Abstract
Objective:
To determine whether an intervention to reduce eveningness chronotype improves sleep, circadian, and health (emotional, cognitive, behavioral, social, physical) outcomes.
Method:
Youth aged 10 to 18 years with an evening chronotype and who were “at risk” in 1 of 5 health domains were randomized to: (a) Transdiagnostic Sleep and Circadian Intervention for Youth (TranS-C; n = 89) or (b) Psychoeducation (PE; n = 87) at a university-based clinic. Treatments were 6 individual, weekly 50-minute sessions during the school year. TranS-C addresses sleep and circadian problems experienced by youth by integrating evidence-based treatments derived from basic research. PE provides education on the interrelationship between sleep, stress, diet, and health.
Results:
Relative to PE, TranS-C was not associated with greater pre–post change for total sleep time (TST) or bed time (BT) on weeknights but was associated with greater reduction in evening circadian preference (pre-post increase of 3.89 points, 95% CI = 2.94–4.85, for TranS-C, and 2.01 points, 95% CI = 1.05–2.97 for PE, p = 0.006), earlier endogenous circadian phase, less weeknight–weekend discrepancy in TST and wakeup time, less daytime sleepiness, and better self-reported sleep via youth and parent report. In terms of functioning in the five health domains, relative to PE, TranS-C was not associated with greater pre–post change on the primary outcome. However, there were significant interactions favoring TranS-C on the Parent-Reported Composite Risk Scores for cognitive health.
Conclusion:
For at-risk youth, the evidence supports the use of TranS-C over PE for improving sleep and circadian functioning, and improving health on selected outcomes.
Clinical trial registration information:
Triple Vulnerability? Circadian Tendency, Sleep Deprivation and Adolescence. (https://clinicaltrials.gov; NCT01828320.
Keywords: sleep, circadian, risk, treatment
Evening chronotype adolescents (“night-owls”) follow a delayed sleep–wake schedule, compared to morning chronotypes (“larks”).1 The onset of puberty triggers an evening preference among approximately 40% of youth,2,3 which is compounded by social changes (eg, less parental control, technology). Eveningness, particularly among youth who have an early school start time, results in sleep deprivation.2,3 This pattern is of concern because sleep is crucial for brain development.4 Although the biological shift toward eveningness during puberty may be difficult to modify, we test the hypothesis that psychosocial, behavioral, and cognitive contributors are modifiable.
Eveningness is associated with adverse consequences across five health domains. In the emotional domain, eveningness is associated with depression, anxiety, and attention problems,5 emotional instability,6 and suicidality.6 In the cognitive domain, it is associated with problems at school7,8 and on cognitive assessments.9 In the behavioral domain, it is associated with the use of caffeine, alcohol, and nicotine at higher amounts,10,11 impulsivity,12 substance use,13 and poorer self-regulation.14 In the social domain, it is associated with aggression, antisocial behavior, and rule breaking.9,15 Finally, in the physical domain, it is associated with less exercise,16 obesity, and inflammation.17,18 Longitudinal studies indicate that eveningness predicts and predates heightened risk.19–21 Despite occasional nonreplications,22,23 the combined picture is alarming. Indeed, the sleep–risk pathway has been identified as a priority in adolescent health.24
Our goal was to modify the psychosocial, behavioral and cognitive processes that contribute to eveningness and a broad range of other sleep and circadian problems often associated with eveningness (eg, insomnia) via the Transdiagnostic Sleep and Circadian Intervention for Youth (TranS-C).25 Targeting research and treatment at a transdiagnostic process is a relatively new approach,26–28 and sleep and circadian problems are plausible transdiagnostic processes.29 Also, prior youth sleep treatment studies have tended to be disorder focused, in that they have treated a specific sleep problem (eg, insomnia) in a specific diagnostic group (eg, depression). However, real life sleep and circadian problems are not so neatly categorized. TranS-C aims to address the need for one short protocol (comprising six sessions) that addresses a range of the most common real-life sleep and circadian problems. TranS-C is transdiagnostic in two senses: it is designed to address the common core of a range sleep and circadian problems, and to be useful for the sleep and circadian problems that are common across health domains.
In the present study, adolescents were selected for reporting an evening chronotype and falling into an “at risk” range on measures of at least one of the five health domains reviewed above (emotional, cognitive, behavioral, social, or physical). Participants were randomly allocated to either (a) TranS-C (n = 89) or (b) Psychoeducation (PE) (n = 87). TranS-C, relative to PE, was hypothesized to improve sleep and circadian function, advance the timing of the endogenous circadian phase via the dim light melatonin onset protocol (DLMO), and decrease risk across the five health domains (emotional, cognitive, behavioral, social, and physical).
METHOD
Study Design
Based in a university clinic, from March 2013 to March 2016, youth were randomly assigned, stratified by sex and age (10–14 years, 15–18 years), in a 1:1 parallel group design, to receive either TranS-C or PE. Sibling pairs (n = 3) were randomized to the same condition.
Assessors were blinded to treatment allocation. Sequentially numbered, opaque, sealed envelopes were generated using a computer-generated random number list. A project coordinator conducted randomization after all eligibility assessments were completed. Assessments were conducted at baseline and at the end of treatment. The Committee for the Protection of Human Subjects approved the study.
Participants
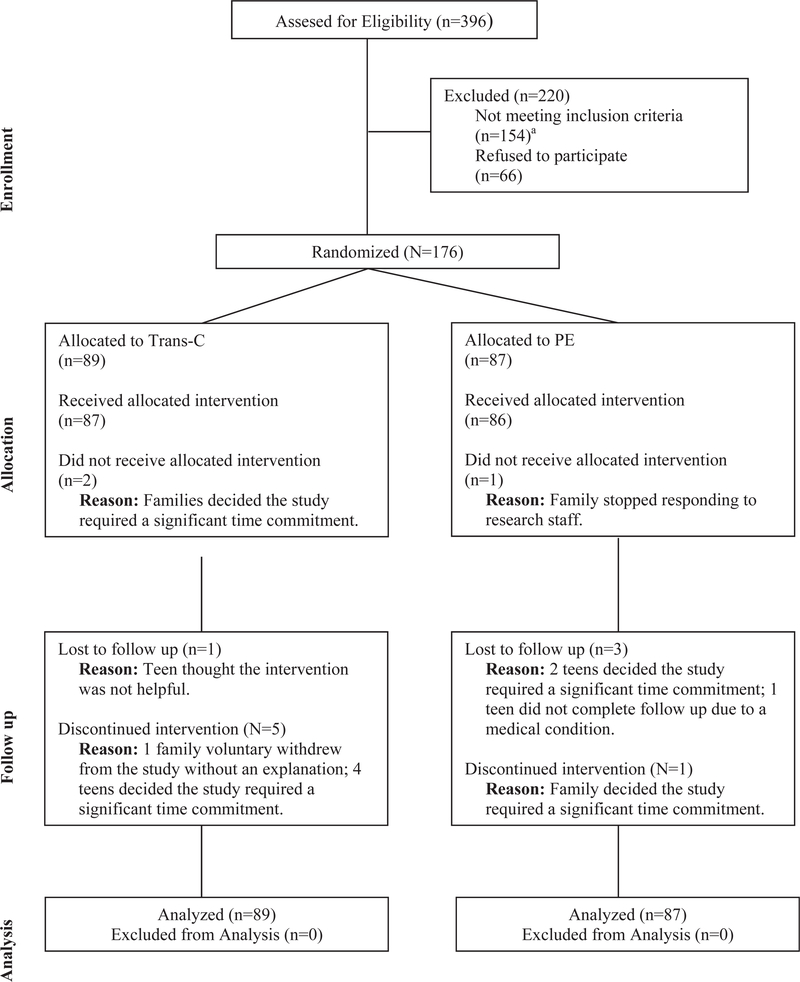
Participant flow is illustrated in Figure 1. Participants included 176 youth recruited through clinicians or advertisements.
FIGURE 1.
Consolidated Standards Of Reporting Trials (CONSORT) Diagram, Illustrating the Flow of Participants Through the Study
Note: aOf 154 individuals who were ineligible, 87 did not meet criteria for eveningness chronotype, 6 had eveningness chronotype but no risk, and 61 did not meet criteria for inclusion because of medical reasons, substance use, suicidality, trauma, or other.
Individuals were eligible if they (a) were between 10 and 18 years old, living with a parent or guardian, and attending a class/job by 9 am at least 3 days per week; (b) were fluent in English; (c) were able and willing to give informed assent; and (d) reported eveningness as demonstrated by scoring within the lowest quartile of the Children’s Morningness–Eveningness Preferences Scale (CMEP; 27 or lower) and had a 7-day sleep diary showing a sleep onset time of 10:40 pm or later for 10- to 13-year- olds, 11:00 pm or later for 14- to 16-year-olds, and 11:20 pm or later for 17- to 18-year-olds at least 3 nights per week.30,31 In addition, this sleep pattern had to have been present for the past 3 months. Finally, participants had to fall into an “at-risk” range on measures of at least one of the five health domains (Table 1).32–39
TABLE 1.
Inclusion Criteria Operationalizing “At Risk” for the Five Health Domains
| Risk Domain | Criteria for Inclusion |
|---|---|
| Emotional | ≥4 on any of the following items on the CDRS: Difficulty Having Fun, Social Withdrawal, Irritability, Depressed Feelings, Excessive Weeping, or a T-score of 61 or above on the MASC-10, based on age group (10–11 years, 12–15 years, or 16–19 years) using the MASC-10 Profile |
| Behavioral | A SSS score >3.93 for males aged 10–13 years, >3.19 for females aged 10–13 years, >4.07 for males aged 14–18 years, or >3.19 for females aged 14–18 years or taking ADHD medication or the K-SADS indicating a diagnosis of ADHD or current alcohol or substance abuse assessed with the K-SADS |
| Social | A parent rating their child as “worse” than others the participant’s age on one or more of the social behavior items (Section VI) from the CBCL |
| Cognitive | A parent rating their child as “failing” in one or more academic classes from CBCL Section VII |
| Physical | A score of ≥4 on the PHQ-15, ≥6 days of school absences, or a BMI above the 85th percentile for the participant’s sex and age |
Note: ADHD = attention-deficit/hyperactivity disorder; BMI = body mass index (the cutoff corresponds to 1 SD above the mean); CBCL = Child Behavior Checklist,32 which asks the parent if their child does “worse than,” “average,” or “better than” other teens their age or if the teen is “failing,” “below average,” “average,” or “above average”; CDRS = Child Depression Rating Scale33 (the cutoff is commensurate with “clinical symptoms”)34; K-SADS = Schedule for Affective Disorders and Schizophrenia for School-Age Children35; MASC-10 = Multidimensional Anxiety Scale for Children (the cutoff T score was selected to capture the “slightly elevated” through to the “very elevated” range)36; PHQ-15 = Physical Health Ques- tionnaire-15 (the cutoff corresponds to “minimal somatic symptom severity” through to the “high somatic symptom severity” range)37; SSS = Sensation Seeking Scale38 (the cutoff correspond to at or above 1 SD over the normative average).39
Exclusion criteria were (a) an active, progressive physical illness or neurological degenerative disease directly related to the onset and course of the sleep disturbance; (b) evidence of obstructive sleep apnea, restless legs syndrome, or periodic limb movement disorder (youth presenting with provisional diagnoses of any of these disorders were referred for a nonstudy polysomnography evaluation at the parent’s discretion and were enrolled only if the diagnosis was disconfirmed); (c) significantly impairing pervasive developmental disorder; (d) bipolar disorder, schizophrenia, or another current Axis I disorder if there was a risk of harm if treatment were delayed. Participants ceased taking medications that alter sleep (eg, hypnotics) 4 weeks prior to the assessment (2 weeks for melatonin) or were excluded. Finally, history of substance dependence in the past 6 months or current suicide risk sufficient to preclude treatment on an outpatient basis was exclusionary.
In other words, receipt of another sleep treatment was the only type of treatment excluded. We wanted to be sure that any improvements in sleep could be attributed to TranS-C or PE, not the other sleep treatment. Other medications were allowed. A medication-free group would have been nonrepresentative. We also included youth with Axis I psychiatric comorbidity (except for bipolar disorder and schizophrenia), even if they were receiving treatment for that comorbidity.
Treatments
Therapists were doctoral or master’s level. Weekly super- vision was conducted by AGH for TranS-C and by NZ and AGH for PE. Sessions were audio recorded. Treatment integrity was evaluated by AGH for TranS-C with the Cognitive Therapy Rating Scale (CTRS)40 and by NZ for PE on a scale of 1 to 100, on which higher scores were awarded for alliance building, reflective listening, and providing PE without emphasizing behavior change.
A checklist of elements specific to TranS-C and PE was used to rate presence/absence and focus on behavior change (a distinguishing feature of the treatments) for a random subset of tapes (49 TranS-C, 58 PE). A total of 182 TranS-C elements were coded in TranS-C, relative to 16 TranS-C elements in PE. In all, 77 PE elements were coded in PE, relative to 20 PE elements in TranS-C sessions. In TranS-C, 88 instances of actions taken by the therapist to promote behavior change were present with 0 instances in PE.
Treatment involved 6 individual, weekly, 50-minute sessions delivered during the school year to minimize the impact of summer schedule variability.41 A total of 149 participants (85%) attended all 6 sessions (18% had 1 double session to complete treatment within the school semester).
Transdiagnostic Sleep and Circadian Intervention for Youth
Transdiagnostic Sleep and Circadian Intervention for Youth (TranS-C)25 is grounded in sleep and circadian basic science and combines elements from four evidence-based interventions: Cognitive Behavior Therapy for Insomnia,42–44 Interpersonal and Social Rhythm Therapy,45 Chronotherapy,46 and Motivational Interviewing.47 TranS-C is a modular approach that allows the treatment sessions to be focused on the specific sleep problem experienced by each patient. The goal is to reverse maintaining psychosocial, behavioral and cognitive processes via four cross-cutting modules, four core modules, and seven optional modules (also see Supplement 1, available online). The four Cross Cutting Modules are as follows: case formulation; education; behavior change and motivation; goal setting. The four Core Modules are as follows: establishing regular sleep–wake times including learning a wind-down and wake-up routine; improving daytime functioning; correcting unhelpful sleep-related beliefs; and maintenance of behavior change. The Optional Modules are as follows: improving sleep efficiency; reducing time in bed; dealing with delayed or advanced phase; reducing sleep-related worry/vigilance; promoting compliance with continuous positive airway pressure or exposure therapy for claustrophobic reactions to continuous positive airway pressure; and negotiating sleep in a complicated environment and reducing nightmares.
Psychoeducation (PE) is an active comparison treatment associated with sleep improvement.48 Sessions focus on the interrelationship among sleep, stress, diet, health, exercise, accidents, and mood. Participants were also given the choice of sampling meditation, yoga, and/or outdoors appreciation. The emphasis was on providing information but not on specifically facilitating behavior change (also see Supplement 1, available online).
Measures
Except where specified, assessments were administered before and after treatment. Assessments were audio recorded. A random subset (10%) were reviewed. Interrater reliabilities were as follows: Schedule for Affective Disorders and Schizophrenia (K-SADS) diagnosis: κ= 0.78; Child Depression Rating Scale (CDRS)-Parent: κ = 0.46; CDRS-Teen: κ = 0.56). More detailed procedures are available in Supplement 2, available online.
Diagnosis
The K-SADS35 was administered separately to youth and one parent/caregiver to assess for participant current and lifetime Axis I disorders at the pretreatment assessment.
Sleep Outcomes
Sleep Diary.
An a priori decision was made to investigate weeknight average total sleep time (TST) and bedtime (BT) average as primary outcomes to best capture the sleep problems of interest.2 Secondary outcomes were the discrepancy between weeknight and weekends for TST, BT, and waketime (WUP). A sleep diary was used.49 Calculation of sleep diary variables is described in Table S1, available online.
Other secondary outcomes were scores on the Sleepiness Scale,50 Pittsburgh Sleep Quality Index (PSQI),51,52 and CBCL Sleep Composite (parent report).32
Circadian Outcomes
Children’s Morningness-Eveningness Preferences Scale (CMEP)53 was a primary outcome. Scores range from 10 (Extreme evening preference) to 43 (Extreme morning preference).
Dim Light Melatonin Onset (DLMO) was a secondary outcome. DLMO is the gold standard index of the endogenous circadian phase.54 It is assessed with the serial saliva sampling method one night before and after treatment in an overnight stay in the Psychology Department at UC Berkeley based on established protocol.55 Thirteen saliva samples were collected for each participant before and after treatment, beginning 5.5 hours before average bedtime (computed from 7 nights of sleep diary) and ending 30 minutes after average bedtime. Saliva (~1 ml) samples were collected in 30-minute intervals in dim light (<50 lux) using untreated Salivettes (Sardtedt; Nümbrecht, Germany) and assayed for melatonin (SolidPhase, Inc.; Portland, Maine) using radioimmunoassay test kits (APLCO Diagnostics, Windham, NH). DLMO was defined as the interpolated time at which melatonin exceeded 3.0 pg/ml. The selection of this threshold was based upon prior experience with melatonin as a marker of circadian phase and the visual inspection of each participant’s DLMO record.55 Saliva samples were collected from all randomized participants. There were 15 (8.5%) and 29 (16.5%) participants with missing DLMO data at pretreatment and posttreatment, respectively. Further detail is provided in Supplement 2, available online.
Functioning in Five Health Domains
For each of the five health domains (ie, emotional, cognitive, behavioral, social, and physical health), three clusters of composite scores were calculated using the cumulative risk index.56,57 The first cluster is the Youth Self-Report Composite Risk Score (primary), which was derived from psychometrically validated questionnaires representing each of the five health domains (Supplement 2, available online, lists the questionnaires). The second cluster is the Youth Ecological Momentary Assessment (EMA) Composite Risk Score, which was derived from telephone calls twice per day on weekdays and four times per day on weekends. The questions asked during the calls were adapted from Silk et al.58 The rationale for including EMA was to index “real world” functioning in each of the five health domains. The third cluster is the Parent-Reported Composite Risk Score, comprising a composite score of parent responses to CBCL subscales representing the five health domains. Further detail on the calculation of these three clusters of composite scores is provided in Supplement 2, available online.
A Medications Tracking Log was completed.
A credibility/expectancy questionnaire (CEQ)59 was administered at the end of the second therapy session to index expectation of improving and credibility of treatment.
Timeline for Assessments and Treatment
Adolescents and parents/guardians were screened via telephone. Eligible adolescents completed a sleep diary for 7 nights to ascertain the presence of eligibility critiera (d). If met, an in-person assessment was conducted during which the K-SADS and questionnaires were completed. If the participant continued to meet criteria, the activities from this point forward were conducted within the school semester because holiday schedules differ markedly during adolescence.41
To determine DLMO collection times and wake-up times, an additional 7 nights of sleep diary were collected immediately prior to the overnight stay in the laboratory.
After the overnight stay, the participant was randomized and completed a further 7-night sleep diary and answered phone calls to collect Ecological Momentary Assessment (EMA) data. This additional sleep diary was collected so that sleep diary data were concurrent with EMA. These measures were the basis of the primary and secondary outcomes. Compensation is described in Supplement 3, available online. Treatment commenced 1 week later.
After treatment, the procedures for the overnight assessment to collect DLMO were repeated, along with an in-person interview and 7 days of EMA and sleep diary.
Data Analysis
Sample size was determined via power analysis. G*Power 3.1 was used to estimate sample size using Cohen’s d = 0.48 for primary outcomes, assuming significance of 0.05 and power of at least 80%. A total of 69 participants were needed for each condition. The final recruitment allowed for at least 20% more for potential attrition (more detail in Supplement 4, available online). There were no interim analyses. The final sample size for the analysis was 176. To reach the target number of participants for each semester, we had to recruit more eligible participants than we needed, because participants sometimes decided not to participate during the eligibility assessment. Therefore, when recruiting the final cohort, we allowed extra eligible participants to proceed, all of whom decided to participate. Hence, the total sample size is larger than indicated by the power analysis.
Data analysis was conducted in Stata 14 (StataCorp, College Station, TX). All analyses were adjusted for age and sex, which were the stratification factors used during randomization. Using intent-to-treat,60 multilevel modeling with maximum likelihood estimation with the assumption of missing at random was used to examine continuous outcomes. The fixed component of the model included stratification factors (age and sex), an indicator for time period (Time = 0 pretreatment, Time = 1 posttreatment), an indicator for treatment condition (Treatment = 1 TranS-C, Treatment = 0 PE), and a Time by Treatment interaction term. The random part of the model included a subject-specific random intercept and a time- and subject-specific error term. The treatment effect of interest was the interaction, representing the difference in mean change from pretreatment to posttreatment between TranS-C and PE. The model also provided estimates of the mean pre–post change in the PE condition (coefficient of Time) and in the TranS-C condition (coefficient of Time plus interaction coefficient), and the significance of these changes was also reported regardless of the significance of the interaction because PE may be an active control and can be beneficial. Using Hochberg’s61 procedure, the outcomes are considered two subfamilies of analyses. The error rate in each subfamily was controlled under 0.025 using the Hochberg’s step-up procedure, so that the overall family wise error rate did not exceed 0.05.
In all, 27 participants (15%) completed their pretreatment assessment prior to starting the school semester. Of these, 13 participants did not continue to meet eligibility critiera (d) once the semester started. The analyses included these participants, as there was no change to the findings when they were excluded.
RESULTS
Participant Characteristics
There were no differences in demographic or clinical characteristics between treatment groups at pretreatment (Table 2). There were no significant differences between participants randomized versus excluded for age (p = 0.27) or sex (p = 0.19). There was no group difference in the rate of dropouts during treatment (TranS-C =7.9%, PE = 2.3%; p = 0.09) or follow-up (TranS-C = 1.1%, PE = 3.4%; p = 0.30). The two treatment groups also did not differ with respect to time between treatment sessions (p = 0.34), engagement in other types of treatment (p = 0.25), and season in which treatment was provided (p = 0.69).
TABLE 2.
Baseline Demographic and Clinical Characteristics of Patients Treated with Transdiagnostic Sleep and Circadian Intervention (TranS-C) and Psychoeducation (PE)
| TranS-C (n = 89) |
PE (n = 187) |
|||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Female | 49 | 55 | 53 | 61 |
| Ethnicity | ||||
| Hispanic or Latino | 14 | 16 | 13 | 15 |
| Not Hispanic or Latino | 75 | 84 | 74 | 85 |
| Race (adolescent) | ||||
| White | 58 | 65 | 56 | 64 |
| African American/Black | 4 | 4 | 8 | 9 |
| American Indian or Alaskan Native | 0 | 0 | 0 | 0 |
| Asian | 11 | 12 | 7 | 8 |
| Native Hawaiian or Other Pacific Islander | 2 | 2 | 0 | 0 |
| Refused to answer | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 | 0 |
| Mixed race | 14 | 16 | 16 | 18 |
| Family annual income ($) | ||||
| ≤20,000 | 2 | 2 | 4 | 5 |
| 20,001–50,000 | 11 | 12 | 10 | 11 |
| 50,001–100,000 | 26 | 29 | 16 | 18 |
| 100,000 + | 47 | 53 | 55 | 63 |
| Refused to answer/missing | 3 | 3 | 2 | 2 |
| Current grade (at baseline) | ||||
| 5 | 4 | 4 | 1 | 1 |
| 6 | 4 | 4 | 3 | 3 |
| 7 | 6 | 7 | 8 | 9 |
| 8 | 11 | 12 | 14 | 16 |
| 9 | 16 | 18 | 12 | 14 |
| 10 | 22 | 25 | 24 | 28 |
| 11 | 13 | 15 | 12 | 14 |
| 12 | 12 | 13 | 13 | 15 |
| College | 1 | 1 | ||
| Any current K-SADS Diagnosis (teen report) | 34/87a | 39 | 29/84a | 35 |
| Any past K-SADS Diagnosis (teen report) | 40/86 | 47 | 37/85 | 44 |
| Any current K-SADS Diagnosis (parent report) | 21/85 | 25 | 28/83 | 34 |
| Any past K-SADS Diagnosis (parent report) | 24/84 | 29 | 31/84 | 37 |
| Mean | SD | Mean | SD | |
| Age (y) | 14.76 | 1.94 | 14.78 | 1.74 |
Note: K-SADS = Schedule for Affective Disorders and Schizophrenia for School-Age Children.
The values 82 and 78 are the total sample without missing on this variable. TranS-C and PE did not differ on any baseline demographic and clinical characteristics.
Medications
At study entry, medication use was as follows: antidepressants, n = 19 (11%); stimulants, n = 22 (13%); and antipsychotics, n = 1 (1%). The doses of 70% of antidepressants, 73% of stimulants, and 100% of antipsychotics remained stable across treatment. There were no group differences in discontinuation during the treatment phase (TranS-C = 0%; PE = 1%). There was no between-group difference on medication dosage change during the treatment phase (p = 0.24).
Sleep Outcomes
Sleep outcomes data are provided in Table 362 and in Table S2, available online. There was no Treatment by Time interaction for TST or BT on weeknights. However, TranS-C yielded longer TST on weeknights at posttreatment relative to pretreatment, and there was no pre–post difference for PE. There was no pre–post change in BT on weeknights for either treatment.
TABLE 3.
Raw Means and SDs as Well as Treatment Effects for Primary and Secondary Sleep and Circadian Outcomes for Transdiagnostic Sleep and Circadian Intervention (TranS-C) (n = 89) vs. Psychoeducation (PE) (n = 87) From Baseline to Posttreatment
| TranS-C |
PE |
Treatment by Time Interaction |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Posttreatment |
Pre–Post Change |
Baseline |
Posttreatment |
Pre–Post Change |
|||
| Outcome | Mean (SD) | Mean (SD) | Coeff. (95% CI) | Mean (SD) | Mean (SD) | Coeff. (95% CI) | P | d |
| Sleep and Circadian Outcomes | ||||||||
| SD-TST weeknightsa | 459.06 (64.92) | 482.76 (82.55) | 24.72 (7.81, 41.63) | 454.96 (61.51) | 464.81 (76.01) | 13.02 (−4.03, 30.07) | .34 | −0.08 |
| SD-BT weeknightsa | 22.87 (1.07) | 22.85 (0.98) | −0.03 (−0.22, 0.15) | 22.99 (1.05) | 23.04 (1.12) | −0.05 (−0.23, 0.14) | .93 | −0.06 |
| SD-TST weeknight—weekend discrepancy | −70.39 (113.10) | −31.16 (115.19) | 39.43 (9.03, 69.84) | −48.91 (89.28) | −56.46 (106.25) | −8.67 (−38.74, 21.40) | .03 | −0.44 |
| SD-BT weeknight—weekend discrepancy | −0.79 (1.23) | −0.68 (1.41) | 0.12 (−0.25, 0.48) | −0.58 (1.13) | −0.51 (1.09) | 0.07 (−0.29, 0.43) | .85 | −0.11 |
| SD-WUP weeknight—weekend discrepancy | −1.90 (1.36) | −1.13 (1.29) | 0.78 (0.37, 1.19) | −1.42 (1.28) | −1.32 (1.55) | 0.08 (−0.33, 0.48) | .02 | −0.55 |
| Sleepiness | 6.20 (4.52) | 4.83 (4.03) | −1.33 (−2.28, −0.37) | 6.15 (4.01) | 6.37 (4.71) | 0.26 (−0.67, 1.19) | .02 | 0.35 |
| PSQI | 7.58 (2.99) | 5.85 (2.56) | −1.51 (−2.10, −0.92) | 7.58 (3.03) | 6.75 (3.48) | −0.62 (−1.20, −0.05) | .04 | 0.29 |
| CBCL Sleep Composite | 3.32 (2.03) | 1.84 (1.86) | −1.43 (−1.83, −1.03) | 3.24 (2.13) | 2.51 (1.91) | −0.67 (−1.07, −0.26) | .01 | 0.40 |
| CMEPa | 21.11 (3.79) | 25.08 (4.86) | 3.89 (2.94, 4.85) | 21.52 (3.86) | 23.61 (4.60) | 2.01 (1.05, 2.97) | .01b | −0.50 |
| DLMO | 21.30 (1.10) | 21.14 (0.92) | −0.01 (−0.02, 0.00) | 21.30 (1.07) | 21.47 (1.13) | 0.01 (0.00, 0.02) | .04 | 0.28 |
Note: All sleep outcome variables were not significantly different at baseline comparing two treatment conditions, except for waketime (WUP) weeknight–weekend discrepancy (p = .02). Treatment by time interaction term is estimated using multilevel model and is interpreted as the difference in the pre-post difference scores comparing TranS-C Youth versus Psychoeducation. CBCL = Child Behavior Checklist (parent-report); CMEP = Children’s Morningness-Eveningness Preferences; Coeff. = Coefficient; DLMO = Dim Light Melatonin Enset; PSQI = Pittsburgh Sleep Quality Index; SD-BT = Sleep diary bedtime; SD-TST = Sleep diary total sleep time; SD-WUP = Sleep diary wakeup time; Sleepiness = Sleepiness subscale from School Sleep Habits Survey.
Indicates primary outcome.
Note that the exact p = .006 here was still significant after correcting for multiple comparisons for the primary outcomes. d Indicates effect size for the treatment effect on pre—post change of each outcome and is calculated using the mean change scores and pretest raw SDs from each treatment condition, based on Feingold62 equation 5.
Compared to PE, TranS-C was associated with greater reduction in the weeknight–weekend discrepancy for TST from pretreatment to posttreatment. There was no pre-post change in BT weeknight–weekend discrepancy for either treatment. Compared to PE, TranS-C was associated with greater pre–post reduction in the weeknight–weekend discrepancy for WUP. In addition, compared to PE, TranS-C exhibited greater pre–post reduction in Sleepiness, PSQI, and parent-reported CBCL Sleep Composite.
Circadian Outcome
Circadian outcome data are provided in Table 3. Compared to PE, TranS-C exhibited a significantly greater pre–post increase in CMEP (d = −0.50, medium effect) and earlier DLMO at posttreatment compared to pretreatment. It should be noted that the phase advance for participants who received TranS-C (DLMO from 21.30 to 21.14) should be interpreted in the context of the phase delay for participants who received PE (DLMO from 21.30 to 21.47).
Health Domains
Health domain data are listed in Table 4. For the Youth Self-Report Composite Risk Scores, none of the Treatment by Time interactions attained statistical significance. Both treatments exhibited significant pre–post improvement in the Emotional domain. As shown in Table 4, both CDRS and PHQ were significantly reduced from pretreatment and posttreatment in both conditions.
TABLE 4.
Raw Means and SDs As Well As Treatment Effects for Primary and Secondary Health Domain Outcomes (Youth Self-Report and Ecological Momentary Assessment [EMA]) for Transdiagnostic Sleep and Circadian Intervention (TranS-C) (n = 89) vs. Psychoeducation (PE) (n = 87) From Baseline to Posttreatment
| TranS-C |
PE |
Treatment by Time Interaction |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Posttreatment |
Pre-Post Change |
Baseline |
Posttreatment |
Pre-Post Change |
|||
| Outcome | Mean (SD) | Mean (SD) | Coeff. (95% CI) | Mean (SD) | Mean (SD) | Coeff. (95% CI) | p | d |
| Youth Self-Report Composite Risk | ||||||||
| Scorea | ||||||||
| Emotional health | ||||||||
| CDRS | 33.90 (9.34) | 27.01 (8.72) | −6.49 (−8.42, −4.55) | 33.08 9.90) | 27.00 (8.16) | −5.85 (−7.77, −3.93) | .65 | 0.11 |
| MASC | 46.51 (17.73) | 45.45 (17.10) | −2.11 (−4.96, 0.74) | 45.98 (15.99) | 44.74 (18.03) | −0.90 (−3.71, 1.92) | .55 | 0.09 |
| Composite | 0.25 (0.91) | −0.18 (0.80) | −0.38 (−0.53, −0.24) | 0.17 (0.82) | −0.21 (0.81) | −0.36 (−0.50, −0.21) | .80 | 0.001 |
| Cognitive health | ||||||||
| ACS | 50.56 (8.23) | 52.18 (8.09) | 0.91 (−0.55, 2.37) | 51.24 7.22) | 51.29 (7.77) | −0.31 (−1.73, 1.12) | .24 | −0.15 |
| YSAS (school/cognitive items) | 11.68 (2.95) | 11.69 (3.14) | 0.23 (−0.37, 0.82) | 11.90 (2.83) | 12.49 (2.94) | 0.51 −0.08, 1.11) | .50 | 0.02 |
| Composite | 0.02 (0.85) | −0.09 (0.89) | −0.04 (−0.19, 0.12) | −0.01 (0.71) | 0.09 (0.87) | 0.11 (−0.04, 0.27) | .17 | 0.18 |
| Behavioral health | ||||||||
| Sensation Seeking Scale | 27.28 (5.97) | 27.35 (6.61) | 0.15 (−0.75, 1.06) | 26.36 6.22) | 27.51 (7.04) | 0.84 (−0.04, 1.72) | .29 | 0.09 |
| Alcohol and Substance Use | 5.76 (8.24) | 5.51 (8.10) | 0.07 (−0.76, 0.91) | 5.67 6.62) | 6.26 (8.37) | 0.23 (−0.59, 1.06) | .79 | 0.01 |
| Composite | 0.01 (0.81) | −0.01 (0.83) | 0.02 (−0.07, 0.11) | −0.07 (0.78) | 0.06 (0.92) | 0.08 (−0.01, 0.17) | .31 | 0.07 |
| Social health | ||||||||
| YSAS: Friends | 18.53 (4.58) | 17.73 (3.69) | −0.62 (−1.51, 0.26) | 18.81 (4.98) | 18.68 (4.82) | 0.13 (−0.75, 1.02) | .24 | 0.18 |
| YSAS: Family | 11.92 (3.50) | 11.33 (3.56) | −0.39 (−1.11, 0.32) | 12.34 3.67) | 11.68 (4.17) | −0.54 (−1.25, 0.17) | .78 | −0.05 |
| YSAS: Romantic | 7.34 (2.03) | 7.62 (1.78) | 0.17 (−0.28, 0.62) | 7.59 (1.69) | 7.62 (1.85) | 0.04 (−0.40, 0.48) | .69 | 0.02 |
| Composite | −0.04 (0.60) | −0.09 (0.62) | −0.04 (−0.17, 0.09) | 0.09 (0.71) | 0.02 (0.69) | −0.03 (−0.17, 0.10) | .96 | 0.04 |
| Physical health | ||||||||
| MAQ | 3.36 (5.35) | 4.20 (8.22) | 0.82 (−0.54, 2.17) | 2.83 (4.31) | 3.40 (5.11) | 0.51 (−0.83, 1.84) | .75 | −0.04 |
| PHQ | 9.30 (5.37) | 7.97 (5.01) | −1.15 (−2.05, −0.25) | 8.58 (4.40) | 7.01 (4.33) | −1.56 (−2.44, −0.67) | .53 | −0.15 |
| Composite | 0.02 (0.77) | 0.04 (0.83) | 0.02 (−0.13, 0.18) | 0.00 (0.66) | −0.01 (0.56) | 0.00 (−0.15, 0.15) | .83 | −0.05 |
| Youth EMA Composite Risk Score | ||||||||
| Emotional health (Positivity Ratio) | 1.47 (0.53) | 1.46 (0.65) | −0.03 (−0.12, 0.06) | 1.45 (0.42) | 1.44 (0.49) | −0.01 (−0.10, 0.09) | .71 | 0.07 |
| Cognitive health | 6.78 (0.98) | 6.86 (0.87) | 0.12 (−0.12, 0.35) | 7.09 (1.16) | 6.91 (1.06) | −0.20 (−0.43, 0.02) | .05 | −0.33 |
| Behavioral health | 2.82 (2.79) | 2.28 (2.67) | −0.28 (−0.49, −0.08) | 3.21 (3.15) | 2.34 (2.53) | −0.30 (−0.50, −0.11) | .88 | 0.04 |
| Social health | ||||||||
| Alone | 1.45 (0.52) | 1.50 (0.66) | 0.04 (−0.05, 0.13) | 1.40 (0.42) | 1.43 (0.48) | 0.03 (−0.06, 0.12) | .88 | 0.01 |
| With a family member | 1.72 (0.68) | 1.59 (0.85) | −0.13 (−0.30, 0.03) | 1.67 (0.60) | 1.60 (0.62) | −0.04 (−0.21, 0.13) | .43 | −0.18 |
| With a peer | 2.10 (0.75) | 2.25 (0.84) | 0.12 (−0.16, 0.41) | 2.01 (0.76) | 1.93 (0.59) | −0.06 (−0.31, 0.20) | .37 | 0.21 |
| Physical health | 1.47 (0.31) | 1.59 (0.35) | 0.11 (0.04, 0.19) | 1.44 (0.32) | 1.53 (0.34) | 0.11 (0.03, 0.19) | .97 | 0.04 |
Note: Treatment by time interaction term is estimated using multilevel model and is interpreted as the difference in the pre–post difference scores comparing TranS-C Youth vs. Psychoeducation. d Indicates effect size for the treatment effect. ACS = Attention Control Scale; CDRS = Children’s Depression Rating Scale; Coeff. = Coefficient; MASC = Multidimensional Anxiety Scale for Children; MAQ = Modifiable Activity Questionnaire for Adolescents; PHQ = Physical Health Questionnaire–15; YSAS = Youth Social Adjustment Scale.
Indicates primary outcome.
For the Youth EMA Composite Risk Score, there was no Treatment by Time interaction for emotional health via EMA. There was a marginally significant effect for the Treatment by Time interaction for cognitive health via EMA, such that there was a pre–post increase for TranS-C and a decrease for PE. There were no Treatment by Time interactions for behavioral or physical health. However, there was a significant pre–post decrease for behavioral health and an increase for physical health in both conditions. No Treatment by Time interaction or pre–post change was observed for the social health composite.
For the Parent-Reported Composite Risk Scores (Table 5), relative to PE, TranS-C had greater reduction in problems related to Cognitive Health, Thought Problems, and Rule-Breaking Behavior pre–post, as indicated by the Treatment by Time interactions. For the other CBCL subscales and composites, there were no Treatment by Time interactions.
TABLE 5.
Raw Means and SDs As Well As Treatment Effects for Parent-Reported Health Domain Outcomes for Transdiagnostic Sleep and Circadian Intervention (TranS-C) (n = 89) vs. Psychoeducation (PE) (n = 87) From Baseline to Posttreatment
| TranS-C |
PE |
Treatment by Time Interaction |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Post-treatment |
Pre–Post Change |
Baseline |
Posttreatment |
Pre–Post Change |
|||
| Outcome | Mean (SD) | Mean (SD) | Coeff. (95% CI) | Mean (SD) | Mean (SD) | Coeff. (95% CI) | P | d |
| Parent-Reported Composite Risk | ||||||||
| Score | ||||||||
| Emotional Health | ||||||||
| Anxious/Depressed | 3.13 (3.48) | 2.61 (2.97) | −0.31 (−0.86, 0.23) | 4.11 (3.78) | 3.61 (3.56) | −0.26 (−0.81, 0.30) | .89 | 0.02 |
| Withdrawn/Depressed | 2.83 (2.84) | 2.49 (2.54) | −0.16 (−0.66, 0.34) | 3.14 (2.77) | 2.99 (2.72) | −0.07 (−0.57, 0.44) | .80 | 0.02 |
| Composite | −0.04 (0.93) | −0.18 (0.79) | −0.07 (−0.22, 0.08) | 0.16 (0.92) | 0.06 (0.87) | −0.05 (−0.20, 0.10) | .83 | 0.02 |
| Cognitive Health | ||||||||
| Thought problems | 3.56 (2.59) | 2.38 (2.31) | −1.13 (−1.60, −0.65) | 3.75 (2.73) | 3.60 (2.90) | −0.08 (−0.56, 0.40) | .002 | 0.40 |
| Attention problems | 4.23 (3.61) | 4.01 (3.85) | −0.11 (−0.71, 0.49) | 4.17 (4.13) | 4.33 (4.30) | 0.21 (−0.40, 0.81) | .47 | 0.08 |
| Composite | 0.05 (0.81) | −0.20 (0.81) | −0.22 (−0.36, −0.09) | 0.08 (0.90) | 0.07 (0.97) | 0.01 (−0.13, 0.15) | .02 | 0.29 |
| Behavioral Health | ||||||||
| Rule-Breaking Behavior | 1.91 (2.31) | 1.39 (1.87) | −0.36 (−0.81, 0.09) | 1.98 (2.16) | 2.31 (2.61) | 0.35 (−0.10, 0.81) | .03 | 0.29 |
| Aggressive Behavior | 3.84 (4.02) | 3.62 (4.22) | 0.05 (−0.59, 0.68) | 4.54 (4.52) | 3.76 (3.73) | −0.57 (−1.22, 0.07) | .18 | −0.15 |
| Composite | −0.01 (0.91) | −0.15 (0.86) | −0.07 (−0.22, 0.08) | 0.09 (0.88) | 0.07 (0.92) | 0.01 (−0.15, 0.16) | .49 | 0.07 |
| Social Health | ||||||||
| Social Problems | 1.36 (1.52) | 1.24 (1.81) | −0.09 (−0.48, 0.30) | 1.86 (2.15) | 1.83 (2.49) | 0.06 (−0.33, 0.45) | .60 | 0.11 |
| Composite | −0.10 (0.75) | −0.16 (0.89) | −0.04 (−0.24, 0.15) | 0.14 (1.06) | 0.13 (1.23) | 0.03 (−0.16, 0.22) | .60 | 0.11 |
| Physical Health | ||||||||
| Somatic Complaints | 2.89 (3.11) | 2.14 (2.75) | −0.66 (−1.12, −0.19) | 2.49 (2.74) | 2.01 (2.43) | −0.38 (−0.86, 0.09) | .42 | 0.08 |
| Composite | 0.18 (1.12) | −0.09 (0.99) | −0.24 (−0.40, −0.07) | 0.03 (0.99) | −0.14 (0.87) | −0.14 (−0.31, 0.03) | .42 | 0.08 |
Note: Parent-report composite risk score was based on Child Behavior Checklist (CBCL). Treatment by time interaction term is estimated using multilevel model and is interpreted as the difference in the pre-post difference scores comparing TranS-C Youth versus Psychoeducation. d Indicates effect size for the treatment effect. Coeff. = Coefficient.
Treatment Integrity and Credibility
Both CTRS (n = 69, mean = 45.43, SD = 4.45) and PE treatment integrity scores (n = 77, mean = 92.44, SD = 10.13) indicate that both treatments were delivered with fidelity. There were no significant group differences on the CEQ (all p > 0.05).
DISCUSSION
Relative to PE, TranS-C was associated with a greater decrease in evening circadian preference, an earlier endogenous circadian phase, greater decrease in daytime sleepiness, and greater increase in sleep via the PSQI. This pattern of findings was also observed on the parent-reported CBCL Sleep Composite and on two sleep diary outcomes: weeknight–weekend discrepancy for TST and WUP. In addition, youth who received TranS-C demonstrated significantly longer TST on weeknights (23 minutes) from before to after treatment, which was not observed in PE. This pattern of findings is consistent with research documenting the effectiveness of sleep interventions for youth,42–44 extending prior research by documenting that a transdiagnostic approach improves selected sleep outcomes and changes a biological marker of circadian functioning. The improvement observed for TranS-C on DLMO should be interpreted in terms of the circadian phase delay that occurred for PE. TranS-C may buffer against a phase delay that would otherwise have occurred, had youth not received TranS-C. We were surprised that there was no treatment effect for three sleep diary variables (TST, BT and the weeknight–weekend discrepancy in BT). Indeed, the results are more positive for global, retrospective questionaires. We believe that these provide more comprehensive coverage of sleep problems relative to the individual sleep parameters derived from the sleep diary, which were the primary outcomes. In hindsight, the three sleep diary variables may have suffered from the wide transdiagnostic inclusion gates. For example, TST that is either too long or too short is problematic.63 Thus, when we combine short sleepers (eg, insomnia) and long sleepers (eg, hypersomnia), the calculation of TST is not reflective of treatment change. Sleep diary reporting standards may need revision given the complexity of “real world” sleep problems and sleep health.63 Also, in terms of BT, “shuteye time” may be the more appropriate primary outcome given the current tech- nological age.64 Interestingly, reducing the discrepancy between weekday and weekend WUP and TST was more malleable than BT. Indeed, many youth had a non modifiable number of tasks that had to be completed—such as homework, sports, dinner—in the hours between school ending and bedtime. Finally, consideration should be given to including sleep extension in TranS-C, as it effectively advances BTs in adolescents.65
In terms of functioning in the five health domains, contrary to prediction, TranS-C was not associated with greater pre–post change on the primary outcome relative to PE. For secondary outcomes, there was a marginally significant effect for TranS-C to show improvement on the cognitive health Youth EMA Composite Risk Score relative to PE. There were also Treatment by Time interactions favoring TranS-C on the Parent-Reported Composite Risk Scores for cognitive health, thought problems, and rule breaking. In sum, select health domains appear to be mitigated by TranS-C. Yet it is surprising that the other domains were not affected, given the evidence that sleep deprivation can adversely affect these domains. Relevant literature tends to use full-night sleep deprivation, whereas youth are better characterized as partially sleep deprived. Perhaps the measures were insufficiently sensitive to the health problems associated with youth eveningness. Finally, a large change on TST and BT may be required before strong downstream health effects will be observed.
PE and TranS-C exhibited significant improvement in the emotional domain on the Youth Self-Reported Composite Risk Scores as well as the behavioral health domain on the Youth EMA Composite Risk Score. These findings are consistent with evidence that PE is an active treatment that provides real benefits.48 It is also important to note the marginally significant effect of pre–post reduction in cognitive functioning via EMA associated with PE. Also, for the physical health domain on the Youth EMA Composite Risk Score, youth in both groups became more inactive after the intervention. These results are surprising and require future research.
There are several limitations in the current investigation. This study is more toward the efficacy than the effectiveness end of the continuum. Future research is needed to test the generalizability of these findings with fewer exclusion criteria. To the best of our knowledge, this is the first study to examine the five health domains that we included; as such, there was minimal pre-existing guidance as to how to optimally assess these outcomes. Interrater reliabilities for the diagnostic measures were acceptable for KSAD but low for CDRS. The outcomes reported are those listed as primary and secondary on https://clinicaltrials.gov. These prioritize youths’ sleep experience. Future reports will include objective measures and additional sleep diary outcomes. In addition, a no-treatment control was not included. Also, the income of the sample was relatively high, raising the need to assess generalizability of the findings to lower-income families. Although not significant, dropout was higher in TranS-C relative to PE. Outcomes immediately posttreatment are presented; follow-up data are currently being collected.
In summary, this study used multiple methods and multiple informers and provides a test of a treatment that addresses an important and understudied mechanism—the role of dysregulated sleep and circadian rhythms contrib- uting to vicious cycles of escalating vulnerability and risk— in youth. The stated primary outcomes were not significant except for CMEP (the primary circadian outcome). As such, formally this is a negative trial for most of the primary outcomes. However, important secondary outcomes are significant, including a biological marker of the endogenous circadian phase. As such, for at-risk youth, the evidence tentatively supports the use of TranS-C over PE for improving sleep and circadian functioning and for improving health. Potential advantages of the transdiagnostic approach for the practice of behavioral sleep medicine is that it contributes to reducing the “Too many empirically supported treatments problem” (p. 68)66 and the associated burden on clinicians. Also, the modular approach ensures that the intervention targets are individualized and address a range of separate sleep and circadian mechanisms.
Supplementary Material
Acknowledgments
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD071065).
This study was presented in a keynote at the 47th Annual Congress of the European Association for Behavioural and Cognitive Therapies in Ljubljana, Slovenia, September 13–16, 2017 and World Sleep in Prague, Czech Republic, October 7–11, 2017.
Drs. Dong and Rabe-Hesketh served as the statistical experts for this research.
The authors are grateful to the team for their assistance, particularly Emily M. Clark, BA, Brenden Mei, BA, Xin Zhao, BA, Leah M. Miller, BA, O’Min Kwon, BA, Aaron T. Daley, BA, Armando Martinez, BA, Emily Pfannenstiel, BA, Shay O’Brien, MSW, and Jie Jane Chen, BA, of the University of California, Berkeley, for assistance with project co-ordination; Eve Fine, MSW, Davin Duval, MSW, Annie Liang, MSW, Caitlin Eggleston, BA, Deidre Abrons, MFT, and Ania Foster, MFT, of the University of California, Berkeley, for assistance with conducting treatment; Elizabeth Mason, PhD, of the University of New South Wales, Lauren Asarnow, PhD, of Stanford University, and Adriane Soehner, PhD, of the University of Pittsburgh, for assistance with project set-up.
Disclosure: Dr. Harvey has received research support from the National Institutes of Health and book royalties from American Psychological Association, Guilford Press, and Oxford University Press. Dr. Rabe-Hesketh has received book royalties from Stata Press and Springer. Dr. Wyatt has served as a consultant to Philips-Respironics and has received royalties from UpToDate. Dr. Hinshaw has received book royalties from Cambridge University Press, St. Martin’s Press, Ballantine Books, and Oxford University Press. Dr. Silk has received research support from the National Institutes of Health. Dr. Blum has received research support from Pau Innovation Gift Fund Seed Grant; has served as a consultant to Circadia Technologies; and has received book royalties from Parallax Press. Ms. Dolsen has received research support from the National Institutes of Health. Ms. Gumport has received research support from the National Institutes of Health.
Footnotes
Drs. Dong, Kanady, Smith, Thompson and Mss. Hein and Zannone report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Allison G. Harvey, University of California, Berkeley..
Kerrie Hein, University of California, Berkeley..
Emily A. Dolsen, University of California, Berkeley..
Lu Dong, University of California, Berkeley..
Sophia Rabe-Hesketh, University of California, Berkeley..
Nicole B. Gumport, University of California, Berkeley..
Jennifer Kanady, University of California, Berkeley..
James K. Wyatt, Rush University, Chicago, IL..
Stephen P. Hinshaw, University of California, Berkeley..
Jennifer S. Silk, University of Pittsburgh, PA..
Rita L. Smith, University of California, Berkeley..
Monique A. Thompson, University of California, Berkeley..
Nancee Zannone, University of California, Berkeley..
Daniel Jin Blum, University of California, Berkeley..
REFERENCES
- 1.Horne JA, Ӧstberg OA. A self-assessment questionnaire to determine morningness- eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 2.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12: 110–118. [DOI] [PubMed] [Google Scholar]
- 3.Carskadon MA, Mindell JA, Drake C. Contemporary sleep patterns of adolescents in the USA: results of the 2006 National Sleep Foundation Sleep in America poll. J Sleep Res. 2006;5(Suppl 1):1–93. [Google Scholar]
- 4.Dang-Vu TT, Desseilles M, Peigneux P, Maquet P. A role for sleep in brain plasticity. Pediatr Rehabil. 2006;9:98–118. [DOI] [PubMed] [Google Scholar]
- 5.Caci H, Bouchez J, Bayle FJ. Inattentive symptoms of ADHD are related to evening orientation. J Atten Disord. 2009;13:36–41. [DOI] [PubMed] [Google Scholar]
- 6.Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adoles- cents. J Biol Rhythms. 2007;22:268–274. [DOI] [PubMed] [Google Scholar]
- 7.Short MA, Gradisar M, Lack LC, Wright HR. The impact of sleep on adolescent depressed mood, alertness and academic performance. J Adolesc. 2013;36:1025–1033. [DOI] [PubMed] [Google Scholar]
- 8.Clarisse R, Le Floc’h N, Kindelberger C, Feunteun P. Daily rhythmicity of attention in morning- vs. evening-type adolescents at boarding school under different psychosocio- logical testing conditions. Chronobiol Int. 2010;27:826–841. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein D, Hahn C, Hasher L, Wiprzycka U, Zelazo PD. Time of day, intellectual performance, and behavioral problems in morning versus evening type adolescents: Is there a synchrony effect? Personal Indiv Diff. 2007;42:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adan A Chronotype and personality factors in the daily consumptions of alcohol and psychostimulants. Addiction. 1994;89:455–462. [DOI] [PubMed] [Google Scholar]
- 11.Randler C Differences between smokers and non-smokers in morning-eveningness. Social Behav Personal. 2008;36:565–575. [Google Scholar]
- 12.Adan A, Natale V, Caci H, Prat G. Relationship between circadian typology and functional and dysfunctional impulsivity. Chronobiol Int. 2010;27:606–619. [DOI] [PubMed] [Google Scholar]
- 13.Negriff S, Dorn LD, Pabst SR, Susman EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Res. 2011;185:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Digdon NL, Howell AJ. College students who have an eveningness preference report lower self-control and greater procrastination. Chronobiol Int. 2008;26:1029–1046. [DOI] [PubMed] [Google Scholar]
- 15.Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morning- ness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Dev Psychol. 2007;43:811–822. [DOI] [PubMed] [Google Scholar]
- 16.Campos-Hirata F, Lima MCO, de Bruin VMS, Nobrega PR, Wenceslau GP, de Bruin PFC. Depression in medical school: the influence of morning-eveningness. Chronobiol Int. 2007;24:939–946. [DOI] [PubMed] [Google Scholar]
- 17.Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of car- diovascular disease. Ann Behav Med. 2011. [DOI] [PubMed] [Google Scholar]
- 18.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asarnow LD, McGlinchey E, Harvey AG. The effects of bedtime and sleep duration on academic and emotional outcomes in a nationally representative sample of adolescents. J Adolesc Health. 2014;54:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGlinchey EL, Harvey AG. Risk behaviors and negative health outcomes for adoles- cents with late bedtimes. J Youth Adolesc. 2015;44:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asarnow LD, McGlinchey E, Harvey AG. Evidence for a possible link between bedtime and change in body mass index. Sleep. 2014;38:1523–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts RD, Kyllonen PC. Morningness-eveningness and intelligence: early to bed, early to rise will likely make you anything but wise! Personal Indiv Diff. 1999;27: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 23.Giampietro M, Cavallera GM. Morning and evening types and creative thinking. Personal Indiv Diff. 2007;42:453–463. [Google Scholar]
- 24.Parthasarathy S, Carskadon MA, Jean-Louis G, et al. Implementation of sleep and circadian science: recommendations from the Sleep Research Society and National Institutes of Health workshop. Sleep. 2016;39:2061–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey AG, Buysse DJ. Treating Sleep Problems: A Transdiagnostic Approach. New York: Guilford; 2017. [Google Scholar]
- 26.Harvey A, Watkins E, Mansell W, Shafran R. Cognitive Behavioural Processes Across Psychological Disorders: A Transdiagnostic Approach to Research and Treatment. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 27.Fairburn CG, Cooper Z, Shafran R. Cognitive behaviour therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav Res Ther. 2003;41:509–528. [DOI] [PubMed] [Google Scholar]
- 28.Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behav Therapy. 2004;35:205–230. [DOI] [PubMed] [Google Scholar]
- 29.Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev. 2011;31:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014;54: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianotti F, Cortesi F. Sleep patterns and daytime function in adolescence: an epidemiological survey of an Italian high school student sample. In: Carskadon MA, ed. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge, UK: Cambridge University Press; 2002:132–147. [Google Scholar]
- 32.Becker SP, Ramsey RR, Byars KC. Convergent validity of the Child Behavior Checklist sleep items with validated sleep measures and sleep disorder diagnoses in children and adolescents referred to a sleep disorders center. Sleep Med. 2015;16:79–86. [DOI] [PubMed] [Google Scholar]
- 33.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. J Am Acad Child Psychiatry. 1984;23:191–197. [DOI] [PubMed] [Google Scholar]
- 34.Poznanski E, Freeman L, Mokros H. Childrens Depression Rating Scale–Revised. Psychopharmacol Bull. 1985;21:979–989. [Google Scholar]
- 35.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizo- phrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 36.March JS, Sullivan K, Parker J. Test-retest reliability of the Multidimensional Anxiety Scale for Children. J Anxiety Disord. 1999;13:349–358. [DOI] [PubMed] [Google Scholar]
- 37.Russo MF. A sensation seeking scale for children: further refinement and psychometric development. J Psychopathol Behav Assess. 1993;15:69–86. [Google Scholar]
- 38.Stephenson MT, Hoyle RH, Palmgreen P, Slater MD. Brief measures of sensation seeking for screening and large-scale surveys. Drug Alcohol Depend. 2003;72: 279–286. [DOI] [PubMed] [Google Scholar]
- 39.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. [DOI] [PubMed] [Google Scholar]
- 40.Young J, Beck A. Cognitive Therapy Scale: Rating Manual. Philadelphia: University of Pennsylvania; 1980. [Google Scholar]
- 41.Bei B, Allen NB, Nicholas CL, Dudgeon P, Murray G, Trinder J. Actigraphy-assessed sleep during school and vacation periods: a naturalistic study of restricted and extended sleep opportunities in adolescents. J Sleep Res. 2014;23:107–117. [DOI] [PubMed] [Google Scholar]
- 42.Schlarb A, Liddle C, Hautzinger M. JuSt–a multimodal program for treatment of insomnia in adolescents: a pilot study. Nat Sci Sleep. 2010;3:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gradisar M, Dohnt H, Gardner G, et al. A randomized controlled trial of cognitive behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep. 2011;34:1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bruin EJ, Oort FJ, Bögels SM, Meijer AM. Efficacy of Internet and group administered cognitive behavioral therapy for insomnia in adolescents: a pilot study. Behav Sleep Med. 2014;12:235–254. [DOI] [PubMed] [Google Scholar]
- 45.Frank E, Kupfer DJ, Thase ME, et al. Two year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62: 996–1004. [DOI] [PubMed] [Google Scholar]
- 46.Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light & Wake Therapy. 1 ed. Basel: Karger; 2009. [Google Scholar]
- 47.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. New York: Guilford Press; 2002. [Google Scholar]
- 48.Harvey AG, Soehner AM, Kaplan KA, et al. Treating insomnia improves sleep, mood and functioning in bipolar disorder: a pilot randomized controlled trial. J Consult Clin Psychology. 2015;83:564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 51.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 52.de la Vega R, Tomé-Pires C, Solé E, et al. The Pittsburgh Sleep Quality Index: validity and factor structure in young people. Psychol Assess. 2015;27:e22. [DOI] [PubMed] [Google Scholar]
- 53.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. [DOI] [PubMed] [Google Scholar]
- 54.Lewy AJ. Circadian rhythms and mood disorders: a guide for the perplexed. J Clin Psychiatry. 2015;76(1):478–664. [DOI] [PubMed] [Google Scholar]
- 55.Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29:1075–1080. [DOI] [PubMed] [Google Scholar]
- 56.Burchinal MR, Roberts JE, Hooper S, Zeisel SA. Cumulative risk and early cognitive development: a comparison of statistical risk models. Dev Psychol. 2000;36:793–807. [DOI] [PubMed] [Google Scholar]
- 57.Rutter M Protective factors in children’s responses to stress and disadvantage. Ann Acad Med Singapore. 1979;8:324–338. [PubMed] [Google Scholar]
- 58.Silk JS, Forbes EE, Whalen DJ, et al. Daily emotional dynamics in depressed youth: a cell phone ecological momentary assessment study. J Exp Child Psychology. 2011;110:241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. [DOI] [PubMed] [Google Scholar]
- 60.Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hochberg Y A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 62.Feingold A Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods. 2009;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Exelmans L, Van den Bulck J. Bedtime, shuteye time and electronic media: sleep displacement is a two-step process. J Sleep Res. 2017;26:364–370. [DOI] [PubMed] [Google Scholar]
- 65.Dewald-Kaufmann J, Oort F, Meijer A. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: a randomized controlled trial. J Child Psychol Psychiatry. 2014;55:273–283. [DOI] [PubMed] [Google Scholar]
- 66.Weisz JR. Building robust psychotherapies for children and adolescents. Perspect Psychol Sci. 2014;9:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.