Abstract
Objective
This surveillance study was conducted to verify the post-market safety and effectiveness of bevacizumab, which was approved in Japan in 2013 for the treatment of patients with newly diagnosed and or recurrent malignant glioma.
Methods
This was a prospective, observational, multicenter post-marketing surveillance study. Patients with newly diagnosed or recurrent malignant glioma scheduled for bevacizumab treatment were enrolled. The incidence and severity of adverse drug reactions were calculated. The effectiveness of bevacizumab was assessed by the 1-year survival rate and the overall survival rate.
Results
The safety analysis set and the effectiveness analysis set each comprised 258 of the 268 enrolled patients: tumours were newly diagnosed in 80 patients (31%) and recurrent in 178 patients (68.9%). The incidence of grade ≥ 3 adverse drug reactions was 15.1%. Adverse drug reactions of special interest included 14 cerebral bleeding events and 11 infections. Of the 80 patients with newly diagnosed malignant glioma, 44 (55%) were alive throughout the 18-month observation period. The 1-year survival rate for patients with newly diagnosed glioblastoma was 78%. Median overall survival was not calculated, but 51.2% of patients were alive at the last date of observation of the last observed patient. In patients with recurrent glioblastoma, the 1-year survival rate was 38.9%, and the median overall survival was 10.2 months.
Conclusions
The results suggest no new safety concerns, and the effectiveness might be similar to previously reported data in clinical trials. Therefore, bevacizumab is considered as one of the treatment options for patients with malignant glioma in real-world clinical practice.
Keywords: bevacizumab, malignant glioma, newly diagnosed, post-marketing surveillance, recurrent
The post-marketing surveillance study of bevacizumab demonstrated the safety and effectiveness for the treatment of Japanese patients with newly diagnosed and recurrent malignant glioma.
Introduction
Malignant gliomas, including glioblastoma (GBM), anaplastic astrocytoma and anaplastic oligodendroglioma, are the most common subtypes of primary brain tumours. These aggressive, highly invasive and neurologically destructive tumours (1) have an incidence of 5–20 cases per 1 00 000 population in Japan (2) and the United States (3). The standard treatment for patients with newly diagnosed GBM is safe, maximal surgical resection, followed by radiotherapy with concurrent temozolomide and maintenance temozolomide (4). Tumour-treating field therapy has recently been introduced as an effective antimitotic treatment with minimal toxicity in patients with newly diagnosed GBM (5). However, most of the patients experience tumour progression with a median survival of <15 months from the initial diagnosis (6–9), a 1-year survival rate of 61–65% (8,10) and a 5-year survival rate of 16% (10). Furthermore, there is no standard therapy for recurrent GBM, and the estimated 6-month progression-free survival (PFS) rate is 11–16% (6,9).
A promising alternative therapeutic approach is the inhibition of angiogenesis through vascular endothelial growth factor (VEGF), which is a key regulator of angiogenesis. Overexpressed VEGF and angiogenesis, prominent features of many cancers including GBM, are characterized by tortuous blood vessels, vascular permeability and hypoxia, which lead to necrosis (11–13). Bevacizumab is a recombinant humanized monoclonal antibody targeting VEGF that blocks the binding of human VEGF to its receptors (14–16); it is known to induce significant changes in glioma vascular physiology and improves intratumoural oxygenation, thus inducting and enhancing the antitumour activity of ionizing radiation (17,18).
The US Food and Drug Administration granted accelerated approval to bevacizumab for the treatment of recurrent GBM in 2009 on the basis of the BRAIN trial (AVF3708g) (19), a phase II open-label, non-comparative trial of bevacizumab alone and bevacizumab plus irinotecan, and full approval for this indication was granted in 2017. Based on this trial, the phase II JO22506 study (20) was conducted in Japan to confirm the efficacy and safety of bevacizumab monotherapy in Japanese patients (n = 31) with recurrent malignant glioma. For newly diagnosed GBM, the AVAglio study (BO21990) (21), a phase III trial of bevacizumab in combination with the Stupp regimen (8) followed by maintenance bevacizumab until progression, was conducted including Japanese patients. In 2013, bevacizumab was approved in Japan for the treatment of newly diagnosed or recurrent malignant glioma on the basis of these three studies. The approval for treating newly diagnosed glioma was based on clinically meaningful prolongation of PFS and the non-detrimental effect on overall survival (OS) in combination with the standard regimen in the AVAglio study. The Japanese health authority assessed that the improvement of PFS would maintain patients’ general condition longer and allow the reduction or cessation of corticosteroid use (22).
Patients with poor performance status (PS) were excluded from the AVAglio study, and other clinical trials did not generally include many patients with large tumours. Thus, we conducted a post-marketing surveillance (PMS) study to evaluate the safety and effectiveness of bevacizumab in Japanese patients with newly diagnosed or recurrent malignant glioma in real-world clinical practice.
In particular, because the incidence proportion of cerebral bleeding events, including tumour-related bleeding, and infectious diseases in the AVAglio study was higher in the bevacizumab group than in the placebo group (21,22), cerebral bleeding events and infections were assessed as adverse drug reactions (ADRs) of special interest in this PMS study.
Materials and methods
Study design
This was a prospective, observational, multicenter PMS study of the safety and effectiveness of bevacizumab (Avastin®, Chugai Pharmaceutical Co., Ltd, Tokyo, Japan) in Japanese patients with malignant glioma. Registered patients received intravenous infusions of bevacizumab at a dose of 10 mg/kg every 2 weeks or 15 mg/kg every 3 weeks according to the package insert (http://www.info.pmda.go.jp/go/pack/4291413A1022_1_20/). Investigators chose the dose and regimen on the basis of each patient’s condition.
The study was conducted in accordance with the Japanese regulatory requirements stipulated in good post-marketing study practice (GPSP). The need for Institutional Review Board/Ethics Committee approval was waived under GPSP. Informed consent was obtained from all patients receiving bevacizumab according to the package insert of bevacizumab, but the need for informed consent to participate in this PMS study was waived under GPSP.
Patients and data assessment
Patients planning to receive bevacizumab for the treatment of newly diagnosed or recurrent malignant glioma in Japan were registered using a central registration system. Newly diagnosed patients who had GBM with tumour at baseline were the patients with GBM harbouring residual disease prior to bevacizumab administration, which was defined as the presence of tumour regardless of whether patients had surgery or not. The observation period was from September 2013 to November 2016. After starting treatment, each patient was followed up for 18 months or until the discontinuation period of bevacizumab, which was set to cover the onset times of cerebral bleeding events (4–555 days after starting treatment) observed in the AVAglio study. The clinical data collected included patient characteristics, treatment history of the primary disease, details of bevacizumab treatment, concurrent treatment, outcome and adverse events (AEs). The patient characteristics included height, weight, pregnancy status, in-patient/out-patient status, Karnofsky PS, newly diagnosed/recurrent status, classification of malignant glioma, tumour location, tumour size, maximal cross-sectional area of the tumour at baseline, history of allergy and medical history. AEs that occurred during the study were collected regardless of causal relationship to bevacizumab. Physicians reported AEs and their date of onset, grade, seriousness, interventions (including how bevacizumab treatment was handled), outcome, date of outcome and relationship to bevacizumab.
Reported events were graded by physicians according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0 and were coded and grouped according to the Medical Dictionary for Regulatory Activities/Japanese edition ver. 19.1. In case of cerebral bleeding events, additional information including the site of the event and the method of diagnosis (computed tomography or magnetic resonance imaging) was recorded. Cerebral bleeding events and infections were studied as ADRs of special interest. Cerebral bleeding events including cerebral haemorrhage, haemorrhage intracranial, intracranial tumour haemorrhage, tumour haemorrhage and cerebral microhaemorrhage were defined as ‘cerebral bleeding events’. The severity of cerebral bleeding events was assessed according to the CTCAE grade score of haemorrhage intracranial. Infection events including anal fistula, breast abscess, cholecystitis acute, herpes zoster, pneumonia, pneumonia aspiration, septic shock and wound infection were defined as ‘infections’. An ADR was defined as an AE for which a causal relationship with the drug could not be ruled out. The effectiveness of bevacizumab was assessed by examining the 1-year survival rate and the OS rate.
Statistical analysis
Sample size was based on the proportion of cerebral bleeding events (4.3%) observed in the bevacizumab group of the AVAglio study. If the proportion of cerebral bleeding events was similar to that in the AVAglio study, a sample size of 240 patients would show that the upper limit of the 95% confidence interval was ≤7.7%. A sample size of 240 patients provided 95% power to detect at least one event occurring in 1.25% of patients.
Thus, infections that occurred more frequently in the bevacizumab group than in the placebo group in the AVAglio study, such as nasopharyngitis (12.9%), urinary tract infection (9.5%), upper respiratory infection (6.5%) and sepsis (1.3%), could also be detected in 240 patients. Therefore, in this study, case report forms (CRFs) would be collected for 264 patients, taking into account the patient dropout rate.
The safety analysis set was defined as all patients for whom CRFs were collected, excluding those who did not receive bevacizumab. The effectiveness analysis set included all patients in the safety analysis set, excluding those who did not have any effectiveness data. The χ2 test was used to examine the association between ADR incidence and patient characteristics, and the significance level was set at 5% (P < 0.05). The 1-year survival rate and the OS rate were calculated using the Kaplan–Meier method. OS rate was defined as the time from the first dose of bevacizumab to the date of death (regardless of the cause of death) or censoring on the last date of survival.
Results
Patient disposition and characteristics
CRFs were collected for 263 of 268 patients registered at 74 contracted sites in Japan. The safety analysis set comprised 258 patients after the exclusion of those registered after the start of administration (n = 4) and those who started administration after the end of registration (n = 1). The effectiveness analysis set also comprised 258 patients (Fig. 1). The patient characteristics are summarized in Table 1. Of the 258 patients, 80 (31%) were newly diagnosed and 178 (68.9%) had recurrent tumours. GBM was confirmed in 57 patients (71.2%) with newly diagnosed malignant glioma and in 98 patients (55%) with recurrent malignant glioma. The median (range) ages of patients with newly diagnosed or recurrent disease were 63.5 years (4–86 years) and 58.5 years (3–92 years), respectively. The ratio of male patients was slightly higher than that of female patients with both newly diagnosed disease (55%) and recurrent disease (52.2%). Tumours remained in 67 patients (83.7%) with newly diagnosed malignant glioma and in 156 patients (87.6%) with recurrent malignant glioma. The distribution of the maximal cross-sectional area of the tumours at baseline in patients with newly diagnosed GBM is shown in the Online Resource Figure. The mean maximal cross-sectional area ± SD of the tumours at baseline in patients with newly diagnosed GBM (n = 41) was 951.7 ± 1092.9 mm2. Most of the patients (90.6%) underwent surgery, and 89.1% of the patients received radiotherapy before starting bevacizumab treatment (Table 1). Among 19 patients with newly diagnosed glioma who had not received radiotherapy before starting bevacizumab, 9 started radiotherapy concurrently with bevacizumab and 10 received no initial radiotherapy with bevacizumab due to age and tumour conditions.
Figure 1.
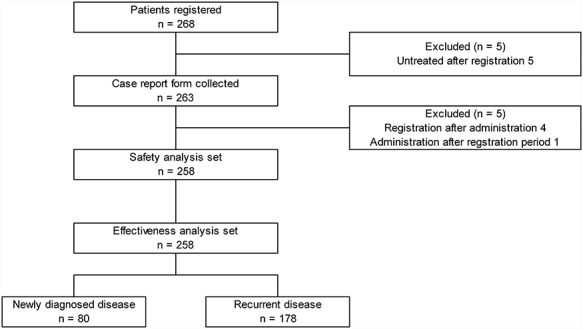
Flow diagram of patients.
Table 1.
Patient characteristics
| All N = 258 | Newly diagnosed 80 (31%) |
Recurrent 178 (68.9%) |
||
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 137 (53.1) | 44 (55) | 93 (52.2) | |
| Female | 121 (46.8) | 36 (45) | 85 (47.7) | |
| Age (years), n (%) | ||||
| <15 | 4 (1.5) | 1 (1.2) | 3 (1.6) | |
| ≥15, <65 | 160 (62) | 42 (52.5) | 118 (66.2) | |
| 65≤ | 94 (36.4) | 37 (46.2) | 57 (32) | |
| Median (range), years | 61 (3–92) | 63.5 (4–86) | 58.5 (3–92) | |
| Karnofsky PS, n (%) | ||||
| 100~70 | 145 (56.2) | 46 (57.5) | 99 (55.6) | |
| 60~50 | 84 (32.5) | 22 (27.5) | 62 (34.8) | |
| 40~10 | 29 (11.2) | 12 (15) | 17 (9.5) | |
| Histology of primary tumour, n (%) | ||||
| Glioblastoma | 155 (60) | 57 (71.2) | 98 (55) | |
| Anaplastic astrocytoma or anaplastic | ||||
| oligodendroglioma or anaplastic | 74 (28.6) | 13 (16.2) | 61 (34.2) | |
| oligoastrocytoma | ||||
| Other | 21 (8.1) | 2 (2.5) | 19 (10.6) | |
| Not specified | 8 (3.1) | 8 (10) | 0 (0) | |
| Tumour at baseline, n (%) | ||||
| No | 34 (13.1) | 12 (15) | 22 (12.3) | |
| Yes | 223 (86.4) | 67 (83.7) | 156 (87.6) | |
| Unknown | 1 (0.3) | 1 (1.2) | 0 (0) | |
| Median (range), mm2 | 691.5 (0–7560) | 696.5 (0–7000) | 686 (2.2–7560) | |
| Treatment before starting bevacizumab, n (%) | ||||
| Surgery | No | 24 (9.3) | 10 (12.5) | 14 (7.8) |
| Yes | 234 (90.6) | 70 (87.5) | 164 (92.1) | |
| Radiotherapy | No | 28 (10.8) | 19 (23.7) | 9 (5) |
| Yes | 230 (89.1) | 61 (76.2) | 169 (94.9) | |
| Chemotherapy | No | 53 (20.5) | 13 (16.2) | 40 (22.4) |
| Yes | 205 (79.4) | 67 (83.7) | 138 (77.5) | |
| Temozolomide | No | 63 (24.4) | 14 (17.5) | 49 (27.5) |
| Yes | 195 (75.5) | 66 (82.5) | 129 (72.4) | |
PS, performance status.
Treatment status
Bevacizumab treatment was discontinued in 225 patients (87.2%) during the observation period. The reasons for discontinuation were disease progression (n = 104, 46.2%) and AEs (n = 27, 12%). The median (range) duration of bevacizumab treatment was 191 days (1–783 days) for newly diagnosed disease and 127 days (1–767 days) for recurrent disease. Some patients were observed for longer than 18 months. The median dose was 10 mg/kg (range: 9.2–15 mg/kg; Online Resource Table). Concurrent temozolomide was given to 81.2% of patients with newly diagnosed disease and 53.9% of patients with recurrent disease. During the study, radiotherapy was administered to 51.2% of patients with newly diagnosed disease and 14% of patients with recurrent disease. Concomitant anticoagulants were given to 5.4% of patients (n = 14). Nearly half of the patients (41%) had concomitant diseases, including hypertension (19.7%), hyperlipidaemia (10.4%), diabetes mellitus (5%) and thromboembolism (3.4%).
Safety results
A total of 173 ADRs were reported in 77 patients (29.8%). ADRs with incidence proportions >2% included proteinuria (n = 12, 4.6%), hypertension (n = 10, 3.8%), platelet count decreased (n = 8, 3.1%), cerebral haemorrhage (n = 7, 2.7%), malaise (n = 6, 2.3%) and lymphocyte count decreased (n = 6, 2.3%) (Table 2).
Table 2.
Adverse drug reactions (ADRs)
| Number of patients (%), N = 258 | |||
|---|---|---|---|
| Grade ≤ 2 | Grade ≥ 3 | Total | |
| ADRs with incidence >2% | |||
| Proteinuria | 9 (3.4) | 4 (1.5) | 12 (4.6) |
| Hypertension | 8 (3.1) | 2 (0.7) | 10 (3.8) |
| Platelet count decreased | 5 (1.9) | 4 (1.5) | 8 (3.1) |
| Cerebral haemorrhage | 5 (1.9) | 2 (0.7) | 7 (2.7) |
| Malaise | 5 (1.9) | 1 (0.3) | 6 (2.3) |
| Lymphocyte count decreased | 2 (0.7) | 4 (1.5) | 6 (2.3) |
| ADRs of special interest | |||
| Cerebral bleeding events | 9 (3.4) | 5 (1.9) | 14 (5.4) |
| Infections | 3 (1.1) | 7 (2.7) | 10 (3.8) |
Medical Dictionary for Regulatory Activities/Japanese edition version (19.1).
A total of 59 grade ≥ 3 ADRs were observed in 39 patients (15.1%) including proteinuria, platelet count decreased and lymphocyte count decreased (each n = 4, 1.5%). A total of 61 serious ADRs were observed in 41 patients (15.8%). The major serious ADRs were cerebral haemorrhage (n = 7, 2.7%), cerebral infarction (n = 4, 1.5%), tumour haemorrhage (n = 3, 1.1%) and platelet count decreased (n = 3, 1.1%). During the study, there were seven deaths due to tumour haemorrhage, pneumonia, lung cancer metastatic, hepatic neoplasm, gallbladder cancer, pulmonary infarction or cerebral haemorrhage (one each), which were considered to be related to bevacizumab treatment.
Regarding ADRs of special interest, 14 cerebral bleeding events occurred in 14 patients (5.4%; 95% CI: 2.9–8.9; grade ≤ 2, n = 9; grade ≥ 3, n = 5) (Table 2), comprising tumour-related cerebral bleeding events (n = 13, 5%, 95% CI: 2.7–8.4) and tumour-unrelated cerebral bleeding events (n = 1, 0.3%, 95% CI: 0–2.1). The cerebral bleeding events comprised cerebral haemorrhage (grade ≤ 2, n = 5; grade ≥ 3, n = 2), haemorrhage intracranial (grade ≥ 3, n = 1), intracranial tumour haemorrhage (grade ≤ 2, n = 1), tumour haemorrhage (grade ≤ 2, n = 2; grade ≥ 3, n = 2) and cerebral microhaemorrhage (grade ≤ 2, n = 1). The outcomes of cerebral bleeding events were recovered/improved (n = 7), not recovered (n = 3), fatal (n = 2), recovered with sequelae (n = 1) and unknown (n = 1). The onset times were 2–324 days after the start of treatment and 10 events occurred within 4 months.
A total of 11 infections occurred in 10 patients (3.8%; 95% CI: 1.8–7; grade ≤ 2, n = 3; grade ≥ 3, n = 7) (Table 2), comprising pneumonia (grade ≤ 2, n = 2; grade ≥ 3, n = 1), wound infection (grade ≥ 3, n = 2), anal fistula (grade ≤ 2, n = 1), breast abscess (grade ≥ 3, n = 1), herpes zoster (grade ≤ 2, n = 1), pneumonia aspiration (grade ≥ 3, n = 1), septic shock (grade ≥ 3, n = 1) and cholecystitis acute (grade ≥ 3, n = 1). They included eight serious ADRs, of which seven were grade ≥ 3 ADRs. The outcomes of patients with grade ≥ 3 infections were recovered/improved (n = 5), not recovered (n = 1) and fatal (n = 1). The onset times were 7–514 days after the start of treatment, and infections were observed throughout the observation period. Five of the patients with infections received concomitant corticosteroids.
Efficacy
Of the 80 patients with newly diagnosed malignant glioma, 44 (55%) were alive throughout the 18-month observation period, excluding patients with unknown outcome (n = 10, 12.5%). The reasons for death (n = 26) were disease progression (n = 24), ADRs (n = 1) and other (n = 1). The periods when the deaths occurred were 27–546 days after the start of treatment. The 1-year survival rate was 73.5% for patients with newly diagnosed malignant glioma (Table 3). Median OS was not calculated, but 56% of the patients were still alive at the last date of observation of the last observed patient (870 days after the start of the study). The 1-year survival rate was 78% for patients with newly diagnosed GBM (Table 3, Fig. 2(a)). Median OS was not calculated, and 51.2% of the patients were still alive at the last date of observation of the last observed patient (870 days after the start of the study). The 1-year survival rate was 72% for patients with newly diagnosed GBM with the tumour at baseline, and the median OS was 546 days (18.2 months) (Table 3, Fig. 2(b)). Of the 178 patients with recurrent malignant glioma, 52 (29.2%) were alive throughout the 18-month observation period, excluding unknown patients (n = 24, 13.4%). The reasons for death (n = 102) were disease progression (n = 96), ADRs (n = 4), other (n = 1) and unknown (n = 1). The periods when the deaths occurred were 19–715 days after the start of treatment. The 1-year survival rate was 45.7% for patients with recurrent malignant glioma, and the median OS was 324 days (10.8 months). In patients with recurrent GBM, the 1-year survival rate was 38.9% and the median OS was 307 days (10.2 months) (Table 3, Fig. 2(c)). The Kaplan–Meier survival curve of patients with newly diagnosed GBM classified according to Karnofsky performance status (KPS) (≥70 vs. <70) is shown in Supplementary Fig. S2.
Table 3.
One-year survival rate and median overall survival (OS)
| Number of patients survived 1 year | 1-Year survival rate (%)* | Median OS (days) | |
|---|---|---|---|
| Newly diagnosed malignant glioma (N = 80) | 41 | 73.5 | Not reached |
| Newly diagnosed GBM (N = 57) | 29 | 78 | Not reached |
| Newly diagnosed GBM with the tumour at baseline (N = 46) | 21 | 72 | 546 |
| Recurrent malignant glioma (N = 178) | 55 | 45.7 | 324 |
| Recurrent GBM (N = 98) | 22 | 38.9 | 307 |
GBM, glioblastoma.
*Each 1-year survival rate was estimated using the Kaplan–Meier method.
Figure 2.
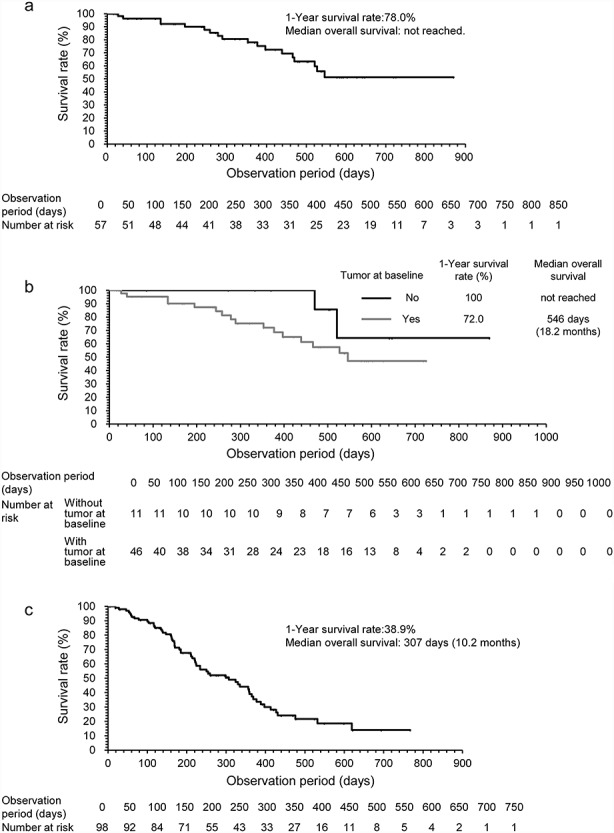
Kaplan–Meier analyses. (a) Overall survival (OS) in patients with newly diagnosed glioblastoma (GBM), (b) in patients with newly diagnosed GBM with/without the tumour at baseline and (c) OS in patients with recurrent GBM.
Discussion
We designed this prospective, observational, multicenter PMS study to evaluate the safety and effectiveness of bevacizumab in patients with newly diagnosed or recurrent malignant glioma in clinical practice. Randomized controlled trials can provide the highest levels of clinical evidence with the least bias but cannot collect data on patients with poor PS or with large tumours, which is relevant for use in real-world applications.
In the AVAglio study, the incidence proportions of cerebral bleeding events and infections in the bevacizumab group were higher than those in the placebo group (21,22). Therefore, both ADRs were assessed as ADRs of special interest in this study. In the AVAglio study, the incidence proportion of cerebral bleeding events was 2.5% in the placebo group and 4.3% in the bevacizumab group (22), suggesting that bevacizumab may contribute to intracranial haemorrhage as an ADR. This should be considered when bevacizumab is used for treatment of patients with malignant glioma, since cerebral bleeding also occurred in our study with an incidence proportion of 5.4% (95% CI: 3–8.9), which was higher than that found in the placebo group in the AVAglio study. Furthermore, of the 14 patients with cerebral bleeding in our study, not a few experienced this AE of high grade; five patients had grade ≥ 3 and two patients died of this event. Therefore, occurrence of cerebral bleeding events after administration of bevacizumab should be taken into consideration. The incidence of infections in the current study was 3.8%, which was less than that reported in the AVAglio study (51.9%). In the current study, there were no occurrences of the following ADRs for which the incidence of corresponding events was higher in the bevacizumab group than in the placebo group in the AVAglio study: nasopharyngitis (12.9%), urinary tract infection (9.5%) and upper respiratory infection (6.5%). It should be noted that our real-world study included patients with poor PS who were excluded from the AVAglio study, but the incidence of ADRs was similar between the studies. Only ADRs requiring treatment were reported because this was a study of the real-world setting, which suggests that reporting bias is a limitation.
In the AVAglio study, 67–69.7% of patients had a Karnofsky PS ≥90 and 41–42.3% of patients had a complete resection. Median PFS, a co-primary endpoint, was longer in the bevacizumab group than in the placebo group (10.6 vs 6.2 months; hazard ratio for progression or death = 0.64; 95% CI: 0.55–0.74; P < 0.001), but OS, the other co-primary endpoint, was not different between the two groups. The respective OS rates with bevacizumab and placebo were 72.4 and 66.3% at 1 year (P = 0.049).
In this study, although patients with low Karnofsky PS or large tumour volumes were included, the 1-year survival rate in patients with newly diagnosed GBM was 78%, suggesting that effectiveness might be similar to the efficacy reported in clinical trials.
In the future, it will be necessary to clearly identify the Japanese patient groups that can benefit from bevacizumab. In the ongoing Phase II study of BevacIzumab beyOnd progression disease for GlioblastoMA tReated with Key therapeutics (BIOMARK) study, bevacizumab treatment is continued even after disease progression, and a comprehensive genetic analysis is planned. We eagerly await the results of that study.
Conclusions
These results suggest no new safety concerns, and effectiveness might be similar to previously reported data in clinical trials. Therefore, bevacizumab is considered as one of the treatment options for patients with malignant glioma in real-world settings.
Supplementary Material
Acknowledgements
The authors would like to thank the physicians, clinical staff and patients who participated in this study. The authors would also like to thank Dr Tetsuji Asao (SunFlare Co., Ltd) for writing and editing support for the manuscript.
Funding
This study was supported by Chugai Pharmaceutical Co., Ltd. The study sponsor contributed to the study design; the data collection, analysis and interpretation and draft manuscript writing.
Conflict of interest
Dr Motoo Nagane received a consulting fee from Chugai Pharmaceutical Co., Ltd as a medical adviser of the Bevacizumab Appropriate Use Committee. He received research grants and consulting fees from Eisai Co., Ltd, Ono Pharmaceutical Co., Ltd, MSD K. K., Daiichi Sankyo Co., Ltd, UCB Japan Co., Ltd, AbbVie GK, Otsuka Pharmaceutical Co., Ltd, Nippon Kayaku Co., Ltd and Chugai Pharmaceutical Co., Ltd; research grants from Toray Industries Inc., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd, Shionogi & Co., Ltd, Pfizer Japan Inc., Astellas Pharma Inc., Tsumura & Co. and Sanofi K. K. and consulting fees from Novocure and Dainippon Sumitomo Pharma Co., Ltd. Yasuko Hayashi, Ayaka Shimizu and Masako Ura are employees of Chugai Pharmaceutical Co., Ltd. Dr Ryo Nishikawa received a consulting fee from Chugai Pharmaceutical Co., Ltd as a medical adviser of the Bevacizumab Appropriate Use Committee. He received research grants and consulting fees from MSD K. K., Eisai Co., Ltd and Chugai Pharmaceutical Co., Ltd and consulting fees from Ono Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Novocure, AbbVie GK and Nippon Kayaku Co., Ltd.
References
- 1. Narita Y, Shibui S. Committee of Brain Tumor Registry of Japan supported by the Japan neurosurgical society: trends and outcomes in the treatment of gliomas based on data during 2001–2004 from the brain tumor registry of Japan. Neurol Med Chir (Tokyo) 2016;55:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakamura H, Makino K, Yano S, Kuratsu J. Kumamoto brain tumor research group. Epidemiological study of primary intracranial tumors: a regional survey in Kumamoto prefecture in southern Japan—20-year study. Int J Clin Oncol 2011;16:314–21. [DOI] [PubMed] [Google Scholar]
- 3. National Cancer Institute SEER Cancer Statistics Factsheets: Brain and Other Nervous System Cancer. Bethesda, MD: https://seer.cancer.gov/statfacts/html/brain.html (3 August 2018, date last accessed). [Google Scholar]
- 4. Fernandes C, Costa A, Osório L, et al. . Chapter 11. Current standards of care in glioblastoma therapy. In: De Vleeschouwer S, editor. Glioblastoma [Internet]. Brisbane (AU): Codon Publications, 2017. https://www.ncbi.nlm.nih.gov/books/NBK469987/ (3 August 2018, date last accessed). [PubMed] [Google Scholar]
- 5. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamborn KR, Yung WK, Chang SM, et al. . Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 2008;10:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. [DOI] [PubMed] [Google Scholar]
- 8. Stupp R, Mason WP, Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 9. Wick W, Puduvalli VK, Chamberlain MC, et al. . Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 2010;28:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Committee of Brain Tumor Registry of Japan Report of brain tumor registry of Japan (2005–2008) 14th edition. Neurol Med Chir (Tokyo) 2017;57(Suppl 1):9–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lund EL, Spang-Thomsen M, Skovgaard-Poulsen H, Kristjansen PE. Tumor angiogenesis—a new therapeutic target in gliomas. Acta Neurol Scand 1998;97:52–62. [DOI] [PubMed] [Google Scholar]
- 12. Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992;359:845–8. [DOI] [PubMed] [Google Scholar]
- 13. Thomas AA, Omuro A. Current role of anti-angiogenic strategies for glioblastoma. Curr Treat Options Oncol 2014;15:551–66. [DOI] [PubMed] [Google Scholar]
- 14. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004;3:391–400. [DOI] [PubMed] [Google Scholar]
- 15. Okamoto S, Nitta M, Maruyama T, et al. . Bevacizumab changes vascular structure and modulates the expression of angiogenic factors in recurrent malignant gliomas. Brain Tumor Pathol 2016;33:129–36. [DOI] [PubMed] [Google Scholar]
- 16. Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem 2006;13:1845–57. [DOI] [PubMed] [Google Scholar]
- 17. Matuschek C, Bölke E, Nawatny J, et al. . Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther Onkol 2011;187:135–9. [DOI] [PubMed] [Google Scholar]
- 18. McGee MC, Hamner JB, Williams RF, et al. . Improved intratumoral oxygenation through vascular normalization increases glioma sensitivity to ionizing radiation. Int J Radiat Oncol Biol Phys 2010;76:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moen MD. Bevacizumab: In previously treated glioblastoma. Drugs 2010;70:181–9. [DOI] [PubMed] [Google Scholar]
- 20. Nagane M, Nishikawa R, Narita Y, et al. . Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol 2012;42:887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chinot OL, Wick M, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–22. [DOI] [PubMed] [Google Scholar]
- 22. Pharmaceuticals and Medical Devices Agency Review Report http://www.pmda.go.jp/drugs/2013/P201300096/450045000_21900AMX00910_A100_1.pdf. (22 August 2018, data last accessed) (in Japanese).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


