Abstract
The present study investigated the sensitization of 5-fluorouracil (5-FU)-resistant colon cancer cells in vitro, using oxymatrine, a Chinese herb, and a quinolizidine alkaloid compound extracted from the root of Sophora flavescens. The HCT-8 colon cancer cell line and its 5-FU-resistant subline HCT-8/5-FU were treated with 5-FU and oxymatrine, alone or in combination, at various doses. The cells were subsequently assessed for changes in cell viability, apoptosis and morphology and analyzed by fluorescence microscopy and western blotting. The data demonstrated that HCT-8/5-FU markedly increased the dose of 5-FU required for the suppression of tumor cell viability (78.77±1.90 µg/ml vs. 9.20±0.96 µg/ml in parental HCT-8 cells), whereas HCT-8/5-FU induced the tumor cell epithelial-mesenchymal transition (EMT). By contrast, oxymatrine alone and in combination with 5-FU altered HCT-8/5-FU cell morphology, apoptosis and EMT phenotypes. The combination of oxymatrine and 5-FU reduced the protein expression of snail family transcriptional repressor 2 and vimentin, phosphorylated p65 and induced the expression of E-cadherin, by inhibiting the nuclear factor κB (NF-κB) signaling pathway. In conclusion, the data from the present study demonstrated that EMT was associated with 5-FU chemoresistance in HCT-8/5-FU colon cancer cells, and that oxymatrine treatment was able to reverse such resistance. Oxymatrine may regulate tumor cell EMT and inactivate the NF-κB signaling pathway, and may therefore serve as a potential therapeutic drug to reverse 5-FU resistance in colon cancer cells.
Keywords: colon cancer, 5-fluorouracil resistance, oxymatrine, epithelial-mesenchymal transition, nuclear factor κB
Introduction
Colorectal cancer is one of the most commonly diagnosed types of cancer worldwide, and was the second and third most prevalent cancer in women and men in 2014, respectively (1). The global colorectal cancer incidence, particularly in China, has continued to increase by 2- to 4-fold in recent years (2,3). To date, the treatment of colorectal cancer consists of a combination of surgery, chemotherapy, radiotherapy and/or immunotherapy. For example, treatment with 5-fluorouracil (5-FU) was shown to improve the survival of patients with various types of cancer, including colorectal cancer, rectal cancer, gastric cancer, breast cancer (4–6), compared with untreated patients. 5-FU directly inhibits the activity of the thymidylate synthetase enzymes in tumor cells (7). Since the introduction of 5-FU into clinical practice in the 1950s, it remains the most widely used chemotherapeutic drug for cancer treatment, including colorectal cancer (8). However, 5-FU resistance develops in ~50% of patients with colorectal cancer, leading to a poor long-term outcome and prognosis (9). Thus, the elucidation of the molecular mechanism(s) underlying 5-FU resistance may prevent or reverse resistance in colorectal cancer, thereby benefiting patients.
Matrine and oxymatrine are biologically active compounds extracted from Sophora flavescens, a leguminous plant. The two compounds exhibit similar molecular structures and pharmacological activities, and are often converted into each other (10). Previous studies demonstrated the anticancer activities of Sophora flavescens (11–13). Another study revealed that oxymatrine exerts an anticancer effect in colorectal cancer via the inhibition of the nuclear factor κB (NF-κB) signaling pathway (14). Furthermore, oxymatrine reversed vincristine, paclitaxel and doxorubicin chemoresistance in various cancer cell lines by inhibiting the expression of lung resistance-related protein, P-glycoprotein (P-gp) and multidrug resistance (MDR) proteins (15). Moreover, an association between chemotherapy resistance and the epithelial mesenchymal transition (EMT) in tumor cells in various types of human cancer has been documented, including lung, breast and colon cancers (16–18). Therefore, targeting the EMT in cancer cells may improve the effects of anticancer agents. Indeed, the EMT is a key step in several biological processes in the human body, including early embryonic differentiation and development, wound healing, tissue fibrosis, and cancer invasion and metastasis (19,20). Major EMT characteristics in tumor cells include increased cell migratory ability, altered cellular morphology and the generation of cancer stem cells (21). These characteristics were observed in certain chemoresistant cancers, including colorectal cancer (22), nasopharyngeal cancer (23), hepatocellular carcinoma (24) and breast cancer (25). Tumor cell chemoresistance was associated with EMT phenotypes, including decreased expression of the epithelial marker E-cadherin and the increased expression of mesenchymal markers (vimentin and N-cadherin) and other associated transcription factors [twist family bHLH transcription factor 1, snail family transcriptional repressor 2 (SNAI2) and snail family transcriptional repressor 1] (26).
A previous study reported that the NF-κB signaling pathway was associated with the EMT and played an important role in 5-FU resistance in various types of cancer, includign colon, rectum and breast cancers (27). Oxymatrine was revealed to inhibit the EMT in colon cancer cells by targeting the NF-κB signaling pathway (14), whereas activation of NF-κB signaling led to P-gp upregulation, which was associated with drug resistance (28). Therefore, the present study investigated the effects of oxymatrine on 5-FU resistance in colorectal cancer cells in vitro. Furthermore, the potential synergism between oxymatrine and 5-FU was explored. The results of the present study suggested that the combination of 5-FU and oxymatrine may be a novel therapeutic strategy for colorectal cancer, particularly 5-FU-resistant colon cancer.
Materials and methods
Cell culture
The HCT-8 human colon cancer cell line and its 5-FU-resistant subline HCT-8/5-FU were obtained from The iCell Bioscience Inc. Cells were cultured in RPMI-1640 medium (Gibco; Life Technologies Corporation) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) and 1% penicillin in a humidified incubator with 5% CO2 at 37°C. For drug treatment, 2×104/ml HCT-8/5-FU cells were seeded into cell culture dishes or plates and grown overnight. The cells were subsequently treated with 2 mg/ml oxymatrine (Chengdu Push Bio-Technology Co., Ltd.), 20 ng/ml tumor necrosis factor-α (TNF-α; NF-κB activator; Sigma-Aldrich; Merck KGaA), alone or in combination, for up to 24 h at 37°C. The cells were then subjected to western blot analyses.
Flow cytometry analysis of apoptosis
Early and late apoptosis were assessed using an annexin V-fluorescein isothiocyanate (FITC)/propidium iodide staining kit (cat. no. WLa001; Wanleibio Co., Ltd.) according to the manufacturer's instructions (BD FACSCanto II; BD Biosciences). Briefly, 5-FU and oxymatrine were dissolved in sterile distilled water. HCT-8/5-FU cells (4×105/well) were treated with 1 µg/ml 5-FU and 2 mg/ml oxymatrine, alone or in combination, for 24 h at 37°C. Cells were subsequently collected and analyzed using a flow cytometer (BD FACSCanto II). Data were analyzed using FlowJo software version 10.4 (Flowjo LLC).
Cell morphology
HCT-8 and HCT-8/5-FU cells in the logarithmic phase were cultured for 24 h at 37°C. Cells were subsequently treated with 1 µg/ml 5-FU (Push Bio-technology Co., Ltd.) for 24 h at 37°C. Changes in tumor cell morphology were monitored (at least 3 random microscopic fields) and images were captured using a light microscope (AE31; Motic Incorporation, Ltd.; magnification, ×100).
Fluorescence microscopy
HCT-8/5-FU cells (5×105) were seeded onto glass coverslips, grown for 24 h at 37°C, and treated with 2 mg/ml oxymatrine or 1 µg/ml 5-FU, alone or in combination, for 24 h at 37°C. Next, tumor cells were fixed in 4% paraformaldehyde in PBS for 15 min and permeabilized in 0.1% Triton X-100 (Roche Diagnostics) for 30 min at 37°C. Subsequently, cells were blocked in 10% goat serum (cat. no. SL038; Beijing Solarbio Science & Technology Co., Ltd.) in PBS for 50 min at room temperature and then further incubated with anti-E-cadherin antibody (1:1,000; cat. no. Ab1416; Abcam) or anti-vimentin antibody (1:1,000; cat. no. Ab92547; Abcam) both from Abcam overnight at 4°C. Subsequently, the cells were washed three times with PBS and then incubated with FITC-(cat. no. A0568; Beyotime Institute of Biotechnology) or Cy3-congujated secondary antibodies (cat. no. A0516; Beyotime Institute of Biotechnology) at 37°C for 60 min. Cells were stained with DAPI Vectashield (Wanleibio Co., Ltd.) according to the manufacturer's instructions, covered with coverslips using a fluorescent mounting medium (Wanleibio Co., Ltd.), and examined (at least 3 random microscopic fields; magnification, ×400) using a fluorescence microscope (BX53; Olympus Corporation).
Western blot analysis
HCT-8/5-FU cells were cultured and treated with 1 µg/ml 5-FU and 2 mg/ml oxymatrine, alone or in combination, or with-20 ng/ml TNF-α or 1% DMSO for 24 h at 37°C. Total cellular protein was extracted on ice using a cell lysis buffer containing 150 mM NaCl, 1% sodium deoxycholate, 50 mM Tris (pH 7.5), 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, 1% Triton X-100, 1 mM EDTA and 1 mM Na3VO4. The cell lysates were centrifuged at 12,000 × g for 15 min at 4°C, and the protein concentration was determined using the BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific, Inc.). Subsequently, the protein samples (35 µg) were separated by SDS-PAGE on 5–10% gels and transferred onto polyvinylidene fluoride membranes (EMD Millipore). After blocking in 5% nonfat milk in PBS at 4°C for 1 h, each membrane was incubated with primary antibodies against E-cadherin (1:1,000; cat. no. 60335-1-1g; ProteinTech Group), vimentin (1:500; cat. no. WL00742; Wanleibio Co., Ltd.), SNAI2 (1:500; cat. no. WL01863; Wanleibio Co., Ltd.), phosphorylated (p)-p65 (1:500; cat. no. WL02169; Wanleibio Co., Ltd.), p65 (1:500; cat. no. WL01273b; Wanleibio Co., Ltd.), MDR1 (1:500; cat. no. WL02395; Wanleibio Co., Ltd.or β-actin (1:1,000; cat. no. WL01372; Wanleibio Co., Ltd.) at 4°C overnight. The following day, the membranes were washed three times with PBS-Tween 20 and then incubated with a peroxidase-conjugated secondary antibody (1:5,000; cat. no. WLA023; Wanleibio Co., Ltd.) at room temperature for 1 h. The protein signals were detected using an enhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol, and visualized using X-ray films. β-actin was used as the loading control. Changes in EMT markers were determined in two groups: i) The negative control cells (HCT-8 cells); and ii) the Experience group cells (HCT-8/5-FU cells) (Fig. 2A). Changes in EMT markers following drug treatments were assayed in four groups: i) The negative control cells (HCT-8/5-FU cells); ii) the oxymatrine-treated cells (HCT-8/5-FU + 2 mg/ml oxymatrine); iii) the TNF-α-treated cells (HCT-8/5-FU + 20 ng/ml TNF-α); and iv) the combined treatment (MIX) cells (HCT-8/5-FU + 2 mg/ml oxymatrine +20 ng/ml TNF-α) (Fig. 4A). Changes in EMT markers following drug treatments were determined in four groups: i) The negative control cells (HCT-8/5-FU cells); ii) the 5-FU-treated cells (HCT-8/5-FU + 1 µg/ml 5-FU); iii) the oxymatrine-treated cells (HCT-8/5-FU + 2 mg/ml oxymatrine); and iv) the combined treatment (MIX) cells (HCT-8/5-FU + 2 mg/ml oxymatrine +1 µg/ml 5-FU) (Fig. 6A).
Figure 2.
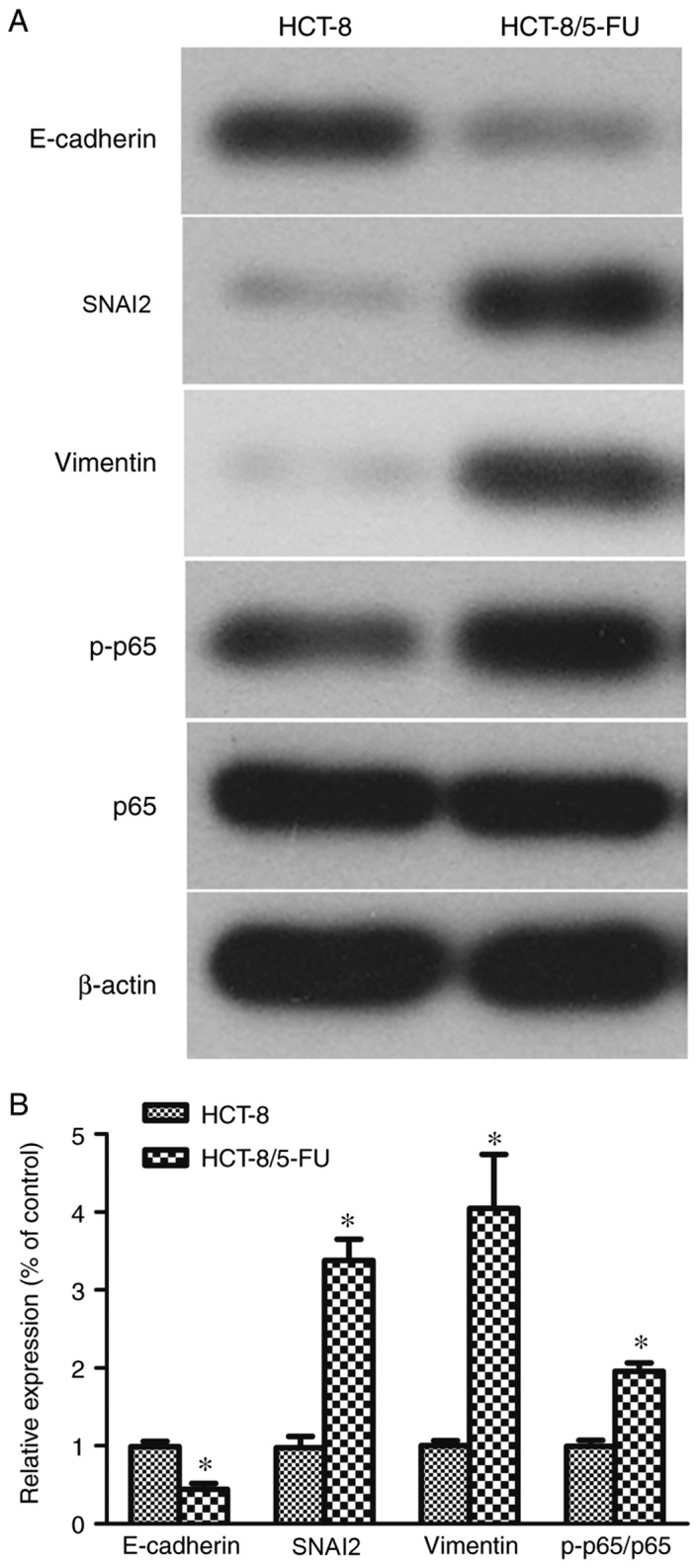
Expression of epithelial-mesenchymal transition markers. (A) Western blot analysis of protein lysates collected from HCT-8 and HCT-8/5-FU cells treated with 1 µg/ml 5-FU for 24 h and (B) the quantification. *P<0.05 vs. parental HCT-8 cells. p-p65, phosphorylated p65; 5-FU, 5-fluorouracil; SNAI2, snail family transcriptional repressor 2.
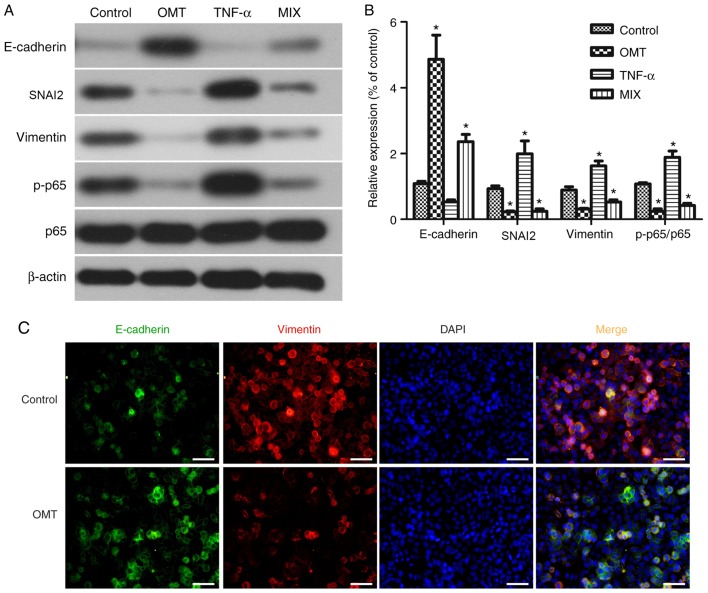
Figure 4.
Effects of oxymatrine on the modulation of epithelial-mesenchymal transition proteins in HCT-8/5-FU cells. (A) Western blot analysis of proteins extracted from HCT-8/5-FU cells treated with 2 mg/ml oxymatrine and 20 ng/ml TNF-α, alone or in combination, for 24 h. (B) Quantification of western blot analysis. *P<0.05 vs. control cells. (C) Immunofluorescence staining of HCT-8/5-FU cells treated with 2 mg/ml oxymatrine for 24 h, and then subjected to immunofluorescence staining of E-cadherin (green) and vimentin (red) proteins, and DAPI nuclei staining (blue). Scale bar, 50 µm. Magnification, ×400. p-p65, phosphorylated p65; OMT, oxymatrine; MIX, combined treatment; TNF-α, tumor necrosis factor-α; SNAI2, snail family transcriptional repressor 2.
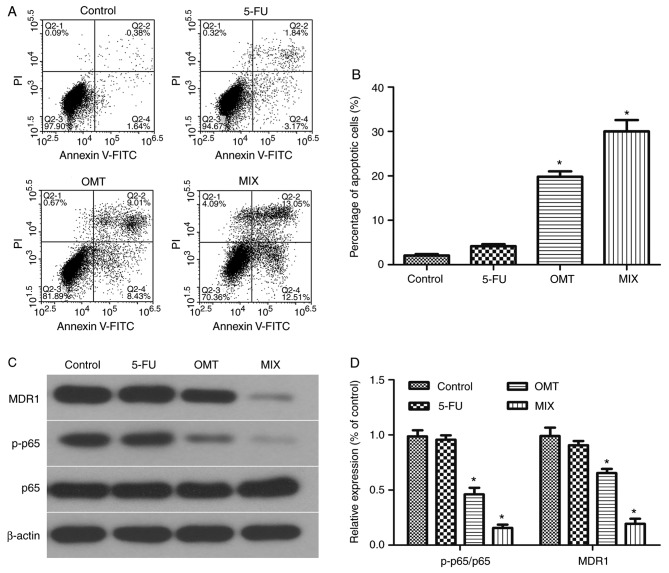
Figure 6.
Oxymatrine induced HCT-8/5-FU cell apoptosis via inactivation of the NF-κB signaling pathway. HCT-8/5-FU cells were treated with 1 µg/ml 5-FU and 2 mg/ml oxymatrine, alone or in combination, for 24 h and then subjected to (A) flow cytometry analysis and (B) quantification of apoptosis. Additionally, proteins were extracted for (C) western blot analysis and (D) quantification. *P<0.05 vs. control cells. MDR1, multi-drug resistance protein; OMT, oxymatrine; MIX, combined treatment; 5-FU, 5-fluorouracil; p-p65, phosphorylated.
Assessing the effects of oxymatrine on the drug resistance of HCT-8/5-FU cells
Oxymatrine (0, 2, 4 and 8 mg/ml) was added into cell culture medium (RPMI-1640 supplemented with 10% fetal bovine serum) containing different concentrations of 5-FU (0, 0.5, 1, 2, 4, 8, 16 and 32 µg/ml). HCT-8/5-FU cells (4×103) were grown in the aforementioned medium for 24 h and subjected to the MTT assay. DMSO (150 µl/well) was used to dissolve the formzan crystals, and the optical density was measured at 570 nm using a microplate reader. The drug resistance index (RI) was calculated as follows: RI=half maximal inhibitory concentration (IC50) of drug-resistant cells/IC50 of the control cells.
Statistical analysis
The data are presented as the mean ± standard deviation or were quantified as gray levels, where applicable, from three independent experiments with duplicate readings. The control and experimental groups were compared using the one-way analysis of variance followed by the Dunnett's post hoc test. The data between two groups were compared using the Student's t-test. All statistical analyses were performed using SPSS software version 21.0 (IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
Assessment of the cancer cell RI
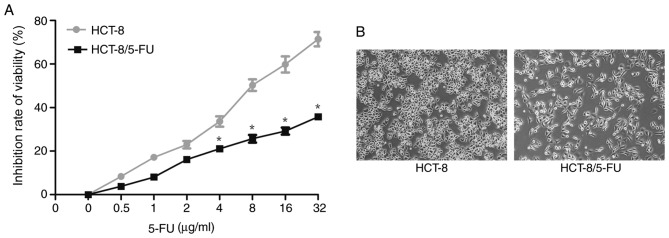
Compared with parental HCT-8 cells, HCT-8/5-FU cells exhibited increased survival in culture medium containing high doses of 5-FU (>4 µg/ml) for 24 h. In particular, the IC50 values of HCT-8/5-FU and HCT-8 cells were 78.77±1.90 and 9.20±0.96 µg/ml, respectively (P<0.05; Fig. 1A), and the RI of HCT-8/5-FU cells was 8.56.
Figure 1.
Effects of 5-FU on the inhibition of colon cancer cell viability. (A) HCT-8 and HCT-8/5-FU cells were cultured, treated with 5-FU at various doses for 24 h, and then subjected to the MTT assay. Compared with parental HCT-8 cells, HCT-8/5-FU cells exhibited increased viability following treatment with high doses of 5-FU (>4 µg/ml). *P<0.05 vs. parental HCT-8 cells. (B) Changes in cell morphology were monitored following treatment of HCT-8 and HCT-8/5-FU cells with 1 µg/ml 5-FU for 24 h. Magnification, ×100. 5-FU, 5-fluorouracil.
Changes in morphology of HCT-8 and HCT-8/5-FU cells following 5-FU treatment
Both cell lines were treated with 1 µg/ml 5-FU for 24 h and were assessed under an inverted microscope for changes in cell morphology. HCT-8 cells exhibited typical epithelial morphology characterized by a round shape and distinct epithelial clusters, whereas HCT-8/5-FU cells displayed an elongated and irregular fibroblast-like morphology (Fig. 1B), indicating that HCT-8/5-FU cells underwent EMT following treatment with 1 µg/ml 5-FU for 24 h.
Induction of EMT in HCT-8/5-FU and HCT-8 cells
Differences in the protein expression levels of EMT markers were detected in untreated HCT-8/5-FU and HCT-8 cells (Fig. 2A). Compared with HCT-8 cells, HCT-8/5-FU cells expressed significantly increased levels of SNAI2, vimentin and p-p65, and significantly decreased levels of E-cadherin (P<0.05; Fig. 2B).
Effects of oxymatrine on HCT-8/5-FU cell viability
HCT-8/5-FU cell viability was significantly reduced following treatment with oxymatrine compared with treatment with 5-FU alone (P<0.05; Fig. 3). However, there was no significant difference between cells treated with any dose of 5-FU (0, 0.5, 1, 2, 4, 8, 16 and 32 µg/ml) and 4 or 8 mg/ml oxymatrine (all P>0.05; Fig. 3). The data demonstrated a favorable inhibition of HCT-8/5-FU cell viability following treatment with 2 mg/ml oxymatrine and 1 µg/ml 5-FU; this dose combination was therefore used for subsequent experiments.
Figure 3.
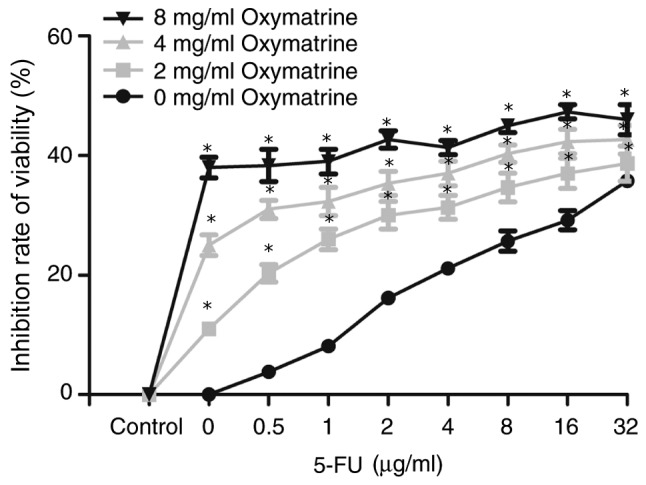
Effects of the combination of oxymatrine and 5-FU on the suppression of 5-FU-resistant colon cancer cell viability. HCT-8/5-FU cells were treated with 5-FU in combination with oxymatrine at various doses for 24 h, and then subjected to the MTT cell viability assay. HCT-8/5-FU cell viability was significantly reduced following treatment with oxymatrine compared with treatment with 5-FU alone. *P<0.05 vs. parental HCT-8/5-FU cells treated with 5-FU alone. Data demonstrated favorable inhibition of HCT-8/5-FU cell viability with 2 mg/ml oxymatrine and 1 µg/ml 5-FU; thus, this dose combination was used for subsequent experiments. 5-FU, 5-fluorouracil.
Oxymatrine inhibits EMT in HCT-8/5-FU cells via the NF-κB signaling pathway
In order to explore whether oxymatrine inhibits the EMT in HCT-8/5-FU cells, the expression of EMT-associated biomarkers was assessed in HCT-8/5-FU cells, following treatment with 20 ng/ml TNF-α and 2 mg/ml oxymatrine, alone or in combination for 24 h (Fig. 4A). The mesenchymal marker vimentin and the epithelial marker E-cadherin were downregulated and upregulated following treatment with 2 mg/ml oxymatrine for 24 h. Moreover, oxymatrine significantly inhibited the expression of the NF-κB signaling pathway protein p-p65 compared with the other three groups (P<0.05; Fig. 4B), suggesting that NF-κB signaling may mediate the effects of oxymatrine in HCT-8/5-FU cells and that oxymatrine may inhibit EMT via the NF-κB signaling pathway in HCT-8/5-FU. Oxymatrine treatment significantly inhibited p-p65 protein expression. However, this did not exclude the fact that oxymatrine in combination with 5-FU may also possess synergistic antitumor activity in HCT-8 cells, or that oxymatrine alone may exhibit antitumor effects on HCT-8/5-FU cells. In addition, fluorescence microscopy showed that E-cadherin expression (green) was upregulated, whereas vimentin expression (red) was downregulated, following the incubation of HCT-8/5-FU cells with 2 mg/ml oxymatrine for 24 h compared with HCT-8/5-FU without oxymatrine (Fig. 4C).
Oxymatrine induces HCT-8/5-FU cells to undergo apoptosis
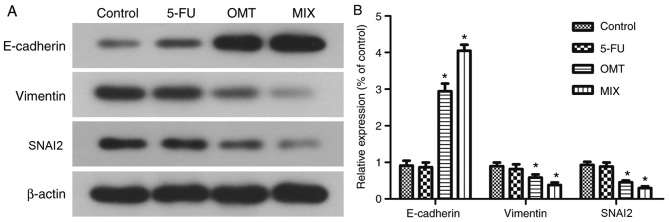
The mesenchymal marker vimentin and the epithelial marker E-cadherin were downregulated and upregulated, respectively, following treatment with 2 mg/ml oxymatrine for 24 h (Fig. 5A and B). The results from flow cytometry (Fig. 6A) demonstrated that there was no significant changes in the apoptotic rate of 5-FU-treated HCT-8/5-FU cells compared with the control group (HCT-8/5-FU+0 µg/ml 5-fluorouracil; P>0.05; Fig. 6B). However, the apoptotic rates were significantly increased in the OMT- and MIX-treated groups compared with the control group (both P<0.05; Fig. 6B), with the MIX-treated group exhibiting the highest apoptotic rate. Additionally, the protein expression levels of MDR1 and p-p65 were significantly downregulated in the OMT- and MIX-treated groups compared with the control group (both P<0.05; Fig. 6C and D), whereas no significant differences were observed between the 5-FU-treated and control groups (P>0.05; Fig. 6C and D).
Figure 5.
Effects of 5-FU, oxymatrine and their combination on the regulation of epithelial-mesenchymal transition markers in tumor cells. (A) Western blot analysis of HCT-8/5-FU cells treated with 1 µg/ml 5-FU, 2 mg/ml oxymatrine, alone or in combination, for 24 h and (B) the quantification. *P<0.05 vs. control cells. OMT, oxymatrine; MIX, combined treatment; 5-FU, 5-fluorouracil; SNAI2, snail family transcriptional repressor 2.
Discussion
Despite recent advancements in the treatment of patients with colon cancer, overcoming 5-FU resistance remains clinically challenging (8). Therefore, it is important to elucidate the underlying mechanisms of chemotherapy resistance in order to improve patient outcomes. It was reported that oxymatrine not only had inhibitory effects on the regulation of cancer cell metastasis, but was also involved in inducing the EMT, in vitro, in colon cancer, rectum, breast and lung cancers (11). Furthermore, oxymatrine reversed chemotherapy resistance in colorectal cancer in vitro (15). Thus, oxymatrine may serve as a potential therapeutic agent by reversing EMT in tumor cells. Indeed, the present study assessed oxymatrine sensitization of 5-FU-resistant colon cancer cells in vitro and explored the underlying molecular events. HCT-8/5-FU cells significantly increased the 5-FU concentration required to decrease tumor cell survival (8.56-fold increase compared with parental HCT-8 cells), and HCT-8/5-FU cells induced tumor cell EMT phenotypes and the expression of mesenchymal markers. Furthermore, oxymatrine alone and in combination with 5-FU altered HCT-8/5-FU cell morphology, induced tumor cell apoptosis and upregulated E-cadherin expression by suppressing the NF-κB signaling pathway. The results obtained in the present study revealed that the EMT was involved in 5-FU chemoresistance in HCT-8/5-FU colon cancer cells in vitro. Furthermore, oxymatrine reversed 5-FU chemoresistance by modulating the EMT through inactivation of the NF-κB signaling pathway. Therefore, oxymatrine may serve as a novel therapeutic agent to reverse 5-FU resistance in colon cancer cells.
Oxymatrine is a quinolizidine alkaloid compound extracted from the root of Sophora flavescens. Previous studies have demonstrated that oxymatrine exhibits a wide range of anticancer activities (11,14), including inhibition of tumor cell proliferation, invasion and metastasis in gallbladder, cervical, lung and ovarian cancer (29–32). Additionally, recent studies have revealed that oxymatrine may reverse chemoresistance, including resistance to paclitaxel in lung cancer cells (15) and in human squamous cells (33), and resistance to cisplatin in lung cancer cells (34). However, to date, the precise mechanisms underlying the effects of oxymatrine on 5-FU resistance in colorectal cancer remains unclear. Tumor cells undergo EMT in order to increase migration, invasion and anoikis tolerance during cancer development and progression (20). In particular, during EMT, tumor cells lose cell-cell adhesion and gain migratory and invasive properties, which are required for tumor initiation and metastasis (35,36). At the gene level, tumor cells involved in the EMT downregulate E-cadherin expression, upregulate vimentin and SNAI2 expression and gain cancer stem cell-like properties (21,37,38). A previous study revealed that an increase in the EMT was considered to be a novel mechanism associated with chemotherapy resistance in cancer of epithelial origin (39). In order to assess the validity of this hypothesis, the present study compared the EMT phenotype of HCT-8/5-FU cells with HCT-8 cells and demonstrated the association between the EMT and 5-FU resistance. In particular, the results revealed that HCT-8/5-FU cells exhibited an irregular fibroblastic morphology, whereas the parental HCT-8 cells displayed an epithelial morphology. Compared with HCT-8 cells, HCT-8/5-FU cells had increased levels of vimentin, SNAI2 and p-p65 proteins, but lower levels of E-cadherin protein, indicating that the EMT may be associated with 5-FU resistance.
In order to explore the effects of oxymatrine on the EMT and 5-FU resistance in colon cancer cells in the present study, HCT-8/5-FU cells were treated with oxymatrine. It was revealed that oxymatrine (≥2 mg/ml) significantly inhibited tumor cell viability, which suggest that oxymatrine reversed the chemoresistance of HCT-8/5-FU cells. In addition, oxymatrine upregulated E-cadhern expression and downregulated SNAI2 and vimentin expression, indicating that oxymatrine reverse the EMT phenotype of HCT-8/5-FU cells. The activation of the NF-κB signaling pathway is important for the regulation of cell proliferation, differentiation, survival, migration, invasion and the EMT process (40–42). The NF-κB signaling pathway is involved in transforming growth factor β-induced EMT and the 5-Fu chemotherapy resistance in colon cancer (43,44). In the present study, the expression of p-p65 was significantly increased in HCT-8/5-FU cells compared with HCT-8 cells. Moreover, oxymatrine treatment significantly suppressed the expression of the p-p65 protein. However, this did not rule out the fact that oxymatrine in combination with 5-FU may also possess synergistic antitumor activity in HCT-8 cells or that oxymatrine alone exhibits antitumor effects on HCT-8/5-FU cells, which may be addressed by future studies on different 5-FU-sensitive and resistant cancer cell lines. In addition, the present study lacked an invasion assay, which may determine effects on the EMT process more conclusively. Thus, future investigations using alternative cell lines in vitro and in vivo are required to validate the current findings. In conclusion, the results of the present study demonstrated that the colon cancer cell EMT was involved in the chemoresistance of HCT-8/5-FU cells to 5-FU, and that oxymatrine treatment was able to reverse this resistance. Oxymatrine may regulate the EMT process and inactivate the NF-κB signaling pathway in tumor cells. The findings of the present study provide a novel theoretical basis for the sensitization of 5-FU-resistant colon cancer cells in vitro.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by grants from The Natural Science Foundation of Guangxi Zhuang Autonomous Region (grant no. 2018GXNSFBA050072), The National Natural Science Foundation of China (grant no. 8176110028), The 2018 Innovation Project of Guangxi Graduate Education (grant no. YCBZ2018046) and The Guangxi Zhuang Autonomous Region Health and Family Planning Commission Self-Financing Research Project (grant no. Z20170086).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
LL, JAH and ZWC designed the study. LL, JW, JL, LW, ZXC, CLH and TQG performed the experiments and provided technical support. LL, JAH and ZWC analyzed the data. LL prepared and revised the manuscript. JAH and ZWC supervised the work. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, corp-author. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakih MG. Metastatic colorectal cancer: Current state and future directions. J Clin Oncol. 2015;33:1809–1824. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 5.Boland P, Ma W. Immunotherapy for colorectal cancer. Cancers (Basel) 2017;9(pii):E50. doi: 10.3390/cancers9050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200. doi: 10.1002/14651858.CD002200.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Shigeta K, Ishii Y, Hasegawa H, Okabayashi K, Kitagawa Y. Clinical usefulness of 5-FU metabolic enzymes as predictive markers of response to chemotherapy in colorectal cancer. World J Surg. 2016;40:1019–1020. doi: 10.1007/s00268-015-3286-z. [DOI] [PubMed] [Google Scholar]
- 8.Sobrero A, Guglielmi A, Grossi F, Puglisi F, Aschele C. Mechanism of action of fluoropyrimidines: relevance to the new developments in colorectal cancer chemotherapy. Semin Oncol. 2000;27(5 Suppl 10):S72–S77. [PubMed] [Google Scholar]
- 9.Iqbal A, George TJ. Randomized clinical trials in colon and rectal cancer. Surg Oncol Clin N Am. 2017;26:689–704. doi: 10.1016/j.soc.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Wen S, Zhan Y, He Y, Liu X, Jiang J. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med. 2008;74:245–251. doi: 10.1055/s-2008-1034304. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, You RL, Qin WJ, Hai LN, Fang MJ, Huang GH, Kang RX, Li MH, Qiao YF, Li JW, Li AP. Anti-tumor activities of active ingredients in compound kushen injection. Acta Pharmacol Sin. 2015;36:676–679. doi: 10.1038/aps.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou YJ, Guo YJ, Yang XL, Ou ZL. Anti-cervical cancer role of matrine, oxymatrine and Sophora Flavescens alkaloid gels and its mechanism. J Cancer. 2018;9:1357–1364. doi: 10.7150/jca.22427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nourmohammadi S, Aung TN, Cui J, Pei JV, De Ieso ML, Harata-Lee Y, Qu Z, Adelson DL, Yool AJ. Effect of compound kushen injection, a natural compound mixture, and its identified chemical components on migration and invasion of colon, brain, and breast cancer cell lines. Front Oncol. 2019;9:314. doi: 10.3389/fonc.2019.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang L, Huang J. Oxymatrine inhibits epithelial-mesenchymal transition through regulation of NF-κB signaling in colorectal cancer cells. Oncol Rep. 2016;36:1333–1338. doi: 10.3892/or.2016.4927. [DOI] [PubMed] [Google Scholar]
- 15.Joshi P, Vishwakarma RA, Bharate SB. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur J Med Chem. 2017;138:273–292. doi: 10.1016/j.ejmech.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6:10697–10711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21(pii):E965. doi: 10.3390/molecules21070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: Mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5(pii):E17. doi: 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: A central regulator of cancer progression. Trends Cell Biol. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao Y, Lu Y, Wang X, Feng W, Sun X, Guo H, Tang C, Zhang X, Shi Q, Yu H. Eukaryotic translation initiation factor 5A2 (eIF5A2) regulates chemoresistance in colorectal cancer through epithelial mesenchymal transition. Cancer Cell Int. 2015;15:109. doi: 10.1186/s12935-015-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Zhang L, Xie B, Wang X, Yang X, Ding N, Zhang J, Liu Q, Tan G, Feng D, Sun LQ. FOXC2 promotes chemoresistance in nasopharyngeal carcinomas via induction of epithelial mesenchymal transition. Cancer Lett. 2015;363:137–145. doi: 10.1016/j.canlet.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Ma JL, Zeng S, Zhang Y, Deng GL, Shen H. Epithelial-mesenchymal transition plays a critical role in drug resistance of hepatocellular carcinoma cells to oxaliplatin. Tumour Biol. 2016;37:6177–6184. doi: 10.1007/s13277-015-4458-z. [DOI] [PubMed] [Google Scholar]
- 25.Yang Q, Huang J, Wu Q, Cai Y, Zhu L, Lu X, Chen S, Chen C, Wang Z. Acquisition of epithelial-mesenchymal transition is associated with Skp2 expression in paclitaxel-resistant breast cancer cells. Br J Cancer. 2014;110:1958–1967. doi: 10.1038/bjc.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–431. doi: 10.1016/S0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Sun Y, Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayo MW, Baldwin AS. The transcription factor NF-kappaB: Control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Su BS, Chang LH, Gao Q, Chen KL, An P, Huang C, Yang J, Li ZF. Oxymatrine induces apoptosis in human cervical cancer cells through guanine nucleotide depletion. Anticancer Drugs. 2014;25:161–173. doi: 10.1097/CAD.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Han Q, Zhu Y. Oxymatrine inhibited cell proliferation by inducing apoptosis in human lung cancer A549 cells. Biomed Mater Eng. 2015;26(Suppl 1):S165–S172. doi: 10.3233/BME-151302. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Bi T, Dai W, Wang G, Qian L, Gao Q, Shen G. Effects of oxymatrine on the proliferation and apoptosis of human hepatoma carcinoma cells. Technol Cancer Res Treat. 2016;15:487–497. doi: 10.1177/1533034615587616. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Jiang K, Zhao F. Oxymatrine suppresses proliferation and facilitates apoptosis of human ovarian cancer cells through upregulating microRNA-29b and downregulating matrix metalloproteinase-2 expression. Mol Med Rep. 2015;12:5369–5374. doi: 10.3892/mmr.2015.3977. [DOI] [PubMed] [Google Scholar]
- 33.Luo SX, Deng WY, Wang XF, Lü HF, Han LL, Chen BB, Chen XB, Li N. Molecular mechanism of indirubin-3′-monoxime and Matrine in the reversal of paclitaxel resistance in NCI-H520/TAX25 cell line. Chin Med J (Engl) 2013;126:925–929. [PubMed] [Google Scholar]
- 34.Wang HQ, Jin JJ, Wang J. Matrine induces mitochondrial apoptosis in cisplatin-resistant non-small cell lung cancer cells via suppression of β-catenin/survivin signaling. Oncol Rep. 2015;33:2561–2566. doi: 10.3892/or.2015.3844. [DOI] [PubMed] [Google Scholar]
- 35.Tse JC, Kalluri R. Mechanisms of metastasis: Epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem. 2007;101:816–829. doi: 10.1002/jcb.21215. [DOI] [PubMed] [Google Scholar]
- 36.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Kong D, Li Y, Wang Z, Sarkar FH. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: Are they cousins or twins? Cancers (Basel) 2011;3:716–729. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 39.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaltschmidt B, Greiner JFW, Kadhim HM, Kaltschmidt C. Subunit-specific role of NF-κB in cancer. Biomedicines. 2018;6(pii):E44. doi: 10.3390/biomedicines6020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–334. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett. 2010;295:214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao C, Zhao Q, Zhang C, Wang G, Yao Y, Huang X, Zhan F, Zhu Y, Shi J, Chen J, et al. miR-15b-5p resensitizes colon cancer cells to 5-fluorouracil by promoting apoptosis via the NF-κB/XIAP axis. Sci Rep. 2017;7:4194. doi: 10.1038/s41598-017-04172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.