Abstract
The use of systemic glucocorticoids (GCs), as well as local injections, continues to be a controversial issue in the sport/anti-doping community. There is widespread and legitimate use of GCs for numerous health conditions, yet there are concerns about side effects and the possibility of enhanced athletic performance in limited settings. This is compounded by the uncertainty regarding the prevalence of GC use, mechanisms underlying physiological effects and complex pharmacokinetics of different formulations. While WADA continues to promote research in this complex area, some international sporting federations, major event organisers and professional sports leagues have introduced innovative rules such as needle policies, mandatory rest periods and precompetition guidelines to promote judicious use of GCs, focusing on athlete health and supervision of medical personnel. These complementary sport-specific rules are helping to ensure the appropriate use of GCs in athletes where overuse is a particular concern. Where systemic GCs are medically necessary, Therapeutic Use Exemptions (TUEs) may be granted after careful evaluation by TUE Committees based on specific and strict criteria. Continued vigilance and cooperation between physicians, scientists and anti-doping organisations is essential to ensure that GC use in sport respects not only principles of fairness and adherence to the rules but also promotes athlete health and well-being. The purpose of this narrative review is to summarise the use and management of GCs in sport illustrating several innovative programmes by sport leagues and federations.
Keywords: athlete, doping, drug use, elite performance, sport
Introduction
The use of systemic glucocorticoids (GCs) in sport remains a vexing issue. There are some people in the sport community who view GC treatment as perfectly acceptable for athletes if clinically indicated, while others believe that athletes with either chronic or acute medical conditions should be prevented from competing rather than be allowed to use systemic GCs or local injections. There are proponents of removing GCs entirely from the WADA List of Prohibited Substances (List), while others want to increase the number of prohibited routes and not allow Therapeutic Use Exemptions (TUEs).
Are GCs a scourge in sport or a therapeutic product that is relatively well controlled? This paper examines (1) the prevalence of GC use in the general and athletic populations, (2) anti-doping regulations, (3) the science around whether systemic GCs can enhance performance, (4) health risks, adverse effects and negative effects on performance, (5) strategies and policies by sporting federations to ensure appropriate use of GCs and (6) current management of the TUE process for GCs.
Prevalence of GC use in the general and athletic populations
GCs are one of the most widely used and effective medication classes in the general population and are available in a variety of pharmaceutical formulations (eg, injections, tablets, creams, eye-drops, ear drops, inhalers and nasal sprays).1 Administered for both their systemic and local effects, GCs are used globally in a wide array of clinical specialities, primarily for their anti-inflammatory and immunosuppressive properties. In some settings, the medical use of oral GCs appears to have increased in recent years as GCs are an accessible and affordable alternative to targeted but costlier medications. Prevalence of systemic use predominately for short-term use varies between 1% and 3%2 3 although ranged as high as 17.1% in a recent study of adults in France.4 Oral GCs are commonly used in many countries as part of first-line treatment in some infectious disease settings (acute otitis media, pharyngitis) although determining efficacy is still an area of active investigation.5 6 A large survey of adults in 48 western European centres found that a median 3.5% of respondents were currently using asthma medications (many of which include inhaled GCs), with a prevalence of 80% in those experiencing recent asthma attacks.7 The Global Initiative for Asthma GINA 2018 report recommends early use of inhaled GCs in asthma management8 although there is no accompanying prevalence data.
In athlete populations, there is an increased prevalence of musculoskeletal injuries and asthma9 10 and therefore frequent legitimate therapeutic GC use would not be surprising. Nevertheless, there is a scarcity of prevalence estimates in athlete populations. An analysis of abbreviated TUEs where the IOC was notified of GC use by athletes in advance of Olympic Games in the 1990s and early 2000s suggests that at least 5% to 12% of competitive elite athletes were treated with GCs by all routes, predominantly inhaled.11 In a recent unpublished international survey of medical doctors working with elite athletes, over 85% reported that they have at least occasionally administered injectable GCs as part of their normal practice (personal communication, Dr David Hughes, Australian Institute of Sport).
Anti-doping regulations
GCs, administered by certain routes, were first prohibited in sport by the IOC in 1985 and have been prohibited by WADA since its initial List, published in 2004. Substances or methods are considered for inclusion in the List if they meet two out of the following three criteria as stated in the World Anti-Doping Code: (1) potential to enhance, or enhances sport performance; (2) represents an actual or potential health risk to the athlete; (3) violates the spirit of sport.12 GCs are prohibited in-competition when administered by ‘systemic’ (oral, rectal, intramuscular or intravenous) routes.13 Administration by all other routes (including intra-articular and other periarticular injections) is regarded as local administration and are not prohibited in-competition. GC administration by any route is not prohibited out-of-competition (OOC).
Regardless of the specific GC substance and their individual pharmacological characteristics, a presumptive adverse analytical finding (AAF) is reported by WADA-accredited laboratories when the urinary levels of in-competition samples exceed a 30 ng/mL reporting level. Pharmacokinetics of GCs is complex and influenced by the formulation, type of esterification and salt, administration route, site and method of administration. Accordingly, while the laboratory reporting limit may demonstrate the presence of a GC, it cannot necessarily indicate if the administration was in-competition or OOC or likely to have a pharmacological or ergogenic effect. Any physician or athlete will be unsure when to stop using systemic GCs before the in-competition period to avoid exceeding the reporting limit. To further complicate the pharmacokinetic picture, intra-articular injections may give rise to systemic levels and physicians may inadvertently mischaracterise the site of injection in the absence of radiological or ultrasound guidance. Substance-specific reporting limit refinements are an area of active discussion and research among WADA-appointed experts and are beyond the scope of this paper.
Do systemic GCs enhance performance?
Some athletes have undoubtedly attempted to harness the purported performance-enhancing effects of systemic GCs that they perceive to be beneficial in their particular sporting discipline. However, the complex and pleiotropic mechanisms of GC action suggest that these medications are an unwieldy tool for the athlete seeking to gain a performance advantage and are considered to be a less popular component of doping regimens than in the past.14 Some patients and athletes reported experiencing euphoria following systemic administration.15 However, the scientific evidence supporting measurable euphoria in clinical populations is equivocal and the interpretation of the data is complicated by the association of confounding chronic pain.16 17
There is no evidence of performance-enhancing effects from short-term use of systemic GCs.18–22 There are randomised double-blind cross-over studies which suggest that athletes can exploit high-dose week-long courses of oral GCs to improve their submaximal intensity exercise performance for brief periods of time.23 24 These dosages would be easily detected during anti-doping tests, if taken in-competition. The precise mechanism for this effect is unclear but suggested to result from a combination of effects on metabolism, muscle, inflammation and the nervous system. This drug effect was shown in one study of male athletes whose training was tightly periodised alongside oral GC use.24 Exploiting this type of performance-enhancing regimen while effectively avoiding adrenal insufficiency and detection by standard in-competition doping controls would require meticulous medical supervision. It may also require more complex and exotic pharmacological manipulation of the hypothalamic–pituitary–adrenal axis than that offered by prescription GCs.25
Athletes and doctors have described inappropriate methods whereby systemic GC use, restricted nutrition and low-intensity training might be combined OOC to lose weight and preserve muscle mass.26 However, given the widely understood protein-catabolic functions of GCs,27 28 this doping mechanism remains speculative and controversial. Furthermore, efficacy may be dependent on the use of GCs as part of a complex cocktail that includes other prohibited but poorly detected hormones such as insulin.29
Recent accounts of systemic GC’s supposed potency have come from athletes who have also confessed to concurrent use of other performance-enhancing methods and substances, including anabolic agents such as testosterone.14 30 31 Such GC regimens may only have relevance in a small subset of sporting disciplines, such as in the steep mountain stages of cycling’s Grand Tours, where athletes might be willing to accept compromise in their training regimens or absolute power output in the pursuit of a superior power to weight ratio. OOC use would still necessitate a prolonged continuation of GC use into the competition period to avoid adrenal insufficiency due to feedback mechanisms. Prolonged GC use carries well-known medical risks, some of which could permanently diminish athletic performance.32
Health risks, adverse events and negative effects on performance
Treatment with GCs for many conditions has a long history and a reasonable safety profile. High doses or chronic use of systemic GCs pose some risk to the health of the athlete. Careful examination, diagnosis and deliberation by the physician is paramount and the benefits of treatment need to be weighed against potential risks and adverse effects. Potential performance enhancement use, described above and thought to be restricted to specific sport contexts with high-dose GC use, is also potentially associated with significant risks to the health of an athlete.
Adverse events with well-established causal associations to clinically appropriate GC use touch on virtually every human system, range from acute to chronic negative health outcomes and include adrenal insufficiency, immunodeficiency, osteoporosis, muscle wasting, tendon/fascia failure, avascular necrosis of the femoral head, various electrolyte, nutrient and other metabolic imbalances, glaucoma and cataracts. Perhaps because GCs are such common and clinically versatile medications, some physicians may overestimate their therapeutic value and underestimate the severity of associated adverse events.33 34 Even a single intra-articular injection could result in clinically significant adrenal insufficiency leading to malaise, electrolyte imbalance and immunosuppression for several weeks.35–37
Importantly, the aetiology of these symptoms may be unrecognised by the athlete and medical personnel, particularly in a sporting context where athletes train at high intensity and symptoms can masquerade as fatigue related to overtraining. Furthermore, an athlete who suffers trauma or serious injury may be at increased risk for adrenal crisis due to the hypothalamic–pituitary–adrenal suppression from prior GC use. This could be particularly problematic if the athlete fails to disclose this prior use.
Both the efficacy and potential harm of intra-articular injections are highly debated. Evidence from a recent prospective sham-controlled study in patients with osteoarthritis suggested that frequent triamcinolone knee injections, delivered according to a predetermined schedule, failed to effectively manage long-term pain and led to a statistically significant reduction in cartilage thickness.38 Nevertheless, medical society recommendations, as well as a comprehensive meta-analysis support the efficacy and safety of the very same intervention,39 40 strongly suggesting that judicious use of intra-articular injections in appropriate patients and circumstances can yield positive outcomes. There is a lack of published evidence on safety or harm of intra-articular GC use in athletic populations, and further research is urgently required due to the ubiquitous use of intra-articular GCs.
Policies to ensure the appropriate use of GCs
Despite concerns of possible abuse for a competitive advantage or potential detrimental effects on athlete health, GCs are widely used in sport for legitimate therapeutic reasons. Understanding that the List is harmonised across all sports from Archery to Wakeboarding, doping with GCs is not a concern where the purported benefits of high-dose GC use (prolonged power output at submaximal exercise intensities or aggressive catabolic weight management) are unlikely to be performance enhancing. Therefore, an AAF for GC would not likely be associated with any doping intent. The use of systemic GCs in many sports must be considered in a different light than in high-risk sports such as cycling, where abuse is well-documented and scientific evidence provides some support.
Mindful of the specific challenges posed by GC use in sport, sporting and anti-doping organisations have introduced innovative policies and are strengthening existing regulations to address the reasonable therapeutic use of GCs.
Needle policies
In cycling, there have been years of anecdotal stories concerning GC abuse and its use as a doping agent. Interestingly, many of these athletes neglected to mention the multiple other potent anabolic and erythropoietic doping agents that they were using concurrently.31 41 Historically, the number of AAFs and athlete testimonies have supported the assertion of widespread abuse of GCs in cycling, although there is evidence to suggest that its abuse has declined in recent years. Nevertheless, the Union Cycliste Internationale (UCI) enacted revisions to its medical rules in 2011 prohibiting the use of needles 48 hours prior to competition, for any purpose, unless strict medical criteria were met. This was modified in 2013 requiring 8 days rest prior to competition following any GC injection.42 The response of doctors to these rule changes has been mainly positive because they help obviate pressure from athletes and team personnel to administer injections. The intent was also to protect athletes’ health, obliging them to rest and recover properly. According to the UCI’s own statistics, these rule changes effectively prohibiting all GC injections in-competition have further helped to reduce the occurrence of GC AAFs. Since the reporting values were established at 30 ng/mL, the number of AAFs in-competition declined steadily from 72 per year in 2005 down to 9 AAFs in 2016 (personal communication, Francesca Rossi, Director, Cycling Anti-Doping Foundation). The UCI’s use of distinct and specific rules to support the List and World Anti-Doping Code and to address abuse problems that have disproportionately affected cycling appears to be effective.
The IOC has also adopted a needle policy for all participating athletes at the Olympic Games.43 Needles must not be used except by: (1) medically qualified practitioners for the clinically justified treatment of injury, illness or other medical conditions or (2) those requiring autoinjection therapy for an established medical condition with a valid TUE, for example, insulin treatment for diabetes. This policy helps the IOC identify potential abuse of GC injections and focus on identifying and regulating physicians who may be engaged in aberrant medical practice, rather than sanctioning athletes who are following their doctor’s advice. This needle policy has been implemented by several International Sport Federations to strengthen controls on the use of substance injections, including GC injections.
Needle policies are constructive approaches to curbing abuse of potentially dangerous or unnecessary GC injection practices that neither stigmatise nor penalise athletes and responsible physicians. Each sport should decide whether a needle policy is warranted to control potential abuse.
League GC policies
Australian Football League (AFL) athletes, like many professional athletes, face strenuous and often short careers marked by multiple injuries and significant load management issues resulting in wear and stress on joints. The judicious use of GC is not infrequently part of an overall therapeutic strategy. When considering whether GCs may confer deliberate or inadvertent performance advantages, data collected in the AFL provide an illuminating context. A review of doping control forms and mandated documentation of all injections collected over an 8-month period from over 800 athletes found that 25% of athletes received a local (usually intra-articular) injection during the season. Of those who received injections, the average number received during the season was 2.2 injections. Further analysis revealed that use of GC injections actually declined during the finals period, supporting a pattern of clinically driven use consistent with injury management rather than one of abuse (personal communication, Dr Peter Harcourt, AFL Medical Director). While not without risk, intra-articular GC injections carried out judiciously do not contravene principles of good medical practice. All GC use in the AFL is annually reviewed and forms part of the league’s medical auditing of team medical personnel using a peer review format. When GC use exceeds the recommendations, the league authorities act to ensure athlete welfare (see table 1).
Table 1.
League sport recommendations for precompetition glucocorticoid use
| Route | Oral and rectal | Intramuscular, intravenous | Intra-articular, intrabursal, periarticular and other ‘local’ tissue injections |
| Recommendation | Only under medical control; cease 1 week precompetition |
Avoid altogether; consider requesting a TUE if use is less than 1 month precompetition | Avoid 3 days precompetition; avoid long-acting formulations (triamcinolone); follow manufacturer’s dose recommendations; retain full medical documentation for AAF investigation purposes |
AAF, adverse analytical finding; TUE, Therapeutic Use Exemption.
With good administration systems, professional sports are able to implement regulatory controls similar to the successful ‘needle’ and ‘athlete health’ policies of the IOC, UCI and a number of other international sport federations. Physician control is easier in the setting of team sports, particularly with professional teams where there are contracted team physicians and auditing of healthcare practices.
TUEs: a critical part of GC regulation
Athletes subject to doping control who are legitimately treated with prohibited routes of GCs in or very near to sporting competition may be permitted to use the medication if granted a TUE. However to avoid abuse, specific and strictly enforced rules are described in the International Standard for TUEs (ISTUE) and the World Anti-Doping Code for athletes seeking a TUE.12 44 ISTUE Article 4.1 lays out four strict criteria, all of which must be met in the estimation of a TUE Committee (TUEC) composed of physicians with expertise in the areas of medicine relevant to the application. Contrary to occasional reports in the media of rampant TUE abuse, TUEs are seldom sought by elite athletes.45 The number of TUEs in the last four Olympic Games has remained steady at approximately 1.2% (WADA data, confirmed by the IOC). Importantly, when granted, a TUE for a GC directly relates to individual medical needs and generally is narrowly limited in dose, frequency and duration of use. Anecdotal stories of cheating with GC TUEs are mostly reported in cycling and have been less common in the last 5 years. This may be due to a better understanding by athletes of the medication’s benefit–risk relationship, the more stringent application of the rules and investigative follow-up of AAFs.
Due to the value of systemic GCs in the treatment of many medical conditions common in athletes (eg, exacerbations of asthma, sinusitis, musculoskeletal injuries, acute allergic reactions and inflammatory bowel disease (IBD)), this class of prohibited substances consistently makes up the largest share of granted TUEs, according to recent statistics collected from WADA’s Anti-Doping Administration and Management System (ADAMS) database (see figure 1).
Figure 1.
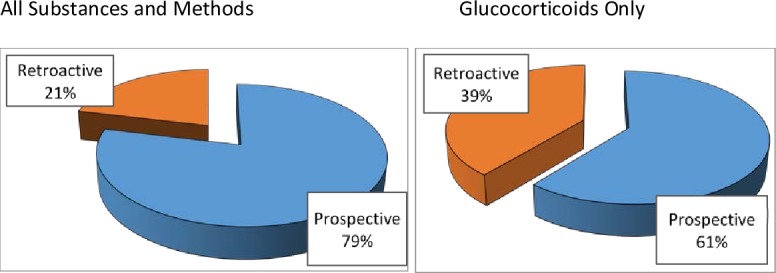
The percentage of TUEs that were entered in the WADA’s ADAMS database prospectively and retroactively. It also compares TUEs for all substances with glucocorticoids only. ADAMS, Anti-Doping Administration and Management System; TUEs, Therapeutic Use Exemptions.
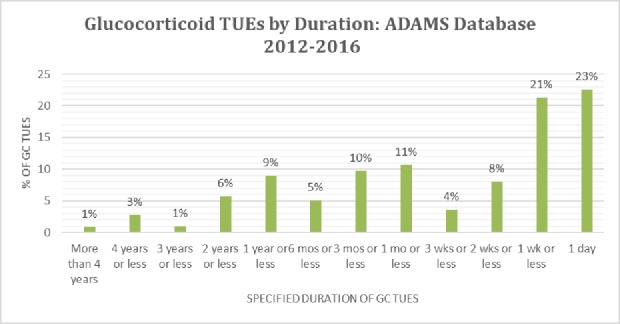
A prospective TUE application is preferred both for control and to provide permission certainty for athletes. However, GC TUE applications are often requested retroactively (~40%). This is consistent with clinical practices in the management of acute situations where elite athletes face challenges of travel and demanding training schedules. An athlete will tend to follow their doctor’s advice and accept immediate medical treatment rather than waiting and applying for a TUE. A retroactive TUE application scenario can lead to significant anxiety and uncertainty for clean athletes and their doctors who know that rejection of a retroactive TUE application may result in an anti-doping rule violation and significant consequences for the athlete. Promoting clean sport requires both careful clinical decision-making and diligent medical documentation. Consistent with indications typical for athlete populations and responsible treatment decisions, most GC TUEs registered in the ADAMS are for a duration of 1 week or less (see figure 2). TUEs granted for longer durations are more often prospective and are typically granted for inflammatory arthritis, IBD and other chronic autoimmune conditions.
Figure 2.
The duration of granted GC TUEs from the ADAMS database for the period 2012–2016. ADAMS, Anti-Doping Administration and Management System; GC, glucocorticoid; TUEs, Therapeutic Use Exemptions.
TUECs evaluate applications based on the ISTUE and WADA’s TUE Physician Guidelines. These guidelines, regularly updated by sport and specialist physicians, address various health conditions, including a number where systemic GCs are considered an important element of treatment (eg, musculoskeletal conditions, IBD, adrenal insufficiency and asthma).
Summary
The use of GCs in sport is a highly complex issue due to their widespread use in medicine, the many formulations and routes of administration with varying pharmacokinetics, negative health effects and potential doping associations. The mechanisms of action and ergogenicity in different sporting disciplines remain to be fully elucidated. Anti-doping regulations as well as sport policies monitoring physician behaviour have been implemented to control the use of GCs in sport. Further research is necessary to continue to refine thresholds as well as monitor potential abuse and judge the effectiveness of present regulatory programmes. A careful application of the TUE process will allow injured or ill athletes the right to compete while maintaining fairness within sport.
What is already known.
Historically, there has been considerable tension between anti-doping rules predicated on the evidence of potential for performance enhancement and the acceptance of the use of systemic glucocorticoids (GCs) for the treatment of medical conditions in elite athletes.
The prevalence of GC use in both the general and athlete population remains challenging to quantify and mechanisms of action promoting performance enhancement in sports have yet to be fully elucidated.
What are the new findings.
Where GC abuse (or overuse) is a potential concern, some sport federations, major event organisations and professional sport leagues have developed rules and recommendations to govern the use of GC, taking into account current knowledge of risks and benefits to strike a balance between fair play and athlete health.
Acknowledgments
The authors acknowledge the contribution of David Healy in the preparation of this manuscript.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: Each of the authors confirms that this manuscript has not been previously published and is not currently under consideration by any other journal. Neither have the Working Group conclusions and recommendations contained in the review ever been made public in any previous forum. Additionally, all of the authors have approved the contents of this paper and have agreed to abide by the British Journal of Sports Medicine’s submission policies.
Competing interests: AV works for the WADA. RB, PH, AK are members of the WADA List Expert Group and KM is a member of the WADA TUE Expert Group. The content of this manuscript does not represent any official views of WADA.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Fuentes A, Pineda M, Venkata K. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharmacy 2018;6 10.3390/pharmacy6020043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology 2011;50:1982–90. 10.1093/rheumatology/ker017 [DOI] [PubMed] [Google Scholar]
- 3. Laugesen K, Jørgensen JOL, Sørensen HT, et al. Systemic glucocorticoid use in Denmark: a population-based prevalence study. BMJ Open 2017;7:e015237 10.1136/bmjopen-2016-015237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bénard-Laribière A, Pariente A, Pambrun E, et al. Prevalence and prescription patterns of oral glucocorticoids in adults: a retrospective cross-sectional and cohort analysis in France. BMJ Open 2017;7:e015905 10.1136/bmjopen-2017-015905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cope D, Bova R. Steroids in otolaryngology. Laryngoscope 2008;118:1556–60. 10.1097/MLG.0b013e31817c0b4d [DOI] [PubMed] [Google Scholar]
- 6. Sadeghirad B, Siemieniuk RAC, Brignardello-Petersen R, et al. Corticosteroids for treatment of sore throat: systematic review and meta-analysis of randomised trials. BMJ 2017;358 10.1136/bmj.j3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ECRHS Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European community respiratory health survey (ECRHS). Eur Respir J 1996;9:687–95. 10.1183/09031936.96.09040687 [DOI] [PubMed] [Google Scholar]
- 8. GINA Global strategy for asthma management and prevention. global initiative for asthma. Available: https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/
- 9. Bennell KL, Crossley K. Musculoskeletal injuries in track and field: incidence, distribution and risk factors. Aust J Sci Med Sport 1996;28:69–75. [PubMed] [Google Scholar]
- 10. Burns J, Mason C, Mueller N, et al. Asthma prevalence in Olympic summer athletes and the general population: an analysis of three European countries. Respir Med 2015;109:813–20. 10.1016/j.rmed.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 11. Fitch K. Glucocorticoids at the Olympic games: state-of-the-art review. Br J Sports Med 2016;50:1267 10.1136/bjsports-2016-096664 [DOI] [PubMed] [Google Scholar]
- 12. WADA World anti-doping code, 2018. Available: https://www.wada-ama.org/en/resources/the-code/world-anti-doping-code
- 13. WADA The prohibited list, 2018. Available: https://www.wada-ama.org/en/what-we-do/the-prohibited-list
- 14. Stokes S. Jaksche on Sky’s TUE controversy; ‘We used the same excuse in my era.’. Available: https://cyclingtips.com/2016/09/jaksche-on-skys-tue-controversy-we-used-the-same-excuse-in-my-era/ [Accessed 7 Dec 2018].
- 15. Cyclingnews Westra admits using TUEs for performance enhancement. Available: http://www.cyclingnews.com/news/westra-admits-using-tues-for-performance-enhancement/ [Accessed 7 Dec 2018].
- 16. Lotan I, Fireman L, Benninger F, et al. Psychiatric side effects of acute high-dose corticosteroid therapy in neurological conditions. Int Clin Psychopharmacol 2016;31:224–31. 10.1097/YIC.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 17. Ozdel O, Kara CO, Kara IG, et al. Does corticosteroid usage in rhinoplasty cause mood changes? Adv Ther 2006;23:809–16. 10.1007/BF02850322 [DOI] [PubMed] [Google Scholar]
- 18. Arlettaz A, Collomp K, Portier H, et al. Effects of acute prednisolone intake during intense submaximal exercise. Int J Sports Med 2006;27:673–9. 10.1055/s-2005-872826 [DOI] [PubMed] [Google Scholar]
- 19. Arlettaz A, Collomp K, Portier H, et al. Effects of acute prednisolone administration on exercise endurance and metabolism. Br J Sports Med 2008;42:250–4. 10.1136/bjsm.2007.039040 [DOI] [PubMed] [Google Scholar]
- 20. Arlettaz A, Portier H, Lecoq A-M, et al. Effects of acute prednisolone intake on substrate utilization during submaximal exercise. Int J Sports Med 2008;29:21–6. 10.1055/s-2007-964994 [DOI] [PubMed] [Google Scholar]
- 21. Petrides JS, Gold PW, Mueller GP, et al. Marked differences in functioning of the hypothalamic-pituitary-adrenal axis between groups of men. J Appl Physiol 1997;82:1979–88. 10.1152/jappl.1997.82.6.1979 [DOI] [PubMed] [Google Scholar]
- 22. Petrides JS, Mueller GP, Kalogeras KT, et al. Exercise-Induced activation of the hypothalamic-pituitary-adrenal axis: marked differences in the sensitivity to glucocorticoid suppression. J Clin Endocrinol Metab 1994;79:377–83. 10.1210/jcem.79.2.8045951 [DOI] [PubMed] [Google Scholar]
- 23. Arlettaz A, Portier H, Lecoq A-M, et al. Effects of short-term prednisolone intake during submaximal exercise. Med Sci Sports Exerc 2007;39:1672–8. 10.1249/mss.0b013e3180dc992c [DOI] [PubMed] [Google Scholar]
- 24. Collomp K, Arlettaz A, Portier H, et al. Short-Term glucocorticoid intake combined with intense training on performance and hormonal responses. Br J Sports Med 2008;42:983–8. 10.1136/bjsm.2007.043083 [DOI] [PubMed] [Google Scholar]
- 25. Duclos M. Glucocorticoids: a doping agent? Endocrinol Metab Clin North Am 2010;39:107–26. 10.1016/j.ecl.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 26. Millar D. How to get away with doping. Available: https://www.nytimes.com/2016/10/16/opinion/sunday/how-to-get-away-with-doping.html [Accessed 7 Dec 2018].
- 27. Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol 2015;6:12 10.3389/fphys.2015.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schakman O, Kalista S, Barbé C, et al. Glucocorticoid-Induced skeletal muscle atrophy. Int J Biochem Cell Biol 2013;45:2163–72. 10.1016/j.biocel.2013.05.036 [DOI] [PubMed] [Google Scholar]
- 29. Barel M, Perez OAB, Giozzet VA, et al. Exercise training prevents hyperinsulinemia, muscular glycogen loss and muscle atrophy induced by dexamethasone treatment. Eur J Appl Physiol 2010;108:999–1007. 10.1007/s00421-009-1272-6 [DOI] [PubMed] [Google Scholar]
- 30. Fotheringham A. The end of the road: the festina affair and the tour that almost wrecked cycling: Bloomsbury sport, 2016: 352. [Google Scholar]
- 31. Dekker T. Descent: my EPIC fall from cycling Superstardom to doping dead end. Boulder, CO USA: VeloPress, 2017: 224. [Google Scholar]
- 32. Simunkova K, Jovanovic N, Rostrup E, et al. Effect of a pre-exercise hydrocortisone dose on short-term physical performance in female patients with primary adrenal failure. Eur J Endocrinol 2016;174:97–105. 10.1530/EJE-15-0630 [DOI] [PubMed] [Google Scholar]
- 33. Curtis JR, Westfall AO, Allison J, et al. Population-Based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006;55:420–6. 10.1002/art.21984 [DOI] [PubMed] [Google Scholar]
- 34. Nassar K, Janani S, Roux C, et al. Long-Term systemic glucocorticoid therapy: patients' representations, prescribers' perceptions, and treatment adherence. Joint Bone Spine 2014;81:64–8. 10.1016/j.jbspin.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 35. Borresen SW, Klose M, Rasmussen AK, et al. Adrenal insufficiency caused by locally applied Glucocorticoids-Myth or fact? Curr Med Chem 2015;22:2801–9. 10.2174/0929867322666150716113003 [DOI] [PubMed] [Google Scholar]
- 36. Duclos M, Guinot M, Colsy M, et al. High risk of adrenal insufficiency after a single articular steroid injection in athletes. Med Sci Sports Exerc 2007;39:1036–43. 10.1249/mss.0b013e31805468d6 [DOI] [PubMed] [Google Scholar]
- 37. Johnston PC, Lansang MC, Chatterjee S, et al. Intra-articular glucocorticoid injections and their effect on hypothalamic-pituitary-adrenal (HPA)-axis function. Endocrine 2015;48:410–6. 10.1007/s12020-014-0409-5 [DOI] [PubMed] [Google Scholar]
- 38. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA 2017;317:1967–75. 10.1001/jama.2017.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ 2004;328 10.1136/bmj.38039.573970.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465–74. 10.1002/acr.21596 [DOI] [PubMed] [Google Scholar]
- 41. Millar D. Racing through the dark: the fall and rise of David Millar. United Kingdom: Orion, 2011: 368. [Google Scholar]
- 42. UCI New UCI anti-doping rules introduced to reflect 2015 World Anti-Doping Code and further strengthen cycling’s anti-doping procedures. Union Cycliste Internationale, 2015. Available: https://www.uci.org/inside-uci/press-releases/new-uci-anti-doping-rules-introduced-to-reflect-2015-world-anti-doping-code-and-further-strengthen-cycling-s-anti-doping-procedures-168056-8a67
- 43. IOC IOC needle policy for the games of the XXXI Olympiad in Rio in 2016. Report dated: 2016-05-02. Available: https://www.antidoping.ee/wp-content/uploads/2016/05/02_ioc_needle_policy_for_the_games_of_the_xxxi_olympiad_in_rio_in_2016_eng.pdf
- 44. WADA International Standard for Therapeutic Use Exemptions (ISTUE). Report dated: 2015-12-10. Available: https://www.wada-ama.org/en/resources/therapeutic-use-exemption-tue/international-standard-for-therapeutic-use-exemptions-istue
- 45. WADA 2018 Pyeongchang Olympic Games 10 report. Report dated: 2018-05-10. Available: https://www.wada-ama.org/en/resources/world-anti-doping-program/2018-pyeongchang-olympic-games-io-report