Abstract
Background
Between 1975 and 1985 a total of 91 Danish patients with moderate and severe hemophilia (PWH) was infected with HIV constituting a major scandal in the Danish health care system. This study describes the burden of HIV infection among Danish PWH by evaluating changes from 1988 to 2012 in well-being, social function, experiencing stigma and openness about disease among Danish HIV+ PWH.
Methods
Three anonymous surveys were conducted in 1988, 2001 and 2012 targeting all Danish patients with moderate to severe hemophilia. Survey responses were received from 53, 21 and 18 HIV+ PWH respectively. A matched comparison sample of HIV− PWH was identified for each survey-year, using propensity score matching. Differences for each survey-year and trends over time were analyzed using ordinal logistic regression.
Results
In 1988, HIV+ PWH had more psychosomatic symptoms than HIV− PWH, but in 2001 life satisfaction was higher among HIV+ PWH than among HIV− PWH. Tests of differences in trend over time showed larger improvements in life satisfaction among HIV+ PWH than HIV− PWH, while HIV− PWH showed an increase in educational level compared to HIV+ PWH. Analysis restricted to HIV+ PWH showed an increase in perceived stigmatization.
Conclusions
Differences between Danish HIV+ and HIV− PWH regarding well-being and psychosomatic symptoms seem to have evened out between 1988 and 2012. However, results suggest that HIV+ PWH still experience stigmatization and lower levels of education.
Keywords: HIV, Hemophilia, Well-being, Stigma, Social conditions, Employment
Background
The bleeding disorders Hemophilia A and B, and von Willebrand’s Disease are chronic diseases that requires lifelong and costly treatment. These rare and innate diseases are caused by a deficiency of coagulation factor causing longer bleeds when injured than persons with normal coagulation factors. Classification of hemophilia is based on plasma procoagulant levels, and classified as mild, moderate or severe [1]. In spite of being a rare disease, hemophilia has gained a high level of awareness due to the hemophilia scandal. Before heat-treatment of factor products was introduced in 1984, patients with moderate or severe hemophilia (PWH) were infected with both HIV and Hepatitis C worldwide through contaminated blood products used as part of their hemophilia-treatment. In Denmark, a total of 91 PWH were infected with HIV between 1975 and 1985, in some incidents even after the risks of this treatment became known. Historically, patients with hemophilia (PWH) have been burdened with increased risk of bleeds, increased mortality risk, chronic pain and reduced mobility due to hemophilic arthropathy [2]. Improvements of hemophilia treatment – particularly treatment with factor products - during the1970s were accompanied by expectations that PWH would experience reduced mortality, reduced morbidity and increased well-being. However, the HIV epidemic caused a setback for PWH, as HIV infection had devastating impact on the quality of life, quality of care and longevity of PWH [3]. HIV+ PWH were described as one of the major risk groups for AIDS along with intravenous drug users and homosexuals [4]. In the 1980s being infected with HIV was associated with social stigma and both physical and psychological morbidity [5]. At this point in time, there was no effective treatment for HIV and HIV+ PWH had a short life expectancy. Many HIV+ PWH retired early and more than 20 countries, including Denmark, established compensation programs for the patients infected through contaminated blood products [6, 7]. From the 1980s and to the 2010s the lives of HIV+ PWH have undergone improvements in terms of prognosis, disease consequences, and treatment regimens, but also concerning the social stigma around HIV+ patients [8, 9]. However, the consequences of these major improvements for well-being among HIV+ PWH are not well described [10].
Previous studies have shown varying results when comparing HIV+ PWH and HIV− PWH on quality of life (QoL), well-being, social function, and psychosomatic symptoms. Some studies have found increased psychological distress [11–14], although less so for adolescents being open about their HIV status [15]. Other studies found no significant differences between HIV+ PWH and HIV− PWH [16–18]. Studies using prospective data regarding the development in well-being and social function are scarce and to the best of our knowledge there have been no recent studies on symptom burden among HIV+ PWH.
To evaluate the future needs of PWH and in particular HIV+ PWH, the Danish Hemophilia Society collected survey data on all Danish patients with moderate to severe hemophilia in 1988. These surveys were repeated twice, in 2001 and 2012. Spanning 24 years, the data enable us to study the development in well-being, social function, and stigmatization among HIV+ PWH.
The present study has three aims: 1) To compare well-being and social function between Danish HIV+ and HIV− PWH at three time points, 2) To assess the trend in well-being and social function over time in HIV+ as compared to HIV− PWH, 3) to assess the trend over time in stigmatization and openness about HIV for HIV+ PWH (these questions were only asked HIV+ PWH). We hypothesized a positive development in well-being and social function, as well as reduced stigmatization due to improved treatment and changes regarding the public attitude and knowledge about HIV.
Methods
Three-wave panel study
The Danish Hemophilia Society collected survey data through anonymous questionnaires in 1988, 2001 and 2012. The surveys were developed by the Danish Hemophilia Society. The two Danish hemophilia centers located at Aarhus University Hospital and Copenhagen University Hospital Rigshospitalet, identified all Danish patients with moderate-to-severe hemophilia A or B (factor VIII or IX ≤5%) or type 3 von Willebrand’s disease. The centers also registered HIV status. For 1988, there were 85 HIV+ PWH out of a total of 212 PWH (6 HIV+ PWH were diseased before start of the study). In 2001, there were 30 surviving HIV+ PWH out of 190 PWH. In 2012, there were 27 HIV+ PWH out of 240 PWH. Since no new cases of HIV infection occurred after 1985, the HIV+ PWH registered in 1988, 2001 and 2012 are subsamples of the 91 PWH originally infected. The hemophilia centers distributed the 1988 and the 2001 surveys by mail to all identified PWH. In 2012, the survey was administered online with the opportunity to receive a paper version of the questionnaire. In 1988, 53 responses were received from HIV+ PWH out of a total of 135 responses (response rates 62 and 64% respectively). In 2001, 21 responses were received from HIV+ PWH out of a total of 164 responses (response rates 70 and 86%). In 2012, 18 responses were received from HIV+ PWH out of a total of 166 responses (response rates 67 and 69%).
Variables
The study variables are presented in Table 1. Data was self-reported except for yearly factor use; severity of hemophilia; inhibitor; hepatitis B and C; and HIV infection. These variables were assessed by self-report in 1988 and 2012 but from patient charts in 2001. Hemophilia severity was not assessed in 1988. Some of the variables are summarized as two scales regarding joint mobility and psychosomatic symptoms. Joint mobility was assessed by five questions on the range of motion in periods with no bleeds and summed into a scale ranging from 0 (reduced mobility for all types of joint) to 5 (full mobility for all types of joint) [19]. Psychosomatic symptoms were measured by four questions concerning discomforts within the last 2 weeks: headache, anxiety, depression, and fatigue. The responses to the questions were summed into a scale ranging from 0 (no symptoms) to 4 (symptoms within every category). A detailed description of the questionnaires is provided as Additional file 1.
Table 1.
Description of study variables
| Variable | Definition/Question | |
|---|---|---|
| Background and clinical variables | Age | Age at January 1st of 1988, 2001 and 2012 |
| Number of bleeding episodes treated with factor |
1988: 5 response categories 2001–2012: # of episodes |
|
| Yearly factor usea | Units per year | |
| Severity of hemophiliab | Moderate; Severe | |
| Inhibitor (ever)a | Never; Current or previous | |
| Hepatitis B or C (ever)a | Never; Current or previous | |
| HIV Infectiona | Yes; No | |
| Joint mobility | Questions on range of motion in periods with no bleeds in the following joints: hips, knees, ancles, shoulders, and elbows | |
| Social function | Education | Highest education completed |
| Work | Questions on current employment, work hours and social benefits. | |
| Family type | Living with spouse or partner; Living alone; Other family type (e.g. living with parents or house sharing) | |
| Social activities | “Do you attend meeting, clubs, or other activities outside work or school, including sports, evening school or the like?” | |
| Well-being | Life satisfaction | “All in all, how satisfied or dissatisfied are you with your life as it stands today?” |
| Psychosomatic symptoms | cHeadache; cAnxiety, nervousness, unrest; cDepressed, in low spirit, unhappy; cTiredness. | |
| Worries | “Patients with hemophilia have a certain risk of developing life-threatening bleeds. Do you ever think about that?” | |
| Being alone | “Are you ever alone, but want to be together with other people?” | |
| Stigma | Stigma | “How often have you felt like people look down upon you, avoid you or in any way react negatively about your HIV positive status?” |
| Openness | “Who knows that you are HIV positive?” |
a1988 and 2012: Self-reported, 2001: Extracted from charts
b1988: not recorded, 2001: Extracted from charts, 2012: Self-reported
c “Have you, within the last 2 weeks, been bothered with following pain or discomforts.”
Statistical analyses
To enable comparisons between HIV+ PWH and HIV− PWH, a matched comparison sample was identified for each of the 3 years, using propensity score matching [20]. Matching variables were age group, yearly factor use and hepatitis infection. Hepatitis infection was not used as matching variable in 1988, since a large proportion of patients in 1988 did not know whether they had been infected. After controlling for age, factor use and hepatitis, other background variables (number of bleeding episodes, presence of inhibitor and joint mobility) were not significantly associated with HIV status and were not used as matching variables. The probability of being HIV infected was calculated from age, factor use, and hepatitis using a logistic regression model. HIV+ PWHs were matched with HIV− PWH of similar risk for HIV infection using 1:1 optimal matching (R package MatchIt [21]). Comparisons between HIV+ and HIV− PWH were conducted for each year using ordinal logistic regression. Comparison of HIV+ PWH and HIV− PWH showed that the propensity score matching did not provide a perfect match on age and factor use in 1988 and 2012. These variables were therefore included as covariates in subsequent statistical testing. Trend over time was evaluated in a combined data set by including a year times HIV interaction in an ordinal logistic regression model. Comparisons between 1988, 2001 and 2012 were done at group level, since the anonymous questionnaires did not permit tracing of individual patients.
Results
Treatment related variables and joint mobility
The proportion of HIV+ PWH who had ever experienced Hepatitis infection increased from 54% in 1988 to 95% in 2001 (Table 2). Because Hepatitis C was not identified until 1989, the measure from 1988 included Hepatitis B only. For HIV+ PWH, frequency of bleeding episodes tended to decrease from 1988 to 2012, but no significant trend was found compared to HIV− PWH. In 2001 and 2012, almost all patients had severe hemophilia. The proportion of PWH in the sample having had inhibitor was below 25% in 1988 and 2001 but 33% in 2012. Generally, joint mobility declined over time for both HIV− PWH and HIV+ PWH with only one patient from each group having full joint mobility (level 5) in 2012.
Table 2.
Data characteristics by survey year and HIV status (%)
| 1988 | 2001 | 2012 | |||||
|---|---|---|---|---|---|---|---|
| HIV+ | HIV− | HIV+ | HIV− | HIV+ | HIV− | ||
| Age distribution | 0–15 years | 8 | 23 | 0 | 0 | 0 | 0 |
| 16–24 years | 26 | 21 | 6 | 6 | 6 | 6 | |
| 25–34 years | 38 | 25 | 39 | 33 | 0 | 0 | |
| 35–44 years | 11 | 21 | 39 | 39 | 44 | 33 | |
| 45–54 years | 17 | 11 | 17 | 22 | 22 | 33 | |
| 55–88 years | 0 | 0 | 0 | 0 | 28 | 28 | |
| N | (53) | (53) | [18] | [18] | [18] | [18] | |
| Factor use (per year) | 0–25.000 units | 8 | 12 | 0 | 0 | 6 | 6 |
| 25.001–75.000 units | 10 | 20 | 20 | 19 | 28 | 22 | |
| 75.001–125.000 units | 22 | 16 | 35 | 38 | 11 | 22 | |
| 125.001–250.000 units | 46 | 37 | 35 | 33 | 17 | 22 | |
| 250.001–500.000 units | 8 | 12 | 10 | 5 | 33 | 22 | |
| 500.001- units | 6 | 2 | 0 | 5 | 6 | 6 | |
| N | (49) | (50) | [21] | [20] | [18] | [18] | |
| Hepatitis B or C | Never | 46a | 63a | 5 | 5 | 6 | 6 |
| Current or previous | 54a | 37a | 95 | 95 | 94 | 94 | |
| N | (46) | (46) | [21] | [21] | [18] | [18] | |
| Bleeding episodes | No episodes | 6 | 4 | 5 | 0 | 6 | 6 |
| 1–10 episodes | 34 | 53 | 53 | 50 | 61 | 33 | |
| 11–25 episodes | 30 | 8 | 26 | 15 | 17 | 39 | |
| 26–50 episodes | 19 | 21 | 16 | 25 | 11 | 11 | |
| 51+ episodes | 11 | 15 | 0 | 10 | 6 | 11 | |
| N | (53) | (53) | [20] | [19] | [18] | [18] | |
| Severity of hemophilia | Severe | – | – | 100 | 100 | 89 | 100 |
| Moderate | 0 | 0 | 11 | 0 | |||
| N | [21] | [21] | [18] | [18] | |||
| Inhibitor (ever) | Never | 77 | 84 | 81 | 90 | 67 | 67 |
| Current or previous | 23 | 16 | 19 | 10 | 33 | 33 | |
| N | (50) | (52) | [21] | [21] | [18] | [18] | |
| Joint mobility | 0 | 13 | 9 | 24 | 14 | 17 | 22 |
| 1 | 17 | 17 | 10 | 5 | 6 | 22 | |
| 2 | 25 | 9 | 19 | 24 | 44 | 17 | |
| 3 | 15 | 21 | 10 | 24 | 17 | 22 | |
| 4 | 17 | 13 | 38 | 24 | 11 | 11 | |
| 5 | 12 | 30 | 0 | 10 | 6 | 6 | |
| N |
aOnly HBV
Social function and well-being
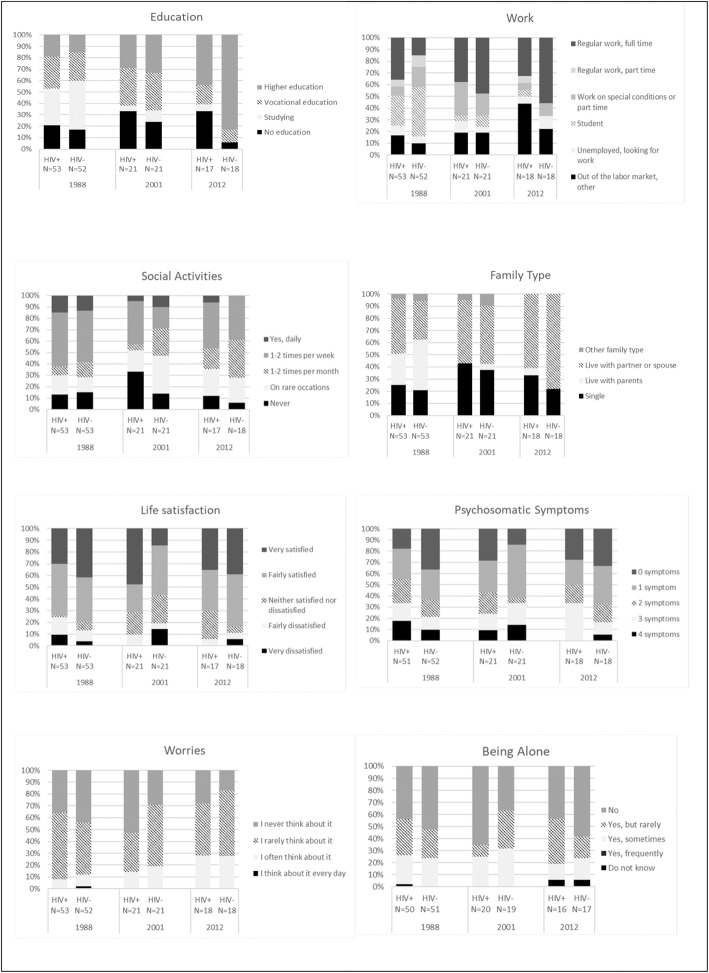
Table 3 shows tests of differences between HIV+ and HIV− PWH for each of the 3 years and tests for trends over time for HIV+ PWH compared to HIV− PWH. Figure 1 shows descriptive information for these variables. As shown in Table 3, three comparisons of HIV+ and HIV− PWH were statistically significant at a 5% level; psychosomatic symptoms, life satisfaction and level of education. In 1988, HIV+ PWH had more psychosomatic symptoms than HIV− (p = 0.026, Table 3, Fig. 1). In 2001, life satisfaction was higher among HIV+ compared to HIV− PWH (p = 0,049, Table 3). In 2012, the level of education was higher among HIV− compared to HIV+ PWH (p = 0,015, Table 3). Tests of differences in trend over time found two significant results: the trend in life satisfaction was more positive for HIV+ PWH compared to HIV− PWH (p = 0,046, Table 3). Across years, HIV− PWH attained higher levels of education than HIV+ PWH (p = 0,024, Table 3).
Table 3.
P-values of trends statistically significant trends in italic
| HIV+/HIV−a | Trends (HIV+/HIV−)b | |||
|---|---|---|---|---|
| 1988 | 2001 | 2012 | ||
| Education | 0,717 | 0,622 | 0,015 | 0,024 |
| Work | 0.075 | 0.534 | 0.328 | 0.113 |
| Social activities | 0.752 | 0.679 | 0.901 | 0.884 |
| Family type | 0,333 | 0,753 | 0,459 | 0,383 |
| Life satisfaction | 0.179 | 0.049 | 0.655 | 0.046 |
| Psychosomatic symptoms | 0.026 | 0.552 | 0.395 | 0.173 |
| Worries | 0.996 | 0.169 | 0.649 | 0.379 |
| Being Alone | 0.535 | 0.166 | 0.561 | 0.121 |
aComparison between HIV+ and HIV− for each year
bTrend over time for HIV+ compared to HIV
Fig. 1.
Descriptive information on variables
While no other statistically significant HIV-related differences were found, some trends in the data are worth noticing. While the proportion of HIV+ PWH with a regular full-time job was steady throughout the 24 years (range 33–38%), the proportion of HIV− PWH with a regular full-time job increased from 15 to 56%. A decrease was seen in social activities, but this decrease was seen for both HIV+ PWH and HIV− PWH. Similarly, an increase was seen in the number of both HIV+ PWH and HIV− PWH living with partner or spouse, more prominently among HIV− PWH.
Stigmatization and openness about HIV
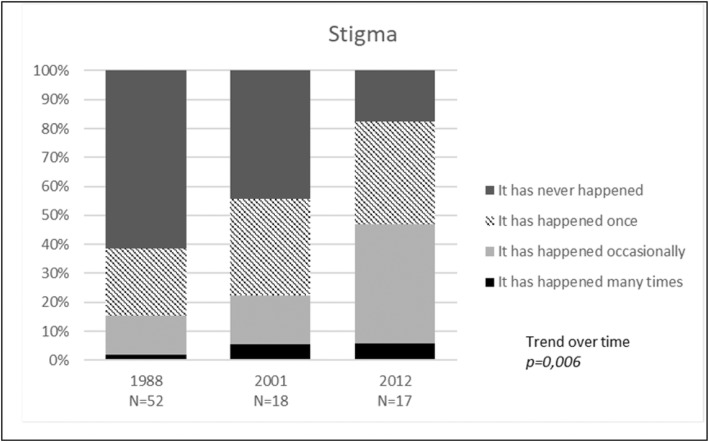
Figures 2 and 3 show the analyses of the experienced stigmatization and openness towards spouse/partner, children, families and colleagues. There was a significant increase in experienced stigmatization among HIV+ PWH from 1988 to 2012 (p = 0,006, Fig. 2), with larger proportions of HIV+ PWH in 2012 that had experienced stigmatization many times or occasionally.
Fig. 2.
Perceived stigmatization of HIV status among HIV-positive PWH
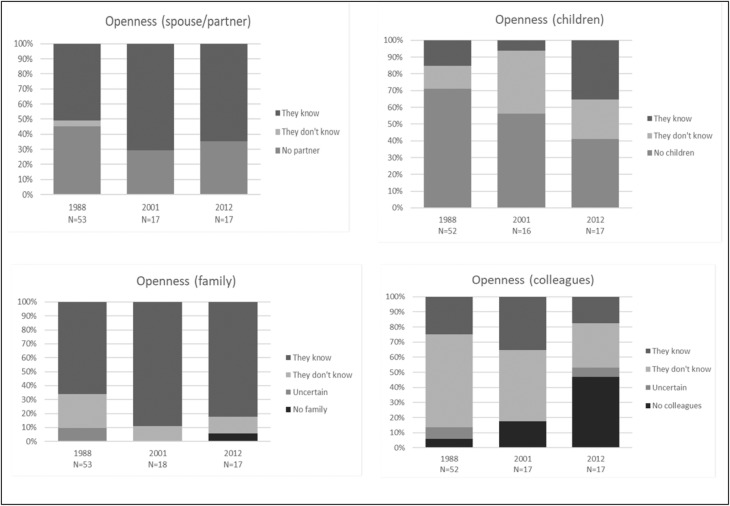
Fig. 3.
Openness about disease
While no changes over time regarding openness were statistically significant, the figures suggest some trends: The proportion of HIV+ PWH being open about their HIV status to spouse/partner and children saw an overall increase from 1988 to 2012 (Fig. 3). In 2012 24% of HIV+ PWH indicated that their children did not know about their HIV status. Openness about HIV status to other family increased, whereas openness towards colleagues decreased.
Discussion
This study found a higher burden of psychosomatic symptoms such as anxiety, headache, tiredness and depression among HIV+ PWH compared to HIV− PWH in 1988. We also found higher levels of life satisfaction among HIV+ PWH in 2001 compared to HIV−, and a significantly more positive trend over time in life satisfaction among HIV+ compared to HIV− PWH. In 2012, a higher level of education was seen among HIV− PWH compared to HIV+ PWH and a significant trend over time towards higher education for HIV− PWH. No differences between HIV+ and HIV− PWH in social activities, feelings of loneliness, or worries about bleeding were found. Finally, in analyses restricted to HIV+ PWH, we found an increase in perceived stigmatization from 1988 to 2012.
Our findings on experienced stigma contradicted our hypotheses and were inconsistent with previous studies on stigma among HIV+ patients in general [9]. There are several possible explanations for these findings. A qualitative study from 2015 found HIV-related stigma within health contexts to be a broad and complex phenomenon [8]. Our questions may have been too simple to reflect this complexity. We asked about ever having experienced stigmatization, and the results from 2001 and 2012 may therefore reflect accumulation of experience through many years of being HIV infected. The fact that HIV+ PWH still recalls experience of stigmatization may reflect a persistent stigma associated with HIV infection and points to an issue of importance to HIV+ PWH.
We found weak and statistically insignificant trends towards greater openness about HIV status. A study from 2002 found that openness about HIV status among adolescents was positively associated with both social support, self-competence and decreased problem behavior [15]. The direction of an association between HIV openness and support is unclear. It is possible that openness about HIV induce sympathy and social support. On the other hand, HIV+ PWH may only choose to be open about HIV if they trust the environment to be supportive.
Our results regarding increased psychosomatic symptoms in 1988 are in line with a 1990 US study [11] and a 1992 Canadian study [5] finding elevated scores of depression and anxiety among HIV+ PWH. In relation to our findings on increased well-being among HIV+ PWH in 2001, results from other studies are mixed. Some studies show low satisfaction [5, 12, 13] and QoL [14]. An Italian study from 1995 [18] found worse psychological problems among 21 HIV− PWH than among 24 HIV+ PWH and a study from 1999 found no association between HIV-status and health related QoL among individuals with severe hemophilia [16]. Qualitative data suggest that HIV+ PWH suffer from a psychological and physiological burden of HIV [22], as expressed by one Danish HIV+ PWH (authors translation) [23]:
I still don’t dare to look far ahead into the future. For many years, my life revolved on getting used to dying soon, and now one suddenly has to live. In certain contexts, life is harder than death when you have grown up hand in hand with death.
All results must also be interpreted in the light of scientific and social developments regarding hemophilia and HIV/AIDS over the 24-year span. In 1988, there was no curative treatment for HIV, thus HIV+ PWH were influenced by numerous uncertainties on their prognosis. Following the introduction of Highly Active Antiretroviral Treatment (HAART) in the late 1990s the prognosis for HIV+ PWH improved considerably which may explain our results showing increased life satisfaction among HIV+ PWH in 2001. The somewhat similar level of well-being between HIV+ PWH and HIV− PWH in 2012 might be due to the two groups’ comparable disabilities and possibilities in life.
The differences between HIV− and HIV+ PWH regarding educational level in 2012 may indicate that being co-infected with HIV has been a barrier for completing further education. The explanations for this barrier may be complex. HIV+ PWH, who were in the educational system in 1988 may have dropped out or decided not to pursue higher education in light of their perceived low life expectancy. In subsequent years where life expectancy dramatically improved for HIV+ PWH, they may have experienced ‘survivors guilt’ [24] and felt undeserved of pursuing opportunities such as higher education, despite their life expectancy being the same as for HIV− PWH.
In general, our original hypotheses on differences regarding well-being and social function between HIV+ PWH and HIV− PWH were only partly met. While qualitative studies from 1985 to 1991 describe severe psychological impact of HIV-infection among PWH [5, 11], several other authors have found surprisingly small differences between HIV+ PWH and HIV− PWH [14, 16–18]. Similarities between HIV+ PWH and HIV− PWH may have several explanations.
First, results may be biased by non-response from the HIV+ PWH with the largest impact of HIV infection, causing us to underestimate the impact of HIV.
Second, from 1988 to 2001 mortality was high among HIV+ PWH and may have been particularly high for the HIV+ PWH with worst health and lowest quality of life.
Third, even when responding, PWH may underreport symptoms and problems. Qualitative studies have found that some PWH downplay their symptoms and problems as a coping mechanism [25].
Fourth, lack of statistical power due to small sample size may cause us to overlook differences in well-being that are important to patients.
Fifth, Danish HIV+ PWH received an economic compensation for having been infected with HIV [26] and psychological and social counseling services for HIV+ PWH were established by the Danish Hemophilia Society. The compensations and opportunities for support may have reduced the psychological consequences of HIV infection.
Sixth, in light of the multiple burdens and health risks experienced by PWH, the incremental impact of HIV infection after the introduction of HAART might have been smaller than anticipated by outsiders. A limitation in understanding the differences between HIV+ PWH and HIV− PWH is that we do not have person-level information about HIV-related comorbidities or antiretrovival treatment. On population level we know that HAART was offered to all HIV+ PWH in 2001 and 2012 and accepted by nearly all.
Finally, analyses of openness and perceived stigma were carried out in the HIV+ group only. The average age of HIV+ PWH increased from 1988 to 2012, which may have biased the results, if perceptions of stigma vary between the young and the old.
There are several strengths to be noted in the current study. First, a large proportion of the Danish HIV+ PWH from 1988 to 2012 were included, providing knowledge from the group of Danish patients. Second, the information was obtained over a long time span providing more extensive insight than previous studies. Third, propensity score matching enabled us to identify the best possible comparison groups of HIV− PWH.
Conclusion
The differences between Danish HIV+ and HIV− PWH regarding both well-being and psychosomatic symptoms seem to have evened out from 1988 to 2012, even though there are still differences regarding employment and education. Despite great improvements concerning treatment, public attitude and knowledge about HIV, HIV+ PWH still experience challenges. The health risks and the psychosomatic burdens associated with both hemophilia and HIV should be held in mind when reviewing the need for social, psychological and financial support in this patient group.
Supplementary information
Additional file 1. Description of study questionnaire.
Acknowledgements
The authors want to thank the nurses in the hemophilia centers and the staff at the Danish Hemophilia Society for sending out and receiving letters from all participants, Susan Cowan and Tyra Grove Krause, Statens Serum Institut, for data on vital status of Danish Hemophilia Patients, and Annegrethe Hansen, Section of Social Medicine, University of Copenhagen, for comments on a previous version of this paper.
Abbreviations
- HIV
Human Immunodeficiency Virus
- PWH
Patients with hemophilia
- QoL
Quality of life
Authors’ contributions
The process of producing the manuscript was by initiative from EBI, CS and JBB followed by an iterative production from EBI, CS, JBB, TA, LL, EF, LHP, KBH, ALL and JG; all authors have made substantial contributions to the conception and design of the work, JBB have performed the analysis in collaboration with EBI and CS, JBB, EBI and CS have drafted the main part of the work and all authors have revised, commented and approved the submitted version. All authors have agreed to be personally accountable for the work and questions related to the accuracy and integrity of any part of the work.
Funding
This project has been supported by an unrestricted grant from Gilead Sciences to the position as research assistant for EBI to write the manuscript. Gilead had no influence on data collection, analysis, or interpretation.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due the protection of personal information among the group of anonymous participants. Tabled data are available from JBB on reasonable request.
Ethics approval and consent to participate
The 1988 study was approved by the Danish Institutional Review Board. Subsequently, Danish Law specified that questionnaire studies did not require ethical approval [27], so the 2001 and 2012 surveys did not require and could not obtain renewed approval. The questionnaires were accompanied by information regarding the participation being voluntary and having no impact on treatment. For children, parents filled out the questionnaire together with the participant or on behalf of the participant. Informed consent was given by returning a completed version of the questionnaire to the Danish Hemophilia Society, thus written consent was not applicable. For children, informed consent was provided by parents when participating. The Danish National Committee on Health Research Ethics approve consent in this form.
Consent for publication
Not applicable.
Competing interests
TA received a speaker fee at Novo-Nordisk Workshop in 2017. The other authors stated that they had no interest which might be perceived as posing a conflict or bias.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emilie B. Ingvorsen, Email: embi@sund.ku.dk
Christina Schnohr, Email: cwsc@sund.ku.dk.
Terkel Andersen, Email: mail@terkelandersen.dk.
Lars Lehrmann, Email: larslehrmann@live.dk.
Eva Funding, Email: eva.funding@regionh.dk.
Lone H. Poulsen, Email: lonpouls@rm.dk
Karen B. Holm, Email: kbh@bloderforeningen.dk
Alex L. Laursen, Email: alexlaur@rm.dk
Jan Gerstoft, Email: Jan.Gerstoft@regionh.dk.
Jakob B. Bjorner, Email: jacob.bjorner@sund.ku.dk
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12889-019-8062-9.
References
- 1.White GC, 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85(3):560. doi: 10.1055/s-0037-1615621. [DOI] [PubMed] [Google Scholar]
- 2.World Federation of Hemophilia . Symptoms and diagnosis. 2012. [Google Scholar]
- 3.Evatt B. Infectious disease in the blood supply and the public health response. Semin Hematol. 2006;43(2 Suppl 3):S4–S9. doi: 10.1053/j.seminhematol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Wilkie PA, Markova I, Naji SA, Forbes CD. Daily living problems of people with haemophilia and HIV infection: implications for counselling. Int J Rehabil Res. 1990;13(1):15–25. doi: 10.1097/00004356-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Catalan J, Klimes I, Bond A, Day A, Garrod A, Rizza C. The psychosocial impact of HIV infection in men with haemophilia: controlled investigation and factors associated with psychiatric morbidity. J Psychosom Res. 1992;36(5):409–416. doi: 10.1016/0022-3999(92)90001-I. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg PD, Hounshell J, Sherman LA, Godwin J, Ali S, Tomori C, et al. Legal, financial, and public health consequences of HIV contamination of blood and blood products in the 1980s and 1990s. Ann Intern Med. 2002;136(4):312–319. doi: 10.7326/0003-4819-136-4-200202190-00011. [DOI] [PubMed] [Google Scholar]
- 7.The Danish Haemophilia Society . The hemophilia lawsuit [Blødersagen] 2018. [Google Scholar]
- 8.Chambers LA, Rueda S, Baker DN, Wilson MG, Deutsch R, Raeifar E, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health. 2015;15:848. doi: 10.1186/s12889-015-2197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc. 2013;16(3 Suppl 2):18734. doi: 10.7448/IAS.16.3.18734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira AA, Leite IC, Bustamante-Teixeira MT, Correa CS, da Cruz DT, Rodrigues Dde O, et al. Health-related quality of life in hemophilia: results of the hemophilia-specific quality of life index (Haem-a-Qol) at a Brazilian blood center. Rev Bras Hematol Hemoter. 2013;35(5):314–318. doi: 10.5581/1516-8484.20130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dew MA, Ragni MV, Nimorwicz P. Infection with human immunodeficiency virus and vulnerability to psychiatric distress. A study of men with hemophilia. Arch Gen Psychiatry. 1990;47(8):737–744. doi: 10.1001/archpsyc.1990.01810200045006. [DOI] [PubMed] [Google Scholar]
- 12.Barr RD, Saleh M, Furlong W, Horsman J, Sek J, Pai M, et al. Health status and health-related quality of life associated with hemophilia. Am J Hematol. 2002;71(3):152–160. doi: 10.1002/ajh.10191. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Hachisuka K, Okazaki T, Shirahata A, Ogata H. Health status and satisfaction of asymptomatic HIV-positive haemophiliacs in Kyushu, Japan. Haemophilia. 1999;5(1):56–62. doi: 10.1046/j.1365-2516.1999.00212.x. [DOI] [PubMed] [Google Scholar]
- 14.Djulbegovic B, Goldsmith G, Vaughn D, Birkimer J, Marasa M, Joseph G, et al. Comparison of the quality of life between HIV-positive haemophilia patients and HIV-negative haemophilia patients. Haemophilia. 1996;2(3):166–172. doi: 10.1111/j.1365-2516.1996.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 15.Battles HB, Wiener LS. From adolescence through young adulthood: psychosocial adjustment associated with long-term survival of HIV. J Adolesc Health. 2002;30(3):161–168. doi: 10.1016/S1054-139X(01)00341-X. [DOI] [PubMed] [Google Scholar]
- 16.Miners AH, Sabin CA, Tolley KH, Jenkinson C, Kind P, Lee CA. Assessing health-related quality-of-life in individuals with haemophilia. Haemophilia. 1999;5(6):378–385. doi: 10.1046/j.1365-2516.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 17.Pasqual Marsettin E, Ciavarella N, Lobaccaro C, Ghirardini A, Puopolo M, Cultraro D, et al. Knowledge of HIV/AIDS and emotional adjustment in a cohort of men with haemophilia and HIV infection: final report. Haemophilia. 1998;4(6):820–825. doi: 10.1046/j.1365-2516.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 18.Marsettin EP, Ciavarella N, Lobaccaro C, Ghirardini A, Bellocco R, Schinaia N. Psychological status of men with haemophilia and HIV infection: two-year follow-up. Haemophilia. 1995;1(4):255–261. doi: 10.1111/j.1365-2516.1995.tb00085.x. [DOI] [PubMed] [Google Scholar]
- 19.Schnohr C, Bacher T, Andersen T, Lehrmann L, Funding E, Poulsen LH, et al. Joint mobility and physical function of Danish hemophilia patients: a three-wave panel study spanning 24 years. Acta Haematol. 2018;140(4):240–246. doi: 10.1159/000493783. [DOI] [PubMed] [Google Scholar]
- 20.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15(3):199–236. doi: 10.1093/pan/mpl013. [DOI] [Google Scholar]
- 22.Westersø RS. 91 Danish hemophiliacs were infected with HIV, 26 survived [91 danske blødere blev smittet med HIV: 26 overlevede]: B.T. 2014. [Google Scholar]
- 23.Pagter LK. Now you suddenly have to live [Nu skal man pludselig til at leve]. Bloedernyt. 2008:8–9. [Available from: https://www.bloderforeningen.dk/sites/default/files/mediearkiv/pdf/Bl%C3%B8derNyt%204%202008%20NETversion.pdf].
- 24.Hutson SP, Hall JM, Pack FL. Survivor guilt: analyzing the concept and its contexts. ANS Adv Nurs Sci. 2015;38(1):20–33. doi: 10.1097/ANS.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 25.Brodin E, Sunnerhagen KS, Baghaei F, Tornbom M. Persons with Haemophilia in Sweden- experiences and strategies in everyday life. A Single Centre Study. PLoS One. 2015;10(10):e0139690. doi: 10.1371/journal.pone.0139690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Retsinformation.dk. Law on the hemophilia compensation fund [Lov om Blødererstatningsfonden] [Updated June 6, 2018. Available from: https://www.retsinformation.dk/forms/r0710.aspx?id=46951.
- 27.Retsinformation.dk. Law on the Scientific Ethical Treatment of Health Sciences Research Projects [Lov om videnskabsetisk behandling af sundhedsvidenskabelige forskningsprojekter] [Updated June 6, 2018. Available from: https://www.retsinformation.dk/forms/R0710.aspx?id=192671.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Description of study questionnaire.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due the protection of personal information among the group of anonymous participants. Tabled data are available from JBB on reasonable request.