Abstract
Background
The optimal revascularization technique in diabetic patients with complex coronary artery disease (CAD), including left main CAD and multivessel coronary disease (MVD), remains controversial. The current study aimed to compare adverse clinical endpoints of coronary artery bypass graft (CABG) and percutaneous coronary intervention (PCI) in patients with diabetes mellitus (DM).
Methods
Relevant studies were found from MEDLINE, OVID, Science Direct, Embase and the Cochrane Central database from January 2010 to April 2019. Risk ratio (RR) with 95% confidence interval (CI) was used to express the pooled effect on discontinuous variables. Outcomes evaluated were all-cause mortality, major adverse cardiac/cerebrovascular events (MACCE), cardiac death, myocardial infarction, stroke, and repeat revascularization.
Results
Sixteen studies were included (18,224 patients). PCI was associated with the increase risk for MACCE (RR 1.59, 95% CI 1.38–1.85), cardiac death (RR 1.76, 95% CI 1.11–2.80), MI (RR 1.98, 95% CI 1.53–2.57), repeat revascularization (RR 2.61, 95% CI 2.08–3.29). The risks for all-cause mortality (RR 1.23, 95% CI 1.00–1.52) and stroke (RR 0.71, 95% CI 0.48–1.03) were similar between two strategies. Stratified analysis based on studies design and duration of follow-up showed largely similar findings with the overall analyses, except for a significant increased risk of all-cause mortality (RR 1.32, 95% CI 1.04–1.67) in long-term group, and CABG was associated with a higher stroke rate compared to PCI, which are results that were found in RCTs (RR 0.47, 95% CI 0.28–0.79) and mid-term groups (RR 0.39, 95% CI 0.23–0.66).
Conclusions
CABG was superior to PCI for diabetic patients with complex CAD (including left main CAD and/or MVD), but might be associated with a higher risk of stroke mid-term follow-up.
Number of Protocol registration PROSPERO CRD 42019138505.
Keywords: Diabetes mellitus, Coronary artery bypass surgery, Percutaneous coronary intervention, Meta-analysis
Background
In recent years, the occurrence of diabetes mellitus (DM) in patients worldwide has been increasing rapidly [1]. The total number of patients with DM is expected to rise to nearly 600 million by 2035 [2]. As the critical risk factor for coronary artery disease (CAD), DM typically presents with diffuse comorbid atherosclerosis and multiple-vessel stenosis, which are poor prognostic indicators of revascularization strategies [3, 4]. Currently, coronary artery bypass grafting (CABG) has been recommended as the standard of care for patients with diabetes and complex anatomic diseases, including left main CAD [5]. However, with the application of drug-eluting stents (DESs) and the improvement of interventional technology, the incidence of restenosis and repeat revascularization after percutaneous coronary intervention (PCI) has been significantly reduced [6, 7]. PCI is regarded as an alternative to CABG, as it is less invasive, which is favored by more patients. Thus, a number of clinical studies have been conducted globally to estimate and compare the clinical effects and end-point outcomes of the two approaches in an effort to determine the best revascularization strategy for patients with DM and complex CAD [8–11].
Recently, two meta-analyses based on several published studies (e.g., Dai et al. [12]) found that the incidence of all-cause mortality (1–5 years follow-up) of DM patients underwent PCI did not differ significantly from those who underwent CABG. Similarly, Xin et al. [13] found no clear difference in mortality between CABG and PCI in patients with DM and serious coronary disease. However, these analyses assessed a limited number of studies. Furthermore, the evidence was not examined through stratified analysis of follow-up time and/or study type. These issues are important, as a recently published clinical trial [14] showed that the rate of mortality after PCI was significantly higher than CABG, differing from previous results.
Therefore, this study comprehensively examined research completed in the last 10 years, evaluating and comparing clinical outcomes of PCI or CABG in patients with complex coronary disease (including left main CAD and/or MVD) in an effort to determine the most appropriate revascularization strategy for patients with DM.
Methods
This study was performed according to the Cochrane Collaboration guidelines and is reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analysis extension (PRISMA) statement [15, 16]. The protocol was registered in PROSPERO database (http://www.crd.york.ac.uk/PROSPERO/) under the number CRD42019138505.
Inclusion criteria
Inclusion criteria were as follows: (1) types of studies: we included all randomized controlled trials (RCTs) and observational studies (OS); (2) types of participants: all patients with DM (including type 1 and 2 diabetes) included in studies were diagnosed with left main CAD, MVD, or both; (3) types of interventions: all patients underwent direct percutaneous coronary intervention (PCI) or coronary artery bypass grafting surgery (CABG); and (4) outcomes: the incidence of all-cause mortality of patients underwent PCI, comparison to patients with CABG. Other outcomes included the risk of cardiac death, MACCE, myocardial infarction (MI), stroke, or repeat revascularization. MACCE refers to major adverse cardiac events and cerebrovascular events, including death, MI, stroke or repeat revascularization. Subgroups analyses of the incidence of these endpoints were conducted according to different study designs (included RCTs and observational studies) and duration of follow-up (midterm: 1–3 years, long-term: > 3 years).
Exclusion criteria
Exclusion criteria were the following: (1) overlapping and/or repetitive data; (2) review articles, single case reports, and noncomparable studies; (3) the number of diabetic patients for comparison was less than 50; (4) DESs were not used in interventional therapy; and (5) a follow-up period < 1 year.
Search strategy
We searched for all relevant studies from MEDLINE (including PubMed), OVID, Science Direct, Embase and the Cochrane Central database, from January 2010 to April 2019. The following search terms were used to maximize search sensitivity and specificity: percutaneous coronary intervention, drug-eluting stents, coronary artery bypass graft, coronary bypass, left main coronary artery disease, multivessel disease, diabetes mellitus. Additionally, further relevant studies were identified through the reference list of review articles.
Study selection
In the present study, two reviewers (CN Zhai and K Hou) independently screened the titles and abstracts of articles for eligibility criteria. Then, the full text of studies that potentially met inclusion criteria was inspected to determine which studies were included in analyses. If the two reviewers disagreed regarding the inclusions of a study, a consensus was reached by consulting a third researcher (HL Cong).
Data extraction and quality assessment
Data from all included articles were extracted independently by two investigators (CN Zhai and K Hou). Data included the study title, publication date, authors, studies design, number of patients, coronary lesion, duration of follow-up, and the risk of every endpoint, etc. the corresponding authors of the included studies were contacted to obtain any required information that was missing. The total extracted data were verified by a third investigator (HL Cong). Three reviewers (CN Zhai, K Hou, and YY Zhang) independently evaluated the potential risk of bias of randomized trials by applying the Cochrane Collaboration’s tool [17] and the quality of observational studies by using the Newcastle–Ottawa Scale criteria [18].
Statistical analysis
Data analysis was conducted using the RevMan software, version 5.1 (the Nordic Cochrane Centre, the Cochrane collaboration, Copenhagen, Denmark) and STATA 12.0 software (StataCorp, College Station, TX, USA). The risk ratios (RRs) and 95% confidence intervals (CIs) were used to evaluate the dichotomous outcomes, such as the incidence of all-cause mortality. To combine the separate statistics, the inverse variance and Mantel–Haenszel techniques were used. The heterogeneity was investigated by the use of the Q statistic, and P values < 0.05 was regarded as statistically significant, a random-effects model was used in the above circumstances. Sensitivity analysis was conducted using an exclusion method whereby multiple analyses were performed, with a different study excluded in each analysis of the clinical outcome.
Publication bias was evaluated statistically using Begg funnel plots and Egger’s bias test. The above methods measured the degree of funnel-plot asymmetry statistically [19, 20]. The Begg adjusted rank correlation test was used to evaluate the relationship between the test accuracy estimate and their variances. The deviation of Spearman ρ values from zero provided an estimate of the funnel-plot asymmetry. Positive values indicated a trend toward higher levels of test accuracy in studies with smaller sample sizes. The Egger bias test detects funnel-plot asymmetry by determining whether the intercept deviates significantly from zero in a regression of the standardized effect estimates against their precision values.
Results
Search results and characteristics of included studies
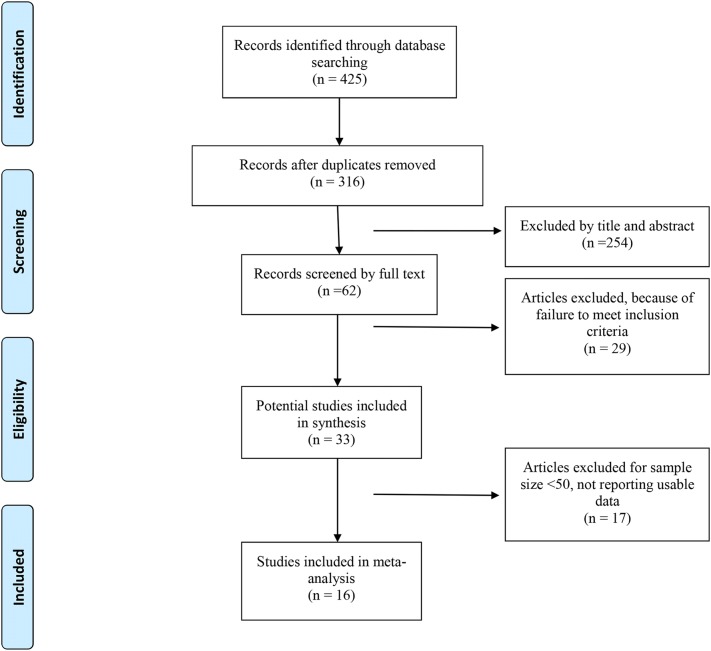
A total of 425 articles were found during the initial electronic search, after removal of duplicate studies. After screening, 409 failed to meet eligibility criteria. Eventually, a total of 16 articles, which included 7 randomized controlled trails [5, 8, 9, 14, 21–23] and 9 observational studies [11, 24–31], met all eligibility criteria (Fig. 1).
Fig. 1.
Flow of studies through the meta-analysis
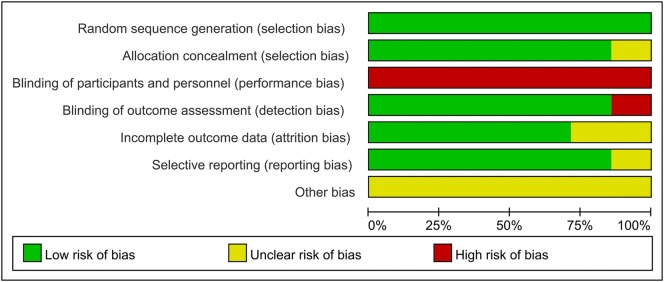
A total of 18,224 patients with DM receiving PCI (n = 9863) and CABG (n = 8361) were included in the present analysis. Table 1 showed the general characteristics of the individuals included in the studies investigated. Some raw data of characteristics were not fully available. Although contact with the authors of the original studies was attempted, no responses were received. The methodological quality of included RCTs was presented in Fig. 2. Judgement about each risk of bias item are presented as percentages across RCTs in Fig. 3. Although the nature of the intervention made trials blinded for patients impossible, this was not considered a source of significant bias. The quality of observational studies is presented in Table 2 and they had high quality in their data outcome and clinical design.
Table 1.
Characteristics of the included studies
| Study | Year | Follow-up years | Samples P/C | Age, years P/C | Male, % P/C | HTN, % P/C | Smoke, % P/C | Dsl, % P/C | Stent type | Study design | Coronary lesion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kapur et al. [21] | 2010 | 1 | 172/178 | 64.3/63.6 | 70.7/77.9 | 20.8/18.7 | 24.6/23.2 | NA | DES | RCT | MVD (2- or 3-vessel disease) |
| Luo et al. [24] | 2012 | 3 | 99/127 | 65/66 | 70/98 | 72/87 | 43.0/54.0 | 15.0/31.0 | DES | OS | Unprotected LM disease (and/or 1-, 2-, or 3-vessel coronary disease) |
| Farkouh et al. [5] | 2012 | 1 | 953/947 | 63.2/63.1 | 73.2/69.5 | NA | 14.8/16.6 | NA | SES or PES | RCT | MVD with stenosis of more than 70% in 2 or more major epicardial vessels |
| Kamalesh et al. [8] | 2013 | 2 | 101/97 | 62.7/62.1 | 99.0/99.0 | 96.0/95.7 | 27.7/20.6 | NA | DES | RCT | MVD (2- or 3-vessel disease) |
| Kappetein et al. [9] | 2013 | 5 | 231/221 | NA | NA | NA | NA | NA | PES | RCT | LM and/or 3-vessel disease |
| Ben-Gal et al. [23] | 2015 | 1 | 1349/423 | 65.0/65.0 | 73.0/66.3 | 79.4/85.9 | 21.0/24.0 | 61.8/72.7 | DES | RCT | LM and/or 2-, 3-vessel disease |
| Bangalore et al. [25, 34] | 2015 | 4 | 773/773 | 64.9/64.7 | 68.0/68.0 | NA | NA | NA | EES | OS | MVD (2-, 3-vessel disease and without LM) |
| Marui et al. [26] | 2015 | 5 | 1065/933 | 68.7/67.8 | 68.0/73.0 | 88.0/84.0 | 25.0/25.0 | NA | DES | OS | LM and/or 3-vessel disease |
| Ahn et al. [22] | 2015 | 5 | 102/90 | NA | NA | NA | NA | NA | SES | RCT | Unprotected LM disease with stenosis of more than 50% |
| Naito et al. [27] | 2015 | 3.8 | 256/227 | 72.7/72.7 | 78.1/68.3 | 77.0/74.0 | 58.6/62.6 | 76.6/68.7 | DES | OS | LM and/or 2-, 3-vessel disease |
| Yu et al. [28] | 2015 | 7.1 | 143/131 | 65.0/66.0 | 72.0/77.9 | 68.5/65.6 | 49.7/45.8 | 53.8/37.4 | DES | OS | Unprotected LM and 1-, 2-, or 3-vessel coronary disease |
| Zheng et al. [29] | 2016 | 3 | 348/806 | NA | NA | NA | NA | NA | DES | OS | LM and/or 1-, 2-, 3-vessel disease |
| Li et al. [30] | 2017 | 10 | 406/406 | 41.9/42.0 | 366/369 | 60.8/63.5 | 66.0/65.3 | NA | DES | OS | LM and/or 1-, 2-, 3-vessel disease |
| Ramanathan et al. [11] | 2017 | 5 | 2710/1865 | 67.3/65.2 | 72.0/73.2 | 88.1/91.8 | NA | 77.5/79.5 | DES | OS | MVD with stenosis of more than 70% in 2 or more major epicardial vessels |
| Nagendran et al. [31] | 2018 | 5 | 869/869 | 65.1/65.1 | 23.0/21.0 | 83.0/85.0 | 18.0/20.0 | 80.0/82.0 | DES | OS | LM and/or 2-, 3-vessel disease |
| Milojevic et al. [14] | 2019 | 3 | 286/268 | NA | NA | NA | NA | NA | EES | RCT | LM and/or 1-, 2-, or 3-vessel coronary disease |
HTN hypertension, Dsl dyslipidemia, P/C percutaneous coronary intervention versus coronary artery bypass surgery, DES drug-eluting stents, but type of the stents is not available, SES sirolimus-eluting stent, PES paclitaxel-eluting stent, EES everolimus-eluting stent, MVD multivessel disease, LM left main, RCT randomized controlled trials, OS observational studies, NA not available
Fig. 2.
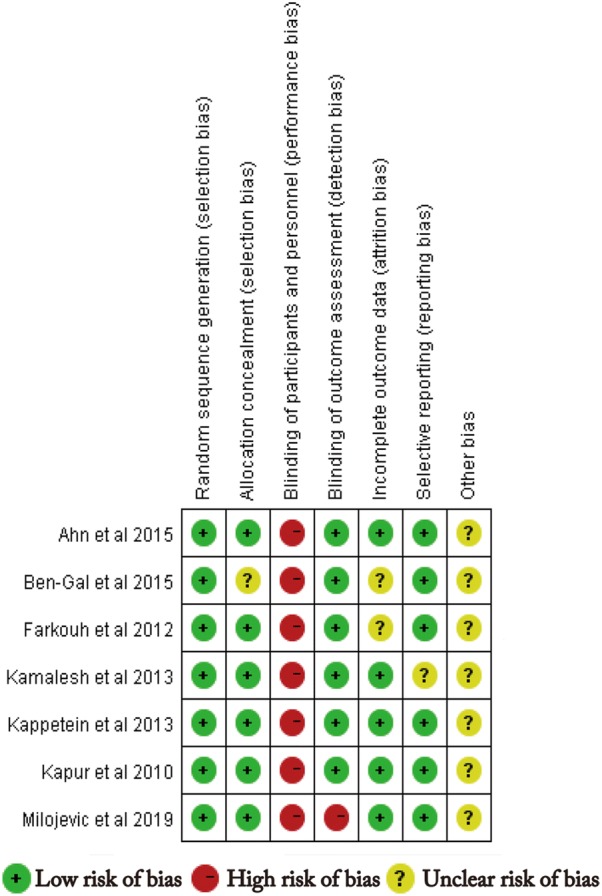
Methodological quality of included RCTs. This risk-of-bias tool incorporates assessment of randomization (sequence generation and allocation concealment), blinding (participants, personnel, and outcome assessors), completeness of outcome data, selection of outcomes reported, and other sources of bias. The items were scored with “yes,” “no,” or “unsure.”
Fig. 3.
Risk of bias. Each risk-of-bias item is presented as percentages across included RCTs, which indicate the proportion of different levels of risk of bias for each item
Table 2.
Newcastle–Ottawa scale (NOS) for assessing quality of observational studies
| Study | Selection | Comparability of the cohort | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Outcome not present at baseline | Assessment of outcome | Enough follow-up duration | Adequate follow-up | |||
| Luo et al. [24] | * | * | * | * | ** | * | * | * | 9 |
| Bangalore et al. [25, 34] | * | * | * | * | ** | * | * | * | 9 |
| Marui et al. [26] | * | * | * | * | ** | * | * | * | 9 |
| Naito et al. [27] | * | * | * | * | ** | – | * | * | 8 |
| Yu et al. [28] | * | * | * | * | ** | * | * | * | 9 |
| Zheng et al. [29] | * | * | * | * | ** | * | * | * | 9 |
| Li et al. [30] | * | * | * | * | ** | – | * | * | 8 |
| Ramanathan et al. [11] | * | * | * | * | ** | * | * | * | 9 |
| Nagendran et al. [31] | * | * | * | * | ** | * | * | * | 9 |
The scale assigns 4 points for selection, 2 points for comparability and 3 points for outcome (* 1 point; ** 2 points). Score of 5 to 6 considered as moderate quality and 7 to 9 as high quality
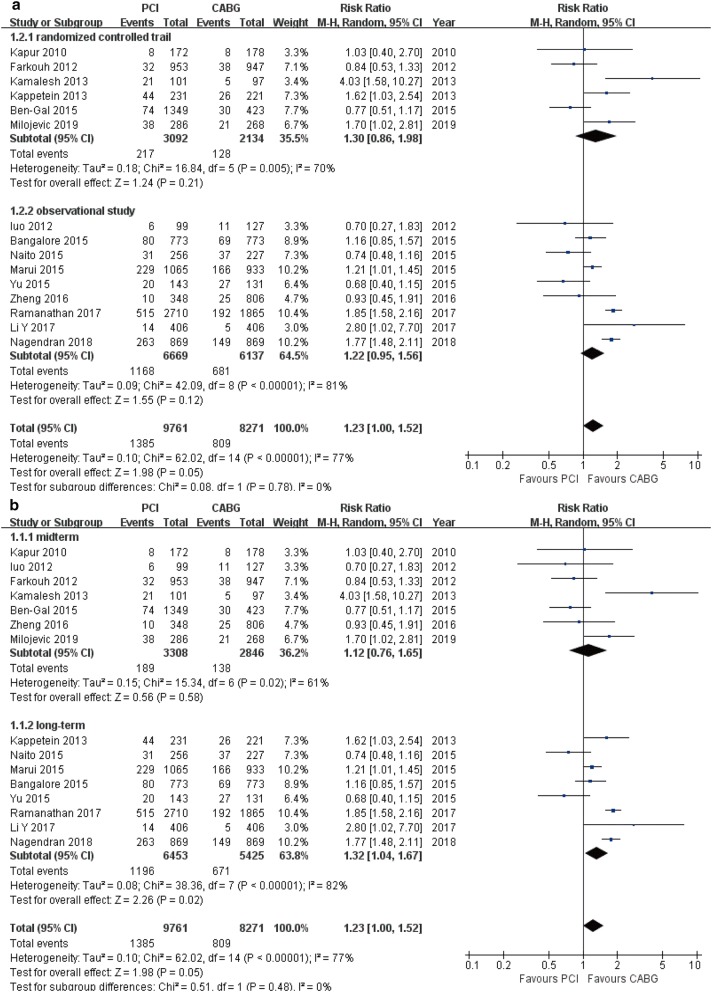
All-cause mortality
All-cause mortality was reported in 15 studies (18,032 patients) (Table 3). Comparing with patients undergoing CABG, all-cause mortality was significantly higher in patients who received PCI (RR 1.23, 95% CI 1.00–1.52, P = 0.05). In analysis stratified by study design and duration of follow-up, consistent findings were observed in the subgroup of long-term follow-up (RR 1.32, 95% CI 1.04–1.67, P = 0.02). A trend toward increased risk was detected in the subgroups of RCTs (RR 1.30, 95% CI 0.86–1.98, P = 0.08), OS (RR 1.22, 95% CI 0.95–1.56, P = 0.12) and mid-term follow-up (RR 1.12, 95% CI 0.76–1.65, P = 0.58; Fig. 4a, b).
Table 3.
Total meta-analysis outcomes and stratified analysis of each endpoint based on study design and duration of follow-up
| Endpoints | Subgroup | Study, n | RR | 95% CI | Pvalue | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|---|---|
| All-cause death | Total | 15 | 1.23 | 1.00–1.52 | 0.05 | 77 | < 0.001 |
| RCT | 6 | 1.30 | 0.86–1.98 | 0.08 | 70 | 0.005 | |
| OS | 9 | 1.22 | 0.95–1.56 | 0.12 | 81 | < 0.001 | |
| Mid-term | 7 | 1.12 | 0.76–1.65 | 0.58 | 61 | 0.02 | |
| Long-term | 8 | 1.32 | 1.04–1.67 | 0.02 | 82 | < 0.001 | |
| MACCE | Total | 8 | 1.59 | 1.38–1.85 | < 0.001 | 68 | 0.003 |
| RCT | 5 | 1.40 | 1.20–1.63 | < 0.001 | 26 | 0.25 | |
| OS | 3 | 1.87 | 1.72–2.03 | < 0.001 | 0 | 0.52 | |
| Mid-term | 3 | 1.31 | 1.11–1.54 | 0.001 | 14 | 0.31 | |
| Long-term | 5 | 1.84 | 1.70–1.99 | < 0.001 | 0 | 0.58 | |
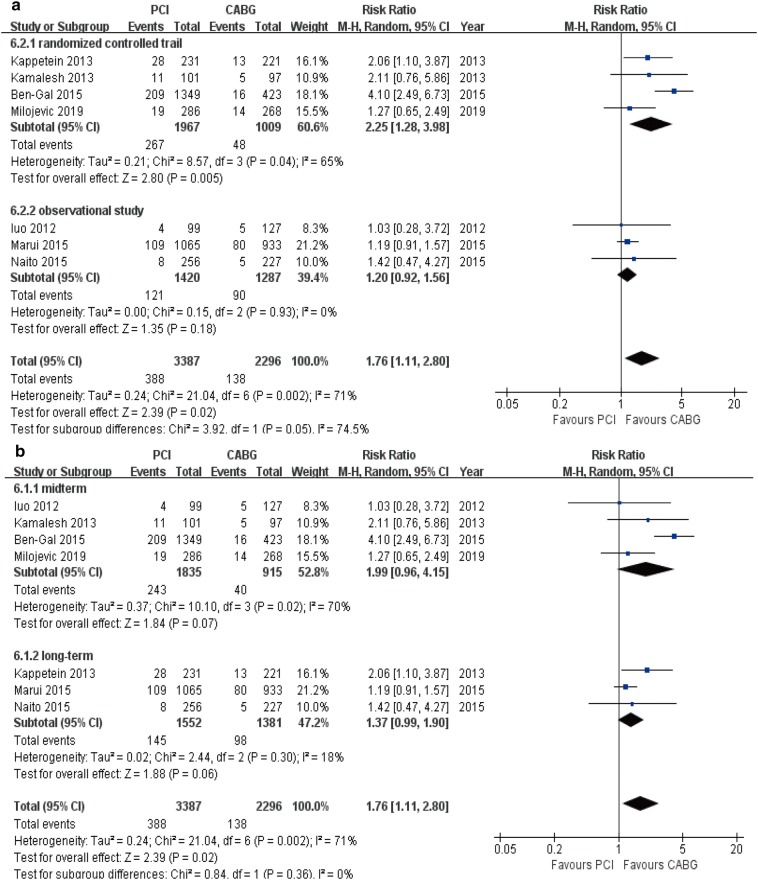
| Cardiac death | Total | 7 | 1.76 | 1.11–2.80 | 0.02 | 71 | 0.002 |
| RCT | 4 | 2.25 | 1.28–3.98 | 0.005 | 65 | 0.04 | |
| OS | 3 | 1.20 | 0.92–1.56 | 0.18 | 0 | 0.93 | |
| Mid-term | 4 | 1.99 | 0.96–4.15 | 0.07 | 70 | 0.02 | |
| Long-term | 3 | 1.37 | 0.99–1.90 | 0.06 | 18 | 0.30 | |
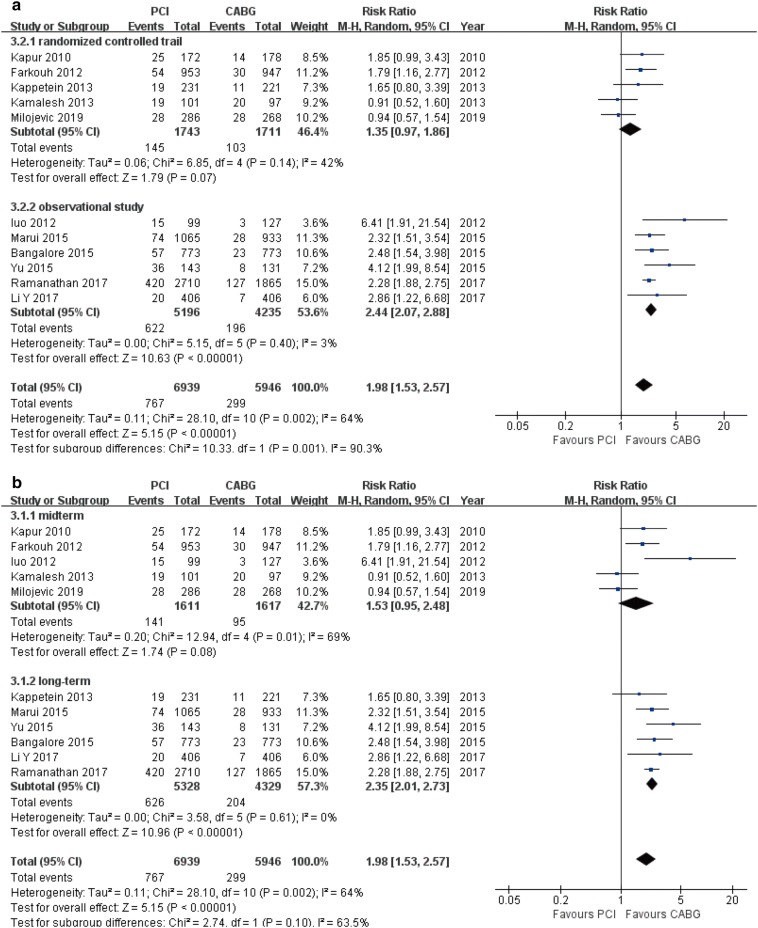
| MI | Total | 11 | 1.98 | 1.53–2.57 | < 0.001 | 64 | 0.002 |
| RCT | 5 | 1.35 | 0.97–1.86 | 0.07 | 42 | 0.14 | |
| OS | 6 | 2.44 | 2.07–2.88 | < 0.001 | 3 | 0.40 | |
| Mid-term | 5 | 1.53 | 0.95–2.48 | 0.08 | 69 | 0.01 | |
| Long-term | 6 | 2.35 | 2.01–2.73 | < 0.001 | 0 | 0.61 | |
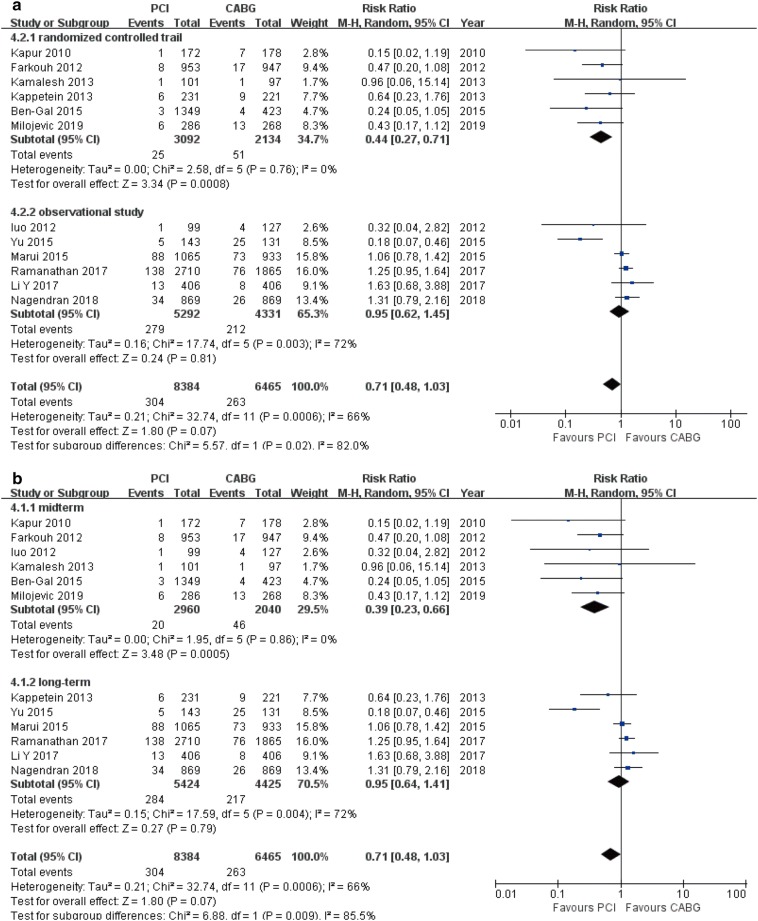
| Stroke | Total | 12 | 0.71 | 0.48–1.03 | 0.07 | 66 | < 0.001 |
| RCT | 6 | 0.44 | 0.27–0.71 | < 0.001 | 0 | 0.76 | |
| OS | 6 | 0.95 | 0.62–1.45 | 0.81 | 72 | 0.003 | |
| Mid-term | 6 | 0.39 | 0.23–0.66 | < 0.001 | 0 | 0.86 | |
| Long-term | 6 | 0.95 | 0.64–1.41 | 0.79 | 72 | 0.004 | |
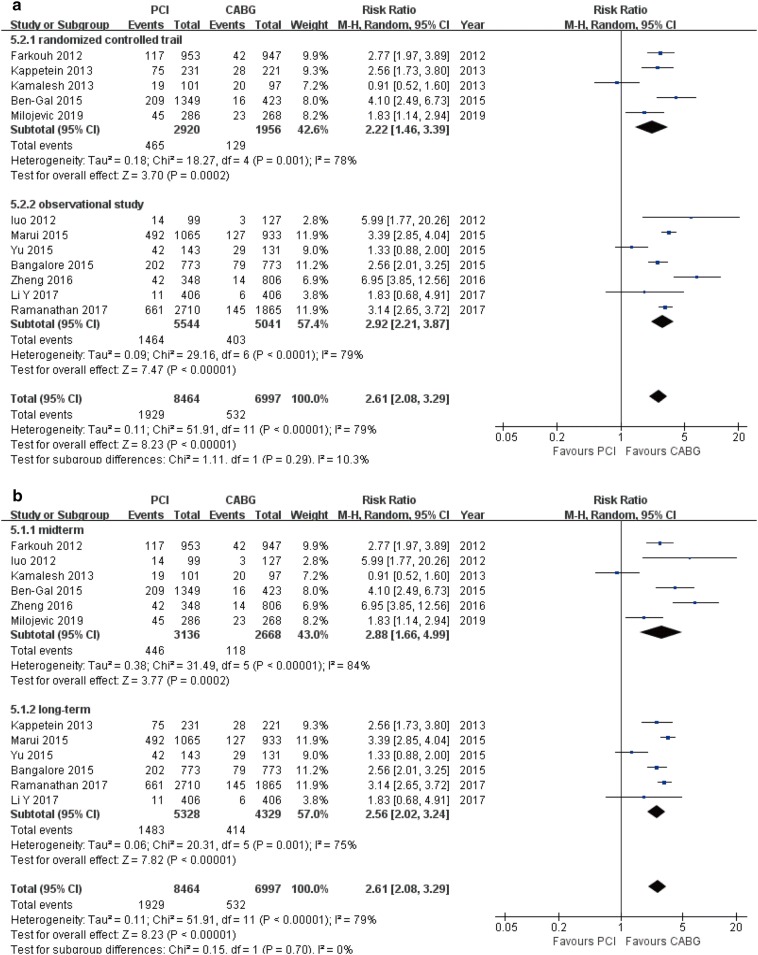
| Repeat revascularization | Total | 12 | 2.61 | 2.08–3.29 | < 0.001 | 79 | < 0.001 |
| RCT | 5 | 2.22 | 1.46–3.39 | < 0.001 | 78 | 0.001 | |
| OS | 7 | 2.92 | 2.21–3.87 | < 0.001 | 79 | < 0.001 | |
| Mid-term | 6 | 2.88 | 1.66–4.99 | < 0.001 | 84 | < 0.001 | |
| Long-term | 6 | 2.56 | 2.02–3.24 | < 0.001 | 75 | 0.001 |
Italic values indicate significance of P value (Pvalue and Pheterogeneity < 0.05)
RR risk ratio, CI confidence intervals, MACCE major adverse cardiac and cerebrovascular event, MI myocardial infarction, RCT randomized controlled trials, OS observational studies, Mid-term 1–3 years follow-up, Long-term > 3 years follow-up
Fig. 4.
Forest plots for all-cause mortality between PCI and CABG patients (a subgroup analysis of study design; b subgroup analysis of follow-up periods)
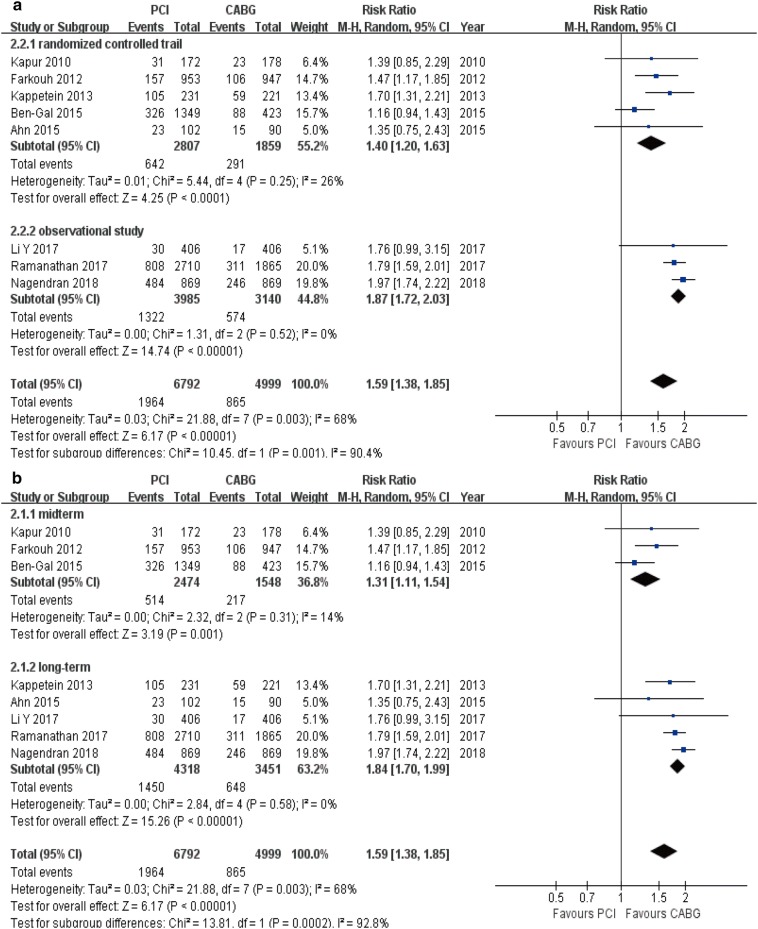
Macce
The overall incidence of MACCE was higher in the PCI group compared with CABG group (8 studies; 11,791 patients; RR 1.59, 95% CI 1.38–1.85, P < 0.001) (Table 3). The same statistically significant differences were found in stratified analyses [RCTs (RR 1.40, 95% CI 1.20–1.63, P < 0.001); OS (RR 1.87, 95% CI 1.72–2.03, P < 0.001); mid-term follow-up (RR 1.31, 95% CI 1.11–1.54, P = 0.001); and long-term follow-up (RR 1.84, 95% CI 1.70–1.99, P < 0.001); Fig. 5a, b].
Fig. 5.
Forest plots for MACCE between PCI and CABG patients (a subgroup analysis of study design; b subgroup analysis of follow-up periods)
Cardiac death
Compared to the CABG group, patients receiving PCI demonstrated a higher risk of cardiac death (7 studies; 5683 patients; RR 1.76, 95% CI 1.11–2.80, P = 0.02) (Table 3). This was seen in the RCTs subgroup (RR 2.25, 95% CI 1.28–3.98, P = 0.005). In other stratified analyses, although there was no statistically significant findings, the similar trend toward increased risk was observed in OS (RR 1.20, 95% CI 0.92–1.56, P = 0.18), mid-term follow-up (RR 1.99, 95% CI 0.96–4.15, P = 0.07), and long-term follow-up (RR 1.37, 95% CI 0.99–1.90, P = 0.06) subgroups (Fig. 6a, b).
Fig. 6.
Forest plots for cardiac death between PCI and CABG patients (a subgroup analysis of study design; b subgroup analysis of follow-up periods)
Myocardial infarction
There was a statistically significant increase in the risk of myocardial infarction in patients undergoing PCI compared to patients undergoing CABG (11 studies, 12,885 patients, RR 1.98, 95% CI 1.53–2.57, P < 0.001) (Table 3). This result was consistent in OS (RR 2.44, 95% CI 2.07–2.88, P < 0.001) and long-term follow-up (RR 2.35, 95% CI 2.01–2.73, P < 0.001) subgroups. A trend toward increased risk was observed in RCTs (RR 1.35, 95% CI 0.97–1.86, P = 0.07) and mid-term follow-up subgroups (RR 1.53, 95% CI 0.95–2.48, P = 0.08; Fig. 7a, b).
Fig. 7.
Forest plots for myocardial infarction between PCI and CABG patients (a subgroup analysis of study design; b subgroup analysis of follow-up periods)
Stroke
There showed no significant difference in incidence of stroke between PCI and CABG (12 studies; 14,849 patients; RR 0.71, 95% CI 0.48–1.03, P = 0.07) (Table 3). Analysis by study design and follow-up time found that the rate of stroke was lower in the PCI patients compared with CABG patients in RCTs (RR 0.44, 95% CI 0.27–0.71, P < 0.001) and mid-term follow-up subgroups (RR 0.39, 95% CI 0.23–0.66, P < 0.001). The risk of stroke was similar in both OS (RR 0.95, 95% CI 0.62–1.45, P = 0.81) and long-term follow-up subgroups (RR 0.95, 95% CI 0.64–1.41, P = 0.79; Fig. 8a, b).
Fig. 8.
Forest plots for stroke between PCI and CABG patients (a subgroup analysis of study design; b subgroup analysis of follow-up periods)
Repeat revascularization
Overall, there was a statistically significant increase in the risk of repeat revascularization in patients undergoing PCI compared with CABG (12 studies; 15,461 patients; RR 2.61, 95% CI 2.08–3.29, P < 0.001) (Table 3). This effect was also demonstrated in all multiple stratified analyses [RCTs (RR 2.22, 95% CI 1.46–3.39, P < 0.001); OS (RR 2.92, 95% CI 2.21–3.87, P < 0.001); mid-term follow-up (RR 2.88, 95% CI 1.66–4.99, P < 0.001); and long-term follow-up (RR 2.56, 95% CI 2.02–3.24, P < 0.001); Fig. 9a, b].
Fig. 9.
Forest plots for repeat revascularization between PCI and CABG patients (a subgroup analysis of study design; b subgroup analysis of follow-up periods)
Sensitivity analyses and publication bias
Publication bias tests were performed for all endpoints of included studies. There was no evidence of publication bias (Table 4). Sensitivity analyses in overall endpoints showed that the results of these analyses were not excessively influenced by any of the included studies.
Table 4.
Publication bias assessment of this meta-analysis
| Endpoints | Egger’s test | Begg’s test | ||
|---|---|---|---|---|
| t-value | p | t-value | p | |
| All-cause mortality | − 1.39 | 0.189 | 0.40 | 0.692 |
| Cardiac death | 0.56 | 0.597 | 0.30 | 0.764 |
| MACCE | − 1.68 | 0.144 | 0.37 | 0.711 |
| MI | − 0.14 | 0.889 | 0.62 | 0.533 |
| Stroke | − 2.06 | 0.066 | 0.89 | 0.373 |
| Repeat revascularization | − 0.89 | 0.395 | 0.34 | 0.732 |
MACCE major adverse cardiac and cerebrovascular event, MI myocardial infarction
Discussion
The optimal revascularization strategy for patients with DM and complex CAD, including left main CAD, is an important issue for cardiovascular experts. In clinical practice, PCI is more acceptable to patients with DM because of less trauma and faster recovery. CABG is more invasive than PCI and has a higher risk of adverse cerebral vascular events during perioperative period [32]. Although PCI is more likely to be associated with higher rate of adverse events after revascularization, previous studies [13] have reported no difference in mortality between PCI and CABG and CABG was associated with excess stroke [33, 34], making it difficult for physicians to assess the two strategies. This study pooled data from 16 studies, which included 18,224 diabetic patients (follow-up ≥ 1 year) with left main CAD and/or MVD, undergoing either PCI or CABG. At the long-term follow-up (> 3 years), we found that PCI was significantly associated with a high risk of all-cause mortality, MACCE, MI, repeat revascularization, although the risk of stroke was lower at the midterm follow-up (1–3 years). CABG was significantly associated with a lower risk of long-term mortality and other adverse clinical endpoints compared to PCI in patients with DM.
Previous studies have compared the two revascularization strategies and found different results than those observed in the current study. Although no separate analysis of diabetic cohorts was performed, a meta-analysis by Zhang et al. [35] suggested that PCI, with newer generation DES, might be a safe alternative revascularization strategy for left main CAD. However, it was noted that this method presented a higher risk of repeat revascularization. Mahmoud et al. [36] compared the clinical outcomes between these two strategies found that PCI was associated with a lower early risk of MACCE, while the risk of all-cause mortality, MI, and stroke were similar to CABG at long-term follow-up. Additionally, an in-depth comparative study of patients with DM [12] found that the risk of mortality (1–5 year follow-up) was not significantly different between PCI and CABG patients with DM; results that were supported by a recent study found no obvious difference in the incidence of all-cause mortality between the two strategies [13].
While the above-mentioned studies differ from the current results, several other studies have reported results similar to those reported here. For example, a study by Bundhun et al. [33], which involved 1297 patients with insulin-treated type 2 DM, found that, compared to PCI, CABG was associated with lower risk of several adverse long-term clinical outcomes, including mortality. However, it was also found that the rate of stroke was higher in patients who received CABG. A meta-analysis by Lee et al. [37] found a lower rate of mortality and MACCE in patients who underwent CABG. These authors also pointed out that insulin dependence had no influence on the clinical endpoints between the two therapy strategies. Smit et al. [38] observed that CABG was associated with a significantly lower risk of mortality and repeat revascularization in patients with DM or MVD, but that stroke was more common after this procedure. However, it should be noted that the studies included in this meta-analysis had relatively few patients with DM.
A previous study [24] identified that there was a prognostic impact of DM on treatment effects by either PCI or CABG, and that, in terms of both safety and efficacy, PCI was inferior to CABG in the diabetic group. CABG is recommended as a more appropriate revascularization strategy in patients with DM and complex coronary lesions. A high risk of restenosis was observed in patients with DM who underwent PCI, which may be associated with the need for more than two stents in MVD, as well as the continuous progression of diffuse atherosclerosis in non-culprit vessels [39]. At present, it is recommended to apply SYNTAX scores to the selection of revascularization strategies [40, 41]. Although the SYNTAX score might be considered useful in interventional cardiology, it has some limitations [42]. Compared with PCI, there was a higher risk of periprocedural stroke in patients undergoing CABG [43]. Moreover, post-operative problems or complications, such excessive sedation, ventilation, the use of intra-aortic balloon pump to help the heart, administration of inotropes, wound infection, hemorrhage, and pneumonia in patients with DM, were also elevated than those in non-diabetic patients [44, 45] and needed to be monitored and reasonably prevented.
Several studies have identified that adverse events were related to the severity of diabetes; for example, glycosylated hemoglobin (HbA1c) level and insulin therapy were independent risk factors for the development of post-surgery complications [46, 47]. Moreover, it is well-known that other risk factors such plasma homocysteine (Hcy) and c-reactive protein (CRP) have also an important role in CVD [48, 49], and it is a modifiable risk factors for restenosis [50]. Intervention for these risk factors and optimal glycemic control are the critical component of diabetes management. A recent study demonstrated that the use of sodium glucose cotransporter 2 inhibitors (SGLT2-is) reduces the risk of major cardiovascular events [51, 52]. In addition, the abnormal platelet activation observed in patients with DM is conducive to the formation of pathological thrombosis and the progression of cardiovascular diseases [53]. Therefore, improve interventional techniques, optimize antiplatelet/glycemic management, and the reduction of long-term adverse prognosis require further research.
In addition, despite subgroup analysis, heterogeneity still exited. We deemed that several clinical heterogeneity could not be eliminated. Among possible reasons for heterogeneity, differences in surgical techniques and differences in procedures of percutaneous transluminal coronary angioplasty (PTCA) (e.g., type of stent, concomitant medication, preparation for the prevention of contrast-induced nephropathy) could account for diversities in results across studies. Furthermore, heterogeneity may also have been caused by study design. Therefore, because of limited information obtained from original studies, heterogeneity cannot be completely resolved. Accordingly, although the results of present meta-analysis should be considered appropriately, methodological quality defects and clinical heterogeneity should be considered when interpreting the findings.
The number of published large-scale studies on patients with DM is currently small. To the best of our knowledge, the current study has the largest sample size of patients left main CAD and/or MVD to date. In addition, the current study compared the mid-term and long-term clinical outcomes between PCI and CABG. We found that CABG is superior to PCI in patients with DM, especially in differences of MACCE and repeat revascularization in all multiple stratified analyses. Although, it should be noted that the risk of stroke in CABG patients was higher than PCI patients at the mid-term follow-up, no statistically significant difference was observed at long-term follow-up. Our meta-analysis has higher accuracy, reliability, and statistical power due to the amplified sample size, resulting from the combination of the original studies. Thus, the current results could be considered stronger evidence than any one individual study. We hope that these results will assist diabetics and physicians in choosing the most appropriate revascularization strategy.
Limitations
Some limitations of this meta-analysis should be considered: (1) because of the limited data available, this study was unable to conduct more in-depth stratified analysis based on the complex lesions of the coronary artery in diabetic patients; (2) we could not conduct a subgroup analysis comparing different type of DES (drug-eluting stent) and in-depth stratification on the basis of diabetic patients’ different drugs or comorbidity, due to limited studies and inadequate access to data; (3) the results of the included observational studies may have been selectively reported; and (4) due to the incomplete demographic data of diabetes patients, we cannot evaluate for heterogeneity by patient-level covariates. Unfortunately, heterogeneity of these variables (e.g., surgical experience, interference of diabetic complications, differences in drugs strategies) can never be completely resolved.
Conclusions
CABG was superior to PCI in patients with DM and complex CAD (including left main CAD and/or MVD) regard to all-cause mortality, MACCE, MI, repeat revascularization, but is associated with a higher risk of stroke at mid-term follow-up. Further rigorous, high-quality research is required to confirm these conclusions, however, due to the several limitations of the current studies.
Acknowledgements
We thank LetPub (https://www.letpub.com) and Professor Gloria Nance for their linguistic assistance during the preparation of this manuscript. Additionally, we thank Tianjin Chest Hospital Labor Union.
Abbreviations
- CAD
coronary artery disease
- MVD
multivessel coronary disease
- CABG
coronary artery bypass graft
- PCI
percutaneous coronary intervention
- DM
diabetes mellitus
- RR
risk ratio
- CI
confidence interval
- MACCE
major adverse cardiac/cerebrovascular events
- MI
myocardial infarction
- RCTs
randomized controlled trials
- OS
observational studies
- DESs
drug-eluting stent
- HbA1c
glycosylated hemoglobin
- Hcy
homocysteine
- CRP
c-reactive protein
- SGLT2-is
sodium glucose cotransporter 2 inhibitors
Authors' contributions
HLC designed the study. CNZ statistical analysis, participated in most of the study steps. CNZ, YCH, and KH prepared the manuscript and assisted in the study processes. KH, YXZ, and YYZ assisted in the data collection, and helped in the, interpretation of the study. All authors read and approved the final manuscript.
Funding
The authors gratefully acknowledge the financial supports by Tianjin Chest Hospital Labor Union (HL Cong’s model worker innovation studio).
Availability of data and materials
Datasets are available through the corresponding author upon reasonable request.
Ethics approval and consent to participate
All analyses were based on previous published studies, thus no ethical approval and patient consent are required. All previous published studies were approved by ethics committee, respectively.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
ChuanNan Zhai, Email: zcndoctor1983@163.com.
HongLiang Cong, Email: hl_cong@126.com.
Kai Hou, Email: kaihou_2018@163.com.
YueCheng Hu, Email: hu_yuecheng@163.com.
JingXia Zhang, Email: zhangjingxia_2019@126.com.
YingYi Zhang, Email: zhangyingyi_2018@163.com.
References
- 1.Setacci C, de Donato G, Setacci F, Chisci E. Diabetic patients: epidemiology and global impact. J Cardiovasc Surg. 2009;50:263–273. [PubMed] [Google Scholar]
- 2.Iminger-Finger I, Kargul J, Laurent GJ. Diabetes: present and future. Int J Biochem Cell Biol. 2017;88:196. doi: 10.1016/j.biocel.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52:255–262. doi: 10.1016/j.jacc.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 4.Mathew V, Gersh BJ, Williams BA, Laskey WK, Willerson JT, Tilbury RT, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2004;109:476–480. doi: 10.1161/01.CIR.0000109693.64957.20. [DOI] [PubMed] [Google Scholar]
- 5.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 6.Moussa I, Leon MB, Baim DS, O’Neill WW, Popma JJ, Buchbinder M, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation. 2004;109:2273–2278. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- 7.Moses JW, Nikolsky E, Mehran R, Cambier PA, Bachinsky WB, Leya F, et al. Safety and efficacy of the 2.25-mm sirolimus-eluting Bx velocity stent in the treatment of patients with de novo native coronary artery lesions: the SIRIUS 2.25 trial. Am J Cardiol. 2006;98:1455–1460. doi: 10.1016/j.amjcard.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 8.Kamalesh M, Sharp TG, Tang XC, Shunk K, Ward HB, Walsh J, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol. 2013;61:808–816. doi: 10.1016/j.jacc.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Kappetein AP, Head SJ, Morice MC, Banning AP, Serruys PW, Mohr FW, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg. 2013;43:1006–1013. doi: 10.1093/ejcts/ezt017. [DOI] [PubMed] [Google Scholar]
- 10.Morice MC, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation. 2014;129:2388–2394. doi: 10.1161/CIRCULATIONAHA.113.006689. [DOI] [PubMed] [Google Scholar]
- 11.Ramanathan K, Abel JG, Park JE, Fung A, Mathew V, Taylor CM, et al. Surgical versus percutaneous coronary revascularization in patients with diabetes and acute coronary syndromes. J Am Coll Cardiol. 2017;70:2995–3006. doi: 10.1016/j.jacc.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Dai X, Luo ZC, Zhai L, Zhao WP, Huang F. Reassessing coronary artery bypass surgery versus percutaneous coronary intervention in patients with type 2 diabetes mellitus: a brief updated analytical report (2015–2017) Diabetes Ther. 2018;9:2163–2171. doi: 10.1007/s13300-018-0504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin X, Wang X, Dong X, Fan Y, Shao W, Lu X, et al. Efficacy and safety of drug-eluting stenting compared with bypass grafting in diabetic patients with multivessel and/or left main coronary artery disease. Sci Rep. 2019;9:7268. doi: 10.1038/s41598-019-43681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milojevic M, Serruys PW, Sabik JF, Kandzari DE, Schampaert E, van Boven AJ, et al. Bypass surgery or stenting for left main coronary artery disease in patients with diabetes. J Am Coll Cardiol. 2019;73:1616–1628. doi: 10.1016/j.jacc.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) BMJ. 2016;354:i4086. doi: 10.1136/bmj.i4086. [DOI] [PubMed] [Google Scholar]
- 17.Bunn F, Trivedi D, Alderson P, Hamilton L, Martin A, Iliffe S. The impact of Cochrane Systematic Reviews: a mixed method evaluation of outputs from Cochrane Review Groups supported by the UK National Institute for Health Research. Syst Rev. 2014;3:125. doi: 10.1186/2046-4053-3-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 21.Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55:432–440. doi: 10.1016/j.jacc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Ahn JM, Roh JH, Kim YH, Park DW, Yun SC, Lee PH, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease: 5-year outcomes of the PRECOMBAT study. J Am Coll Cardiol. 2015;65:2198–2206. doi: 10.1016/j.jacc.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Gal Y, Mohr R, Feit F, Ohman EM, Kirtane A, Xu K, et al. Surgical versus percutaneous coronary revascularization for multivessel disease in diabetic patients with non-ST-segment-elevation acute coronary syndrome: analysis from the Acute Catheterization and Early Intervention Triage Strategy trial. Circ Cardiovasc Interv. 2015;8:e002032. doi: 10.1161/CIRCINTERVENTIONS.114.002032. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Yu X, Chen F, Du X, He J, Gao Y, et al. Impact of diabetes mellitus on patients with unprotected left main coronary artery lesion disease treated with either percutaneous coronary intervention or coronary-artery bypass grafting. Coron Artery Dis. 2012;23:322–329. doi: 10.1097/MCA.0b013e3283564961. [DOI] [PubMed] [Google Scholar]
- 25.Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus eluting stents versus coronary artery bypass graft surgery for patients with diabetes mellitus and multivessel disease. Circ Cardiovasc Interv. 2015;8:e002626. doi: 10.1161/CIRCINTERVENTIONS.115.002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marui A, Kimura T, Nishiwaki N, Mitsudo K, Komiya T, Hanyu M, et al. Five-year outcomes of percutaneous versus surgical coronary revascularization in patients with diabetes mellitus (from the CREDO-Kyoto PCI/CABG Registry Cohort-2) Am J Cardiol. 2015;115:1063–1072. doi: 10.1016/j.amjcard.2015.01.544. [DOI] [PubMed] [Google Scholar]
- 27.Naito R, Miyauchi K, Konishi H, Tsuboi S, Ogita M, Dohi T, et al. Comparing mortality between coronary artery bypass grafting and percutaneous coronary intervention with drug-eluting stents in elderly with diabetes and multivessel coronary disease. Heart Vessels. 2016;31:1424–1429. doi: 10.1007/s00380-015-0746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, He J, Luo Y, Yuan F, Song X, Gao Y, et al. Influence of diabetes mellitus on long-term outcomes of patients with unprotected left main coronary artery disease treated with either drug-eluting stents or coronary artery bypass grafting. Int Heart J. 2015;56:43–48. doi: 10.1536/ihj.14-193. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Z, Xu B, Zhang H, Guan C, Xian Y, Zhao Y, et al. Coronary artery bypass graft surgery and percutaneous coronary interventions in patients with unprotected left main coronary artery disease. JACC Cardiovasc Interv. 2016;9:1102–1111. doi: 10.1016/j.jcin.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Dong R, Hua K, Liu TS, Zhou SY, Zhou N, et al. Outcomes of coronary artery bypass graft surgery versus percutaneous coronary intervention in patients aged 18–45 years with diabetes mellitus. Chin Med J. 2017;130:2906–2915. doi: 10.4103/0366-6999.220305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagendran J, Bozso SJ, Norris CM, McAlister FA, Appoo JJ, Moon MC, et al. Coronary artery bypass surgery improves outcomes in patients with diabetes and left ventricular dysfunction. J Am Coll Cardiol. 2018;71:819–827. doi: 10.1016/j.jacc.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Zhang W, Wang L, Wang S, Yu Y, Chen S, et al. Male patients with diabetes undergoing coronary artery bypass grafting have increased major adverse cerebral and cardiovascular events. Interact Cardiovasc Thorac Surg. 2019;28:607–612. doi: 10.1093/icvts/ivy287. [DOI] [PubMed] [Google Scholar]
- 33.Bundhun PK, Wu ZJ, Chen MH. Coronary artery bypass surgery compared with percutaneous coronary interventions in patients with insulin-treated type 2 diabetes mellitus: a systematic review and meta-analysis of 6 randomized controlled trials. Cardiovasc Diabetol. 2016;15:2. doi: 10.1186/s12933-015-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bangalore S, Toklu B, Feit F. Outcomes with coronary artery bypass graft surgery versus percutaneous coronary intervention for patients with diabetes mellitus: can newer generation drug-eluting stents bridge the gap. Circ Cardiovasc Interv. 2014;7:518–525. doi: 10.1161/CIRCINTERVENTIONS.114.001346. [DOI] [PubMed] [Google Scholar]
- 35.Zhang XL, Zhu QQ, Yang JJ, Chen YH, Li Y, Zhu SH, et al. Percutaneous intervention versus coronary artery bypass graft surgery in left main coronary artery stenosis: a systematic review and meta-analysis. BMC Med. 2017;15:84. doi: 10.1186/s12916-017-0853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoud AN, Elgendy IY, Mentias A, Saad M, Ibrahim W, Mojadidi MK, et al. Percutaneous coronary intervention or coronary artery bypass grafting for unprotected left main coronary artery disease. Catheter Cardiovasc Interv. 2017;90:541–552. doi: 10.1002/ccd.26970. [DOI] [PubMed] [Google Scholar]
- 37.Lee BJ, Herbison P, Wong CK. Is the advantage of coronary bypass graft surgery over percutaneous coronary intervention in diabetic patients with severe multivessel disease influenced by the status of insulin requirement. J Geriatr Cardiol. 2014;11:83–89. doi: 10.3969/j.issn.1671-5411.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smit Y, Vlayen J, Koppenaal H, Eefting F, Kappetein AP, Mariani MA. Percutaneous coronary invervention versus coronary artery bypass grafting: a meta-analysis. J Thorac Cardiovasc Surg. 2015;149:831–838. doi: 10.1016/j.jtcvs.2014.10.112. [DOI] [PubMed] [Google Scholar]
- 39.Head SJ, Mack MJ, Holmes DR, Mohr FW, Morice MC, Serruys PW, et al. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg. 2012;41:535–541. doi: 10.1093/ejcts/ezr105. [DOI] [PubMed] [Google Scholar]
- 40.Bundhun PK, Sookharee Y, Bholee A, Huang F. Application of the SYNTAX score in interventional cardiology: a systematic review and meta-analysis. Medicine. 2017;96:e7410. doi: 10.1097/MD.0000000000007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bundhun PK, Bhurtu A, Huang F. Worse clinical outcomes following percutaneous coronary intervention with a high SYNTAX score: a systematic review and meta-analysis. Medicine. 2017;96:e7140. doi: 10.1097/MD.0000000000007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bundhun PK, Yanamala CM, Huang F. Percutaneous coronary intervention, coronary artery bypass surgery and the SYNTAX score: a systematic review and meta-analysis. Sci Rep. 2017;7:43801. doi: 10.1038/srep43801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mack MJ, Head SJ, Holmes DR, Ståhle E, Feldman TE, Colombo A, et al. Analysis of stroke occurring in the SYNTAX trial comparing coronary artery bypass surgery and percutaneous coronary intervention in the treatment of complex coronary artery disease. JACC Cardiovasc Interv. 2013;6:344–354. doi: 10.1016/j.jcin.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Santos KA, Berto B, Sousa AG, Costa FA. Prognosis and complications of diabetic patients undergoing isolated coronary artery bypass surgery. Braz J Cardiovasc Surg. 2016;31:7–14. doi: 10.5935/1678-9741.20160008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raza S, Sabik JF, Ainkaran P, Blackstone EH. Coronary artery bypass grafting in diabetics: a growing health care cost crisis. J Thorac Cardiovasc Surg. 2015;150(304–2):e2. doi: 10.1016/j.jtcvs.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamaria S, Bhatheja S, Vengrenyuk Y, Sweeny J, Choudhury H, Barman N, et al. Prognostic relation between severity of diabetes mellitus (on or off insulin) ± chronic kidney disease with cardiovascular risk after percutaneous coronary intervention. Am J Cardiol. 2018;121:168–176. doi: 10.1016/j.amjcard.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Hermanides RS, Kennedy MW, Kedhi E, van Dijk PR, Timmer JR, Ottervanger JP, et al. Impact of elevated HbA1c on long-term mortality in patients presenting with acute myocardial infarction in daily clinical practice: insights from a ‘real world’ prospective registry of the Zwolle Myocardial Infarction Study Group. Eur Heart J Acute Cardiovasc Care. 2019 doi: 10.1177/2048872619849921. [DOI] [PubMed] [Google Scholar]
- 48.Bellia C, Bivona G, Scazzone C, Ciaccio M. Association between homocysteinemia and metabolic syndrome in patients with cardiovascular disease. Ther Clin Risk Manag. 2007;3:999–1001. [PMC free article] [PubMed] [Google Scholar]
- 49.Vivona N, Bivona G, Noto D, Sasso BL, Cefalù AB, Chiarello G, et al. C-reactive protein but not soluble CD40 ligand and homocysteine is associated to common atherosclerotic risk factors in a cohort of coronary artery disease patients. Clin Biochem. 2009;42:1713–1718. doi: 10.1016/j.clinbiochem.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Xiao S, Yang C, Ye R, Hu X, Chen X. Association of elevated plasma homocysteine level with restenosis and clinical outcomes after percutaneous coronary interventions: a systemic review and meta-analysis. Cardiovasc Drugs Ther. 2019;33:353–361. doi: 10.1007/s10557-019-06866-0. [DOI] [PubMed] [Google Scholar]
- 51.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 52.Sezai A, Sekino H, Unosawa S, Taoka M, Osaka S, Tanaka M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol. 2019;18:76. doi: 10.1186/s12933-019-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santilli F, Liani R, Di FP, Formoso G, Simeone P, Tripaldi R, et al. Increased circulating resistin is associated with insulin resistance, oxidative stress and platelet activation in type 2 diabetes mellitus. Thromb Haemost. 2016;116:1089–1099. doi: 10.1160/TH16-06-0471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available through the corresponding author upon reasonable request.