Abstract
This study was designed to evaluate the antioxidative status of serum by measuring its total antioxidant capacity, as well as the antioxidant enzyme activity (superoxide dismutase, catalase, and glutathione reductase), in dogs with various stages of degenerative mitral valve disease (DMVD) compared to healthy controls. In total, 71 client-owned dogs in different stages of DMVD, which included healthy controls, took part in the study. Following an anamnesis, clinical examination, standard transthoracic echocardiograpic examination, chest X-ray, complete blood (cell) count, and serum biochemistry, dogs were divided into 2 study groups. Blood was drawn from each dog once at the time of presentation and selected antioxidant parameters were measured using commercially available assay kits. The activity of superoxide dismutase gradually decreased in the more advanced stages of DMVD, while the activity of catalase was significantly higher in the group of dogs with asymptomatic DMVD compared to healthy controls and dogs with symptomatic DMVD. No significant changes were noted in total antioxidant capacity and the activity of glutathione reductase. Results suggested that DMVD has a significant impact on the activity of superoxide dismutase and catalase in the serum of the tested dogs. Knowledge of changes in the activity of antioxidative enzymes may warrant further studies, possibly to evaluate the potential role of compounds with antioxidative properties in the clinical outcome of dogs with DMVD.
Résumé
La présente étude a été conçue afin d’évaluer le statut antioxydant du sérum en mesurant sa capacité antioxydante totale, ainsi que l’activité antioxydante enzymatique (superoxyde dismutase, catalase, et glutathion réductase), chez des chiens avec des degrés divers de maladie dégénérative de la valvule mitrale (DMVD) comparativement à des témoins en santé. Au total, 71 chiens appartenant à des clients à différents stades de DMVD, qui incluaient des témoins en santé, ont pris part à cette étude. À la suite de la prise d’anamnèse, d’un examen clinique, d’un examen échocardiographie transthoracique standard, de radiographie thoracique, d’un comptage cellulaire sanguin complet, et d’analyse biochimique sérique, les chiens étaient séparés en deux groupes d’étude. Du sang fut prélevé de chaque chien une fois au moment de la présentation et les paramètres antioxydants sélectionnés furent mesurés à l’aide d’une trousse disponible commercialement. L’activité de la superoxyde dismutase diminuait graduellement dans les stades plus avancés de DMVD, alors que l’activité de la catalase était significativement plus élevée dans le groupe de chiens avec une DMVD asymptomatique comparativement aux témoins en santé et aux chiens avec une DMVD symptomatique. Aucun changement significatif n’était noté dans la capacité antioxydante totale et dans l’activité de la glutathion réductase. Les résultats suggèrent que la DMVD a un impact significatif sur l’activité de la superoxyde dismutase, et de la catalase dans le sérum des chiens testés. Des connaissances sur les changements dans l’activité des enzymes antioxydantes pourraient justifier des études additionnelles, possiblement pour évaluer le rôle potentiel de produits avec des propriétés antioxydantes dans le devenir clinique de chiens avec DMVD.
(Traduit par Docteur Serge Messier)
Introduction
Degenerative mitral valve disease (DMVD) is the most common acquired cardiovascular disease in dogs and accounts for approximately 75% of cases of chronic heart failure (1,2). The macroscopic lesions on the atrioventricular valves in the course of DMVD consist of small thickened nodules on the leaflets and rupture of the chordae tendineae (3). This leads to poor coaptation of the mitral valve, its insufficiency, and eventually the development of congestive heart failure. This disease is most prevalent in older, small-breed dogs, although it may occasionally occur in larger breeds (4). Cavalier King Charles spaniels are particularly predisposed to this condition and develop degenerative lesions at an earlier age (5).
The disease is characterized by a long asymptomatic period that progresses into a clinical stage observed only in some dogs. The goal of therapy is to prolong the asymptomatic stage of the disease, which is currently achieved using pharmacotherapy (6). There are single reports suggesting that the pathophysiology of DMVD might be associated with oxidative stress and that administering antioxidants may positively affect the patient clinical outcome. Significant differences were found between dogs with chronic heart failure (CHF) and healthy controls in plasma 8-F2α-isoprostane and total antioxidant capacity (7). Moreover, higher erythrocytic glutathione peroxidase activity was reported in dogs with CHF secondary to DMVD than in dogs with dilated cardiomyopathy (DCM) (8). It was also found that serum paraoxonase-1 activity was lower in dachshunds with asymptomatic DMVD than in those with a symptomatic stage of the disease and healthy controls (9). On the other hand, no differences were found either in the plasma coenzyme Q10 concentration or the erythrocyte superoxide dismutase (SOD) and whole blood glutathione peroxidase activity among dogs with different classes of heart failure (10). Furthermore, it was found that plasma malondialdehyde, oxidized low-density lipoprotein, and vitamin E were not associated with a clinical stage of DMVD (7,11).
Oxidative stress has recently been defined as “an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signalling and control and/or molecular damage” (12). The most common reactive oxygen species (ROS) include the superoxide anion radical (O2•−), hydrogen peroxide (H2O2), the hydroxyl radical (HO•), as well as singlet oxygen (12). An uncontrolled increase in the levels of ROS may cause structural cellular injury, damaging deoxyribonucleic acid (DNA), proteins, and lipids. Biological compounds, such as carotenoids, vitamins C and E, uric acid, glutathione, and a number of enzymes that catalyze the degradation of reactive forms of oxygen provide protection against ROS. The superoxide anion radical is neutralized to H2O2 by superoxide dismutase, while H2O2 is converted into water by catalase and glutathione peroxidase. The latter reaction uses glutathione, which serves as an electron donor, converting it to its oxidized form, glutathione disulfide. Finally, glutathione reductase (GR) reduces the oxidized glutathione to the monomeric molecule, completing the cycle (13). These enzymes act intra- and extracellularly and play an important role in protecting the organism from oxidative injury.
To date, no reports exist on the activity of major antioxidative enzymes in serum during the course of DMVD. Therefore, the aim of this study was to evaluate the antioxidative status of serum by measuring its total antioxidant capacity as well as the antioxidative enzyme activity in dogs with various stages of degenerative mitral valve disease compared to healthy controls.
Materials and methods
A total of 71 dogs were included in the study. All dogs underwent a detailed anamnesis, clinical examination, standard transthoracic echocardiograpic examination (Aloka F36 or Aloka Arietta V60; Hitachi-Aloka, Tokyo, Japan), chest X-rays (GIERTH HF 200A; Gierth X-Ray International, Riesa, Germany), as well as a complete blood (cell) count (CBC) (LaserCyte Dx; IDEXX Laboratories, Westbrook, Maine, USA), and serum chemistry analysis (Konelab Prime 30ISE; Thermo Scientific, Waltham, Massachusetts, USA). Only animals with no changes in the echocardiogram (M-mode, B-mode, color doppler) and no degenerative lesions typical for DMVD, as well as no abnormalities found during the various examinations, were included in the control group (group A). Blood samples used as controls were collected from healthy animals presenting to the Faculty’s Small Animal Teaching Hospital for preventive screening. An additional cardiac examination was offered to these individuals at no further cost.
The main inclusion criterion for the study group was the presence of an echocardiographically confirmed mitral valve insufficiency (or an additional tricuspid valve insufficiency) formed secondary to degenerative lesions of its leaflets, diagnosed using color doppler as well as continuous-wave doppler. Group B contained animals in stage B1 or B2 of DMVD according to the American College of Veterinary Internal Medicine (ACVIM) classification, including dogs with DMVD and echocardiographic features of cardiomegaly or without cardiomegaly, without clinical signs of chronic heart failure (14). Group C included animals with stage Cc disease according to the ACVIM classification. These were dogs with compensated chronic heart failure caused by DMVD. Animals with acute DMVD were excluded from the study. In addition, animals with abnormalities in their CBC or serum biochemistry (up to a 2-fold increase in the reference range values of aminotransferase activity and urea was considered acceptable) and clinical, ultrasound, or X-ray signs of any other disease apart from chronic DMVD were excluded from the study.
Blood from all the studied animals was drawn from a peripheral vein into serum and ethylenediaminetetraacetic acid (EDTA) tubes. All the animals fasted for at least 12 h before the examinations. The blood collected for serum was centrifuged after 15 min at room temperature (2000 × g, 15 min, 4°C) and transferred to a laboratory for biochemical analysis, while the remaining blood was divided and frozen at −80°C until analysis. All samples were collected within a 4-month period and stored no longer than 6 mo in total. Written informed consent was obtained from all the owners.
Determination of activity of antioxidant enzymes in serum
The activity of extracellular superoxide dismutase-3 (SOD-3), catalase (CAT), and glutathione reductase (GR) was measured in the serum of the studied animals using commercial kits (Cayman Chemical, Ann Arbor, Michigan, USA and BioVision, Milpitas, California, USA) (SOD: 706002; CAT: 707002; GR: #K761-200). One unit of SOD was defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide anion radical. The activity of CAT was defined as the amount of enzyme leading to the formation of 1 nmol of formaldehyde per minute at room temperature. One unit of GR was defined as the amount of enzyme that generates 1.0 μmol of 5-thio-2-nitrobenzoic acid (TNB) per minute at room temperature.
Determination of total antioxidant capacity
The total antioxidant capacity of serum was measured using the cupric-reducing antioxidant capacity (CUPRAC) method that is based on a single electron transfer (SET) mechanism. A commercially available reagent kit was used to carry out the assay (OxiSelect Total Antioxidant Capacity; Cell Biolabs, San Diego, California, USA). The results of the total antioxidant capacity were expressed as copperreducing equivalents (CRE). The absorbance of the colored enzymatic reaction products was measured using a multi-plate spectrophotometer (Tecan Spark 10M; Tecan, Austria). All the samples were run in duplicate and analyzed simultaneously by 1 examiner (AT) who was blinded to the sample origin, while the final result was an average value of 2 measurements.
Statistical analysis
The data underwent statistical analysis using the GraphPad Prism 5.0 (GraphPad Software, San Diego, California, USA) and Statistica 13 software. The Kolmogorov-Smirnov normality test was used to assess the normally distributed data. The oxidative stress parameters with a skewed distribution were natural logarithm (ln) transformed to normality. Based on the data distribution, either a 1-way analysis of variance (ANOVA) with the Bonferroni post-hoc test or the Kruskal-Wallis with the post-hoc Dunns test were used to compare more than 2 groups of data. Two groups of data were compared using the unpaired t-test or the nonparametric Mann-Whitney test. As the groups differed in terms of age, multivariable linear regression analyses, followed by analyses of covariance (ANCOVA), were conducted to evaluate potential bias. Correlations among the groups were determined using the Spearman correlation coefficient with P < 0.05 considered statistically significant.
Results
In total, 71 dogs were included in the study. The mean (± standard deviation) body weight of the animals was 10.2 ± 3.7 kg and the mean age was 10.7 ± 2.7 y. Forty of the dogs were male and 31 were female. The control group (group A) consisted of 16 healthy dogs, 9 male and 7 female, with a mean age of 8.1 ± 2.6 y and a mean body weight of 11.2 ± 3 kg and included 7 mixed-breed dogs, 3 beagles, 3 miniature schnauzers, a Cavalier King Charles spaniel, a dachshund, and a Nova Scotia duck tolling retriever. Group B included 23 dogs with asymptomatic mitral valve insufficiency with or without cardiomegaly, 13 males and 10 females, with a mean age of 10.3 ± 2.6 y and a mean body weight of 10 ± 3.5 kg. There were 8 mixed-breed dogs, 4 dachshunds, 3 miniature schnauzers, 2 Shih Tzus, 2 Cavalier King Charles spaniels, 1 bichon frise, 1 fox terrier, 1 Yorkshire terrier, and an English cocker spaniel. Group C consisted of 32 dogs with chronic heart failure caused by DMVD, with a mean age of 11.9 ± 1.7 y and a mean weight of 9.95 ± 4 kg. There were 18 male and 14 female dogs, which included 16 mixed-breed dogs, 4 Cavalier King Charles spaniels, 4 dachshunds, 2 miniature schnauzers, 2 Pekingese, 2 Shih Tzus, 1 bull terrier, and a medium poodle. The groups did not differ with respect to body weight, although the dogs with heart failure were significantly older than the healthy dogs (P < 0.001). The results of the CBC and serum chemistry of the dogs are included in Table I, while the results of the heart ultrasound are presented in Table II.
Table I.
Results of complete blood count and blood chemistry of dogs.
| Variable | A | B | C | P-value |
|---|---|---|---|---|
| White blood cells (109/L) | 7.25 ± 2.28 | 7.98 ± 2.45 | 8.88 ± 2.61 | 0.08 |
| Red blood cells (1012/L) | 6.85 ± 0.52 | 7.24 ± 0.82 | 7.14 ± 0.81 | 0.4 |
| Hemoglobin (g/L) | 162.3 ± 13.6 | 169.3 ± 15.6 | 162.4 ± 19.9 | 0.33 |
| Hematocrit (%) | 48 ± 6 | 51 ± 5 | 49 ± 5 | 0.25 |
| AST (U/L) | 27.58 ± 6.73 | 26.96 ± 7.05 | 28.72 ± 8.8 | 0.72 |
| ALT (U/L) | 42.08 ± 18.99C | 58.43 ± 29C | 91.72 ± 53.2A,B | 0.001 |
| Urea (mmol/L) | 5.63 ± 1.76C | 5.96 ± 2.07C | 8.68 ± 4.02A,B | 0.002 |
| Creatinine (μmol/L) | 91.5 ± 24.02 | 71.26 ± 31.17C | 93 ± 30.81B | 0.03 |
| Total protein (g/L) | 60.83 ± 5.44 | 59.74 ± 4.76 | 61.78 ± 7.03 | 0.49 |
| Albumin (g/L) | 30.25 ± 2.42 | 30.61 ± 2.93 | 32.19 ± 2.84 | 0.06 |
| Magnesium (mmol/L) | 0.7 ± 0.09 | 0.74 ± 0.09 | 0.74 ± 0.11 | 0.51 |
| Sodium ion (mmol/L) | 147.44 ± 2.9C | 144.4 ± 3.09 | 143.62 ± 3.86A | 0.01 |
| Potassium (mmol/L) | 4.43 ± 0.2 | 4.45 ± 0.35 | 4.39 ± 0.36 | 0.79 |
| Chloride (mmol/L) | 111.02 ± 1.3 | 110.7 ± 2.69 | 109.24 ± 3.23 | 0.09 |
| Calcium ion (mmol/L) | 1.36 ± 0.07C | 1.29 ± 0.08 | 1.27 ± 0.08A | 0.02 |
| Glucose (mmol/L) | 5.46 ± 0.44 | 6.05 ± 0.67 | 5.69 ± 0.8 | 0.13 |
Data are presented as mean ± SD. Statistical significance among the groups is shown in the upper indexes (A,B,C).
AST — aspartate aminotransferase; ALT — alanine aminotransferase.
Table II.
Echocardiographic and treatment characteristics of study groups.
| Variable | A | B | C | P-value |
|---|---|---|---|---|
| LA/Ao | 1.33 ± 0.14C | 1.51 ± 0.3C | 2.39 ± 0.49A,B | 0.0001 |
| LVIDd | 30.93 ± 3.23C | 33.37 ± 4.34C | 40.87 ± 7.23A,B | 0.0001 |
| LVIDdN | 1.54 ± 0.13C | 1.7 ± 0.21C | 2.11 ± 0.28A,B | 0.0001 |
| Heart rate | 111 ± 12.25C | 103.1 ± 18.89C | 159.5 ± 46.51A,B | 0.0004 |
| TR | 0/16 | 1/23 | 14/32 | |
| Cardiac treatment | 0/16 | 6/23 | 22/32 | |
| Benazepril | 0/16 | 6/23 | 20/32 | |
| Spironolactone | 0/16 | 1/23 | 17/23 | |
| Pimobendan | 0/16 | 0/23 | 17/23 | |
| Furosemide | 0/16 | 0/23 | 18/23 |
Data are presented as mean ± SD. Statistical significance among the groups is shown in the upper indexes (A,B,C).
LA/Ao — left-atrium-to-aorta ratio; LVIDd — left ventricular inner diameter in diastole; LVIDdN — normalized left ventricular end-diastolic diameter (34); TR — tricuspid regurgitation.
Mode heart dimensions were measured in the subvalvular region.
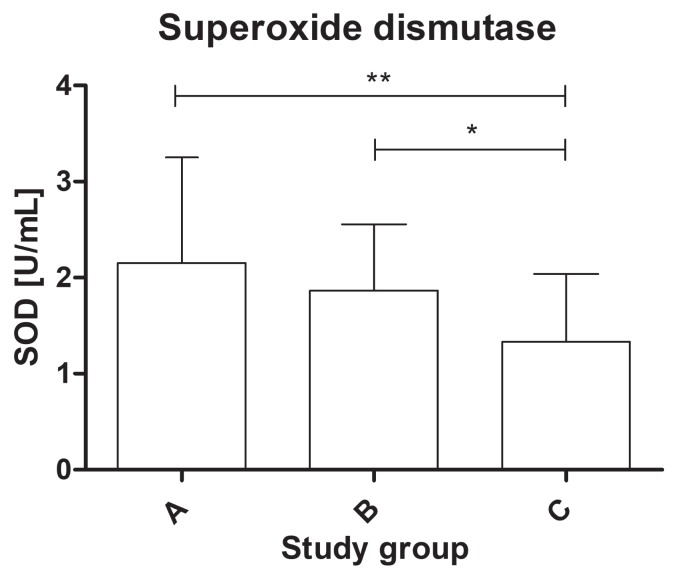
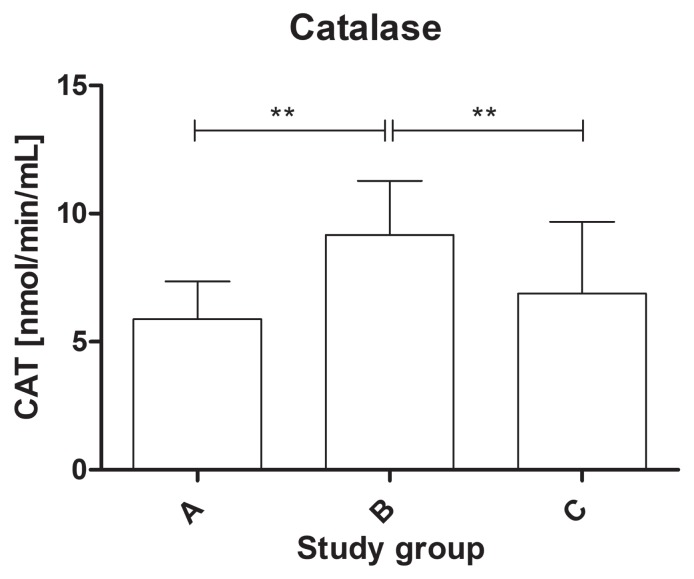
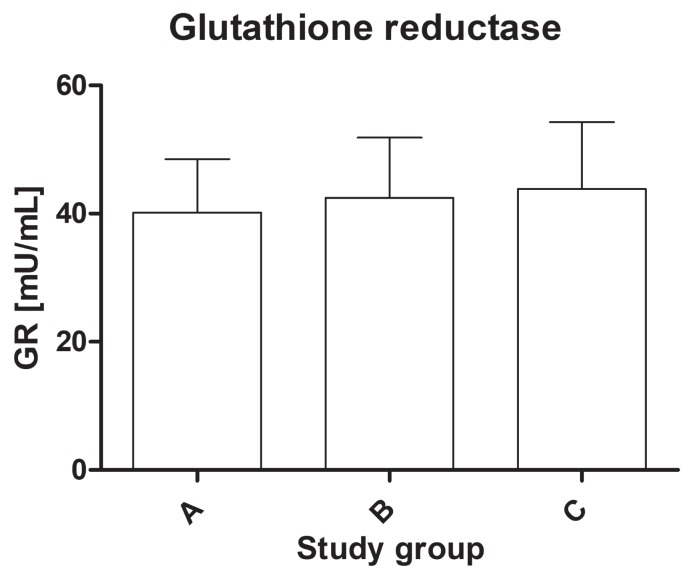
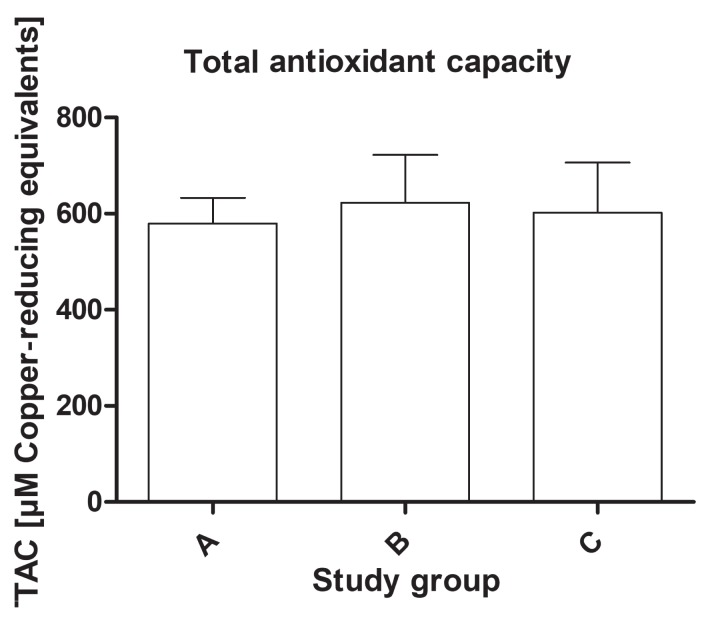
The study found a statistically significant difference in the activity of the antioxidant enzymes among the groups. The activity of SOD-3 was significantly lower in the dogs from group C (mean value: 1.33 ± 0.71 U/mL) compared to group B (mean value: 1.86 ± 0.69 U/mL) and the healthy dogs (mean value: 2.15 ± 1.1 U/mL) (P < 0.01) (Figure 1). The serum activity of catalase was significantly higher in group B (mean value: 9.98 ± 3.32 nmol/ min/mL) than in group A (mean value: 5.89 ± 1.46 nmol/min/mL) and group C (mean value: 7.69 ± 3.62 nmol/min/mL) (P < 0.01) (Figure 2). The activity of glutathione reductase in group A was 40.2 ± 8.26 mU/mL, 42.5 ± 9.38 mU/mL in group B, and 43.9 ± 10.6 mU/mL in group C, and this value did not differ significantly among the groups (P = 0.57) (Figure 3). The total antioxidant capacity of serum in group A was 579 ± 54 copper-reducing equivalents (CRE), 623 ± 99 CRE in group B, and 601 ± 106 CRE in group C and did not differ significantly among the groups (P = 0.61) (Figure 4). There were no associations between age and studied oxidative stress parameters in the multivariable linear regression model. The following intra-assay coefficients of variation (CV) measured for canine samples were obtained: CAT — 23.5%; total oxidant capacity (TAC) — 13.3%; GR — 20.2%; and SOD — 22%.
Figure 1.
The superoxide dismutase (SOD) activity of serum in healthy dogs and dogs with degenerative mitral valve disease (DMVD). * P < 0.05; ** P < 0.01. A — healthy controls; B — asymptomatic DMVD; C — symptomatic, stable DMVD.
Figure 2.
The catalase (CAT) activity of serum in healthy dogs and dogs with degenerative mitral valve disease (DMVD). ** P < 0.01. A — healthy controls; B — asymptomatic DMVD; C — symptomatic, stable DMVD.
Figure 3.
The glutathione reductase (GR) activity of serum in healthy dogs and dogs with degenerative mitral valve disease (DMVD). A — healthy controls; B — asymptomatic DMVD; C — symptomatic, stable DMVD.
Figure 4.
The total antioxidant capacity (TAC) of serum in healthy dogs and dogs with degenerative mitral valve disease (DMVD). A — healthy controls; B — asymptomatic DMVD; C — symptomatic, stable DMVD.
In addition, the symptomatic group of dogs was divided into 2 subgroups: dogs treated pharmacologically (n = 22) and dogs before pharmacological treatment (n = 10) (Table II). There were no significant differences in any of the parameters between the groups.
The correlation analysis revealed a moderate positive correlation between the CAT activity and TAC (r = 0.42, P = 0.001). The activity of catalase weakly correlated with age (r = 0.31, P = 0.012).
Discussion
This study proved that the serum activity of superoxide dismutase and catalase differs between healthy animals and those with asymptomatic and symptomatic DMVD. To the authors’ knowledge, this is the first study to focus on the serum activity of antioxidant enzymes in dogs with DMVD.
Most dogs in this study were small and miniature breeds due to a higher prevalence of DMVD in those breeds than in larger breeds. Despite the fact that Cavalier King Charles spaniels are particularly predisposed to DMVD, they constituted a very small group of subjects due to their low popularity in Poland (15). The mean age of dogs in the groups increased with more advanced heart failure. This is consistent with the authors’ expectations, as the prevalence and severity of the disease increase with age (14).
Mammals have developed enzymatic mechanisms that directly protect the organism from excessive oxidative stress. In the current study, the activity of extracellular isoform of superoxide dismutase (SOD) decreased gradually and significantly. In veterinary literature, there are conflicting reports of SOD activity in dogs with cardiovascular disease. For example, studies on dogs with surgically induced mitral valve insufficiency found a lower cellular activity of SOD in the cardiac tissue from the left ventricle (16). Another study assessing the activity of SOD in an erythrocyte lysate from dogs with idiopathic dilated cardiomyopathy did not confirm this relationship (17). A study on human patients with congestive heart failure found a lower catalytic activity of this enzyme compared to healthy subjects and a negative correlation between its activity and the serum concentration of uric acid, which has an antioxidative effect (18). It may therefore be assumed that the gradual decrease in extracellular SOD activity is a result of declining efficacy of the antioxidant mechanisms caused by an increased oxidative assault. Measuring the SOD activity in blood serum may thus serve as an indicator for evaluating antioxidant status in the circulation of patients with DMVD.
Catalase, which is another parameter analyzed in this study, is considered one of the main intracellular antioxidant enzymes, although it was also reported to catalyze reactions associated with H2O2 metabolism in blood serum (19). Despite the fact that most studies focus on the intracellular activity of this enzyme, mainly in an erythrocyte lysate, there are single reports of its activity in the blood serum of cardiac patients (20). In humans with ischemic heart disease, the activity of serum catalase decreased with the increasing number of vessels affected by atherosclerosis (21). Our findings may suggest therefore that the increase in catalase activity in the asymptomatic stage of the disease was caused by an activation of compensatory mechanisms in response to an excessive formation of free radicals. These mechanisms were most likely exhausted in the symptomatic stage of the disease.
Glutathione reductase (GR) plays a key role in regenerating oxidized glutathione, which is a tripeptide with antioxidant properties, helping it to maintain its function. A study carried out on humans revealed that the activity of that enzyme significantly increases in patients with unstable angina or myocardial infarction, although there was no difference between patients with stable angina and the control group (22). This observation was confirmed by another study that reported increased GR activity in the blood plasma of patients admitted to hospital with unstable angina compared to patients at discharge and in the control group (23). Those results suggest that an acute stage of cardiovascular disease significantly disrupts glutathione metabolism. This is in accordance with our findings as we did not find significant differences in the GR activity among the studied groups of dogs. The patients with symptomatic DMVD in our study were in a stable stage of the disease, which may have significantly affected the activity of glutathione reductase.
The total antioxidant capacity (TAC) is measured in order to determine the ability of the organism to neutralize free radicals (24). The cupric-reducing antioxidant capacity method (CUPRAC) used in our study enables the measurement of the total antioxidant capacity formed by thiol-group antioxidants, vitamins (ascorbic acid, α-tocopherol, β-carotene), bilirubin, albumin, and uric acid (25). It is also known that TAC is not associated with age and sex in healthy dogs (26). This parameter has been repeatedly analyzed in human and canine cardiovascular diseases, with conflicting results. Dogs with chronic heart failure secondary to DMVD and DCM have significantly higher plasma antioxidant capacity than healthy dogs (7). In another study, dogs with cardiac disease (DMVD and DCM) had a significantly higher TAC, measured based on the ferric-reducing ability of the plasma, than the control group, although an analysis using the ABTS method did not reveal differences between the 2 study groups (27). There were no significant differences in the TAC values between dogs with DMVD and DCM in either of the 2 studies (7,27).
Interestingly, another recent study reported a significantly lower plasma TAC in dogs with an asymptomatic phase of spontaneous cardiovascular disease compared to healthy dogs, although no differences were found between dogs with chronic heart failure and the control group (10). In humans, a strong correlation was found between TAC and the degree of dilated cardiomyopathy expressed in ejection fractions (28). In our study, there was no significant difference in the total antioxidant capacity of serum among the groups and this parameter did not correlate with the stage of the disease [expressed in left-atrium-to-aorta ratio (LA/Ao), left ventricular inner diameter in diastole (LVIDd)]. In addition, there was no correlation of this parameter with fractional shortening (FS). This may be caused by the presence of stable and compensated heart failure in the dogs included in our study. Each method used to measure TAC is distinctive, however, and determines different components of the antioxidant defence, which is reflected in the different study results (24).
In this study, there were no differences between the group of dogs with heart failure treated pharmacologically and those without treatment, which is surprising. Several studies reported that the drugs used in standard therapy for chronic heart failure in dogs, such as angiotensin-converting enzyme inhibitors, spironolactone, beta-blockers, or calcium channel blockers, alleviate oxidative stress (29–32). Each drug represents its unique mechanism of free-radical scavenging action, which is usually linked to its chemical structure (33). The results of our study are in accordance with other studies carried out on dogs. For example, no significant difference was found between treated and non-treated dogs with DMVD and with DCM (8). In our study, however, the subgroups were relatively small, the dogs did not receive uniform pharmacotherapy as it was tailored to the needs of each dog, and the treatment duration was not taken into consideration. As a result, the effect of pharmacotherapy for heart failure on oxidative stress in dogs still needs to be studied.
This study had several limitations. In order to obtain practical results, the dogs were divided into groups based on the current ACVIM classification system for DMVD. Dogs with various severity levels of the disease were therefore included in the symptomatic DMVD group, although all were in stable condition. Group B1 and B2 of the ACVIM classification were combined to increase the sample size for the statistical analysis, which should also be considered a limitation. The analysis of a non-standardized group of dogs in terms of the pharmacological treatment may have affected the results. Although all dogs were fed commercial diets with no additional supplementation with antioxidants, the influence of antioxidant additives that might have been included in these diets cannot be excluded.
In summary, degenerative mitral valve disease had a significant impact on the activity of superoxide dismutase and catalase in the serum of the tested dogs. The activity of superoxide dismutase gradually decreased in the more advanced stages of DMVD, while the activity of catalase was significantly higher in the group of dogs with asymptomatic DMVD. Further research should focus on substances that increase the antioxidant capacity in sick animals, which could positively affect the clinical outcome. Other biomarkers of oxidative stress and antioxidative enzymes in other biological material of dogs with DMVD warrant further study.
Acknowledgment
This work was supported by the Wrocław University of Environmental and Life Sciences (Poland) as PhD research program ‘Innowacyjny Doktorat,’ no. D220/0003/17.
References
- 1.Borgarelli M, Häggström J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract. 2010;40:651–663. doi: 10.1016/j.cvsm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Häggström J, Hoglund K, Borgarelli M. An update on treatment and prognostic indicators in canine myxomatous mitral valve disease. J Small Anim Pract. 2009;50:25–33. doi: 10.1111/j.1748-5827.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 3.Whitney JC. Observations on the effect of age on the severity of heart valve lesions in the dog. J Small Anim Pract. 1974;15:511–522. doi: 10.1111/j.1748-5827.1974.tb06529.x. [DOI] [PubMed] [Google Scholar]
- 4.Borgarelli M, Zini E, D’Agnolo G, et al. Comparison of primary mitral valve disease in German Shepherd dogs and in small breeds. J Vet Cardiol. 2004;6:27–34. doi: 10.1016/S1760-2734(06)70055-8. [DOI] [PubMed] [Google Scholar]
- 5.Malik R, Hunt GB, Allan GS. Prevalence of mitral valve insufficiency in cavalier King Charles spaniels. Vet Rec. 1992;130:302–303. doi: 10.1136/vr.130.14.302. [DOI] [PubMed] [Google Scholar]
- 6.Boswood A, Gordon SG, Häggström J, et al. Longitudinal analysis of quality of life, clinical, radiographic, echocardiographic, and laboratory variables in dogs with preclinical myxomatous mitral valve disease receiving pimobendan or placebo: The EPIC study. J Vet Intern Med. 2018;32:72–85. doi: 10.1111/jvim.14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman LM, Rush JE, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with congestive heart failure. J Vet Intern Med. 2005;19:537–541. doi: 10.1892/0891-6640(2005)19[537:asaboo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Verk B, Nemec Svete A, Salobir J, Rezar V, Domanjko Petrič A. Markers of oxidative stress in dogs with heart failure. J Vet Diagn Invest. 2017;29:636–644. doi: 10.1177/1040638717711995. [DOI] [PubMed] [Google Scholar]
- 9.Kulka M, Garncarz M, Parzeniecka-Jaworska M, Kluciński W. Serum paraoxonase 1 activity and lipid metabolism parameter changes in Dachshunds with chronic mitral valve disease. Assessment of its diagnostic usefulness. Pol J Vet Sci. 2017;20:723–729. doi: 10.1515/pjvs-2017-0090. [DOI] [PubMed] [Google Scholar]
- 10.Svete AN, Verk B, Seliškar A, Tomsič K, Križman PJ, Petrič AD. Plasma coenzyme Q10 concentration, antioxidant status, and serum N-terminal pro-brain natriuretic peptide concentration in dogs with various cardiovascular diseases and the effect of cardiac treatment on measured variables. Am J Vet Res. 2017;78:447–457. doi: 10.2460/ajvr.78.4.447. [DOI] [PubMed] [Google Scholar]
- 11.Reimann MJ, Häggström J, Møller JE, Lykkesfeldt J, Falk T, Olsen LH. Markers of oxidative stress in dogs with myxomatous mitral valve disease are influenced by sex, neuter status, and serum cholesterol concentration. J Vet Intern Med. 2017;31:295–302. doi: 10.1111/jvim.14647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutteridge JMC, Halliwell B. Mini-review: Oxidative stress, redox stress or redox success? Biochem Biophys Res Commun. 2018;502:183–186. doi: 10.1016/j.bbrc.2018.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Lei XG, Zhu JH, Cheng WH, et al. Paradoxical roles of antioxidant enzymes: Basic mechanisms and health implications. Physiol Rev. 2016;96:307–364. doi: 10.1152/physrev.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142–1150. doi: 10.1111/j.1939-1676.2009.0392.x. [DOI] [PubMed] [Google Scholar]
- 15.Garncarz M, Parzeniecka-Jaworska M, Jank M, Łój M. A retrospective study of clinical signs and epidemiology of chronic valve disease in a group of 207 Dachshunds in Poland. Acta Vet Scand. 2013;55:52. doi: 10.1186/1751-0147-55-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad K, Gupta JB, Kalra J, Lee P, Mantha SV, Bharadwaj B. Oxidative stress as a mechanism of cardiac failure in chronic volume overload in canine model. J Mol Cell Cardiol. 1996;28:375–385. doi: 10.1006/jmcc.1996.0035. [DOI] [PubMed] [Google Scholar]
- 17.Freeman LM, Brown DJ, Rush JE. Antioxidant status in dogs with idiopathic dilated cardiomyopathy. J Nutr. 1998;128:2768S–2770S. doi: 10.1093/jn/128.12.2768S. [DOI] [PubMed] [Google Scholar]
- 18.Alcaino H, Greig D, Chiong M, et al. Serum uric acid correlates with extracellular superoxide dismutase activity in patients with chronic heart failure. Eur J Heart Fail. 2008;10:646–651. doi: 10.1016/j.ejheart.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Leff JA, Oppegard MA, Terada LS, McCarty EC, Repine JE. Human serum catalase decreases endothelial cell injury from hydrogen peroxide. J Appl Physiol (1985) 1991;71:1903–1906. doi: 10.1152/jappl.1991.71.5.1903. [DOI] [PubMed] [Google Scholar]
- 20.Flores-Mateo G, Carrillo-Santisteve P, Elosua R, et al. Antioxidant enzyme activity and coronary heart disease: Meta-analyses of observational studies. Am J Epidemiol. 2009;170:135–147. doi: 10.1093/aje/kwp112. [DOI] [PubMed] [Google Scholar]
- 21.Serdar Z, Aslan K, Dirican M, Sarandöl E, Yes˛ilbursa D, Serdar A. Lipid and protein oxidation and antioxidant status in patients with angiographically proven coronary artery disease. Clin Biochem. 2006;39:794–803. doi: 10.1016/j.clinbiochem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Zuzak E, Horecka A, Kielczykowska M, et al. Glutathione level and glutathione reductase activity in serum of coronary heart disease patients. J Pre-Clin Clin Res. 2017;11:103–105. [Google Scholar]
- 23.Sapira V, Cojocaru IM, Socoliuc G, et al. Glutathione reductase levels in patients with unstable angina. Rom J Intern Med. 2011;49:197–201. [PubMed] [Google Scholar]
- 24.Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet Res. 2016;12:166. doi: 10.1186/s12917-016-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apak R, Güçlü K, Ozyürek M, Karademir SE, Altun M. Total antioxidant capacity assay of human serum using copper (II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic Res. 2005;39:949–961. doi: 10.1080/10715760500210145. [DOI] [PubMed] [Google Scholar]
- 26.Tomsič K, Seliškar A, Lukanc B, Nemec Svete A. Plasma total antioxidant capacity and activities of blood glutathione peroxidase and superoxide dismutase determined in healthy dogs by using commercially available kits. Acta Veterinaria-Beograd. 2016;66:534–548. [Google Scholar]
- 27.Hetyey CS, Manczur F, Dudás-Györki Z, et al. Plasma antioxidant capacity in dogs with naturally occurring heart diseases. J Vet Med A Physiol Pathol Clin Med. 2007;54:36–39. doi: 10.1111/j.1439-0442.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 28.Demirbag R, Yilmaz R, Erel O, Gultekin U, Asci D, Elbasan Z. The relationship between potency of oxidative stress and severity of dilated cardiomyopathy. Can J Cardiol. 2005;21:851–855. [PubMed] [Google Scholar]
- 29.Chandran G, Sirajudeen KN, Yusoff NS, Swamy M, Samarendra MS. Effect of the antihypertensive drug enalapril on oxidative stress markers and antioxidant enzymes in kidney of spontaneously hypertensive rat. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/608512. 608512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Queisser N, Happ K, Link S, et al. Aldosterone induces fibrosis, oxidative stress and DNA damage in livers of male rats independent of blood pressure changes. Toxicol Appl Pharmacol. 2014;280:399–407. doi: 10.1016/j.taap.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K, Murakami M, Miura D, et al. Beta-blockers and oxidative stress in patients with heart failure. Pharmaceuticals (Basel) 2011;4:1088–1100. doi: 10.3390/ph4081088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anjaneyulu M, Chopra K. Diltiazem attenuates oxidative stress in diabetic rats. Ren Fail. 2005;27:335–344. [PubMed] [Google Scholar]
- 33.Weglicki WB, Mak IT, Simìc MG. Mechanisms of cardiovascular drugs as antioxidants. J Mol Cell Cardiol. 1990;22:1199–1208. doi: 10.1016/0022-2828(90)90083-e. [DOI] [PubMed] [Google Scholar]
- 34.Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J Vet Intern Med. 2004;18:311–321. doi: 10.1892/0891-6640(2004)18<311:asomcm>2.0.co;2. [DOI] [PubMed] [Google Scholar]