Tendinopathy is the most common disorder in sports medicine. Multiple hypotheses have been proposed for the aetiopathogenesis, but many aspects still remain elusive. Microdialysis studies have shown high levels of lactate within tendinosis, even at resting tendons,1 suggesting that hypoxia persists in tendinopathy. The presence of necrotic tenocytes, blocked arteries and anaerobic enzymes within tendinopathy lesions lend further support to the role of hypoxia in the aetiopathogenesis.2 Finally, ‘tendinosis’, the pathognomonic histopathological finding in tendinopathy, is composed of hypoxic, mucoid, hyaline and fibrinoid tissue.2 These tissue types are known to be hypoxia induced.
Tendons are generally poorly vascularised, while certain regions—those most prone to injury—are almost avascular. This can be considered an evolutionary ‘design failure’ that makes tendons susceptible to chronic and acute injuries. As a consequence, healthy tendons have a virtually non-existent tissue turnover throughout adulthood.3 However, somewhat paradoxically, tissue turnover is increased in tendinopathic tendons.3 Given the persisting hypoxia and subsequent anaerobic metabolism,1 2 it comes as no surprise that the enhanced tissue turnover leads to production of poorly organised tissue—tendinosis—in tendinopathy.2
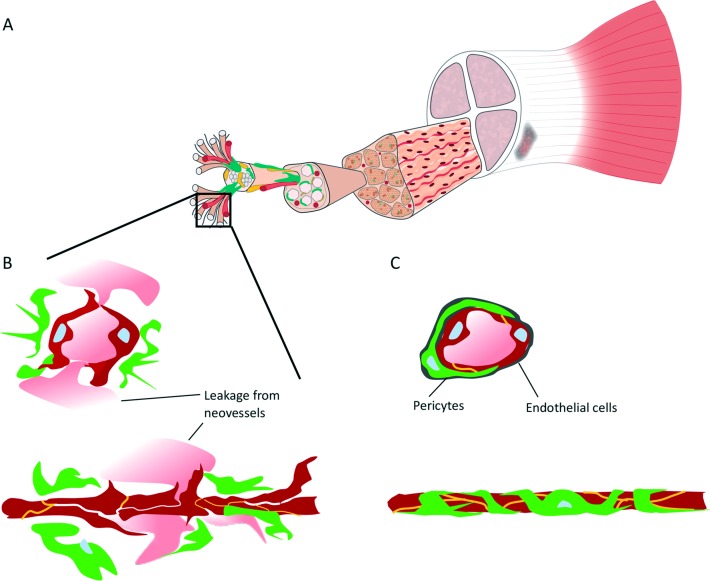
The fundamental survival mechanism of any cell under hypoxia is the activation of hypoxia-inducible factor-1α (HIF-1α),4 a transcription factor that turns on the expression of a large range of genes encoding angiogenic growth factors. 4 5 Characteristic features of both tendinopathic and ruptured tendons are elevated expression of HIF-1α and its target genes, the proangiogenic growth factors, such as vascular endothelial growth factor and abundant neovascularisation.(figure 1).5 The neovascularisation has even been proposed as the origin of tendinopathy-related pain,6 and accordingly, its eradication has been used as a therapy for the condition.6 Given that tissue regeneration requires sufficient supply of oxygen and nutrients, the existence of neovascularisation in tendinopathy should be interpreted as a sign of both persisting hypoxia and failed tissue repair attempt.
Figure 1.
Stabilisation of neovessels in tendinopathy. (A) Tendons respond to hypoxia by secreting angiogenic growth factors that induce the growth of neovessels in tendinopathy. (B) These neovessels are hyperpermeable2; they leak and do not have proper perfusion, failing to deliver oxygen and nutrients required for tissue regeneration. Fibrin-rich exudates leak from the neovessels, which results in fibrinoid degeneration, a typical feature of tendinosis in tendinopathy.2 (C) Future therapies should aim to ‘stabilise’ the neovessels, re-establishing the structural integrity of the vessel walls (lumenisation) and consequently enabling proper perfusion that replenishes supply of oxygen and nutrients. Picture adapted with permission from Taylor and Francis Group.9
Although almost completely ignored in sports medicine, it is well-established in the fields of cancer biology and retinopathy that hypoxia-induced neovessels are hyperpermeable.4 In essence, they leak and do not have proper perfusion4 (figure 1). These hyperpermeable neovessels fail to deliver oxygen and nutrients required for tissue maintenance and possible regeneration. Hyperpermeability also explains the apparent intellectual paradox as to why there is persisting hypoxia within regions of neovascularity.4
As noted, there are some preliminary data to suggest that eradication of neovascularisation has some efficacy in the treatment of tendinopathy. However, from a biological perspective, it is somewhat counterintuitive to assume that eradication of neovessels—those originally induced by cells struggling to survive under hypoxic conditions—could offer a viable long-term solution for tendinopathy. At least, in cancer and retinopathy—conditions with similar vascular changes—antiangiogenic therapies have merely worsened the underlying ischaemia.4 7
A tempting alternative approach is to ‘normalise’ or ‘stabilise’ the neovessels4 (figure 1). These ‘stabilised’ blood vessels with structurally intact vessel walls (lumenisation) and proper perfusion could replenish the supply of oxygen and nutrients and, consequently, enable proper tendon regeneration4 7 (figure 1). Although normalisation of neovascularisations may sound like science fiction, recent discoveries in vascular biology offer new hope, as genes responsible for the stabilisation of neovessels have been identified recently.7 Among them, R-Ras is a small GTPase with a pivotal role in maintaining both proper vascular stabilisation and blood vessel lumenisation.7 It also supports endothelial cell survival.7 Lack of R-Ras, in turn, is associated with hyperpermeable neovessels in neovascular human diseases such as retinopathy and cancer and leads to improper lumenisation of blood vessels in ischaemic skeletal muscle.7 The re-introduction of R-Ras, in turn, stabilised the non-functional blood vessels, restoring proper lumen formation and perfusion and, most importantly, reversing hypoxia.7
The proposed hypoxia-induced pathogenesis for tendinopathy might seem at odds with Doppler ultrasound studies that have shown normal oxygen saturation in tendinopathic tendons.8 However, arterio-venous anastomoses are common in diseased tendons, providing a bypass route for circulation and thus a plausible explanation for the failure to detect reduced oxygen saturation in tendinopathic tendons.2 In the end, hypoxia is the only possible explanation for the reported high levels of lactate1 and particularly for the characteristic histopathological findings in tendinopathic tendons.2
The quest for new therapies in sports medicine should rely on the discoveries of basic science. The novel model presented proposes a pivotal role for hypoxia in the aetiopathogenesis of tendinopathy. Tendons respond to hypoxia by secreting angiogenic growth factors to induce the growth of neovessels (figure 1). Unfortunately, these neovessels are non-functional by nature, failing to deliver oxygen and nutrients required to reverse the prevailing hypoxia. Stabilisation of neovessels could offer a tempting future therapeutic approach for the treatment of tendinopathy.
Footnotes
Contributors: TJ created the concept, wrote the manuscript and is the only author of the manuscript.
Funding: This work was funded by the Academy of Finland, Päivikki and Sakari Sohlberg Foundation, and The Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Alfredson H, Bjur D, Thorsen K, et al. High intratendinous lactate levels in painful chronic Achilles tendinosis. An investigation using microdialysis technique. J. Orthop. Res. 2002;20:934–8. 10.1016/S0736-0266(02)00021-9 [DOI] [PubMed] [Google Scholar]
- 2. Järvinen M, Józsa L, Kannus P, et al. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports 1997;7:86–95. 10.1111/j.1600-0838.1997.tb00124.x [DOI] [PubMed] [Google Scholar]
- 3. Heinemeier KM, Schjerling P, Øhlenschlæger TF, et al. Carbon-14 bomb pulse dating shows that tendinopathy is preceded by years of abnormally high collagen turnover. Faseb J 2018;32:4763–75. 10.1096/fj.201701569R [DOI] [PubMed] [Google Scholar]
- 4. McIntyre A, Harris AL. Metabolic and hypoxic adaptation to anti‐angiogenic therapy: a target for induced essentiality. EMBO Mol Med 2015;7:368–79. 10.15252/emmm.201404271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schneider M, Angele P, Järvinen TAH, et al. Rescue plan for Achilles: therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv Drug Deliv Rev 2018;129:352–75. 10.1016/j.addr.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 6. Pufe T, Petersen WJ, Mentlein R, et al. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports 2005;15:211–22. 10.1111/j.1600-0838.2005.00465.x [DOI] [PubMed] [Google Scholar]
- 7. Li F, Sawada J, Komatsu M. R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature. Nat Commun 2017;8:1720 10.1038/s41467-017-01865-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knobloch K, Grasemann R, Spies M, et al. Midportion Achilles tendon microcirculation after intermittent combined cryotherapy and compression compared with cryotherapy alone. Am J Sports Med 2008;36:2128–38. 10.1177/0363546508319313 [DOI] [PubMed] [Google Scholar]
- 9. Sawada J, Komatsu M. Normalization of tumor vasculature by R-Ras. Cell Cycle 2012;11:4285–6. 10.4161/cc.22465 [DOI] [PMC free article] [PubMed] [Google Scholar]