Abstract
Triple-negative breast cancer (TNBC) is a specific type of breast cancer with poor overall survival (OS) time. Previous studies revealed that microRNAs (miRNAs/miRs) serve important roles in the pathogenesis, progression and prognosis of TNBC. The present study analyzed the miRNA expression and clinical data of patients with TNBC downloaded from The Cancer Genome Atlas. A total of 194 differentially expressed miRNAs were identified between TNBC and matched normal tissues using the cut-off criteria of P<0.05 and |log2 fold change|>2. Of these miRNAs, 65 were downregulated and 129 were upregulated. Using Kaplan-Meier survival analysis, a total of 77 miRNAs that were closely associated with OS time were identified (P<0.05). The intersection of the 77 miRNAs and 194 differentially expressed miRNAs revealed six miRNAs. Log-rank tests based on survival curves were performed and two miRNAs were eliminated. The prognostic value of the remaining four miRNAs was evaluated with a Cox proportional hazards model using multiple logistic regression with forward stepwise selection of variables. Three miRNAs (miR-21-3p, miR-659-5p and miR-200b-5p) were subsequently identified as independent risk factors associated with OS time in the model. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses revealed that the target genes of these three miRNAs were mainly involved in ‘cell protein metabolism’, ‘RNA transcriptional regulation’, ‘cell migration’, ‘MAPK signaling pathway’, ‘ErbB signaling pathway’, ‘prolactin signaling pathway’ and ‘adherens junctions’. Taken together, the results obtained in the present study suggested that the three-miRNA signature may serve as a prognostic biomarker for patients with TNBC.
Keywords: microRNA, breast cancer, triple-negative breast cancer, microRNA-21-3p, microRNA-659-5p, microRNA-200b-5p
Introduction
Breast cancer is one of the most common malignant tumors in females (1). Breast cancer is classified into several subtypes according to the expression of various receptors, such as the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2) (2). In triple-negative breast cancer (TNBC), tumor cells do not express PR, ER or HER-2 (3). The majority of the treatments available to patients with middle- and late-stage TNBC are ineffective (4). Therefore, in order to improve the 5-year survival rate, further research to identify effective TNBC treatments is required (5). It is important to identify individuals at high risk for TNBC and provide appropriate treatment at an early stage (6).
MicroRNAs (miRNAs/miRs) are a family of non-coding RNAs that were discovered in 1993 (7). miRNAs consist of 22–26 nucleotides but have a complex structure that confers the ability induce cleavage or translational repression of target mRNAs (7). miRNAs are therefore important regulators of gene expression (8). Breast cancer arises through the accumulation of genetic mutations and epigenetic modifications; therefore, miRNAs are important factors in breast carcinogenesis (9). A number of miRNAs are expressed at higher or lower levels in tumor tissues compared with normal tissues and may serve as tumor markers in breast cancer (10,11). In fact, miRNAs have attracted great interest as cancer biomarkers in the last decade (12–14).
Certain miRNAs have been identified in previous research as breast cancer biomarkers, including miR-30a, miR-361-5p, miR-27a and miR-193b (12,15–18). However, research based on large sample sizes is lacking, particularly research employing clinical data (19–21). The Cancer Genome Atlas (TCGA) project, which was launched in 2006 by the National Cancer Institute and the National Human Genome Research Institute, contains data on 33 types of cancer from >10,000 patients (22,23). TNBC data available from TCGA consist of tissue miRNA-sequencing (seq) data and clinical information. The present study analyzed the aforementioned data in four steps: i) Identification of differentially expressed miRNAs between breast cancer and adjacent normal tissues; ii) screening of the miRNAs obtained in the first step using the Kaplan-Meier survival method, which yielded a total of six specific miRNAs; iii) identification of three of these six miRNAs that predicted patient survival rate based on statistical analysis; and iv) identification of the biological pathways regulated by these three miRNAs.
Materials and methods
TNBC miRNA-seq dataset and clinical information
The data analyzed in the present study were downloaded from TCGA (up to January 28, 2016). The clinical data for each patient were derived from a variety of methods used to detect HER-2 levels; therefore, ‘patient HER2 immunohistochemistry receptor status’ was used as a standard to determine patient HER-2 status (24). When confirming ER, PR and HER status, only patients with ‘positive’ and ‘negative’ data were enrolled, whereas patients with data deemed ‘close’ or ‘not available’ were excluded. The TNBC miRNA-seq data for miRNA differential expression consisted of data on tumor tissues (n=187) and matched normal tissues (n=12). All of the miRNA expression data were reported as ‘reads-per-million-miRNA-mapped’ and were normalized by log2 transformation. The inclusion criteria regarding clinical information for survival analysis were as follows: i) Complete follow-up data were available for 1–60 months (30-1,825 days); ii) the clinical data were complete (patients with uncertain or missing clinical data, with the exception of metastasis state, which contains various Mx stages, were excluded) and iii) miRNA-seq data integrity was validated (patients without individual miRNA values were exluded). Finally, a total of 151 patients who met these criteria were enrolled in the present study. In order to incorporate as much data as possible, miRNA differential expression and survival analyses were performed independently.
Screening of differentially expressed miRNAs
The Linear Models for Microarray Data (limma) package (version 3.36.5; http://bioconductor.org/packages/release/bioc/html/limma.html) in R (version 3.5.1; http://www.r-project.org/) was used to analyze differentially expressed miRNAs. The comparisons between tumor and normal tissues performed in limma were not conducted using a 1:1 ratio of tumor to healthy tissue, as healthy tissue specimens were not obtained from the majority of patients. Therefore, the tumor tissue from each patient was compared with all healthy tissue specimens. The fold-change (FC) was used to indicate the difference in miRNA expression between tumor and normal tissues. Log|FC|>1.5 and P<0.05 following false discovery rate adjustment were established as the cut-off criteria.
Survival analysis and the miRNA prognostic model
Survival analyses were performed using the survival package (version 2.43-1; http://cran.r-project.org/web/packages/survival/index.html) in R and SPS (version 22.0; IBM Corp.). The samples were sorted according to miRNA expression level and divided into high- and low-expression groups based on the median expression level. Each miRNA was evaluated individually using the Kaplan-Meier method in R. A total of 77 miRNAs that were closely associated with overall survival (OS) time (P<0.05) were identified. The intersection of the 77 miRNAs and 194 differentially expressed miRNAs revealed six miRNAs. After log-rank tests were performed, two miRNAs were eliminated as there was no significant difference in survival time between the low- and high-expression groups. The prognostic value of the remaining four miRNAs was evaluated with a Cox proportional hazards model using multiple logistic regression with forward stepwise selection of variables. Finally, three miRNAs were considered independent risk factors associated with OS time in the model. A risk score was calculated for each patient based on the expression levels of the three miRNAs as determined by the Cox regression coefficients (25). In total, 151 patients were divided into either the high-risk group (n=76) or low-risk group (n=75) based on risk score. The predictive power of the three-miRNA signature and clinical features together was analyzed. Univariate/multivariate Cox regression and the Kaplan-Meier method were used to assess the associations and influence on survival rate of the selected miRNAs. P<0.05 was considered to indicate a statistically significant difference. Data are expressed as the mean ± standard deviation.
Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) analyses
Two online miRNA databases were employed to predict the target genes of the three prognostic miRNAs: TargetScan (version 7.2) (26) and miRDB (27,28). The overlapping set of target genes was depicted in Venn diagrams for subsequent analysis. The overlapping genes were analyzed using the Database for Annotation, Visualization and Integrated Discovery (DAVID; version 6.8; david.ncifcrf.gov). DAVID is a bioinformatics database that integrates biological data and analysis tools, provides systematic and comprehensive bioinformatics annotations for large-scale gene or protein lists and aids users to extract bioinformatics data. The tools in DAVID perform numerous functions, including gene function enrichment, which improves the understanding of the biological roles of specific gene sets (29,30). GO (http://geneontology.org/) and KEGG (https://www.genome.jp/kegg; Release 88.0) pathway enrichment analyses were performed to identify the biological processes and pathways involving target genes. The cut-off criteria were P<0.05 and gene count ≥3 (31).
Results
Identification and analysis of differentially expressed miRNAs in TNBC
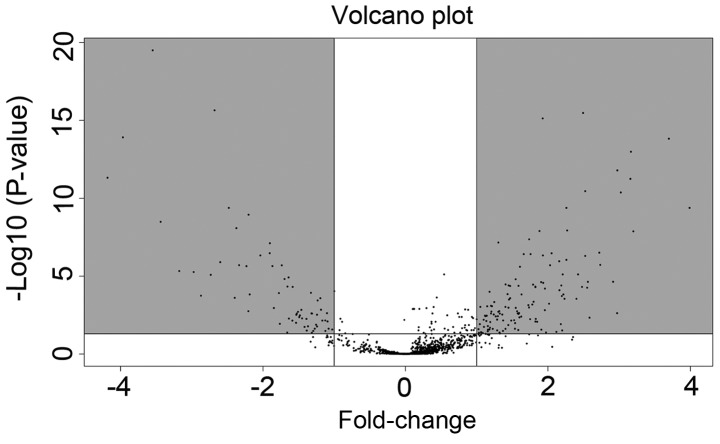
The TNBC miRNA-seq data in the present study included data on 187 tumor tissues and 12 matched normal tissues. A total of 194 differentially expressed miRNAs were identified using the cut-off criteria of P<0.05 and |log2FC|>2. Of these miRNAs, 65 were downregulated and 129 were upregulated (Fig. 1; Table SI).
Figure 1.
Volcano plot of the differentially expressed miRNAs. The dots in the gray regions represent upregulated or downregulated miRNAs. The white spots represent miRNA which has no statistical difference in expression between tumor tissue and normal tissue. miRNA, microRNA.
Identification of three miRNAs associated with OS time in TNBC
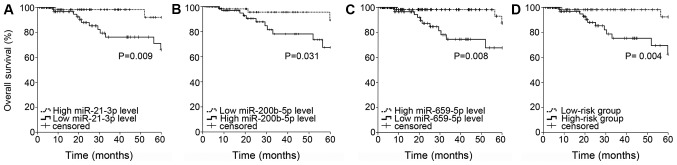
A total of 151 patients with validated data were enrolled in the present study. A total of 77 miRNAs significantly associated with OS time were identified from the survival analysis (Table SII). The intersection of the 77 miRNAs and 194 differentially expressed miRNAs revealed six miRNAs, including miR-4742-3p, miR-21-3p, miR-659-5p, miR-500b-3p, miR-429 and miR-200b-5p. Log-rank tests were performed to evaluate the survival curves. miR-4742-3p and miR-500b-3p were eliminated based on the cut-off criteria (P<0.05; Table SIII). The prognostic value of each of the remaining four miRNAs was evaluated using the Cox proportional hazards model, and miR-21-3p, miR-659-5p and miR-200b-5p were identified as independent risk factors associated with OS time (Fig. 2A-C). Among these miRNAs, miR-200b-5p was identified as a risk factor, whereas miR-21-3p and miR-659-5p were identified as protective factors. Additionally, the association between the aforementioned three miRNAs and clinical features was investigated (Table I). The results revealed that miR-218-1 was associated with metastasis state (P=0.031). A risk score based on the expression levels of the three miRNAs determined using the Cox regression coefficient for each miRNA was calculated as follows: Risk score=(0.627 × expression of miR-200b-5p)-(1.181 × expression of miR-659-5p)-(0.889 × expression of miR-21-3p). The risk score was used to divide the 151 patients into a high-risk group (n=76; risk score=−9.05~-5.99) and a low-risk group (n=75; risk score=−12.96~-9.06). Compared with the low-risk group, the high-risk group exhibited poor survival rate outcomes as assessed using the Kaplan-Meier method (Fig. 2D).
Figure 2.
Kaplan-Meier curve and log-rank test for each miRNA and the three-miRNA signature. (A) miR-21-3p, (B) miR-200b-5p, (C) miR-659-5p and (D) the three-miRNA signature. A total of 151 patients were divided into a high-risk group (n=76; risk score=−9.05~-5.99) and a low-risk group (n=75; risk score=−12.96~-9.06) based on the risk score. The risk score was calculated as follows: (0.627 × expression of miR-200b-5p)-(1.181 × expression of miR-659-5p)-(0.889 × expression of miR-21-3p). miRNA/miR, microRNA.
Table I.
Associations of the three microRNAs with the clinical features of patients with triple-negative breast cancer. The chi-square and t-tests were performed to assess the relationship between miRNA expression and clinical features. Data are expressed as the mean ± standard deviation.
| Variable | Number of patients | miR-659-5p | P-value | miR-21-3p | P-value | miR-200-5p | P-value |
|---|---|---|---|---|---|---|---|
| Patient age at diagnosis | 0.08 | ||||||
| <60 | 100 | 1.40±0.70 | 0.81 | 11.29±0.77 | 0.19 | 4.52±1.10 | |
| ≥60 | 51 | 1.43±0.64 | 11.10±0.92 | 4.16±1.34 | |||
| AJCC clinical stage | 0.33 | ||||||
| GI–II | 123 | 1.43±0.64 | 0.56 | 11.23±0.81 | 0.89 | 4.45±1.17 | |
| GIII–IV | 28 | 1.34±0.83 | 11.21±0.91 | 4.20±1.31 | |||
| AJCC T stage | 0.84 | ||||||
| T1-2 | 134 | 1.42±0.65 | 0.86 | 11.25±0.81 | 0.35 | 4.39±1.22 | |
| T3-4 | 17 | 1.38±0.90 | 11.05±0.93 | 4.45±0.99 | |||
| AJCC N stage | 0.52 | ||||||
| N0-1 | 130 | 1.43±0.66 | 0.32 | 11.23±0.80 | 0.81 | 4.43±1.17 | |
| N2-3 | 21 | 1.27±0.77 | 11.18±0.98 | 4.24±1.36 | |||
| AJCC M stage | 0.03 | ||||||
| M0 | 132 | 1.41±0.67 | 0.88 | 11.21±0.86 | 0.47 | 4.48±1.20 | |
| Mx | 19 | 1.39±0.74 | 11.35±0.54 | 3.84±1.02 | |||
| Histological type | 0.39 | ||||||
| IDC | 136 | 1.41±0.70 | 0.93 | 11.26±0.81 | 0.12 | 4.43±1.21 | |
| Non-IDC | 15 | 1.40±0.43 | 10.91±0.95 | 4.15±1.04 |
miR, microRNA; AJCC, American Joint Committee on Cancer; G, grade; T, tumor; N, node; M, metastasis; IDC, invasive ductile carcinoma.
Univariate and multivariate Cox regression analyses were performed to test the efficacy of the three-miRNA signature (high vs. low risk) in predicting OS time by considering the following clinical features: Age at initial pathological diagnosis (<60 years vs. ≥60 years), histological type [invasive ductile carcinoma (IDC) vs. non-IDC], tumor size [T in the American Joint Committee on Cancer (AJCC; http://cancerstaging.org/) Tumor, Node, Metastasis (TNM) staging system (T1-2 vs. T3-4)], lymph node status (N in the AJCC TNM staging system, N0-1 vs. N2-3), metastasis (M in AJCC TNM staging system, M0 vs. Mx) and AJCC pathological stage (G1-2 vs. G3-4). In the univariate analysis, the three-miRNA signature [hazard ratio (HR)=6.893; P=0.012; 95% confidence interval (CI) 1.52–30.69], pathological stage (HR=5.953; P=0.001; 95% CI 2.04–17.34) and lymph node status (HR=7.850; P=0.001; 95% CI 2.39–25.76) were associated with OS time in patients with TNBC (Table II). In the multivariate analysis, only the three-miRNA signature was identified as an independent prognostic factor (HR=7.396; P=0.011; 95% CI 1.59–34.41; Table II).
Table II.
Univariate and multivariate Cox regression analysis results for patients with triple-negative breast cancer.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variable | P-value | HR (95% CI) | P-value | HR (95% CI) |
| Age (≥60 vs. <60) | 0.538 | 1.494 (0.416–5.366) | 0.740 | 1.256 (0.327–4.831) |
| Histological type (Non-IDC vs. IDC) | 0.686 | 1.366 (0.301–6.190) | 0.969 | 1.034 (0.193–5.545) |
| Tumor size (T3-4 vs. T1-2) | 0.200 | 2.307 (0.643–8.276) | 0.735 | 1.336 (0.250–7.140) |
| Lymph node status (N2-3 vs. N0-1) | 0.001 | 7.850 (2.392–25.761) | 0.051 | 14.666 (0.989–217.523) |
| Metastasis (Mx vs. M0) | 0.477 | 0.042 (0.000–257.128) | 0.984 | <0.001 |
| Pathological stage (G3-4 vs. G1-2) | 0.001 | 5.953 (2.044–17.335) | 0.950 | 0.918 (0.063–13.415) |
| Three-miRNA signature (high-vs. low-risk) | 0.012 | 6.893 (1.524–30.690) | 0.011 | 7.396 (1.590–34.411) |
HR, hazard ratio; CI, confidence interval; IDC, invasive ductile carcinoma; T, tumor; N, node; M, metastasis; G, grade.
KEGG and GO analyses of the target genes of the miRNAs in the three-miRNA signature
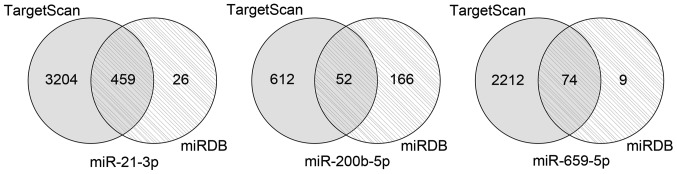
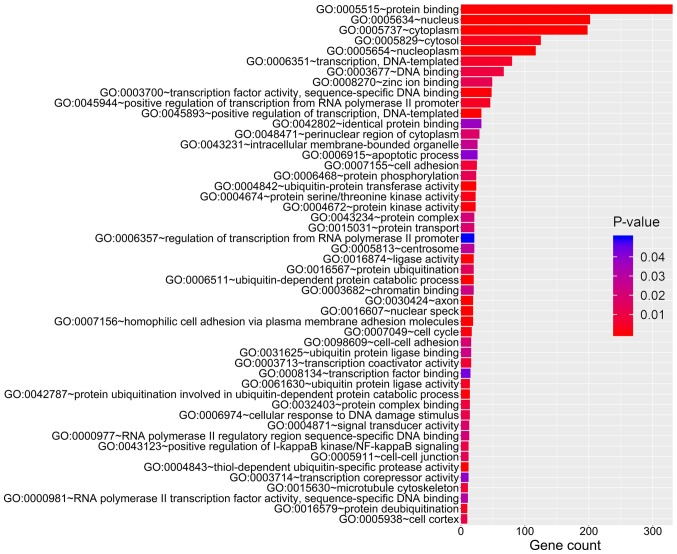
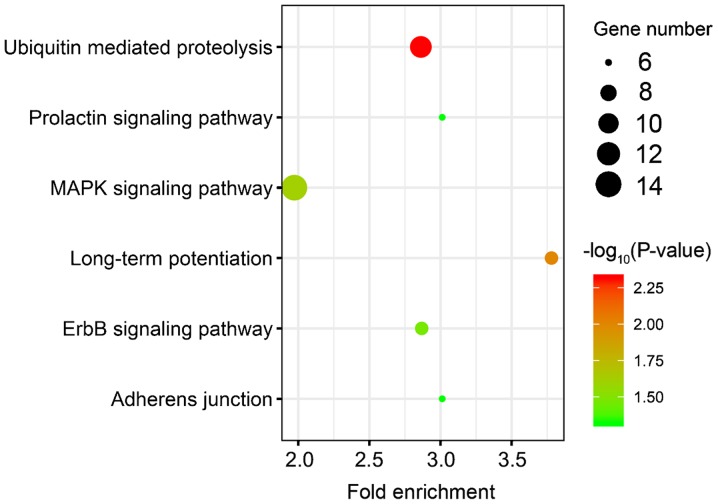
A total of 459 overlapping target genes for miR-21-3p, 52 overlapping target genes for miR-200b-5p and 74 overlapping target genes for miR-659-5p were identified (Fig. 3). The GO results revealed that these overlapping genes were enriched in 79 GO accessions and were mainly involved in ‘cell protein metabolism’, ‘RNA transcriptional regulation’ and ‘cell migration’ (Fig. 4). KEGG pathway analysis revealed enrichment in six pathways, including ‘ubiquitin-mediated proteolysis’, ‘long-term potentiation’, ‘MAPK signaling pathway’, ‘ErbB signaling pathway’, ‘prolactin signaling pathway’ and ‘adherens junction’ (Fig. 5).
Figure 3.
Venn diagrams displaying the overlapping target genes predicted using TargetScan and miRDB online tools. The grey circles represent the target genes of the three prognostic miRNAs predicted by TargetScan, and the circles with the diagonal lines represent the target genes of the three prognostic miRNAs predicted by miRDB. The overlapping areas between the two circles represent the overlapping set of target genes. miR, microRNA.
Figure 4.
GO analysis of the target genes of the miRNAs in the three-mRNA signature. The colors of the bands represent P-values, and the length of the bands represents the number of genes enriched in a particular GO term. GO, Gene Ontology; miRNA, microRNA.
Figure 5.
KEGG pathway analysis of the three-miRNA signature target genes. The color of the dots represents the -log10(P-value), and the dot size represents the number of genes enriched in a particular KEGG pathway. The x-axis represents the fold enrichment of target genes in a particular KEGG pathway. KEGG, Kyoto Encyclopedia of Genes and Genomes.
Discussion
The present study identified a three-miRNA signature associated with the survival rate of patients with TNBC. Patients were divided into two groups according to their risk score based on the expression pattern of the three miRNAs (miR-21-3p, miR-659-5p and miR-200b-5p). Significant differences in OS time were observed between the two groups. In the multivariate Cox model, the three-miRNA signature was identified as an independent prognostic factor of the OS time of patients with TNBC [HR=7.396; 95% CI, 1.59–34.41]. KEGG and GO analyses of the target genes of the three miRNAs indicated that they serve important roles in cell protein metabolism, RNA transcriptional regulation and cell migration, suggesting the aforementioned three miRNAs are implicated in the pathogenesis, progression and prognosis of TNBC. The 95% CI exhibited a wide range, possibly due to the small sample size (n=151). In a similar study, a wide 95% CI interval was also obtained (20). Future studies investigating larger sample sizes are required to assess the accuracy of the three-miRNA signature established in the present study.
The present study investigated the associations between clinical factors, such as age, tumor size and clinical stage, and miRNA levels. However, factors such as co-morbidities, smoking status and treatment received were not taken into account. Databases are unlikely to have records of all of the clinical factors affecting miRNA levels, therefore it is not possible to consider the impact of these clinical factors on patients. In order to circumvent this problem, the limma package in R was used to analyze differentially expressed miRNAs in the present study. The comparisons between tumor and normal tissues performed in limma were not conducted using a 1:1 ratio of tumor to healthy tissue, as healthy tissue specimens were not obtained from the majority of patients. Therefore, the tumor tissue from each patient was compared with all healthy tissue specimens. This approach eliminates the impacts of individual patient treatment options or other diseases (32).
miR-21 is one of the most studied miRNAs in breast cancer, but research focusing on the association between miR-21-3p and TNBC is lacking (33–35). In the present study, miR-21-3p expression was increased in tumor tissues compared with healthy tissues, which is consistent with previous studies (36,37). However, there is conflicting evidence regarding the association between miR-21-3p expression in tumor tissues and OS time. Previous studies revealed that miR-21-3p is an oncogenic factor in breast cancer, which upregulates the expression levels of phosphorylated protein kinase B (AKT) and L1 cell adhesion molecule (37,38). However, another in vitro study suggested that miR-21-3p reduces cancer cell growth (39). Furthermore, Jiao et al (40) reported that miR-21-3p functions as a tumor suppressor; however, further studies are required to investigate the effect of miR-21-3p on the OS time. Jiao et al (40) proposed that miR-21-3p causes transcript decay of miR-21-5p, a typical oncogene. The present study demonstrated that miR-21-3p was a protective factor in TNBC. However, this result requires further corroboration using a larger sample size.
Aberrant expression of miR-200 family members, including miR-200-5p, has been observed in several cancer studies (13,41). The present study revealed that miR-200-5p expression was increased in TNBC tissues compared with healthy tissues, consistent with previous studies (19,21), and that miR-200-5p is a risk factor for OS time. However, previous studies have demonstrated that miR-200b/c activates the focal adhesion kinase/phosphoinositide 3-kinase/AKT/nuclear factor-κB signaling pathway and zinc-finger E-box-binding homeobox factor to inhibit cancer cell invasion and migration (42–44). The aforementioned studies all involved in vitro cell experiments, and one study reported a different conclusion using TCGA data (21). The aforementioned study reported no statistically significant difference in OS time between patients with low vs. high expression of miR-200b/c, highlighting the complex biology of cancer (21). There may be two possibilities to explain the discrepancy between the present study and previously published studies: i) The present study did not analyze a sufficient number of samples to generalize the entire population of patients with TNBC, and ii) in vitro experiments do not reflect the complex microenvironment in vivo, and some unknown factor may influence the function of miR-200-5p in vivo. These key issues require further investigation in future studies.
The present study demonstrated that miR-659-5p is an independent protective factor associated with OS time in patients with TNBC. To the best of our knowledge, the present study was the first to describe an association between miR-659 and breast cancer. Due to the lack of systematic research, little is known about the role of miR-659. One study demonstrated that miR-659 targets the mitogen-activated protein kinase 9 (MAPK9) gene. In patients with nasopharyngeal carcinoma, MAPK9 expression levels influence polymorphic lactotransferrin(LTF) haplotypes, which are positively associated with the incidence of cancer (45). Other studies have focused on the role of miR-659 in muscle development and nervous system and metabolic diseases, such as frontotemporal dementia and diabetes mellitus (46–48). Thus, the mechanism of miR-659-5p in breast cancer requires further investigation.
The present study investigated the biological activities of the selected three miRNAs using KEGG and GO analyses and identified six enriched pathways. Enhanced ubiquitin-mediated proteolysis causes the rapid hydrolysis of proteins, including tumor protein p53 and interferon α inducible protein 27, which are closely associated with invasiveness, malignancy, clinical stage and poor prognosis in breast cancer (49). Ubiquitin-mediated proteolysis has been suggested to be a marker of the risk of breast cancer (50). Zhang et al (51) reported that mitogen-activated protein kinase (MAPK) expression levels in TNBC tissues were significantly increased compared with normal adjacent tissues, indicating an association between MAPK expression and TNBC. In addition, studies have indicated that MAPK protein expression is associated with clinical stage and lymph node metastasis in TNBC (52,53). The epidermal growth factor receptor (EGFR) serves important roles in TNBC (54). Previous studies have revealed the upregulation of EGFR, which affects carcinogenesis, progression and metastasis of breast cancer, in 70% of patients with TNBC (55,56). A previous study reported enrichment of the prolactin (PRL) signaling pathway in TNBC (57). López-Ozuna et al (58) reported that the PRL signaling pathway may be used to predict the response to prodifferentiation therapy in TNBC. The adherens junction pathway is another pathway that was reported to be enriched in TNBC-associated processes (59). Vascular endothelial-cadherin, heparan and the probe for protein kinase C α have previously been implicated in the adherens junction pathway (60–62). The main component of the cell-cell adherens junction is E-cadherin, which plays a major role in maintaining normal breast epithelial cell morphology (63). Breast cancer has been found to downregulate or interfere with the expression of E-cadherin, which is closely associated with the epithelial-to-mesenchymal transition (EMT) (63), a key step in the invasion and metastasis of breast cancer (64,65). The induction of EMT requires the synergy of the MAPK signaling pathway (66,67). The aforementioned observations indicate an association between the MAPK signaling and adherens junction pathways, which requires further investigation.
The present study had a number of limitations. These included a relatively small sample size and a lack of validation experiments, such as reverse-transcription quantitative polymerase chain reaction analysis, for the selected three miRNA transcripts at the tissue and plasma levels. Further research based on a larger cohort of patients with TNBC is required to validate the findings obtained.
In conclusion, the present study identified a three-miRNA signature as a potential prognostic predictor of patients with TNBC. Due to the complex biology of cancer, the molecular mechanisms involving miRNA signatures warrant further investigation.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by The Science and Technology Program of Quanzhou, China (grant no. 2018Z119).
Availability of data and materials
The datasets analyzed during the current study are available in The Cancer Genome Atlas repository, cancergenome.nih.gov.
Authors' contributions
XW wrote the manuscript, designed the study and interpreted the data. MD performed the statistical analysis and interpreted the results. JL was involved in designing the study and critically revising the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Wen YY, Liu WT, Sun HR, Ge X, Shi ZM, Wang M, Li W, Zhang JY, Liu LZ, Jiang BH. IGF-1-mediated PKM2/β- catenin/miR-152 regulatory circuit in breast cancer. Sci Rep. 2017;7:15897. doi: 10.1038/s41598-017-15607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimelis H, LaDuca H, Hu C, Hart SN, Na J, Thomas A, Akinhanmi M, Moore RM, Brauch H, Cox A, et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst. 2018;110:855–862. doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 8.Fu Q, Yang F, Xiang T, Huai G, Yang X, Wei L, Yang H, Deng S. A novel microRNA signature predicts survival in liver hepatocellular carcinoma after hepatectomy. Sci Rep. 2018;8:7933. doi: 10.1038/s41598-018-27641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karsli-Ceppioglu S, Dagdemir A, Judes G, Ngollo M, Penault-Llorca F, Pajon A, Bignon YJ, Bernard-Gallon D. Epigenetic mechanisms of breast cancer: An update of the current knowledge. Epigenomics. 2014;6:651–664. doi: 10.2217/epi.14.59. [DOI] [PubMed] [Google Scholar]
- 10.Hafez MM, Hassan ZK, Zekri AR, Gaber AA, Al Rejaie SS, Sayed-Ahmed MM, Al Shabanah O. MicroRNAs and metastasis-related gene expression in Egyptian breast cancer patients. Asian Pac J Cancer Prev. 2012;13:591–598. doi: 10.7314/APJCP.2012.13.2.591. [DOI] [PubMed] [Google Scholar]
- 11.Chang YY, Lai LC, Tsai MH, Chuang EY. Deep sequencing reveals a MicroRNA expression signature in triple-negative breast cancer. Methods Mol Biol. 2018;1699:99–111. doi: 10.1007/978-1-4939-7435-1_8. [DOI] [PubMed] [Google Scholar]
- 12.Lan H, Lu H, Wang X, Jin H. MicroRNAs as potential biomarkers in cancer: Opportunities and challenges. Biomed Res Int. 2015;2015:125094. doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuberi M, Mir R, Das J, Ahmad I, Javid J, Yadav P, Masroor M, Ahmad S, Ray PC, Saxena A. Expression of serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological features. Clin Transl Oncol. 2015;17:779–787. doi: 10.1007/s12094-015-1355-2. [DOI] [PubMed] [Google Scholar]
- 14.Alunni-Fabbroni M, Majunke L, Trapp EK, Tzschaschel M, Mahner S, Fasching PA, Fehm T, Schneeweiss A, Beck T, Lorenz R, et al. Whole blood microRNAs as potential biomarkers in post-operative early breast cancer patients. BMC Cancer. 2018;18:141. doi: 10.1186/s12885-018-4020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Vazquez R, Ruiz-García E, Meneses García A, Astudillo-de la Vega H, Lara-Medina F, Alvarado-Miranda A, Maldonado-Martínez H, González-Barrios JA, Campos-Parra AD, Rodríguez Cuevas S, et al. A microRNA signature associated with pathological complete response to novel neoadjuvant therapy regimen in triple-negative breast cancer. Tumour Biol. 2017;39:1010428317702899. doi: 10.1177/1010428317702899. [DOI] [PubMed] [Google Scholar]
- 16.Han J, Yu J, Dai Y, Li J, Guo M, Song J, Zhou X. Overexpression of miR-361-5p in triple-negative breast cancer (TNBC) inhibits migration and invasion by targeting RQCD1 and inhibiting the EGFR/ PI3K/Akt pathway. Bosn J Basic Med Sci. 2019;19:52–59. doi: 10.17305/bjbms.2018.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren YQ, Fu F, Han J. MiR-27a modulates radiosensitivity of triple-negative breast cancer (TNBC) cells by targeting CDC27. Med Sci Monit. 2015;21:1297–1303. doi: 10.12659/MSM.893974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wahdan-Alaswad RS, Cochrane DR, Spoelstra NS, Howe EN, Edgerton SM, Anderson SM, Thor AD, Richer JK. Metformin-induced killing of triple-negative breast cancer cells is mediated by reduction in fatty acid synthase via miRNA-193b. Horm Cancer. 2014;5:374–389. doi: 10.1007/s12672-014-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascione L, Gasparini P, Lovat F, Carasi S, Pulvirenti A, Ferro A, Alder H, He G, Vecchione A, Croce CM, et al. Integrated microRNA and mRNA signatures associated with survival in triple negative breast cancer. PLoS One. 2013;8:e55910. doi: 10.1371/journal.pone.0055910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleivi Sahlberg K, Bottai G, Naume B, Burwinkel B, Calin GA, Børresen-Dale AL, Santarpia L. A serum microRNA signature predicts tumor relapse and survival in triple-negative breast cancer patients. Clin Cancer Res. 2015;21:1207–1214. doi: 10.1158/1078-0432.CCR-14-2011. [DOI] [PubMed] [Google Scholar]
- 21.Braicu C, Raduly L, Morar-Bolba G, Cojocneanu R, Jurj A, Pop LA, Pileczki V, Ciocan C, Moldovan A, Irimie A, et al. Aberrant miRNAs expressed in HER-2 negative breast cancers patient. J Exp Clin Cancer Res. 2018;37:257. doi: 10.1186/s13046-018-0920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp Oncol (Pozn) 2015;19:A68–A77. doi: 10.5114/wo.2014.47136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.No authors listed. The future of cancer genomics. Nat Med. 2015;21:99. doi: 10.1038/nm.3801. [DOI] [PubMed] [Google Scholar]
- 24.Dorman SN, Viner C, Rogan PK. Splicing mutation analysis reveals previously unrecognized pathways in lymph node-invasive breast cancer. Sci Rep. 2014;4:7063. doi: 10.1038/srep07063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, Levy R. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 26.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 27.Wong N, Wang X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43((Database Issue)):D146–D152. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X. Improving microRNA target prediction by modeling with unambiguously identified microRNA-target pairs from CLIP-ligation studies. Bioinformatics. 2016;32:1316–1322. doi: 10.1093/bioinformatics/btw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang B, Li Y, Wang T. A three miRNAs signature predicts survival in cervical cancer using bioinformatics analysis. Sci Rep. 2017;7:5624. doi: 10.1038/s41598-017-06032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phipson B, Lee S, Majewski IJ, Alexander WS, Smyth GK. Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. Ann Appl Stat. 2016;10:946–963. doi: 10.1214/16-AOAS920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W, Chang G, Li X, Li Q, Wang S, Wang W. MicroRNA profiling implies new markers of chemoresistance of triple-negative breast cancer. PLoS One. 2014;9:e96228. doi: 10.1371/journal.pone.0096228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mavrogiannis AV, Kokkinopoulou I, Kontos CK, Sideris DC. Effect of vinca alkaloids on the expression levels of microRNAs targeting apoptosis-related genes in breast cancer cell lines. Curr Pharm Biotechnol. 2018;19:1076–1086. doi: 10.2174/1389201019666181112103204. [DOI] [PubMed] [Google Scholar]
- 35.Calvano Filho CM, Calvano-Mendes DC, Carvalho KC, Maciel GA, Ricci MD, Torres AP, Filassi JR, Baracat EC. Triple-negative and luminal A breast tumors: Differential expression of miR-18a-5p, miR-17-5p, and miR-20a-5p. Tumour Biol. 2014;35:7733–7741. doi: 10.1007/s13277-014-2025-7. [DOI] [PubMed] [Google Scholar]
- 36.Farazi TA, Horlings HM, Ten Hoeve JJ, Mihailovic A, Halfwerk H, Morozov P, Brown M, Hafner M, Reyal F, van Kouwenhove M, et al. MicroRNA sequence and expression analysis in breast tumors by deep sequencing. Cancer Res. 2011;71:4443–4453. doi: 10.1158/0008-5472.CAN-11-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aure MR, Leivonen SK, Fleischer T, Zhu Q, Overgaard J, Alsner J, Tramm T, Louhimo R, Alnæs GI, Perälä M, et al. Individual and combined effects of DNA methylation and copy number alterations on miRNA expression in breast tumors. Genome Biol. 2013;14:R126. doi: 10.1186/gb-2013-14-11-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doberstein K, Bretz NP, Schirmer U, Fiegl H, Blaheta R, Breunig C, Müller-Holzner E, Reimer D, Zeimet AG, Altevogt P. miR-21-3p is a positive regulator of L1CAM in several human carcinomas. Cancer Lett. 2014;354:455–466. doi: 10.1016/j.canlet.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Lo TF, Tsai WC, Chen ST. MicroRNA-21-3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One. 2013;8:e75628. doi: 10.1371/journal.pone.0075628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiao W, Leng X, Zhou Q, Wu Y, Sun L, Tan Y, Ni H, Dong X, Shen T, Liu Y, Li J. Different miR-21-3p isoforms and their different features in colorectal cancer. Int J Cancer. 2017;141:2103–2111. doi: 10.1002/ijc.30902. [DOI] [PubMed] [Google Scholar]
- 41.Yeh TS, Wang F, Chen TC, Yeh CN, Yu MC, Jan YY, Chen MF. Expression profile of microRNA-200 family in hepatocellular carcinoma with bile duct tumor thrombus. Ann Surg. 2014;259:346–354. doi: 10.1097/SLA.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Yang D, Xi L, Chen Y, Fu L, Sun K, Yin J, Li X, Liu S, Qin Y, et al. Primed atypical ductal hyperplasia-associated fibroblasts promote cell growth and polarity changes of transformed epithelium-like breast cancer MCF-7 cells via miR-200b/c-IKKβ signaling. Cell Death Dis. 2018;9:122. doi: 10.1038/s41419-017-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pang Y, Liu J, Li X, Xiao G, Wang H, Yang G, Li Y, Tang SC, Qin S, Du N, et al. MYC and DNMT3A-mediated DNA methylation represses microRNA-200b in triple negative breast cancer. J Cell Mol Med. 2018;22:6262–6274. doi: 10.1111/jcmm.13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi SK, Kim HS, Jin T, Hwang EH, Jung M, Moon WK. Overexpression of the miR-141/200c cluster promotes the migratory and invasive ability of triple-negative breast cancer cells through the activation of the FAK and PI3K/AKT signaling pathways by secreting VEGF-A. BMC Cancer. 2016;16:570. doi: 10.1186/s12885-016-2620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo G, Zhou Y, Yi W, Yi H. Expression levels of JNK associated with polymorphic lactotransferrin haplotypes in human nasopharyngeal carcinoma. Oncol Lett. 2016;12:1085–1094. doi: 10.3892/ol.2016.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piscopo P, Albani D, Castellano AE, Forloni G, Confaloni A. Frontotemporal lobar degeneration and MicroRNAs. Front Aging Neurosci. 2016;8:17. doi: 10.3389/fnagi.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zitman-Gal T, Green J, Pasmanik-Chor M, Golan E, Bernheim J, Benchetrit S. Vitamin D manipulates miR-181c, miR-20b and miR-15a in human umbilical vein endothelial cells exposed to a diabetic-like environment. Cardiovasc Diabetol. 2014;13:8. doi: 10.1186/1475-2840-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dmitriev P, Barat A, Polesskaya A, O'Connell MJ, Robert T, Dessen P, Walsh TA, Lazar V, Turki A, Carnac G, et al. Simultaneous miRNA and mRNA transcriptome profiling of human myoblasts reveals a novel set of myogenic differentiation-associated miRNAs and their target genes. BMC Genomics. 2013;14:265. doi: 10.1186/1471-2164-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asher G, Tsvetkov P, Kahana C, Shaul Y. A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev. 2005;19:316–321. doi: 10.1101/gad.319905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Q, Song W, He DY, Li Y. Identification of key gene modules and pathways of human breast cancer by co-expression analysis. Breast Cancer. 2018;25:213–223. doi: 10.1007/s12282-017-0817-5. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Wei L, Yu J, Li G, Zhang X, Wang A, He Y, Li H, Yin D. Targeting of the β6 gene to suppress degradation of ECM via inactivation of the MAPK pathway in breast adenocarcinoma cells. Oncol Rep. 2014;32:1787–1795. doi: 10.3892/or.2014.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartholomeusz C, Gonzalez-Angulo AM, Liu P, Hayashi N, Lluch A, Ferrer-Lozano J, Hortobágyi GN. High ERK protein expression levels correlate with shorter survival in triple-negative breast cancer patients. Oncologist. 2012;17:766–774. doi: 10.1634/theoncologist.2011-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat. 2012;136:331–345. doi: 10.1007/s10549-012-2289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tilch E, Seidens T, Cocciardi S, Reid LE, Byrne D, Simpson PT, Vargas AC, Cummings MC, Fox SB, Lakhani SR, et al. Mutations in EGFR, BRAF and RAS are rare in triple-negative and basal-like breast cancers from Caucasian women. Breast Cancer Res Treat. 2014;143:385–392. doi: 10.1007/s10549-013-2798-1. [DOI] [PubMed] [Google Scholar]
- 56.Park HS, Jang MH, Kim EJ, Kim HJ, Lee HJ, Kim YJ, Kim JH, Kang E, Kim SW, Kim IA, Park SY. High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod Pathol. 2014;27:1212–1222. doi: 10.1038/modpathol.2013.251. [DOI] [PubMed] [Google Scholar]
- 57.Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK, Hilsenbeck SG, Chang JC, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López-Ozuna VM, Hachim IY, Hachim MY, Lebrun JJ, Ali S. Prolactin Pro-differentiation pathway in triple negative breast cancer: Impact on prognosis and potential therapy. Sci Rep. 2016;6:30934. doi: 10.1038/srep30934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martignetti L, Tesson B, Almeida A, Zinovyev A, Tucker GC, Dubois T, Barillot E. Detection of miRNA regulatory effect on triple negative breast cancer transcriptome. BMC Genomics. 2015;16(Suppl):S4. doi: 10.1186/1471-2164-16-S6-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pham TND, Perez White BE, Zhao H, Mortazavi F, Tonetti DA. Protein kinase C α enhances migration of breast cancer cells through FOXC2-mediated repression of p120-catenin. BMC Cancer. 2017;17:832. doi: 10.1186/s12885-017-3827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Modica M, Regondi V, Sandri M, Iorio MV, Zanetti A, Tagliabue E, Casalini P, Triulzi T. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017;384:94–100. doi: 10.1016/j.canlet.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Lim HC, Multhaupt HA, Couchman JR. Cell surface heparan sulfate proteoglycans control adhesion and invasion of breast carcinoma cells. Mol Cancer. 2015;14:15. doi: 10.1186/s12943-014-0279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q, Mattingly RR. Restoration of E-cadherin cell-cell junctions requires both expression of E-cadherin and suppression of ERK MAP kinase activation in Ras-transformed breast epithelial cells. Neoplasia. 2008;10:1444–1458. doi: 10.1593/neo.08968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22:2423–2435. doi: 10.1091/mbc.e11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mali AV, Joshi AA, Hegde MV, Kadam SS. Enterolactone modulates the ERK/NF-κB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-β-induced epithelial-mesenchymal transition. Cancer Biol Med. 2018;15:137–156. doi: 10.20892/j.issn.2095-3941.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, Yu J, Black JD, Tan D, Khoury T. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Ann Surg Oncol. 2007;14:3527–3533. doi: 10.1245/s10434-007-9540-3. [DOI] [PubMed] [Google Scholar]
- 67.Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M, Qin Y, Sun K, Teng Y, Liu M. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 MAPK signalling. Cancer Lett. 2019;442:320–332. doi: 10.1016/j.canlet.2018.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in The Cancer Genome Atlas repository, cancergenome.nih.gov.