Abstract
Attaining thermodynamic stability of colloids in a broad range of concentrations has long been a major thrust in the field of colloidal ligand-capped semiconductor nanocrystals (NCs). This challenge is particularly pressing for the novel NCs of cesium lead halide perovskites (CsPbX3; X = Cl, Br) owing to their highly dynamic and labile surfaces. Herein, we demonstrate that soy lecithin, a mass-produced natural phospholipid, serves as a tightly binding surface-capping ligand suited for a high-reaction yield synthesis of CsPbX3 NCs (6–10 nm) and allowing for long-term retention of the colloidal and structural integrity of CsPbX3 NCs in a broad range of concentrations—from a few ng/mL to >400 mg/mL (inorganic core mass). The high colloidal stability achieved with this long-chain zwitterionic ligand can be rationalized with the Alexander–De Gennes model that considers the increased particle–particle repulsion due to branched chains and ligand polydispersity. The versatility and immense practical utility of such colloids is showcased by the single NC spectroscopy on ultradilute samples and, conversely, by obtaining micrometer-thick, optically homogeneous dense NC films in a single spin-coating step from ultraconcentrated colloids.
Introduction
Colloidal nanocrystals (NCs) of lead halide perovskites (APbX3; A = Cs, formamidinium; X = Cl, Br, I) are new semiconductor NCs which have both unique optical characteristics as well as specific challenges.1 Defect-tolerant photophysics,2 outstanding photoluminescence (PL) characteristics, narrow emitting bands, high quantum yields (QYs), spectral tunability, and facile synthesis make perovskite NCs appealing classical light sources (in LCD and LED displays,3 lasers,4 optical communication,5 scintillators6) as well as single-photon sources.7 Conversely, perovskite NCs are also pursued as promising light harvesters for solar cells8 and photodetectors.5a,9
The surface state of semiconductor NCs has been the dominant factor defining the optical and electronic characteristics of individual NCs and their assemblies.10 The inorganic (NC core)–organic (ligands, solvents) interface is quintessential for the entire life cycle of a semiconductor NC—from its nucleation and growth to integration into devices.11 The surface chemistry of perovskite NCs has been shown to be vastly different from conventional semiconductor NCs, chiefly due to their ionic bonding and low lattice energy within the lead halide NC core (structural lability) and at the inorganic–organic interface.12 Most specific challenges are the susceptibility of the NC cores to water and many other solvents due to their finite solubility and the loose binding of the surface-capping ligands.13 These have been the major obstacles for studying, processing, and applications of perovskite NCs. For instance, rapid ligand desorption renders colloids unstable and eventually causes morphological degradation and coalescence of these NCs. Unsurprisingly, a rapid surge of reports is targeting these difficulties by the judicious choice of the solvents, ligands, and other additives for the synthesis and isolation protocols.1l,1n,12c,12d,14 Alternatively, NC films can be subjected to various surface stabilization and matrix-encapsulation strategies.7j,16
The ideal ligand system is allowing for efficient synthesis and subsequent processing. Common solution-processing techniques—spin coating, inkjet printing, doctor blading, slot die coating, or screen printing—all require concentrated and stable colloids. Obtaining thick, optically homogeneous, and dense films of CsPbBr3 and FAPbBr3 NCs with high PL QYs is of paramount importance in quantum dot-color filter displays,3a,3i,17 wherein the emissive layer must be several micrometers thick in order to completely absorb the blue backlight to reach optimal luminance and a wide color gamut. On the other hand, highly dilute NC systems are required for engineering quantum light sources.7
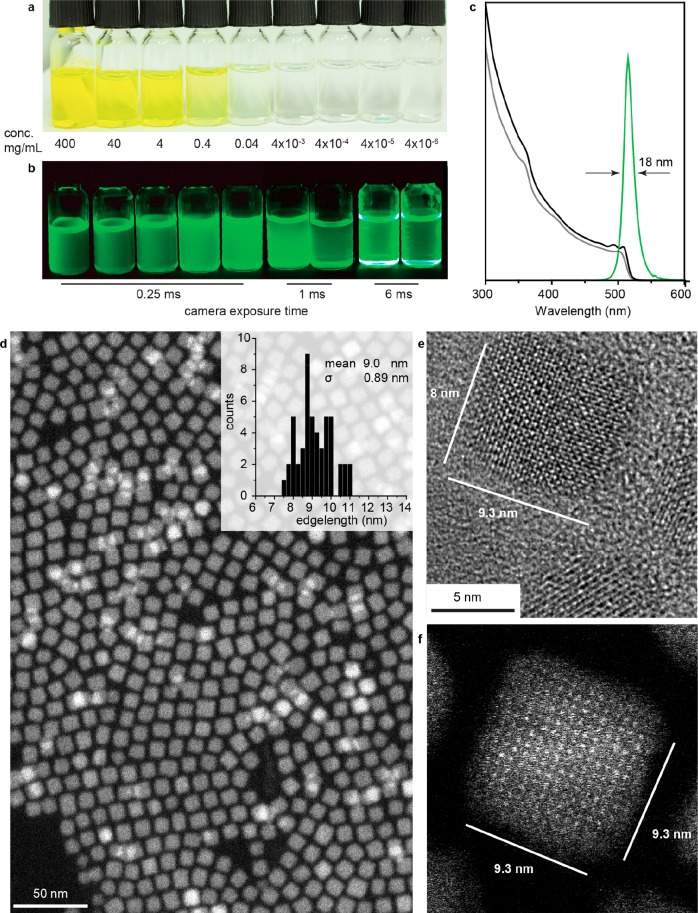
In this study, we sought to devise an effective ligand system that allows retention of the colloidal state, structural integrity, and optical characteristics over a broad range of CsPbBr3 NC concentrations. Using natural soy lecithin as a capping ligand (Figure 1a and 1b), we obtained concentrations of CsPbX3 NCs exceeding 400 mg/mL (inorganic core mass/total volume of the colloid), i.e., approximately 30% by volume (Figure 1c). Furthermore, tight ligand binding allows attaining the colloidal state and maintaining NC integrity in a broad concentration range down to few ng/mL.
Figure 1.
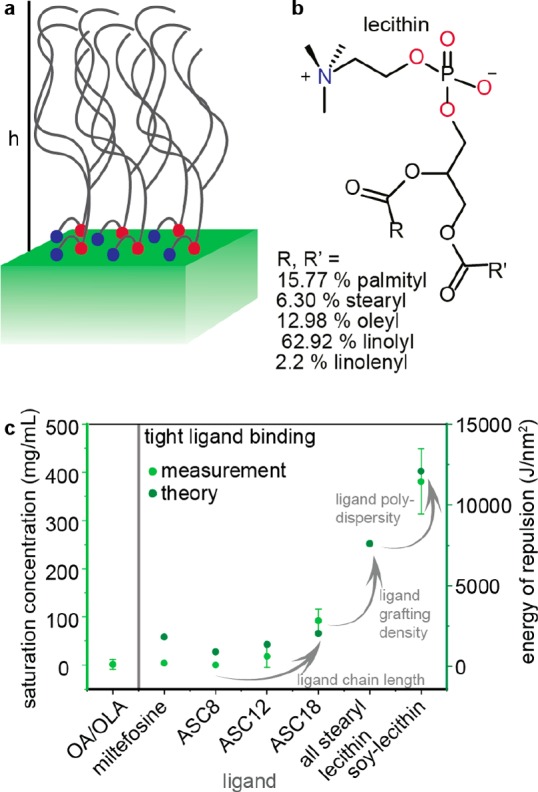
(a) Schematic of a lecithin-ligand brush on a CsPbX3 surface; h indicates the brush height. (b) Chemical structure of lecithin and statistical occurrence of side chains in soy lecithin.15 (c) Plot comparing the saturation concentration CsPbBr3 NCs (ca. 7 nm) capped with different ligands: oleic acid (OA) and oleylamine (OLA), ASC8 [3-(N,N-dimethyloctylammonio)propanesulfonate] and its longer chain analogues (ASC12 and ASC18, having C12 and C18 carbon chains in place of octyl, see also an earlier study in ref (14k)). The highest concentration of above 400 mg/mL (inorganic content per mL of toluene) is obtained with soy lecithin as a combined result of its higher chain length and natural lengths dispersity and, comparatively to all other studied ligands, higher grafting density (two vs one long chains per headgroup; i.e., 3.6 vs 2 tails/nm2). For comparison, ACS18-capped NCs exhibit a saturated concentration of ca. 90 mg/mL.
Results and Discussion
Soy lecithin is an inexpensive, natural, zwitterionic phospholipid which contains a physiological mixture of saturated and unsaturated long hydrocarbon chains (total of five, Figure 1b), two per molecule. Hence, it can be viewed as a polydisperse ligand. The choice of lecithin was inspired, on one hand, by our earlier studies of other zwitterionic long-chain molecules as strongly binding ligands14k and, on the other hand, by insights from polymer physics. To achieve highly concentrated colloids the NC–NC repulsion—typically and in this study of purely steric nature18—needs to be maximized. Ligand-covered NC surfaces can be viewed as brush-like structures (Figure 1a). Details of modeling the ligand–ligand repulsion between two NC surfaces can be found in the SI, Table S1 and Figures S1 and S2. The repulsion between polymer brushes has been studied extensively by De Gennes19 and others.20 The classical model considers these brushes as made up from stretched chains, permanently attached to an infinite, flat surface,20,21 which are valid assumptions for lecithin-capped CsPbBr3 NCs due to static ligand binding (confirmed by NMR, see discussion later in the text) and their cuboidal shape. According to classical, self-consistent mean field theory the factors which increase the energy of repulsion and thus the energy barrier for aggregation are tight binding, long chains, high grafting density,22 and ligand chain polydispersity.21 Calculated energy of repulsion vs number of hydrocarbons reproduces the slope obtained in the logarithmic plot of the experimental saturation concentration vs number of hydrocarbons (Figure S3). We note that we have thus far ignored the effect of the ligand interpenetration (gain in enthalpy upon aggregation due to van der Waals ligand–ligand interaction) that counteracts the steric repulsion.
Interpenetration of ligands explains the lower solubility of stearyl-ASC18 compared to oleyl-ACS18 (Figure S3). Similar effects of the ligand sterics have been observed on smaller, quasispherical NCs (CdSe, Fe2O3, Ag, etc.).23 For sufficiently long chains the overall impact of interdigitation on the system’s free energy will level off, as commonly observed with polymers.24 In fact, both the experiment and the calculations agree on the grafting density and ligand-chain distribution as the factors able to boost the CsPbBr3 NC–NC repulsion well beyond the impact of chain length. Soy lecithin is a natural ligand system that harnesses all three components (Figure 1c). Our results on CsPbBr3 NCs and recent work by others on CdSe NCs25 point to a rather unexplored potential of ligand systems comprising the same headgroup and a mixture of different hydrocarbon tails.
Highly concentrated colloids of lecithin-covered CsPbBr3 NCs retain a fully dispersed state without noticeable aggregation for at least 1 month, as can be readily seen by the Tindall effect (Figure S4) and confirmed by dynamic light scattering (DLS, Figures S5 and S6). They can be centrifuged at 29 500g for 1 h without noticeable precipitation. At the other extreme, in very dilute solutions (Figure 2a and 2b, right), factors such as chain length become less important due to decreased collision probability. The tight binding of the ligand, however, gains a pivotal role as it counteracts dilution-favored desorption of the ligands (leading to NC degradation as well). The PL peak positions and PL QY values are retained upon deep dilution (Figure S7).
Figure 2.
(a and b) Vials containing lecithin-covered ∼8 nm CsPbBr3 NCs at various concentrations (indicated on the figure) under day light and UV light, respectively. (c) Typical absorption spectra (gray, as synthesized; black, fraction 8) and emission spectrum (green, fraction 8), (d) high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) image, (e) HR-TEM image, and (f) high-resolution HAADF-STEM image of CsPbBr3 NCs from fraction 8 obtained by size fractioning; full size-selected series is given in Figure 3a and 3b as well as Figures S15 and S16.
Soy-lecithin-capped CsPbX3 NCs were obtained at 80–150 °C in a hot-injection synthesis (see details in SI) by adapting the methodology that we previously devised for synthetic zwitterionic molecules as capping ligands. In particular, Pb–oleate, Cs–oleate, and trioctylphosphine–halogen adduct (TOPBr2, TOPCl2) were used as precursors, whereas octadecene was employed as a solvent (effect of halide ratio Figrue S8). The NCs crystallize into an orthorhombic perovskite lattice (Pbnm, Figure S9).1d,26 NCs can be isolated by destabilizing the crude solution with acetone followed by centrifugation and redispersion in toluene. The synthesis yield can be ca. 70% (for a 130 °C, synthesis), which is about 800 mg from a 25 mL reaction scale. The loss is primarily due to incomplete precipitation at each stage. We note the practical utility of perovskite NCs is often handicapped by the low synthesis yields caused by the losses at the isolation and purification stages, not by the initial precursor-to-NC conversion. For instance, 90% of NCs are typically lost in conventional OA/OLA synthesis due to the desorption of OA/OLA coating and NC aggregation. With zwitterionic ligands, on the contrary, up to 80% of NCs can be obtained in the form of well-purified colloids.14k In this regard, lecithin is a similarly efficient ligand yet with its own specifics caused by drastically enhanced colloidal stabilization. For instance, running the synthesis at the concentrations of reagents commensurate with conventional OA/OLA synthesis (i.e., five times lower than we report here) yields colloids that are hard to destabilize by acetone.
Lecithin-capped NCs fully retain their spectral characteristics after intense purification by repetitive precipitation with diverse nonsolvents under ambient conditions and after prolonged storage (Figure S10). We note that all results presented in this work are obtained for samples that were at least several weeks old. Efficient ligand binding and ligand retention upon isolation is directly confirmed by NMR spectroscopy and thermal gravimetric analysis (TGA). The 1H and 31P NMR spectra of lecithin-covered NCs show severe line broadening compared to lecithin reference spectra and spectra of decomposed NCs (Figures S11 and S12). Decomposition of NCs was conducted by mixing a concentrated colloid with d6-DMSO. A plausible reason for the line broadening is the increase of the ligand’s rotational correlation time due to association with the NCs. Association of the ligand molecules with each other into micelles, an alternative cause for the reduced molecular tumbling and hence line broadening, can be excluded based on analytical ultracentrifugation (AUC) measurements, as discussed later in the text. Diffusion-ordered NMR spectroscopy (DOSY) shows an increase of the ligand’s diffusion coefficient by a factor of 10 when associated with the NCs (Figure S13). This is in agreement with values previously seen with other zwitterionic ligands (see ref (14k) and Table S2). The NC size received from DOSY, assuming tight binding of the ligands, is commensurate with NC sizes determined by TEM or DLS (Table S2). The combination of these arguments suggests the absence of free organic solutes. With the TGA weight loss of ca. 25% (at 300 °C, Figure S14), one can estimate ligand surface densities of ca. 1.8 nm–2. This value is close to that obtained with single-chain zwitterionic ligands such as ASC18 (ca. 2.0 nm–2 14k), suggesting that bulkier lecithin molecules do not suffer from much increased sterical encumbrance at the NC surface.
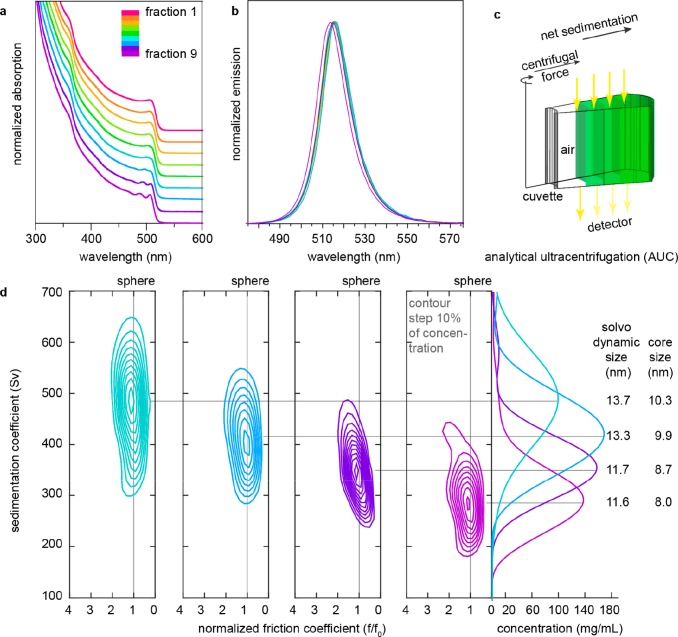
The typical absorption spectrum for CsPbBr3 NCs synthesized at 130 °C is shown in Figure 2c (gray line). Such colloids are characterized by the standard size deviation of ca. 20%. For obtaining practical quantities of narrowly dispersed NCs (size distribution below 10%) we employed a so-called size-selective precipitation technique, extensively used in the past for conventional semiconductor NCs (InP, CdSe, CdTe).27 Size fractioning is conducted by the gradual destabilization of a colloid with a nonsolvent and collection of each fraction by centrifugation. High concentrations and stable surface capping are imperative for an overall practicality of this approach. So-obtained NCs exhibit better-resolved excitonic features in the absorption spectra, as follows from Figure 2c (black curve), showing fraction 8 in this specific case, comprising 16 wt % of the initial ensemble. Electron microscopy reveals a uniform cuboid shape, mean NC size of ∼9 nm, and confirms monodispersity of these NCs (ca. 9.9% of size distribution). Monodisperse NC colloids with a mean NC size precisely adjustable in the range of 6–10 nm can be obtained by combining the effect of the synthesis temperature (higher for larger sizes) and size-selective precipitation (Figures S15 and S16, Table S3). For smaller NCs (<8 nm), which fall into strong and intermediate quantum confinement regimes,28 the benefits of size selection on the sharpening of absorption spectra and narrowing PL lines are most pronounced. At larger sizes, PL bands are already limited by the homogeneous line width,29 as can be seen from the spectral indistinguishability of fractions 1–8 (Figure 3b). Absorbance spectra often are less subjected to homogeneous broadening and hence render a better estimate for the spectral polydispersity of the colloids (Figure 3a).
Figure 3.
(a) Absorption and (b) photoluminescence spectra of size-selected fractions of CsPbBr3 NCs (130 °C synthesis, 20% initial size distribution). NC size decreases with increasing fraction number. (c) Schematic of the AUC principle. (d) C(s,f/f0) distributions estimated by Sedfit from absorbance scans at 500 nm for fractions 6–9 (these fractions together sum up to 59% of the whole ensemble). Unit Sv “Svedberg” is equal to 10–13 s. From the distributions, solvodynamic particle size and core size were calculated and tabulated on the right. Core size refers to the average edge length of the NC. Results are tabulated in Table S4.
The size distribution of the size-selected fractions (for the same 130 °C, synthesis) has been further quantified with two very distinct techniques which can probe the NCs in their native colloidal state: analytical ultracentrifugation (AUC) and small-angle X-ray scattering (SAXS). The results for fractions 6–9 are presented in Table 1 and compared to TEM data (Figure S17).
Table 1. NC Edge Lengths (in nm) Determined by Various Methods.
| fraction | TEM | AUC | SAXS |
|---|---|---|---|
| 6 | 10.4 ± 0.99 | 10.3 | |
| 7 | 10.5 ± 1.2 | 9.9 | |
| 8 | 8.9 ± 0.89 | 8.7 | 7.9 × 9.1 × 9.1, σ = 6.5%, average 8.7 |
| 9 | 8.75 ± 0.87 | 8.0 | 7.2 × 8.2 × 8.3, σ = 6.6%, average 7.9 |
We employed AUC sedimentation velocity (SV) measurement for its capability in determining solvodynamic parameters of the NCs in their original dispersion.30 In an AUC-SV experiment, the dispersion in a cell is spun at a constant speed, causing a net sedimenting movement of the NCs toward the bottom of the cell. The concentration of the nanocrystals is monitored along the cell as time progresses. Absorbance or interference optics can be utilized (Figure 3c). The evolution of the absorbance signal with space and time depends on the sedimentation of the species that absorb at a given wavelength. The evolution of the interference pattern is sensitive to all species present because their sedimentation causes a change in the refractive index of the dispersion. In our AUC-SV experiments we recorded both absorbance and interference simultaneously. A sample composed solely of NCs should have the same distribution of sedimentation coefficients derived from absorbance and interference AUC; any difference would be an indication of optically inactive species in solution (i.e., impurities). The distribution of sedimentation coefficients C(s), which is determined by the particle size, shape, and density, is obtained by numerically fitting the Lamm’s solution to the AUC radial profiles (Figures S18–20).30a,31 We ran AUC on multiple fractions of the NCs. We found that for all fractions the distribution results from absorbance (550 nm) data and interference data were essentially the same (Figure S20), indicating that the particles have a high degree of purity. Representative distributions of the sedimentation coefficients are shown in Figure 3d (and others are in Figure S20). These plots clearly show that each fraction contains NCs of rather narrow size distribution and that fractionation improved size distribution.
In order to better explain the working principle of AUC we can take the example of a NCs-containing sample. The centrifugal forces that act on the NCs determine the movement of the absorbance boundary that is related to the NCs sedimentation coefficient that is proportional to their solvodynamic size. As the experiment progresses, the natural diffusion of the NCs tends to broaden the absorbance boundary whose thickness is effectively infinitely sharp at time zero and progressively can evolve to be sizable. Such evolution of the boundary is used to determine either the particle’s density (assuming spherical shape) or the particle’s frictional coefficient (a parameter related to the shape of the NCs, assuming a known density).30a−30c In the analysis of the NCs described here we assume a frictional coefficient of a cube (f/f0 = 1.05); this allowed us to calculate the density for the NCs. As explained in ref (30a), the simultaneous knowledge of the NCs solvodynamic radius (from the sedimentation coefficient) and the overall particle density allowed us to calculate the size of the inorganic core of the NCs. Overall, the core sizes found in AUC agree quantitatively with the edge lengths retrieved from HR-TEM and SAXS (see Table 1). In addition, we can also conclude that the samples are free from known impurities for these NCs (such as Cs4PbBr6, CsPb2Br5, PbBr2, CsBr, delaminated Ruddleston–Popper phases, organic micelles, etc.) as AUC at 500 nm leads to identical results to AUC at 335 nm or AUC run in interference mode. Any of the impurities named above would have led to additional contributions to the AUC distributions.
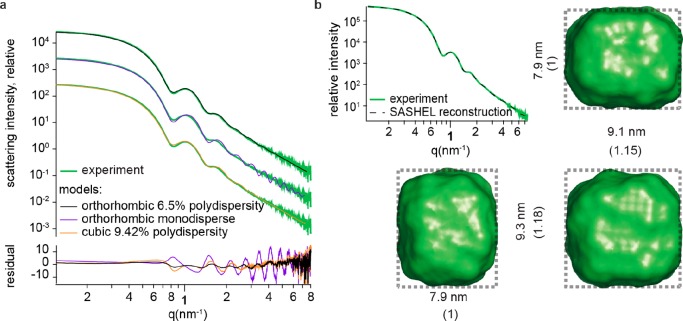
Colloids of NCs with less than 10% polydispersity are highly suited for analysis with small angle X-ray scattering (SAXS). Size- and shape-dependent scattering features can thus be identified and interpreted, as we illustrate here for toluene solutions of lecithin-capped CsPbBr3 NCs (fractions 8 and 9 from 130 °C, synthesis). Colloids were filled in a flow cell capillary, followed by the SAXS measurements at a synchrotron source (see SI section 4.5 for details). After applying intensity and background corrections, the scattering traces all showed clear features of form factor scattering and were hence compared to analytical models.32 Additionally, the shape was reconstructed using SASHEL, a model-free Monte Carlo 3D fitting algorithm, which operates without prior assumptions.33 The results of both methods are shown in Figures 4 and S21 for the NCs of fractions 8 and 9, respectively.
Figure 4.
(a) SAXS traces of NCs from fraction 8 (green) fitted with a monodisperse orthorhombic (violet) and polydisperse cubic (orange) and orthorhombic (black) models. Best fit was found for an orthorhombic model with 6.5% polydispersity on the edge lengths (fitting results Table S5). (b) Model-free fit of the small angle scattering data along with the NC shape that allowed for the a full 3D reconstruction, shown from 3 sides. Shape found from reconstruction of the scattering pattern agrees with the best-fit model, TEM statistics (Figure 2 d–f), and AUC data (Figure 3d). Measurements and fittings for NCs from fraction 9 can be found in Figure S21.
In the analytical model, an oblate cuboid with dimensions 7.99 × 9.27 × 9.27 nm (i.e., aspect ration of 0.86) and 6.43% relative polydispersity was found to most closely represent the NCs of fraction 8. Alternative models such as monodisperse cuboids and cubes with 9.4% relative polydispersity did not reproduce the measured scattering traces as closely, as can be seen from the residuals in Figure 4a. Full reconstruction yielded a cuboidal NC shape of 7.9 × 9.1 × 9.3 nm for NCs of fraction 8 (Figures 4b).
Retention of colloidal, structural, and optical integrity over a broad concentration range of CsPbX3 NCs, i.e., from 400 mg/mL down to few ng/mL, will have diverse practical implications. In the following, we showcase two instances of immense practical utility of ultraconcentrated as well as ultradilute colloids of CsPbX3 NCs.
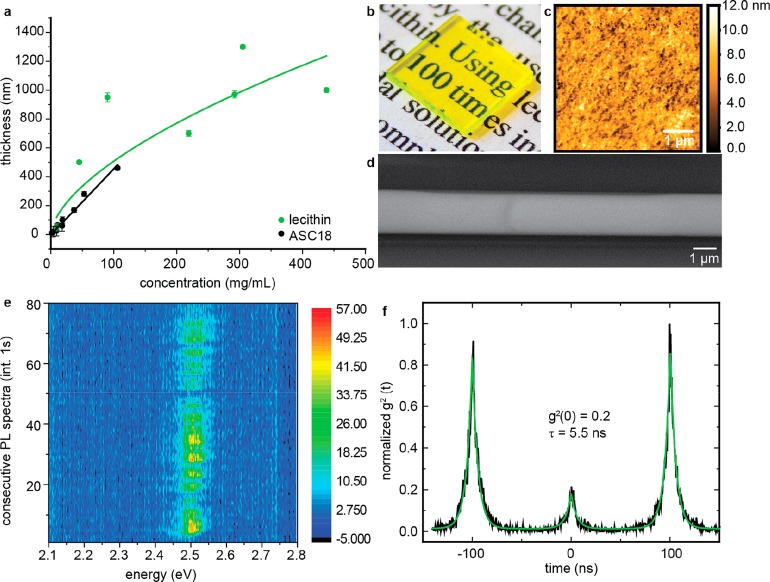
Highly concentrated colloids can be used as inks for one-step deposition of thick, smooth, optically clear films by spin coating. The thickness of the film can be readily adjusted by the concentration of the colloid to above 1 μm while maintaining a roughness of just 15 nm (Figure 5a–d, Figures S22 and S23). Previously, micrometer-thick perovskite NC films were obtained mostly by stepwise deposition with intermittent consolidation steps8d,14c or by filling of a scaffold structure.6a,34 The experimental thickness vs concentration dependence—while linear in ASC18-covered NCs (black)—can be approximated with a square root law for lecithin-covered NCs (green), although an increase with the power of 1/3 would be generally expected. This deviation can be rationalized by the variation of solution properties such as viscosity, vapor pressure, or contact angle at high volumetric loadings,35 which are critical to the spin-coating process. In particular, higher viscosity leads to thicker than expected films. Importantly, these films exhibit excellent optical clarity, indicative of their aggregation-free deposition and high PL QYs of ca. 30% at all thicknesses. Such thick films may find applications in the next-generation displays with NC films serving as both light filters and emitters3a,3i,17 or as scintillators for detecting X-rays and gamma-rays.6,9
Figure 5.
(a) Film thickness (AFM) under identical spin-coating conditions as a function of ink concentration (underlying AFM images Figures S22 and S23). (Green) Films made from toluene solutions of lecithin-capped CsPbBr3 NCs and (black) films from toluene solutions of ASC18-capped CsPbBr3 NCs. Error bars indicate film roughness. Lines are power-law fits. For the lecithin NCs the power is 0.5. (b) Photo of a 1 × 1 cm film 1 μm thick showing optical transparency; (c) AFM scan of a 1 μm thick film with roughness less than 13 nm; (d) SEM image of an identically prepared film showing long-range thickness homogeneity; (e) 80 consecutive spectra of a single lecithin-covered CsPbBr3 NC showing spectral stability due to good ligand passivation; (f) normalized correlogram indicating a single emitter is measured.
Ultradilute colloids are also in great demand. For example, dilution to the ng/mL level is necessary for obtaining sparsely distributed NCs upon deposition and solvent evaporation. In particular, dilutions which result in the average NC-to-NC separation by several micrometers are ideally suited for single NC spectroscopy with typical microphotoluminescence setups. As discussed above, labile ligand shells rapidly lead to the loss of colloidal state and NC aggregation upon such severe dilution due to ligand desorption. We therefore tested the durability of lecithin-capped CsPbBr3 NCs for obtaining single-photon emission at room temperature (Figure 5e and 5f), moreover, by conducting all steps under ambient conditions. Despite the presence of PL blinking as usually reported for these NCs, the PL is stable for several tens of seconds and without significant PL blue shift (typical for perovskite NCs).7j,29a,36 Second-order correlation measurements attest single-photon emission with the characteristic antibunching behavior and a good single-photon purity (g2(0) ≈ 0.2), further confirming a nonaggregated, intact state of these NCs.
Conclusions
We found that soy lecithin–an inexpensive, natural, mass-produced zwitterionic phospholipid–makes for an effective capping ligand for the synthesis of colloidal CsPbX3 NCs. Most importantly, it imparts high colloidal stability in a broad range of NC concentrations (from few ng/mL to above 400 mg/mL). Tight ligand binding, high grafting density, long chains, and ligand polydispersity are the key contributors of the effectiveness of this ligand. High synthesis yield and robustness of the colloids allow for facile size fractioning of the colloids, yielding practical quantities of monodisperse NCs with mean particle size tunable in the range of 6–10 nm. The size, shape, and size distribution of so-obtained NCs have been thoroughly examined with TEM, SAXS, and AUC techniques. In particular, the shape of these NCs have been confirmed to be oblate cuboids with a small aspect ratio of ca. 0.86.
An immense practical utility of such ultraconcentrated and ultradilute colloids has been illustrated by two showcase experiments. First, we have shown a one-step deposition of thick (up to 1.5 μm) NC films by spin coating. Such films exhibit excellent optical clarity while retaining bright fluorescence. Second, such colloids are also versatile inks for obtaining highly dilute NC systems as required for single-dot spectroscopy. Single-photon emission with pronounced photon antibunching has thus been readily observed.
Acknowledgments
This work was financially supported by the Swiss Federal Commission for Technology and Innovation (CTI-No. 18614.1 PFNM-NM) and, in part, by the European Union through the FP7 (ERC Starting Grant NANOSOLID, grant agreement No. [306733]) and through the Horizon 2020 Research and Innovation Programme (grant agreement No. [819740], project SCALE-HALO). Q.O. and F.S. gratefully acknowledge support from the Swiss National Science Foundation (SNSF) (Grant No. 200020_185062). This project received funding from the EU-H2020 Research and Innovation Programme under grant agreement no. 654360 having benefitted from access to the Austrian SAXS beamline provided by TUG@Elettra in Trieste, Italy, within the framework of the NFFA-Europe Transnational Access Activity. The authors thank the Scientific Center for Optical and Electron Microscopy (ScopeM) at ETH Zurich and the Empa Electron Microscopy Center for use of their facilities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b09969.
Experimental methods and supplementary figures (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Schmidt L. C.; Pertegas A.; Gonzalez-Carrero S.; Malinkiewicz O.; Agouram S.; Minguez Espallargas G.; Bolink H. J.; Galian R. E.; Perez-Prieto J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. 10.1021/ja4109209. [DOI] [PubMed] [Google Scholar]; b Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Krieg F.; Caputo R.; Hendon C. H.; Yang R. X.; Walsh A.; Kovalenko M. V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–6. 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Protesescu L.; Yakunin S.; Bodnarchuk M. I.; Bertolotti F.; Masciocchi N.; Guagliardi A.; Kovalenko M. V. Monodisperse Formamidinium Lead Bromide Nanocrystals with Bright and Stable Green Photoluminescence. J. Am. Chem. Soc. 2016, 138, 14202–14205. 10.1021/jacs.6b08900. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Swarnkar A.; Chulliyil R.; Ravi V. K.; Irfanullah M.; Chowdhury A.; Nag A. Colloidal CsPbBr3 Perovskite Nanocrystals: Luminescence beyond Traditional Quantum Dots. Angew. Chem., Int. Ed. 2015, 54, 15424–15428. 10.1002/anie.201508276. [DOI] [PubMed] [Google Scholar]; e Huang H.; Polavarapu L.; Sichert J. A.; Susha A. S.; Urban A. S.; Rogach A. L. Colloidal Lead Halide Perovskite Nanocrystals: Synthesis, Optical Properties and Applications. NPG Asia Mater. 2016, 8, e328–e328. 10.1038/am.2016.167. [DOI] [Google Scholar]; f Kovalenko M. V.; Protesescu L.; Bodnarchuk M. I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. 10.1126/science.aam7093. [DOI] [PubMed] [Google Scholar]; g Zhang J.; Yang X. K.; Deng H.; Qiao K. K.; Farooq U.; Ishaq M.; Yi F.; Liu H.; Tang J.; Song H. S. Low-Dimensional Halide Perovskites and Their Advanced Optoelectronic Applications. Nano-Micro Lett. 2017, 9, 36–62. 10.1007/s40820-017-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Li X.; Cao F.; Yu D.; Chen J.; Sun Z.; Shen Y.; Zhu Y.; Wang L.; Wei Y.; Wu Y.; Zeng H. All Inorganic Halide Perovskites Nanosystem: Synthesis, Structural Features, Optical Properties and Optoelectronic Applications. Small 2017, 13, 1603996–1604020. 10.1002/smll.201603996. [DOI] [PubMed] [Google Scholar]; i Akkerman Q. A.; Raino G.; Kovalenko M. V.; Manna L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. 10.1038/s41563-018-0018-4. [DOI] [PubMed] [Google Scholar]; j Adinolfi V.; Peng W.; Walters G.; Bakr O. M.; Sargent E. H. The Electrical and Optical Properties of Organometal Halide Perovskites Relevant to Optoelectronic Performance. Adv. Mater. 2018, 30, 1700764–1700777. 10.1002/adma.201700764. [DOI] [PubMed] [Google Scholar]; k Zhao Y.; Li J.; Dong Y.; Song J. Synthesis of Colloidal Halide Perovskite Quantum Dots/Nanocrystals: Progresses and Advances. Isr. J. Chem. 2019, 59, 649–660. 10.1002/ijch.201900009. [DOI] [Google Scholar]; l Shamsi J.; Urban A. S.; Imran M.; De Trizio L.; Manna L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. 10.1021/acs.chemrev.8b00644. [DOI] [PMC free article] [PubMed] [Google Scholar]; m Zheng X. P.; Hou Y.; Sun H. T.; Mohammed O. F.; Sargent E. H.; Bakr O. M. Reducing Defects in Halide Perovskite Nanocrystals for Light-Emitting Applications. J. Phys. Chem. Lett. 2019, 10, 2629–2640. 10.1021/acs.jpclett.9b00689. [DOI] [PubMed] [Google Scholar]; n Dutta A.; Pradhan N. Phase-Stable Red-Emitting CsPbI3 Nanocrystals: Successes and Challenges. ACS Energy Lett. 2019, 4, 709–719. 10.1021/acsenergylett.9b00138. [DOI] [Google Scholar]
- a Giansante C.; Infante I. Surface Traps in Colloidal Quantum Dots: A Combined Experimental and Theoretical Perspective. J. Phys. Chem. Lett. 2017, 8, 5209–5215. 10.1021/acs.jpclett.7b02193. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huang H.; Bodnarchuk M. I.; Kershaw S. V.; Kovalenko M. V.; Rogach A. L. Lead Halide Perovskite Nanocrystals in the Research Spotlight: Stability and Defect Tolerance. ACS Energy Lett. 2017, 2, 2071–2083. 10.1021/acsenergylett.7b00547. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Motti S. G.; Meggiolaro D.; Martani S.; Sorrentino R.; Barker A. J.; De Angelis F.; Petrozza A. Defect Activity in Metal-Halide Perovskites. Adv. Mater. 2019, 31, 1901183. 10.1002/adma.201901183. [DOI] [PubMed] [Google Scholar]
- a Kovalenko M. V.; Bodnarchuk M. I. Lead Halide Perovskite Nanocrystals: From Discovery to Self-assembly and Applications. Chimia 2017, 71, 461–470. 10.2533/chimia.2017.461. [DOI] [PubMed] [Google Scholar]; b Zhao X.; Ng J. D. A.; Friend R. H.; Tan Z.-K. Opportunities and Challenges in Perovskite Light-Emitting Devices. ACS Photonics 2018, 5, 3866–3875. 10.1021/acsphotonics.8b00745. [DOI] [Google Scholar]; c Van Le Q.; Jang H. W.; Kim S. Y. Recent Advances toward High-Efficiency Halide Perovskite Light emitting diodes Review and Perspective. Small Methods 2018, 2, 1700419–1700437. 10.1002/smtd.201700419. [DOI] [Google Scholar]; d Chiba T.; Kido J. Lead Halide Perovskite Quantum Dots for Light Emitting Devices. J. Mater. Chem. C 2018, 6, 11868. 10.1039/C8TC03561J. [DOI] [Google Scholar]; e Zhang F.; Song J.; Han B.; Fang T.; Li J.; Zeng H. High-Efficiency Pure-Color Inorganic Halide Perovskite Emitters for Ultrahigh-Definition Displays: Progress for Backlighting Displays and Electrically Driven Devices. Small Methods 2018, 2, 1700382–1700391. 10.1002/smtd.201700382. [DOI] [Google Scholar]; f Kim Y.-H.; Kim S.; Jo S. H.; Lee T.-W. Metal Halide Perovskites: From Crystal Formations to Light-Emitting-Diode Applications. Small Methods 2018, 2, 1800093–1800114. 10.1002/smtd.201800093. [DOI] [Google Scholar]; g Chang S.; Bai Z.; Zhong H. In Situ Fabricated Perovskite Nanocrystals: A Revolution in Optical Materials. Adv. Opt. Mater. 2018, 6, 1800380–1800399. 10.1002/adom.201800380. [DOI] [Google Scholar]; h Chen N.; Bai Z.; Wang Z.; Ji H.; Liu R.; Cao C.; Wang H.; Jiang F.; Zhong H. Low Cost Quantum Dot Film based Wide Color Gamut Backlight Unit for LCD TVs. Dig. Tech. Pap. - Soc. Inf. Disp. Int. Symp. 2018, 49, 1657–1659. 10.1002/sdtp.12303. [DOI] [Google Scholar]; i Yoon H. C.; Lee H.; Kang H.; Oh J. H.; Do Y. R. Highly Efficient Wide-Color-Gamut QD-Emissive LCDs using Red and Green Perovskite Core/Shell QDs. J. Mater. Chem. C 2018, 6, 13023–13033. 10.1039/C8TC04537B. [DOI] [Google Scholar]; j Lu M.; Zhang Y.; Wang S.; Guo J.; Yu W. W.; Rogach A. L. Metal Halide Perovskite Light-Emitting Devices: Promising Technology for Next-Generation Displays. Adv. Funct. Mater. 2019, 29, 1902008–1902043. 10.1002/adfm.201902008. [DOI] [Google Scholar]; k He Z.; Zhang C.; Dong Y.; Wu S.-T. Emerging Perovskite Nanocrystals-Enhanced Solid-State Lighting and Liquid-Crystal Displays. Crystals 2019, 9, 59. 10.3390/cryst9020059. [DOI] [Google Scholar]
- a Yakunin S.; Protesescu L.; Krieg F.; Bodnarchuk M. I.; Nedelcu G.; Humer M.; De Luca G.; Fiebig M.; Heiss W.; Kovalenko M. V. Low-threshold Amplified Spontaneous Emission and Lasing from Colloidal Nanocrystals of Caesium Lead Halide Perovskites. Nat. Commun. 2015, 6, 8056–8064. 10.1038/ncomms9056. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Stylianakis M. M.; Maksudov T.; Panagiotopoulos A.; Kakavelakis G.; Petridis K. Inorganic and Hybrid Perovskite Based Laser Devices: A Review. Materials 2019, 12, 859. 10.3390/ma12060859. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang Y.; Lim C.-K.; Dai Z.; Yu G.; Haus J. W.; Zhang H.; Prasad P. N. Photonics and Optoelectronics using Nano-Structured Hybrid Perovskite Media and their Optical Cavities. Phys. Rep. 2019, 795, 1–51. 10.1016/j.physrep.2019.01.005. [DOI] [Google Scholar]
- a Wu Y.; Li X.; Wei Y.; Gu Y.; Zeng H. Perovskite Photodetectors with both Visible-Infrared Dual-Mode Response and Super-Narrowband Characteristics towards Photo-Communication Encryption Application. Nanoscale 2018, 10, 359–365. 10.1039/C7NR06193E. [DOI] [PubMed] [Google Scholar]; b Mei S.; Liu X.; Zhang W.; Liu R.; Zheng L.; Guo R.; Tian P. High-Bandwidth White-Light System Combining a Micro-LED with Perovskite Quantum Dots for Visible Light Communication. ACS Appl. Mater. Interfaces 2018, 10, 5641–5648. 10.1021/acsami.7b17810. [DOI] [PubMed] [Google Scholar]; c Zhang Y.; Wang L.; Wang K.; Wong K. S.; Wu K. Recent Advances in the Hardware of Visible Light Communication. IEEE Access 2019, 7, 91093–91104. 10.1109/ACCESS.2019.2927054. [DOI] [Google Scholar]; d Cen G.; Liu Y.; Zhao C.; Wang G.; Fu Y.; Yan G.; Yuan Y.; Su C.; Zhao Z.; Mai W. Atomic-Layer Deposition-Assisted Double-Side Interfacial Engineering for High-Performance Flexible and Stable CsPbBr3 Perovskite Photodetectors toward Visible Light Communication Applications. Small 2019, 15, 1902135. 10.1002/smll.201902135. [DOI] [PubMed] [Google Scholar]
- a Chen Q.; Wu J.; Ou X.; Huang B.; Almutlaq J.; Zhumekenov A. A.; Guan X.; Han S.; Liang L.; Yi Z.; Li J.; Xie X.; Wang Y.; Li Y.; Fan D.; Teh D. B. L.; All A. H.; Mohammed O. F.; Bakr O. M.; Wu T.; Bettinelli M.; Yang H.; Huang W.; Liu X. All-Inorganic Perovskite Nanocrystal Scintillators. Nature 2018, 561, 88–93. 10.1038/s41586-018-0451-1. [DOI] [PubMed] [Google Scholar]; b Maddalena F.; Tjahjana L.; Xie A.; Arramel; Zeng S.; Wang H.; Coquet P.; Drozdowski W.; Dujardin C.; Dang C.; Birowosuto M. Inorganic, Organic, and Perovskite Halides with Nanotechnology for High–Light Yield X- and γ-ray Scintillators. Crystals 2019, 9, 88–117. 10.3390/cryst9020088. [DOI] [Google Scholar]
- a Raino G.; Nedelcu G.; Protesescu L.; Bodnarchuk M. I.; Kovalenko M. V.; Mahrt R. F.; Stoferle T. Single Cesium Lead Halide Perovskite Nanocrystals at Low Temperature: Fast Single-Photon Emission, Reduced Blinking, and Exciton Fine Structure. ACS Nano 2016, 10, 2485–2490. 10.1021/acsnano.5b07328. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fu M.; Tamarat P.; Huang H.; Even J.; Rogach A. L.; Lounis B. Neutral and Charged Exciton Fine Structure in Single Lead Halide Perovskite Nanocrystals Revealed by Magneto-optical Spectroscopy. Nano Lett. 2017, 17, 2895–2901. 10.1021/acs.nanolett.7b00064. [DOI] [PubMed] [Google Scholar]; c Fu M.; Tamarat P.; Trebbia J. B.; Bodnarchuk M. I.; Kovalenko M. V.; Even J.; Lounis B. Unraveling Exciton-Phonon Coupling in Individual FAPbI3 Nanocrystals Emitting near-infrared Single Photons. Nat. Commun. 2018, 9, 3318–3328. 10.1038/s41467-018-05876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Tong Y.; Fu M.; Bladt E.; Huang H.; Richter A. F.; Wang K.; Muller-Buschbaum P.; Bals S.; Tamarat P.; Lounis B.; Feldmann J.; Polavarapu L. Chemical Cutting of Perovskite Nanowires into Single-Photon Emissive Low-Aspect-Ratio CsPbX3 (X = Cl, Br, I) Nanorods. Angew. Chem., Int. Ed. 2018, 57, 16094–16098. 10.1002/anie.201810110. [DOI] [PubMed] [Google Scholar]; e Li B.; Huang H.; Zhang G. F.; Yang C. G.; Guo W. L.; Chen R. Y.; Qin C. B.; Gao Y.; Biju V. P.; Rogach A. L.; Xiao L. T.; Jia S. T. Excitons and Biexciton Dynamics in Single CsPbBr3 Perovskite Quantum Dots. J. Phys. Chem. Lett. 2018, 9, 6934–6940. 10.1021/acs.jpclett.8b03098. [DOI] [PubMed] [Google Scholar]; f Becker M. A.; Scarpelli L.; Nedelcu G.; Raino G.; Masia F.; Borri P.; Stoferle T.; Kovalenko M. V.; Langbein W.; Mahrt R. F. Long Exciton Dephasing Time and Coherent Phonon Coupling in CsPbBr2Cl Perovskite Nanocrystals. Nano Lett. 2018, 18, 7546–7551. 10.1021/acs.nanolett.8b03027. [DOI] [PubMed] [Google Scholar]; g Becker M. A.; Vaxenburg R.; Nedelcu G.; Sercel P. C.; Shabaev A.; Mehl M. J.; Michopoulos J. G.; Lambrakos S. G.; Bernstein N.; Lyons J. L.; Stoferle T.; Mahrt R. F.; Kovalenko M. V.; Norris D. J.; Raino G.; Efros A. L. Bright Triplet Excitons in Caesium Lead Halide Perovskites. Nature 2018, 553, 189–193. 10.1038/nature25147. [DOI] [PubMed] [Google Scholar]; h Utzat H.; Sun W.; Kaplan A. E. K.; Krieg F.; Ginterseder M.; Spokoyny B.; Klein N. D.; Shulenberger K. E.; Perkinson C. F.; Kovalenko M. V.; Bawendi M. G. Coherent Single-Photon Emission from Colloidal Lead Halide Perovskite Quantum Dots. Science 2019, 363, 1068–1072. 10.1126/science.aau7392. [DOI] [PubMed] [Google Scholar]; i Tamarat P.; Bodnarchuk M. I.; Trebbia J. B.; Erni R.; Kovalenko M. V.; Even J.; Lounis B. The Ground Exciton State of Formamidinium Lead Bromide Perovskite Nanocrystals is a Singlet Dark State. Nat. Mater. 2019, 18, 717–724. 10.1038/s41563-019-0364-x. [DOI] [PubMed] [Google Scholar]; j Raino G.; Landuyt A.; Krieg F.; Bernasconi C.; Ochsenbein S. T.; Dirin D. N.; Bodnarchuk M. I.; Kovalenko M. V. Underestimated Effect of a Polymer Matrix on the Light Emission of Single CsPbBr3 Nanocrystals. Nano Lett. 2019, 19, 3648–3653. 10.1021/acs.nanolett.9b00689. [DOI] [PubMed] [Google Scholar]
- a NREL Best Research-Cell Efficiency Chart; https://www.nrel.gov/pv/cell-efficiency.html (accessed Aug 26, 2019).; b Swarnkar A.; Marshall A. R.; Sanehira E. M.; Chernomordik B. D.; Moore D. T.; Christians J. A.; Chakrabarti T.; Luther J. M. Quantum Dot–Induced Phase Stabilization of a-CsPbI3 Perovskite for High-Efficiency Photovoltaics. Science 2016, 354, 92–95. 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]; c Sanehira E. M.; Marshall A. R.; Christians J. A.; Harvey S. P.; Ciesielski P. N.; Wheeler L. M.; Schulz P.; Lin L. Y.; Beard M. C.; Luther J. M. Enhanced mobility CsPbI3 Quantum Dot Arrays for Record-Efficiency, High-Voltage Photovoltaic Cells. Sci. Adv. 2017, 3, eaao4204. 10.1126/sciadv.aao4204. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Wheeler L. M.; Sanehira E. M.; Marshall A. R.; Schulz P.; Suri M.; Anderson N. C.; Christians J. A.; Nordlund D.; Sokaras D.; Kroll T.; Harvey S. P.; Berry J. J.; Lin L. Y.; Luther J. M. Targeted Ligand-Exchange Chemistry on Cesium Lead Halide Perovskite Quantum Dots for High-Efficiency Photovoltaics. J. Am. Chem. Soc. 2018, 140, 10504–10513. 10.1021/jacs.8b04984. [DOI] [PubMed] [Google Scholar]; e Song Z.; Chen C.; Li C.; Awni R. A.; Zhao D.; Yan Y. Wide-Bandgap, Low-Bandgap, and Tandem Perovskite Solar Cells. Semicond. Semicond. Sci. Technol. 2019, 34, 093001–093032. 10.1088/1361-6641/ab27f7. [DOI] [Google Scholar]; f Fu H. Colloidal Metal Halide Perovskite Nanocrystals: a Promising Juggernaut in Photovoltaic Applications. J. Mater. Chem. A 2019, 7, 14357–14379. 10.1039/C8TA12509K. [DOI] [Google Scholar]
- Miao J.; Zhang F. Recent Progress on Highly Sensitive Perovskite Photodetectors. J. Mater. Chem. C 2019, 7, 1741–1791. 10.1039/C8TC06089D. [DOI] [Google Scholar]
- a Talapin D. V.; Lee J.-S.; Kovalenko M. V.; Shevchenko E. V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. 10.1021/cr900137k. [DOI] [PubMed] [Google Scholar]; b Boles M. A.; Ling D.; Hyeon T.; Talapin D. V. The Surface Science of Nanocrystals. Nat. Mater. 2016, 15, 141–153. 10.1038/nmat4526. [DOI] [PubMed] [Google Scholar]; c Houtepen A. J.; Hens Z.; Owen J. S.; Infante I. On the Origin of Surface Traps in Colloidal II-VI Semiconductor Nanocrystals. Chem. Mater. 2017, 29, 752–761. 10.1021/acs.chemmater.6b04648. [DOI] [Google Scholar]
- a Weiss E. A. Organic Molecules as Tools To Control the Growth, Surface Structure, and Redox Activity of Colloidal Quantum Dots. Acc. Chem. Res. 2013, 46, 2607–2615. 10.1021/ar400078u. [DOI] [PubMed] [Google Scholar]; b Ling D. S.; Hackett M. J.; Hyeon T. Surface Ligands in Synthesis, Modification, Assembly and Biomedical Applications of Nanoparticles. Nano Today 2014, 9, 457–477. 10.1016/j.nantod.2014.06.005. [DOI] [Google Scholar]; c Kagan C. R.; Lifshitz E.; Sargent E. H.; Talapin D. V. Building Devices from Colloidal Quantum Dots. Science 2016, 353, aac5523. 10.1126/science.aac5523. [DOI] [PubMed] [Google Scholar]; d ten Brinck S.; Infante I. Surface Termination, Morphology, and Bright Photoluminescence of Cesium Lead Halide Perovskite Nanocrystals. ACS Energy Lett. 2016, 1, 1266–1272. 10.1021/acsenergylett.6b00595. [DOI] [Google Scholar]
- a Ravi V. K.; Santra P. K.; Joshi N.; Chugh J.; Singh S. K.; Rensmo H.; Ghosh P.; Nag A. Origin of the Substitution Mechanism for the Binding of Organic Ligands on the Surface of CsPbBr3 Perovskite Nanocubes. J. Phys. Chem. Lett. 2017, 8, 4988–4994. 10.1021/acs.jpclett.7b02192. [DOI] [PubMed] [Google Scholar]; b Nenon D. P.; Pressler K.; Kang J.; Koscher B. A.; Olshansky J. H.; Osowiecki W. T.; Koc M. A.; Wang L. W.; Alivisatos A. P. Design Principles for Trap-Free CsPbX3 Nanocrystals: Enumerating and Eliminating Surface Halide Vacancies with Softer Lewis Bases. J. Am. Chem. Soc. 2018, 140, 17760–17772. 10.1021/jacs.8b11035. [DOI] [PubMed] [Google Scholar]; c Bodnarchuk M. I.; Boehme S. C.; ten Brinck S.; Bernasconi C.; Shynkarenko Y.; Krieg F.; Widmer R.; Aeschlimann B.; Günther G.; Kovalenko M. V.; Infante I. Rationalizing and Controlling the Surface Structure and Electronic Passivation of Cesium Lead Halide Nanocrystals. ACS Energy Lett. 2019, 4, 63–74. 10.1021/acsenergylett.8b01669. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Quarta D.; Imran M.; Capodilupo A.-L.; Petralanda U.; van Beek B.; De Angelis F.; Manna L.; Infante I.; De Trizio L.; Giansante C. Stable Ligand Coordination at the Surface of Colloidal CsPbBr3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 3715–3726. 10.1021/acs.jpclett.9b01634. [DOI] [PubMed] [Google Scholar]
- a De Roo J.; Ibanez M.; Geiregat P.; Nedelcu G.; Walravens W.; Maes J.; Martins J. C.; Van Driessche I.; Kovalenko M. V.; Hens Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–81. 10.1021/acsnano.5b06295. [DOI] [PubMed] [Google Scholar]; b Smock S. R.; Williams T. J.; Brutchey R. L. Quantifying the Thermodynamics of Ligand Binding to CsPbBr3 Quantum Dots. Angew. Chem., Int. Ed. 2018, 57, 11711–11715. 10.1002/anie.201806916. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Grisorio R.; Di Clemente M. E.; Fanizza E.; Allegretta I.; Altamura D.; Striccoli M.; Terzano R.; Giannini C.; Irimia-Vladu M.; Suranna G. P. Exploring the surface chemistry of cesium lead halide perovskite nanocrystals. Nanoscale 2019, 11, 986–999. 10.1039/C8NR08011A. [DOI] [PubMed] [Google Scholar]
- a Koh W.; Park S.; Ham Y. Phosphonic Acid Stabilized Colloidal CsPbX3 (X = Br, I) Perovskite Nanocrystals and Their Surface Chemistry. Chemistry Select 2016, 1, 3479–3482. 10.1002/slct.201600809. [DOI] [Google Scholar]; b Wang C.; Chesman A. S.; Jasieniak J. J. Stabilizing the Cubic Perovskite Phase of CsPbI3 Nanocrystals by Using an Alkyl Phosphinic Acid. Chem. Commun. 2017, 53, 232–235. 10.1039/C6CC08282C. [DOI] [PubMed] [Google Scholar]; c Swarnkar A.; Marshall A. R.; Sanehira E. M.; Chernomordik B. D.; Moore D. T.; Christians J. A.; Chakrabarti T.; Luther J. M. Quantum dot–induced phase stabilization of a-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]; d Lu C.; Li H.; Kolodziejski K.; Dun C.; Huang W.; Carroll D.; Geyer S. M. Enhanced Stabilization of inorganic Cesium Lead Triiodide (CsPbI3) Perovskite Quantum Dots with Tri-Octylphosphine. Nano Res. 2018, 11, 762–768. 10.1007/s12274-017-1685-1. [DOI] [Google Scholar]; e Wu L.; Zhong Q.; Yang D.; Chen M.; Hu H.; Pan Q.; Liu H.; Cao M.; Xu Y.; Sun B.; Zhang Q. Improving the Stability and Size Tunability of Cesium Lead Halide Perovskite Nanocrystals Using Trioctylphosphine Oxide as the Capping Ligand. Langmuir 2017, 33, 12689–12696. 10.1021/acs.langmuir.7b02963. [DOI] [PubMed] [Google Scholar]; f Liu F.; Zhang Y.; Ding C.; Kobayashi S.; Izuishi T.; Nakazawa N.; Toyoda T.; Ohta T.; Hayase S.; Minemoto T.; Yoshino K.; Dai S.; Shen Q. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. 10.1021/acsnano.7b05442. [DOI] [PubMed] [Google Scholar]; g Di Stasio F.; Christodoulou S.; Huo N.; Konstantatos G. Near-Unity Photoluminescence Quantum Yield in CsPbBr3 Nanocrystal Solid-State Films via Postsynthesis Treatment with Lead Bromide. Chem. Mater. 2017, 29, 7663–7667. 10.1021/acs.chemmater.7b02834. [DOI] [Google Scholar]; h Koscher B. A.; Swabeck J. K.; Bronstein N. D.; Alivisatos A. P. Essentially Trap-Free CsPbBr3 Colloidal Nanocrystals by Postsynthetic Thiocyanate Surface Treatment. J. Am. Chem. Soc. 2017, 139, 6566–6569. 10.1021/jacs.7b02817. [DOI] [PubMed] [Google Scholar]; i Woo J. Y.; Kim Y.; Bae J.; Kim T. G.; Kim J. W.; Lee D. C.; Jeong S. Highly Stable Cesium Lead Halide Perovskite Nanocrystals through in Situ Lead Halide Inorganic Passivation. Chem. Mater. 2017, 29, 7088–7092. 10.1021/acs.chemmater.7b02669. [DOI] [Google Scholar]; j Almeida G.; Ashton O. J.; Goldoni L.; Maggioni D.; Petralanda U.; Mishra N.; Akkerman Q. A.; Infante I.; Snaith H. J.; Manna L. The Phosphine Oxide Route toward Lead Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 14878–14886. 10.1021/jacs.8b08978. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Krieg F.; Ochsenbein S. T.; Yakunin S.; ten Brinck S.; Aellen P.; Süess A.; Clerc B.; Guggisberg D.; Nazarenko O.; Shynkarenko Y.; Kumar S.; Shih C.-J.; Infante I.; Kovalenko M. V. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646. 10.1021/acsenergylett.8b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]; l Tan Y.; Zou Y.; Wu L.; Huang Q.; Yang D.; Chen M.; Ban M.; Wu C.; Wu T.; Bai S.; Song T.; Zhang Q.; Sun B. Highly Luminescent and Stable Perovskite Nanocrystals with Octylphosphonic Acid as a Ligand for Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 3784–3792. 10.1021/acsami.7b17166. [DOI] [PubMed] [Google Scholar]; m Dong Y.; Qiao T.; Kim D.; Parobek D.; Rossi D.; Son D. H. Precise Control of Quantum Confinement in Cesium Lead Halide Perovskite Quantum Dots via Thermodynamic Equilibrium. Nano Lett. 2018, 18, 3716–3722. 10.1021/acs.nanolett.8b00861. [DOI] [PubMed] [Google Scholar]; n Imran M.; Caligiuri V.; Wang M.; Goldoni L.; Prato M.; Krahne R.; De Trizio L.; Manna L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. 10.1021/jacs.7b13477. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Bohn B. J.; Tong Y.; Gramlich M.; Lai M. L.; Döblinger M.; Wang K.; Hoye R. L. Z.; Müller-Buschbaum P.; Stranks S. D.; Urban A. S.; Polavarapu L.; Feldmann J. Boosting Tunable Blue Luminescence of Halide Perovskite Nanoplatelets through Postsynthetic Surface Trap Repair. Nano Lett. 2018, 18, 5231–5238. 10.1021/acs.nanolett.8b02190. [DOI] [PubMed] [Google Scholar]; p Uddin M. A.; Mobley J. K.; Masud A. A.; Liu T.; Calabro R. L.; Kim D.-Y.; Richards C. I.; Graham K. R. Mechanistic Exploration of Dodecanethiol-Treated Colloidal CsPbBr3 Nanocrystals with Photoluminescence Quantum Yields Reaching Near 100%. J. Phys. Chem. C 2019, 123, 18103–18112. 10.1021/acs.jpcc.9b05612. [DOI] [Google Scholar]; q Imran M.; Ijaz P.; Goldoni L.; Maggioni D.; Petralanda U.; Prato M.; Almeida G.; Infante I.; Manna L. Simultaneous Cationic and Anionic Ligand Exchange For Colloidally Stable CsPbBr3 Nanocrystals. ACS Energy Lett. 2019, 4, 819–824. 10.1021/acsenergylett.9b00140. [DOI] [Google Scholar]
- Debuch H.Über die enzymatische Spaltung des Lecithins aus Sojabohnen. Hoppe-Seyler's Z. Physiol. Chem. 1957, 306, 279–286 10.1515/bchm2.1957.306.1-2.279 [DOI] [PubMed] [Google Scholar]
- a Zhang X.; Wang H. C.; Tang A. C.; Lin S. Y.; Tong H. C.; Chen C. Y.; Lee Y. C.; Tsai T. L.; Liu R. S. Robust and Stable Narrow-Band Green Emitter: An Option for Advanced Wide-Color-Gamut Backlight Display. Chem. Mater. 2016, 28, 8493–8497. 10.1021/acs.chemmater.6b04107. [DOI] [Google Scholar]; b Zhou Q.; Bai Z.; Lu W. G.; Wang Y.; Zou B.; Zhong H. In Situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights. Adv. Mater. 2016, 28, 9163–9168. 10.1002/adma.201602651. [DOI] [PubMed] [Google Scholar]; c Wang Y.; He J.; Chen H.; Chen J.; Zhu R.; Ma P.; Towers A.; Lin Y.; Gesquiere A. J.; Wu S. T.; Dong Y. Ultrastable, Highly Luminescent Organic-Inorganic Perovskite-Polymer Composite Films. Adv. Mater. 2016, 28, 10710–10717. 10.1002/adma.201603964. [DOI] [PubMed] [Google Scholar]; d Raja S. N.; Bekenstein Y.; Koc M. A.; Fischer S.; Zhang D.; Lin L.; Ritchie R. O.; Yang P.; Alivisatos A. P. Encapsulation of Perovskite Nanocrystals into Macroscale Polymer Matrices: Enhanced Stability and Polarization. ACS Appl. Mater. Interfaces 2016, 8, 35523–35533. 10.1021/acsami.6b09443. [DOI] [PubMed] [Google Scholar]; e Meyns M.; Peralvarez M.; Heuer-Jungemann A.; Hertog W.; Ibanez M.; Nafria R.; Genc A.; Arbiol J.; Kovalenko M. V.; Carreras J.; Cabot A.; Kanaras A. G. Polymer-Enhanced Stability of Inorganic Perovskite Nanocrystals and Their Application in Color Conversion LEDs. ACS Appl. Mater. Interfaces 2016, 8, 19579–19586. 10.1021/acsami.6b02529. [DOI] [PubMed] [Google Scholar]; f Guhrenz C.; Benad A.; Ziegler C.; Haubold D.; Gaponik N.; Eychmüller A. Solid-State Anion Exchange Reactions for Color Tuning of CsPbX3 Perovskite Nanocrystals. Chem. Mater. 2016, 28, 9033–9040. 10.1021/acs.chemmater.6b03980. [DOI] [Google Scholar]; g Huang H.; Chen B.; Wang Z.; Hung T. F.; Susha A. S.; Zhong H.; Rogach A. L. Water resistant CsPbX3 Nanocrystals Coated with Polyhedral Oligomeric Silsesquioxane and their Use as Solid State Luminophores in All-Perovskite White Light-Emitting Devices. Chem. Sci. 2016, 7, 5699–5703. 10.1039/C6SC01758D. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Xu L.; Chen J.; Song J.; Li J.; Xue J.; Dong Y.; Cai B.; Shan Q.; Han B.; Zeng H. Double-Protected All-Inorganic Perovskite Nanocrystals by Crystalline Matrix and Silica for Triple-Modal Anti-Counterfeiting Codes. ACS Appl. Mater. Interfaces 2017, 9, 26556–26564. 10.1021/acsami.7b06436. [DOI] [PubMed] [Google Scholar]; i Li Z.; Kong L.; Huang S.; Li L. Highly Luminescent and Ultrastable CsPbBr3 Perovskite Quantum Dots Incorporated into a Silica/Alumina Monolith. Angew. Chem., Int. Ed. 2017, 56, 8134–8138. 10.1002/anie.201703264. [DOI] [PubMed] [Google Scholar]; j Quan L. N.; Quintero-Bermudez R.; Voznyy O.; Walters G.; Jain A.; Fan J. Z.; Zheng X.; Yang Z.; Sargent E. H. Highly Emissive Green Perovskite Nanocrystals in a Solid State Crystalline Matrix. Adv. Mater. 2017, 29, 1605945–1605951. 10.1002/adma.201605945. [DOI] [PubMed] [Google Scholar]; k Gonzalez-Pedro V.; Veldhuis S. A.; Begum R.; Banuls M. J.; Bruno A.; Mathews N.; Mhaisalkar S.; Maquieira A. Recovery of Shallow Charge-Trapping Defects in CsPbX3 Nanocrystals through Specific Binding and Encapsulation with AminoFunctionalized Silanes. ACS Energy Lett. 2018, 3, 1409–1414. 10.1021/acsenergylett.8b00498. [DOI] [Google Scholar]; l Zhou Y. Y.; Zhao Y. X. Chemical Stability and Instability of Inorganic Halide Perovskites. Energy Environ. Sci. 2019, 12, 1495–1511. 10.1039/C8EE03559H. [DOI] [Google Scholar]
- a Chen H.; He J.; Wu S.-T. Recent Advances on Quantum-Dot-Enhanced Liquid-Crystal Displays. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–11. 10.1109/JSTQE.2017.2649466. [DOI] [Google Scholar]; b Ko Y. H.; Jalalah M.; Lee S. J.; Park J. G. Super Ultra-High Resolution Liquid-Crystal-Display Using Perovskite Quantum-Dot Functional Color-Filters. Sci. Rep. 2018, 8, 12881–2888. 10.1038/s41598-018-30742-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wei Y.; Cheng Z. Y.; Lin J. An Overview on Enhancing the Stability of Lead Halide Perovskite Quantum Dots and their Applications in Phosphor-Converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. 10.1039/C8CS00740C. [DOI] [PubMed] [Google Scholar]
- Yin Y.; Alivisatos A. P. Colloidal Nanocrystal Synthesis and the Organic-Inorganic Interface. Nature 2005, 437, 664–670. 10.1038/nature04165. [DOI] [PubMed] [Google Scholar]
- a De Gennes P. G. Polymer Solutions near an Interface. 1. Adsorption and Depletion Layers. Macromolecules 1981, 14, 1637–1644. 10.1021/ma50007a007. [DOI] [Google Scholar]; b De Gennes P. G. Polymers at an Interface. 2. Interaction between two Plates Carrying Adsorbed Polymer Layers. Macromolecules 1982, 15, 492–500. 10.1021/ma00230a055. [DOI] [Google Scholar]; c De Gennes P. G. Polymers at an Interface: A Simplified View. Adv. Colloid Interface Sci. 1987, 27, 189–209. 10.1016/0001-8686(87)85003-0. [DOI] [Google Scholar]
- Milner S. T. Polymer Brushes. Science 1991, 251, 905–914. 10.1126/science.251.4996.905. [DOI] [PubMed] [Google Scholar]
- Milner S. T.; Witten T. A.; Cates M. E. Effects of Polydispersity in the End-Grafted Polymer Brush. Macromolecules 1989, 22, 853–861. 10.1021/ma00192a057. [DOI] [Google Scholar]
- a Milner S. T.; Witten T. A.; Cates M. E. A Parabolic Density Profile for Grafted Polymers. Europhys. Lett. 1988, 5, 413–418. 10.1209/0295-5075/5/5/006. [DOI] [Google Scholar]; b Milner S. T.; Witten T. A.; Cates M. E. Theory of the Grafted Polymer Brush. Macromolecules 1988, 21, 2610–2619. 10.1021/ma00186a051. [DOI] [Google Scholar]
- a Yang Y.; Qin H.; Peng X. Intramolecular Entropy and Size-Dependent Solution Properties of Nanocrystal-Ligands Complexes. Nano Lett. 2016, 16, 2127–2132. 10.1021/acs.nanolett.6b00737. [DOI] [PubMed] [Google Scholar]; b Yang Y.; Qin H.; Jiang M.; Lin L.; Fu T.; Dai X.; Zhang Z.; Niu Y.; Cao H.; Jin Y.; Zhao F.; Peng X. Entropic Ligands for Nanocrystals: From Unexpected Solution Properties to Outstanding Processability. Nano Lett. 2016, 16, 2133–2138. 10.1021/acs.nanolett.6b00730. [DOI] [PubMed] [Google Scholar]
- a Chakrabarti A.; Nelson P.; Toral R. Interpenetrations in Polymer Brushes. J. Chem. Phys. 1994, 100, 748–749. 10.1063/1.466945. [DOI] [Google Scholar]; b Israelachvili J. N.Repulsive “Steric” or “Overlap” Forces between Polymer-Covered Surfaces. Intramolecular and surface forces, 3rd ed.; Elsevier, 2011; pp 387–393. [Google Scholar]
- Pang Z.; Zhang J.; Cao W.; Kong X.; Peng X. Partitioning Surface Ligands on Nanocrystals for Maximal Solubility. Nat. Commun. 2019, 10, 2454–2462. 10.1038/s41467-019-10389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti F.; Protesescu L.; Kovalenko M. V.; Yakunin S.; Cervellino A.; Billinge S. J. L.; Terban M. W.; Pedersen J. S.; Masciocchi N.; Guagliardi A. Coherent Nanotwins and Dynamic Disorder in Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2017, 11, 3819–3831. 10.1021/acsnano.7b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Murray C. B.; Norris D. J.; Bawendi M. G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. 10.1021/ja00072a025. [DOI] [Google Scholar]; b Guzelian A. A.; Katari J. E. B.; Kadavanich A. V.; Banin U.; Hamad K.; Juban E.; Alivisatos A. P.; Wolters R. H.; Arnold C. C.; Heath J. R. Synthesis of Size-Selected, Surface-Passivated InP Nanocrystals. J. Phys. Chem. 1996, 100, 7212–7219. 10.1021/jp953719f. [DOI] [Google Scholar]; c Poznyak S. K.; Osipovich N. P.; Shavel A.; Talapin D. V.; Gao M.; Eychmüller A.; Gaponik N. Size-Dependent Electrochemical Behavior of Thiol-Capped CdTe Nanocrystals in Aqueous Solution. J. Phys. Chem. B 2005, 109, 1094–1100. 10.1021/jp0460801. [DOI] [PubMed] [Google Scholar]
- Sercel P. C.; Lyons J. L.; Wickramaratne D.; Vaxenburg R.; Bernstein N.; Efros A. L. Exciton Fine Structure in Perovskite Nanocrystals. Nano Lett. 2019, 19, 4068–4077. 10.1021/acs.nanolett.9b01467. [DOI] [PubMed] [Google Scholar]
- a Park Y. S.; Guo S.; Makarov N. S.; Klimov V. I. Room Temperature Single-Photon Emission from Individual Perovskite Quantum Dots. ACS Nano 2015, 9, 10386–10393. 10.1021/acsnano.5b04584. [DOI] [PubMed] [Google Scholar]; b Utzat H.; Shulenberger K. E.; Achorn O. B.; Nasilowski M.; Sinclair T. S.; Bawendi M. G. Probing Linewidths and Biexciton Quantum Yields of Single Cesium Lead Halide Nanocrystals in Solution. Nano Lett. 2017, 17, 6838–6846. 10.1021/acs.nanolett.7b03120. [DOI] [PubMed] [Google Scholar]
- a Carney R. P.; Kim J. Y.; Qian H.; Jin R.; Mehenni H.; Stellacci F.; Bakr O. M. Determination of Nanoparticle Size Distribution Together with Density or Molecular Weight by 2D Analytical Ultracentrifugation. Nat. Commun. 2011, 2, 335–343. 10.1038/ncomms1338. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Demeler B.; Nguyen T. L.; Gorbet G. E.; Schirf V.; Brookes E. H.; Mulvaney P.; El-Ballouli A. O.; Pan J.; Bakr O. M.; Demeler A. K.; Hernandez Uribe B. I.; Bhattarai N.; Whetten R. L. Characterization of size, anisotropy, and density heterogeneity of nanoparticles by sedimentation velocity. Anal. Chem. 2014, 86, 7688–95. 10.1021/ac501722r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Walter J.; Gorbet G.; Akdas T.; Segets D.; Demeler B.; Peukert W. 2D analysis of polydisperse core-shell nanoparticles using analytical ultracentrifugation. Analyst 2017, 142, 206–217. 10.1039/C6AN02236G. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Planken K. L.; Colfen H. Analytical ultracentrifugation of colloids. Nanoscale 2010, 2, 1849–69. 10.1039/c0nr00215a. [DOI] [PubMed] [Google Scholar]
- Schuck P. Size-Distribution Analysis of Macromolecules by Sedimentation Velocity Ultracentrifugation and Lamm Equation Modeling. Biophys. J. 2000, 78, 1606–1619. 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hammersley A. P.; Svensson S. O.; Thompson A.; Graafsma H.; Kvick Å.; Moy J. P. Calibration and Correction of Distortions in Two-Dimensional Detector Systems. Rev. Sci. Instrum. 1995, 66, 2729–2733. 10.1063/1.1145618. [DOI] [Google Scholar]; b Glatter O. Determination of Particle-size Distribution Functions from Small-Angle Scattering Data by means of the Indirect Transformation Method. J. Appl. Crystallogr. 1980, 13, 7–11. 10.1107/S0021889880011429. [DOI] [Google Scholar]
- a Burian M.; Fritz-Popovski G.; He M.; Kovalenko M. V.; Paris O.; Lechner R. T. Considerations on the Model-Free Shape Retrieval of Inorganic Nanocrystals from Small-Angle Scattering Data. J. Appl. Crystallogr. 2015, 48, 857–868. 10.1107/S1600576715006846. [DOI] [Google Scholar]; b Burian M.; Amenitsch H. Dummy-atom modelling of stacked and helical nanostructures from solution scattering data. IUCrJ 2018, 5, 390–401. 10.1107/S2052252518005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kim H.-S.; Lee C.-R.; Im J.-H.; Lee K.-B.; Moehl T.; Marchioro A.; Moon S.-J.; Humphry-Baker R.; Yum J.-H.; Moser J. E.; Grätzel M.; Park N.-G. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. 10.1038/srep00591. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Im J. H.; Lee C. R.; Lee J. W.; Park S. W.; Park N. G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. 10.1039/c1nr10867k. [DOI] [PubMed] [Google Scholar]
- Mooney M. The Viscosity of a Concentrated Suspension of Spherical Particles. J. Colloid Sci. 1951, 6, 162–170. 10.1016/0095-8522(51)90036-0. [DOI] [Google Scholar]
- Yuan G.; Ritchie C.; Ritter M.; Murphy S.; Gómez D. E.; Mulvaney P. The Degradation and Blinking of Single CsPbI3 Perovskite Quantum Dots. J. Phys. Chem. C 2018, 122, 13407–13415. 10.1021/acs.jpcc.7b11168. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.