The simultaneous and independent worldwide outbreaks of Candida auris invasive infections seem to be a puzzling paradox [1, 2]. Since its first isolation, C. auris has risen several questions on how it could have appeared, survived, and thrived [1]. Several speculative hypotheses have been proposed. Although misuse of antimicrobials and over-abuse of azoles have been considered the main contributors to C. auris emergence [2, 3], these do not completely justify its spreading.
One of the most recent theories considers changes in climate conditions as a causative factor altering infectious disease ecology [4, 5] (Fig. 1). Humans and microbes had been influencing each other for decades. Global warming is one of the major components of climate change connected to human activities, having considerable impact on health and indirectly boosting infectious diseases. Only few fungal species can be considered as pathogenetic for humans, as the majority of mammals are remarkably resistant to invasive fungal diseases. Besides immunological responses, humans are characterized by a “thermal restriction zone” that protects against infections. Human-induced climate changes may be responsible for the progressive narrowing of this thermal restriction zone, defined as the difference between human basal temperature and environmental temperature. As C. auris is more thermotolerant if compared to other yeasts, global warming might have played an important role in its emergence [4]. Although the specific ecological niche has not been identified yet, the climatic oscillations effect on wetlands might have contributed to enrich this potential habitat, conferring thermal and salinity tolerance to C. auris non-pathogenetic naïve strains. Acquisition of virulence factors might be explained considering the potential transfer of virulence genes from other pathogenetic Candida spp. to C. auris naïve strains, or by the combination of global warming and UV radiations that might have induced genetic mutations. The upgrade of C. auris strains, from saprophyte to pathogenetic yeasts, has witnessed an intermediate avian host, thus permitting its transmission to humans. Overtime, genetic and epigenetic changes have led to an extreme adaptability of C. auris to different ecological niches, leading to the development of persistent outbreaks in healthcare settings [4, 5].
Fig. 1.
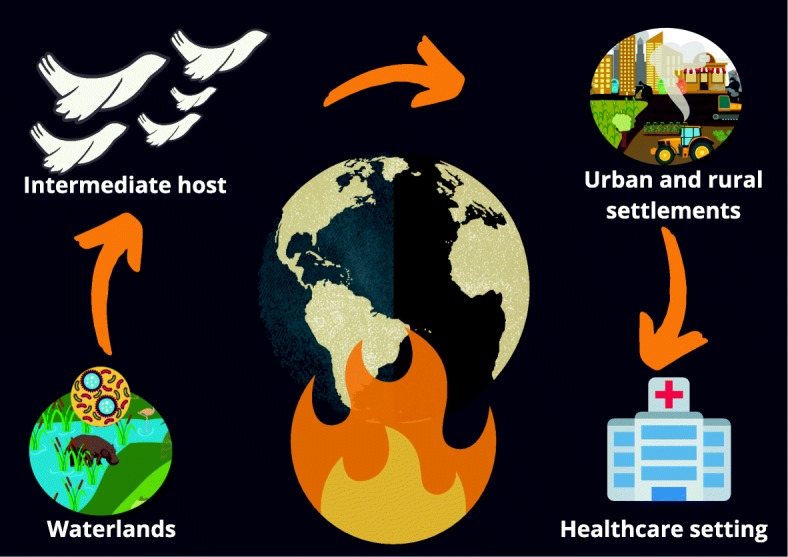
Global warming and the climate changes theory for C. auris emergence and spread. Rising ambient temperatures (caused by human activities) might have selected thermotolerant yeasts in wetlands; subsequently, acquiring opportunistic traits, C. auris might have spread through different ecosystems (wetlands, rural, and urban areas) thanks to intermediate avian hosts; following development of resistance and resilience through interspecies transmission, C. auris invades healthcare settings, leading to persistent outbreaks and causing infections in susceptible critically ill patients
Although global warming seems to be an appealing theory, it is not possible to ignore other factors which might explain C. auris rise. High population densities, poor hygiene, migrations, international travels, and pollution might indeed have contributed to the persistence of C. auris and acquisition of antifungal resistance [4]. Future studies are needed to identify its evolutionary reservoirs and validate the climate changes theory.
Acknowledgements
None.
Abbreviation
- ICU
Intensive care unit
Authors’ contribution
GM, MI, and AC conceived the content, wrote the manuscript, and approved the last version.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
GM and MI declare to have no competing interests. AC is member of the Advisory Board of Critical Care.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giovanni Misseri, Email: giovannimisseri1987@gmail.com.
Mariachiara Ippolito, Email: ippolito.mariachiara@gmail.com.
Andrea Cortegiani, Email: andrea.cortegiani@unipa.it.
References
- 1.Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018;6:69. doi: 10.1186/s40560-018-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortegiani Andrea, Misseri Giovanni, Chowdhary Anuradha. What’s new on emerging resistant Candida species. Intensive Care Medicine. 2018;45(4):512–515. doi: 10.1007/s00134-018-5363-x. [DOI] [PubMed] [Google Scholar]
- 3.Cortegiani A, Misseri G, Giarratano A, Bassetti M, Eyre D. The global challenge of Candida auris in the intensive care unit. Crit Care. 2019;23:150. doi: 10.1186/s13054-019-2449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A, Kontoyiannis DP, Robert V. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio 10:e01397-19. 2019. 10.1128/mBio.01397-19. [DOI] [PMC free article] [PubMed]
- 5.Jackson BR, Chow N, Forsberg K, Litvintseva AP, et al. On the origins of a species: what might explain the rise of Candida auris? J Fungi. 2019;5:58. doi: 10.3390/jof5030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


