]
- Singh M. R.; Kwon Y.; Lum Y.; Ager J. W.; Bell A. T. Hydrolysis of Electrolyte Cations Enhances the Electrochemical Reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 2016, 138, 13006–13012. 10.1021/jacs.6b07612. [DOI] [PubMed] [Google Scholar]
- Gao D.; Scholten F.; Roldan Cuenya B. Improved CO2 Electroreduction Performance on Plasma-Activated Cu Catalysts via Electrolyte Design: Halide Effect. ACS Catal. 2017, 7, 5112–5120. 10.1021/acscatal.7b01416. [DOI] [Google Scholar]
- Varela A. S.; Ju W.; Reier T.; Strasser P. Tuning the Catalytic Activity and Selectivity of Cu for CO2 Electroreduction in the Presence of Halides. ACS Catal. 2016, 6, 2136–2144. 10.1021/acscatal.5b02550. [DOI] [Google Scholar]
- Varela A. S.; Kroschel M.; Leonard N. D.; Ju W.; Steinberg J.; Bagger A.; Rossmeisl J.; Strasser P. pH Effects on the Selectivity of the Electrocatalytic CO2 Reduction on Graphene-Embedded Fe–N–C Motifs: Bridging Concepts between Molecular Homogeneous and Solid-State Heterogeneous Catalysis. ACS Energy Lett. 2018, 3, 812–817. 10.1021/acsenergylett.8b00273. [DOI] [Google Scholar]
- Resasco J.; Chen L. D.; Clark E.; Tsai C.; Hahn C.; Jaramillo T. F.; Chan K.; Bell A. T. Promoter Effects of Alkali Metal Cations on the Electrochemical Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2017, 139, 11277–11287. 10.1021/jacs.7b06765. [DOI] [PubMed] [Google Scholar]
- Kim D.; Resasco J.; Yu Y.; Asiri A. M.; Yang P.. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 10.1038/ncomms5948. [DOI] [PubMed] [Google Scholar]
- Rasul S.; Anjum D. H.; Jedidi A.; Minenkov Y.; Cavallo L.; Takanabe K. A Highly Selective Copper–Indium Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to CO. Angew. Chem., Int. Ed. 2015, 54, 2146–2150. 10.1002/anie.201410233. [DOI] [PubMed] [Google Scholar]
- Ma M.; Hansen H. A.; Valenti M.; Wang Z.; Cao A.; Dong M.; Smith W. A. Electrochemical reduction of CO2 on compositionally variant Au-Pt bimetallic thin films. Nano Energy 2017, 42, 51–57. 10.1016/j.nanoen.2017.09.043. [DOI] [Google Scholar]
- Morales-Guio C. G.; Cave E. R.; Nitopi S. A.; Feaster J. T.; Wang L.; Kuhl K. P.; Jackson A.; Johnson N. C.; Abram D. N.; Hatsukade T.; Hahn C.; Jaramillo T. F. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nature Catal. 2018, 1, 764–771. 10.1038/s41929-018-0139-9. [DOI] [Google Scholar]
- Luc W.; Collins C.; Wang S.; Xin H.; He K.; Kang Y.; Jiao F. Ag–Sn Bimetallic Catalyst with a Core–Shell Structure for CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893. 10.1021/jacs.6b10435. [DOI] [PubMed] [Google Scholar]
- Lu L.; Sun X.; Ma J.; Yang D.; Wu H.; Zhang B.; Zhang J.; Han B. Highly Efficient Electroreduction of CO2 to Methanol on Palladium–Copper Bimetallic Aerogels. Angew. Chem., Int. Ed. 2018, 57, 14149–14153. 10.1002/anie.201808964. [DOI] [PubMed] [Google Scholar]
- Kortlever R.; Peters I.; Koper S.; Koper M. T. M. Electrochemical CO2 Reduction to Formic Acid at Low Overpotential and with High Faradaic Efficiency on Carbon-Supported Bimetallic Pd–Pt Nanoparticles. ACS Catal. 2015, 5, 3916–3923. 10.1021/acscatal.5b00602. [DOI] [Google Scholar]
- Cai Z.; Wu Y.; Wu Z.; Yin L.; Weng Z.; Zhong Y.; Xu W.; Sun X.; Wang H. Unlocking Bifunctional Electrocatalytic Activity for CO2 Reduction Reaction by Win-Win Metal–Oxide Cooperation. ACS Energy Lett. 2018, 3, 2816–2822. 10.1021/acsenergylett.8b01767. [DOI] [Google Scholar]
- Clark E. L.; Hahn C.; Jaramillo T. F.; Bell A. T. Electrochemical CO2 Reduction over Compressively Strained CuAg Surface Alloys with Enhanced Multi-Carbon Oxygenate Selectivity. J. Am. Chem. Soc. 2017, 139, 15848–15857. 10.1021/jacs.7b08607. [DOI] [PubMed] [Google Scholar]
- Sarfraz S.; Garcia-Esparza A. T.; Jedidi A.; Cavallo L.; Takanabe K. Cu–Sn Bimetallic Catalyst for Selective Aqueous Electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851. 10.1021/acscatal.6b00269. [DOI] [Google Scholar]
- Mistry H.; Varela A. S.; Kuhl S.; Strasser P.; Cuenya B. R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 2016, 1, 16009. 10.1038/natrevmats.2016.9. [DOI] [Google Scholar]
- Gao D.; Arán-Ais R. M.; Jeon H. S.; Roldan Cuenya B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. 10.1038/s41929-019-0235-5. [DOI] [Google Scholar]
- Yin G.; Abe H.; Kodiyath R.; Ueda S.; Srinivasan N.; Yamaguchi A.; Miyauchi M. Selective electro- or photo-reduction of carbon dioxide to formic acid using a Cu-Zn alloy catalyst. J. Mater. Chem. A 2017, 5, 12113–12119. 10.1039/C7TA00353F. [DOI] [Google Scholar]
- Yin G.; Sako H.; Gubbala R. V.; Ueda S.; Yamaguchi A.; Abe H.; Miyauchi M. A Cu-Zn nanoparticle promoter for selective carbon dioxide reduction and its application in visible-light-active Z-scheme systems using water as an electron donor. Chem. Commun. 2018, 54, 3947–3950. 10.1039/C8CC00535D. [DOI] [PubMed] [Google Scholar]
- Hu H.; Tang Y.; Hu Q.; Wan P.; Dai L.; Yang X. J. In-situ grown nanoporous Zn-Cu catalysts on brass foils for enhanced electrochemical reduction of carbon dioxide. Appl. Surf. Sci. 2018, 445, 281–286. 10.1016/j.apsusc.2018.03.146. [DOI] [Google Scholar]
- Keerthiga G.; Chetty R. Electrochemical Reduction of Carbon Dioxide on Zinc-Modified Copper Electrodes. J. Electrochem. Soc. 2017, 164, H164–H169. 10.1149/2.0421704jes. [DOI] [Google Scholar]
- Ren D.; Ang B. S.-H.; Yeo B. S. Tuning the Selectivity of Carbon Dioxide Electroreduction toward Ethanol on Oxide-Derived CuxZn Catalysts. ACS Catal. 2016, 6, 8239–8247. 10.1021/acscatal.6b02162. [DOI] [Google Scholar]
- Feng Y.; Li Z.; Liu H.; Dong C.; Wang J.; Kulinich S. A.; Du X. Laser-Prepared CuZn Alloy Catalyst for Selective Electrochemical Reduction of CO2 to Ethylene. Langmuir 2018, 34, 13544–13549. 10.1021/acs.langmuir.8b02837. [DOI] [PubMed] [Google Scholar]
- Lamaison S.; Wakerley D.; Montero D.; Rousse G.; Taverna D.; Giaume D.; Mercier D.; Blanchard J.; Tran H. N.; Fontecave M.; Mougel V. Zn-Cu Alloy Nanofoams as Efficient Catalysts for the Reduction of CO2 to Syngas Mixtures with a Potential-Independent H2/CO Ratio. ChemSusChem 2019, 12, 511–517. 10.1002/cssc.201802287. [DOI] [PubMed] [Google Scholar]
- Li C. W.; Kanan M. W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. 10.1021/ja3010978. [DOI] [PubMed] [Google Scholar]
- Verdaguer-Casadevall A.; Li C. W.; Johansson T. P.; Scott S. B.; McKeown J. T.; Kumar M.; Stephens I. E. L.; Kanan M. W.; Chorkendorff I. Probing the Active Surface Sites for CO Reduction on Oxide-Derived Copper Electrocatalysts. J. Am. Chem. Soc. 2015, 137, 9808–9811. 10.1021/jacs.5b06227. [DOI] [PubMed] [Google Scholar]
- Mariano R. G.; McKelvey K.; White H. S.; Kanan M. W. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science 2017, 358, 1187–1192. 10.1126/science.aao3691. [DOI] [PubMed] [Google Scholar]
- Ma M.; Djanashvili K.; Smith W. A. Controllable Hydrocarbon Formation from the Electrochemical Reduction of CO2 over Cu Nanowire Arrays. Angew. Chem. 2016, 128, 6792–6796. 10.1002/ange.201601282. [DOI] [PubMed] [Google Scholar]
- Schouten K. J. P.; Pérez Gallent E.; Koper M. T. M. The influence of pH on the reduction of CO and to hydrocarbons on copper electrodes. J. Electroanal. Chem. 2014, 716, 53–57. 10.1016/j.jelechem.2013.08.033. [DOI] [Google Scholar]
- Kim B.; Ma S.; Molly Jhong H.-R.; Kenis P. J. A. Influence of dilute feed and pH on electrochemical reduction of CO2 to CO on Ag in a continuous flow electrolyzer. Electrochim. Acta 2015, 166, 271–276. 10.1016/j.electacta.2015.03.064. [DOI] [Google Scholar]
- Favaro M.; Xiao H.; Cheng T.; Goddard W. A.; Yano J.; Crumlin E. J. Subsurface oxide plays a critical role in CO2 activation by Cu(111) surfaces to form chemisorbed CO2, the first step in reduction of CO2. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 6706–6711. 10.1073/pnas.1701405114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.-W.; Scholten F.; Sinev I.; Roldan Cuenya B. Enhanced stability and CO/Formate selectivity of plasma-treated SnOx/AgOx catalysts during CO2 electroreduction. J. Am. Chem. Soc. 2019, 141, 5261–5266. 10.1021/jacs.8b12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y.; Jung H.; Kim N.-K.; Oh H.-S.; Min B. K.; Hwang Y. J. Mixed Copper States in Anodized Cu Electrocatalyst for Stable and Selective Ethylene Production from CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689. 10.1021/jacs.8b02173. [DOI] [PubMed] [Google Scholar]
- Mistry H.; Behafarid F.; Reske R.; Varela A. S.; Strasser P.; Roldan Cuenya B. Tuning catalytic selectivity at the mesoscale via interparticle interactions. ACS Catal. 2016, 6, 1075–1080. 10.1021/acscatal.5b02202. [DOI] [Google Scholar]
- Mistry H.; Reske R.; Strasser P.; Roldan Cuenya B. Size-dependent reactivity of gold-copper bimetallic nanoparticles during CO2 electroreduction. Catal. Today 2017, 288, 30–36. 10.1016/j.cattod.2016.09.017. [DOI] [Google Scholar]
- Ravel B.; Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- Bernal M.; Bagger A.; Scholten F.; Sinev I.; Bergmann A.; Ahmadi M.; Rossmeisl J.; Cuenya B. R. CO2 electroreduction on copper-cobalt nanoparticles: Size and composition effect. Nano Energy 2018, 53, 27–36. 10.1016/j.nanoen.2018.08.027. [DOI] [Google Scholar]
- Biesinger M. C.; Payne B. P.; Grosvenor A. P.; Lau L. W. M.; Gerson A. R.; Smart R. S. C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. 10.1016/j.apsusc.2010.10.051. [DOI] [Google Scholar]
- Jeon H. S.; Sinev I.; Scholten F.; Divins N. J.; Zegkinoglou I.; Pielsticker L.; Cuenya B. R. Operando Evolution of the Structure and Oxidation State of Size-Controlled Zn Nanoparticles during CO2 Electroreduction. J. Am. Chem. Soc. 2018, 140, 9383–9386. 10.1021/jacs.8b05258. [DOI] [PubMed] [Google Scholar]
- Kortlever R.; Shen J.; Schouten K. J. P.; Calle-Vallejo F.; Koper M. T. M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. 10.1021/acs.jpclett.5b01559. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-J.; Sethuraman V.; Michalsky R.; Peterson A. A. Competition between CO2 Reduction and H2 Evolution on Transition-Metal Electrocatalysts. ACS Catal. 2014, 4, 3742–3748. 10.1021/cs5012298. [DOI] [Google Scholar]
- Peterson A. A.; Abild-Pedersen F.; Studt F.; Rossmeisl J.; Norskov J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. 10.1039/c0ee00071j. [DOI] [Google Scholar]
- Kuhl K. P.; Cave E. R.; Abram D. N.; Jaramillo T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. 10.1039/c2ee21234j. [DOI] [Google Scholar]
- Manthiram K.; Beberwyck B. J.; Alivisatos A. P. Enhanced Electrochemical Methanation of Carbon Dioxide with a Dispersible Nanoscale Copper Catalyst. J. Am. Chem. Soc. 2014, 136, 13319–13325. 10.1021/ja5065284. [DOI] [PubMed] [Google Scholar]
- Owen E. A.; Preston G. D. X-ray analysis of zinc-copper alloys. Proc. Phys. Soc. 1923, 36, 49–66. 10.1088/1478-7814/36/1/307. [DOI] [Google Scholar]
- Timoshenko J.; Halder A.; Yang B.; Seifert S.; Pellin M. J.; Vajda S.; Frenkel A. I. Subnanometer Substructures in Nanoassemblies Formed from Clusters under a Reactive Atmosphere Revealed Using Machine Learning. J. Phys. Chem. C 2018, 122, 21686–21693. 10.1021/acs.jpcc.8b07952. [DOI] [Google Scholar]
- Andrews P. T.; Hisscott L. A. X-ray photoelectron spectroscopy of some Cu-Zn alloys. J. Phys. F: Met. Phys. 1975, 5, 1568–1572. 10.1088/0305-4608/5/8/016. [DOI] [Google Scholar]
- Liu K.; Ma M.; Wu L.; Valenti M.; Cardenas-Morcoso D.; Hofmann J. P.; Bisquert J.; Gimenez S.; Smith W. A. Electronic Effects Determine the Selectivity of Planar Au–Cu Bimetallic Thin Films for Electrochemical CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 16546–16555. 10.1021/acsami.9b01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reske R.; Mistry H.; Behafarid F.; Roldan Cuenya B.; Strasser P. Particle Size Effects in the Catalytic Electroreduction of CO2 on Cu Nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. 10.1021/ja500328k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.