Abstract
Screening for genes or markers relevant to bladder cancer (BC) tumorigenesis and progression is of vital clinical significance. The present study used reverse-transcription quantitative PCR reaction assays to examine the expression of mRNA encoding Rho GTPase-activating protein 9 (ARHGAP9) in BC tissue samples and to determine whether ARHGAP9 is an independent prognostic biomarker for non-muscle invasive BC (NMIBC) and muscle invasive BC (MIBC). The results revealed that the downregulation of ARHGAP9 expression in the tissue of patients with NMIBC or MIBC was significantly associated with a poor prognosis. In patients with NMIBC, a high expression of ARHGAP9 was significantly associated with prolonged recurrence-free survival, whereas in MIBC patients, it was significantly associated with an increased progression-free and cancer-specific survival. The risk of cancer-specific death was 2.923 times higher (95% confidence interval, 1.192–7.163) when ARHGAP9 levels were decreased. In conclusion, lower expressions of ARHGAP9 correlated with BC prognosis, indicating that it may be a useful marker for guiding treatment application.
Keywords: Rho GTPase-activating protein 9, non-muscle invasive bladder cancer, muscle invasive bladder cancer, recurrence, progression
Introduction
Bladder cancer (BC), one of the most common malignancies worldwide, is classified into two subtypes based on cancer cell infiltration into the muscle layer of the bladder. Non-muscle invasive BC (NMIBC) is less aggressive but has a high recurrence rate, whereas muscle invasive BC (MIBC) tends to metastasize and has a relatively poor prognosis (1–3). High throughput techniques such as microarray analysis and next generation sequencing, which are used commonly in the fields of genetics and epigenetics, have identified several genes involved in cancer pathogenesis, and have led to identification of cancer biomarkers and to development of novel effective gene targeted therapies (4). In a previous study, we used next generation sequencing and miRNA microarray assays to identify several miRNAs and their target genes that are differentially expressed in BC (5). We found that a novel gene, Rho GTPase-activating protein 9 (ARHGAP9), is down-regulated in BC. In addition, hsa-miR-3620, which interacts with ARHGAP9, is up-regulated.
Rho GTPases are key regulators of the actin cytoskeleton, which plays an important role in cell adhesion and migration. The switch mechanism of Rho GTPases is controlled by binding to GTP or GDP (6–8). ARHGAP9 contains a diverse combination of functional protein domains, including the RhoGAP, SH3, WW, and PH domains (9). Binding of the RhoGAP domain to GTP-bound Rho proteins accelerates GTPase activity, and defective Rho GTPase signaling is implicated in tumorigenesis and metastasis (10,11). Silencing ARHGAP9 inhibits proliferation, migration, and invasion of breast cancer cells (12). Activated ARHGAP9 inhibits adhesion of a human leukemia cell line, KG-1, to fibronectin and collagen through activation of cdc42 and Rac1 but not RhoA (6).
Here, we asked whether ARHGAP9 is a novel prognostic biomarker for BC. We used real-time polymerase chain reaction (PCR) to compare expression of ARHGAP9 mRNA in human BC and control tissues (the latter comprised normal tissue surrounding BC and normal bladder mucosa); and analyzed its ability to predict prognosis of NMIBC and MIBC. ARHGAP9, known as a MAP kinase docking protein, was encoded by ARHGAP9 gene, which shares 16 bases with Gli1 in their 3′ ends (9,13). Accordingly, we asked whether ARHGAP9 plays a role in the MAPK and Hedgehog signaling pathways.
Materials and methods
Patients and tissue samples
The biospecimens used in the present study were provided by the Chungbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare, and Family Affairs. The study was approved by the Institutional Review Board at Chungbuk National University (GR2010-12-010), and the experiments were undertaken with the informed written consents of all participants. The study methodologies conformed with the standards set by the Declaration of Helsinki. The baseline characteristics of the case subjects (n=237 bladder tissue samples) are shown in Table I. Among these, 140 samples were from primary BC patients and were histologically verified as transitional cell carcinomas; the remaining 97 samples used as the control set comprised normal bladder mucosa or normal tissues from the area surrounding BC. To reduce the chances of confounding factors affecting the analyses, patients diagnosed with concomitant carcinoma in situ or carcinoma in situ lesions alone were excluded. Voided urine cytology was tested before surgical treatment to assist BC diagnosis and/or prognosis. Fresh-frozen specimens were obtained during surgical resection of transitional cell carcinoma at Chungbuk National University Hospital. All tumors were macro-dissected, typically within 15 min of surgical resection. Each specimen was confirmed by pathological analysis of a part of fresh-frozen specimens obtained from radical cystectomy and transurethral resection of bladder tumor (TURBT). Tumors were staged (2002 TNM Classification) and graded (2004 WHO Classification), according to standard criteria (14). Clinically metastatic disease and non-cystectomy cases were not excluded from the study. Each patient was followed and managed suggested management according to standard recommendations (15–17). Surveillance was performed by cystoscopic examination and upper urinary tract imaging in accordance with European Association of Urology guidelines (16). Recurrence was defined as relapse of primary NMIBC of the same pathologic stage, and progression of NMIBC and MIBC was defined as TNM stage progression after disease recurrence. The mean follow-up period for NMIBC patients was 72.95 months (range, 3.2–172.2). The mean follow-up period for MIBC patients was 36.18 months (range, 3.0–141.4).
Table I.
Clinicopathological features of primary BC patient and control tissues (surrounding normal tissues and normal bladder mucosae).
| BC (140) | ||||
|---|---|---|---|---|
| Variables | NMIBC | MIBC | Control | P-value |
| No. | 97 | 43 | 97 | |
| Mean age ± SD | 63.45±13.79 | 67.60±9.84 | 61.98±14.32 | 0.083a |
| Sex (%) | 0.975a | |||
| Male | 80 (82.5%) | 36 (83.7%) | 81 (83.5%) | |
| Female | 17 (17.5%) | 7 (16.3%) | 16 (16.5%) | |
| Operation (%) | <0.001b | |||
| TUR-BT | 97 (100.0%) | 17 (39.5%) | ||
| Radical cystectomy | 0 | 26 (60.5%) | ||
| Tumor size (%) | 0.003b | |||
| ≤1 cm | 16 (16.5%) | 2 (4.7%) | ||
| 2–3 cm | 37 (38.1%) | 11 (25.6%) | ||
| >3 cm | 37 (38.1%) | 28 (65.1%) | ||
| Multiplicity (%) | 0.108b | |||
| Single | 52 (53.6%) | 30 (69.8%) | ||
| 2–7 | 28 (28.9%) | 7 (16.3%) | ||
| >7 | 11 (11.3%) | 4 (9.3%) | ||
| Grade, 2004 WHO grading system (%) | <0.001b | |||
| Low | 72 (74.2%) | 8 (18.6%) | ||
| High | 25 (25.8%) | 35 (81.4%) | ||
| Stage (%) | <0.001b | |||
| TaN0M0 | 26 (26.8%) | |||
| T1N0M0 | 71 (73.2%) | |||
| T2N0M0 | 13 (30.2%) | |||
| T3N0M0 | 6 (14.0%) | |||
| T≥4 or N≥1 or M1 | 24 (55.8%) | |||
| Chemotherapy (%) | <0.001b | |||
| No | 97 (100.0%) | 23 (53.5%) | ||
| Yes | 0 | 20 (46.5%) | ||
| BCG therapy (%) | <0.001b | |||
| No | 56 (57.7%) | 38 (88.4%) | ||
| Yes | 40 (41.2%) | 5 (11.6%) | ||
| Recurrence, no. of patients (%) | ||||
| No | 59 (60.8%) | – | ||
| Yes | 38 (39.2%) | – | ||
| Progression, no. of patients (%) | 0.126b | |||
| No | 79 (81.4%) | 30 (69.8%) | ||
| Yes | 18 (18.6%) | 13 (30.2%) | ||
| Survival, no. of patients (%) | 0.009b | |||
| Alive | 64 (66.0%) | 21 (48.8%) | ||
| Non-cancer-specific death | 18 (18.6%) | 3 (7.0%) | ||
| Cancer-specific death | 15 (15.5%) | 19 (44.2%) | ||
| Mean follow-up, months (range) | 72.95 (3.20–172.20) | 36.18 (3.00–141.40) | ||
P-value obtained using Kruskal-Wallis H test (BC compared with control).
P-value obtained using the Mann-Whitney U test (NMIBC compared with MIBC). BC, bladder cancer; BCG, Bacillus Calmette-Guerin; NMIBC, non-muscle invasive bladder cancer; MIBC, muscle invasive bladder cancer; SD, standard deviation.
RNA extraction
Total RNA was extracted from tissues using TRIzol reagent (Invitrogen), as described previously (18), and stored at −80°C. Next, cDNA was synthesized from 1 µg of total RNA using a First Strand cDNA Synthesis kit (Clontech, TAKARA), according to the manufacturer's protocol.
Microarray analysis
Five hundred nanograms of total RNA was used for labeling and hybridization prior to analysis, according to the manufacturer's protocols (Illumina). After the bead chips were scanned with an Illumina Bead Array Reader, the Robust Multiarray Average in R package was used to perform global correction, quantile normalization, and median polish summarization of the microarray data. P-values (t test) were calculated from bead mRNA signal intensities (19–21). The full set of microarray data set are available online at http://www.ncbi.nlm.nih.gov/geo/under data series accession number GSE13507 (21).
mRNA sequencing
Total sequencing reads were subjected to preprocessing as follows: Adapter trimming was performed using cutadapt with default parameters, and quality trimming (Q30) was performed using FastQC with default parameters. Processed reads were mapped to the human reference genome [Ensembl 72 (GRCh37: hg19)] using tophat and cufflink with default parameters (22). Fragments Per Kilobase of exon per million fragments Mapped (FPKM) values were normalized and quantitated using R package Tag Count Comparison (TCC) (23) to determine statistical significance (e.g., P and Q values) and differential expression (e.g., -fold changes).
Quantitative PCR analysis
Tissue mRNAs were amplified by quantitative PCR performed using a Rotor Gene 6000 instrument (Qiagen) and quantified using the 2−∆∆cq method (24). QuantitativePCR reactions were carried out using the SYBR Premix Ex Taq II (Clontech, TAKARA). The following primers were used to amplify candidate genes: ARHGAP9 (Gene ID: ENSG00000123329), sense, 5′-CAGAGCAGTGCCTCTCTC-3′ (18 bp, Tm 58°C); antisense, 5′-CTGCTGGGTCAGATGTCTC-3′ (19 bp, Tm 58°C) and the amplicon size was 179 bp. The control GAPDH (Gene ID: ENSG00000111640) primers were as follows: sense, 5′-CATGTTCGTCATGGGTGTGA-3′ (20 bp, Tm 60°C); antisense, 5′-ATGGCATGGACTGTGGTCAT-3′ (20 bp, Tm 60°C) and the amplicon size was 156 bp. The PCR reaction was performed in a final volume of 10 µl, comprising 5 µl of 2× SYBR Premix EX Taq buffer, 0.5 µl of each 5′and 3′ primer (10 pM/µl), and 2 µl, of sample cDNA. A known concentration of the PCR product was then 10-fold serially diluted from 100 pg/µl to 0.1 pg/µl and used to establish a standard curve. The real-time PCR conditions were as follows: 1 cycle at 96°C for 20 sec, followed by 40 cycles of 3 sec at 96°C for denaturation, 15 sec at 60°C for annealing, and 15 sec at 72°C for extension. The melting program was performed at 72–95°C at a heating rate of 1°C per 45 sec. Rotor-Gene Q software 2.3.1.49 was used for capturing and analyzing spectral data. All samples were run in triplicate. Gene expression was normalized to the expression of GAPDH.
Statistical analysis
To reduce variation among microarrays, the intensity values for each microarray were rescaled using a quantile normalization method (19). Gene expression values were loge-transformed and median-centered across samples. The significance of various clinicopathological variables was evaluated using univariate and multivariate Cox proportional hazard regression models. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to investigate relative risk. Survival curves to determine the prognostic value of the genetic biomarker were plotted using the Kaplan-Meier method and compared using the log-rank test. The Kruskal-Wallis H test and Mann-Whitney U test were used to examine expression of ARHGAP9 in BC tissues versus control tissues. Correlations between ARHGAP9 and genes involved in the MAPK and Hedgehog signaling pathways were examined by calculating non-parametric Spearman's correlation coefficients. Statistical analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM) and GraphPad Prism 7 (GraphPad Software). P-values <0.05 were considered significant.
Results
Expression of ARHGAP9 mRNA in BC tissue
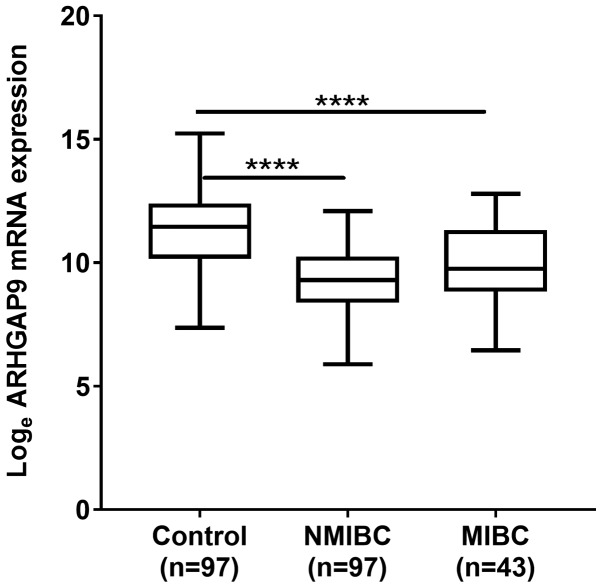
Microarray analysis revealed that expression of mRNA encoding ARHGAP9 in BC tissues was lower than that in control samples. The validation test showed that the real-time PCR results were identical to those of the microarray, i.e., expression of mRNA encoding ARHGAP9 was significantly lower in NMIBC and MIBC tissues than in normal control tissues (P<0.001; Fig. 1).
Figure 1.
Expression of mRNA encoding ARHGAP9 in BC tissue. Expression of ARHGAP9 in NMIBC and MIBC tissue was significantly lower compared with normal control tissue samples. BC, bladder cancer; NMIBC, non-muscle invasive bladder cancer; MIBC, muscle invasive bladder cancer. Control samples represent normal bladder mucosae and normal tissues surrounding bladder cancer. The P-value was calculated using the Mann-Whitney U test. ****P<0.0001. ARHGAP9, Rho GTPase-activating protein 9; BC, bladder cancer; MIBC, muscle invasive BC; NMIBC, non-muscle invasive bladder cancer.
Expression of ARHGAP9 correlates with NMIBC prognosis
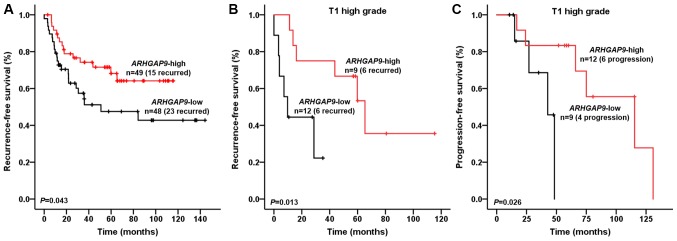
Univariate and multivariate Cox regression analyses revealed that expression of ARHGAP9 in NMIBC patients was an independent predictor of recurrence-free survival (RFS) (HR, 2.436; 95% CI, 1.132–5.243; P=0.023; Table II). Kaplan-Meier analysis demonstrated that NMIBC patients with ARHGAP9 expression levels in the upper 50th percentile experienced less recurrence than those with expression levels in the lower 50th percentile (log-rank test, P=0.043; Fig. 2A). Particularly, for T1 high grade(HG) BC patients, univariate and multivariate Cox regression analysis identified ARHGAP9 expression as an independent risk factor for T1HG BC recurrence (HR, 7.264; 95% CI, 1.291–45.091; P=0.025) and progression (HR, 14.987; 95% CI, 1.093–205.567; P=0.043; Table III). The RFS and progression-free survival (PFS) of T1HG BC patients with ARHGAP9 expression levels in the upper 50th percentile experienced less recurrence and progression than those with expression levels in the lower 50th percentile (log-rank test, P=0.013 and 0.026 respectively; Fig. 2B and C).
Table II.
Univariate and multivariate Cox regression analysis to predict NMIBC recurrence.
| Univariate Cox analysis | Multivariate Cox analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | ||||
| ≤70 (Ref.) vs. >70 | 2.994 (1.579–5.680) | 0.001a | 1.727 (0.820–3.637) | 0.151 |
| Sex | ||||
| Male (Ref.) vs. female | 1.314 (0.577–2.993) | 0.516 | ||
| Tumor size | ||||
| ≤1 cm | Ref. | 0.028a | Ref. | 0.574 |
| 2–3 cm | 1.700 (0.474–6.100) | 0.416 | 1.251 (0.341–4.593) | 0.736 |
| >3 cm | 3.686 (1.093–12.425) | 0.035a | 1.779 (0.484–6.547) | 0.386 |
| Multiplicity | ||||
| Single | Ref. | 0.141 | ||
| 2–7 | 1.071 (0.479–2.395) | 0.867 | ||
| >7 | 2.383 (0.985–5.767) | 0.054 | ||
| 2004 WHO Grade Low (Ref.) vs. high | 2.450 (1.275–4.708) | 0.007a | 1.823 (0.809–3.568) | 0.147 |
| Stage | ||||
| Ta (Ref.) vs. T1 | 2.938 (1.144–7.540) | 0.025a | 2.347 (0.803–6.857) | 0.119 |
| BCG | ||||
| No (Ref.) vs. yes | 1.918 (1.009–3.647) | 0.047a | 1.744 (0.852–3.568) | 0.128 |
| ARHGAP9 expression High expression (Ref.) vs. Low expression | 1.939 (1.009–3.726) | 0.047a | 2.436 (1.132–5.243) | 0.023a |
P<0.05. NMIBC, non-muscle invasive bladder cancer; BCG, Bacillus Calmette-Guerin; CI, confidence interval; HR, hazard ratio; Ref., reference; ARHGAP9, Rho GTPase-activating protein 9.
Figure 2.
Kaplan-Meier curves showing effect of ARHGAP9 on the recurrence-free survival and progression-free survival of NMIBC patients. (A) Recurrence-free survival of patients with NMIBC. (B) Recurrence-free survival of patients with T1 high grade BC. (C) Progression-free survival of patients with T1 high grade BC. BC patients were divided into two groups (upper 50th percentile and lower 50th percentile groups) according to the expression of ARHGAP9. The recurrence-free survival rate of NMIBC patients, particularly in T1HG BC patients, was significantly higher in the high ARHGAP9 expression group (log-rank test; P<0.05). The progression-free survival of T1HG BC patients was significantly higher in the high ARHGAP9 expression group (log-rank test, P<0.05). ARHGAP9, Rho GTPase-activating protein 9; NMIBC, non-muscle invasive bladder cancer; T1HG, T1 high grade; BC, bladder cancer.
Table III.
Univariate and multivariate Cox regression analysis to predict T1 high grade NMIBC recurrence and progression.
| Recurrence | Progression | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Cox analysis | Multivariate Cox analysis | Univariate Cox analysis | Multivariate Cox analysis | |||||||||||||
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||||||||
| Age | ||||||||||||||||
| ≤70 (Ref.) vs. >70 | 2.342 (0.625–8.776) | 0.207 | 1.567 (0.390–6.297) | 0.527 | ||||||||||||
| Sex | ||||||||||||||||
| Male (Ref.) vs. female | 1.327 (0.342–5.154) | 0.682 | 2.748 (0.548–13.781) | 0.219 | ||||||||||||
| Tumor size | ||||||||||||||||
| ≤1 cm | Ref. | 0.976 | Ref. | 0.468 | ||||||||||||
| 2–3 cm | 29604.104 | 0.948 | 9687.884 | 0.968 | ||||||||||||
| (0.000–2.839×10138) | (0.000–3.269×10201) | |||||||||||||||
| >3 cm | 25622.270 | 0.949 | 36480.741 | 0.964 | ||||||||||||
| (0.000–2.454×10138) | (0.000–1.226×10202) | |||||||||||||||
| Multiplicity | ||||||||||||||||
| Single | Ref. | 0.618 | Ref. | 0.850 | ||||||||||||
| 2–7 | 1.450 (0.417–5.040) | 0.559 | 1.548 (0.345–6.943) | 0.568 | ||||||||||||
| >7 | 2.933 (0.296–29.074) | 0.358 | 0.000 (0.000–0.000) | 0.991 | ||||||||||||
| BCG | ||||||||||||||||
| No (Ref.) vs. yes | 1.247 (0.336–4.624) | 0.741 | 0.459 (0.119–1.766) | 0.257 | ||||||||||||
| ARHGAP9 expression High (Ref.) vs. low expression | 5.126 (1.247–21.066) | 0.023a | 7.264 | 0.025a | 6.041 (1.026–35.571) | 0.047a | 14.987 | 0.043a | ||||||||
| (1.291–45.019) | (1.093–205.567) | |||||||||||||||
P<0.05. NMIBC, non-muscle invasive bladder cancer; BCG, Bacillus Calmette-Guerin; CI, confidence interval; HR, hazard ratio; Ref., reference; ARHGAP9, Rho GTPase-activating protein 9.
Expression of ARHGAP9 correlates with MIBC prognosis
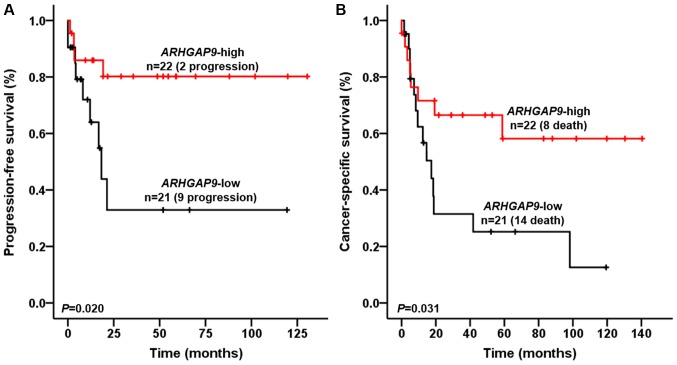
For MIBC patients, univariate and multivariate Cox regression analysis identified ARHGAP9 expression as an independent risk factor for disease progression (HR, 5.241; 95% CI, 1.456–18.870; P=0.011) and cancer-specific death (HR, 2.923; 95% CI, 1.192–7.163; P=0.019) (Tables IV and V). PFS and cancer specific survival (CSS) of patients with ARHGAP9 expression in the upper 50th percentile were significantly higher than those of patients in the lower 50th percentile (log-rank test, P=0.020 and 0.031, respectively; Fig. 3A and B).
Table IV.
Univariate and multivariate Cox regression analysis to predict MIBC progression.
| Univariate Cox analysis | Multivariate Cox analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | ||||
| ≤70 (Ref.) vs. >70 | 1.302 (0.432–3.926) | 0.639 | ||
| Sex | ||||
| Male (Ref.) vs. female | 5.625 (1.766–17.912) | 0.003a | 7.255 (2.062–25.528) | 0.002a |
| Operation | ||||
| TURBT (Ref.) vs. Radical cystectomy | 0.948 (0.309–2.905) | 0.926 | ||
| Tumor size | ||||
| ≤1 cm | Ref. | 0.406 | ||
| 2–3 cm | 12417.036 (0.000–2.033×10143) | 0.954 | ||
| >3 cm | 35009.555 (0.000–5.718×10143) | 0.949 | ||
| Multiplicity | ||||
| Single | Ref. | 0.507 | ||
| 2–7 | 0.358 (0.046–2.800) | 0.328 | ||
| >7 | 1.483 (0.324–6.787) | 0.611 | ||
| 2004 WHO Grade | ||||
| Low (Ref.) vs. high | 31.010 (0.132–7298.224) | 0.218 | ||
| Stage | ||||
| T2 | Ref. | 0.851 | ||
| T3 | 1.630 (0.297–8.958) | 0.574 | ||
| T4 or N1 or M1 | 1.229 (0.358–4.222) | 0.744 | ||
| Chemotherapy | ||||
| No (Ref.) vs. yes | 3.912 (1.076–14.218) | 0.038a | 2.859 (0.752–10.868) | 0.123 |
| ARHGAP9 expression High expression (Ref.) vs. Low expression | 3.818 (1.145–12.733) | 0.029a | 5.241 (1.456–18.870) | 0.011a |
P<0.05. MIBC, muscle invasive bladder cancer; CI, confidence interval; HR, hazard ratio; Ref., reference; ARHGAP9, Rho GTPase-activating protein 9.
Table V.
Univariate and multivariate Cox regression analysis for predicting the cancer-specific survival of patients with MIBC.
| Univariate Cox analysis | Multivariate Cox analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | ||||
| ≤70 (Ref.) vs. >70 | 1.860 (0.791–4.371) | 0.155 | ||
| Gender | ||||
| Male (Ref.) vs. female | 3.379 (1.273–8.967) | 0.014a | 4.046 (1.491–10.976) | 0.006a |
| Operation | ||||
| TURBT (Ref.) vs. Radical cystectomy | 1.026 (0.435–2.417) | 0.954 | ||
| Tumor size | ||||
| ≤1 cm | Ref. | 0.386 | ||
| 2–3 cm | 14923.217 (0.000–1.565E+115) | 0.941 | ||
| >3 cm | 32178.497 (0.000–3.369E+115) | 0.937 | ||
| Multiplicity | ||||
| Single | Ref. | 0.730 | ||
| 2–7 | 0.709 (0.206–2.438) | 0.585 | ||
| >7 | 0.611 (0.137–2.725) | 0.519 | ||
| 2004 WHO Grade | ||||
| Low (Ref.) vs. high | 3.009 (0.699–12.950) | 0.139 | ||
| Stage | ||||
| T2 | Ref. | 0.480 | ||
| T3 | 0.909 (0.181–4.563) | 0.908 | ||
| T4 or N1 or M1 | 1.671 (0.641–4.358) | 0.294 | ||
| Chemotherapy | ||||
| No (Ref.) vs. yes | 1.482 (0.633–3.472) | 0.365 | ||
| ARHGAP9 expression High expression (Ref.) vs. Low expression | 2.554 (1.058–6.163) | 0.037a | 2.923 (1.192–7.163) | 0.019a |
P<0.05. MIBC, muscle invasive bladder cancer; CI, confidence interval; HR, hazard ratio; Ref., reference; ARHGAP9, Rho GTPase-activating protein 9.
Figure 3.
Kaplan-Meier curves demonstrating the effect of ARHGAP9 on the progression-free survival and cancer-specific survival of MIBC patients. Patient (A) progression-free survival and (B), cancer-specific survival rates are presented. BC patients were divided into two groups (upper 50th percentile and lower 50th percentile groups) according to the expression of ARHGAP9. The progression-free survival and cancer-specific survival of MIBC patients were significantly higher in the high ARHGAP9 expression group (log-rank test, P<0.05). ARHGAP9, Rho GTPase-activating protein 9; MIBC, muscle invasive bladder cancer; BC, bladder cancer.
Relationship between ARHGAP9 and genes regulating the MAPK and Hedgehog signaling pathways in BC
To identify whether expression of ARHGAP9 correlates with that of genes regulating the MAPK and Hedgehog signaling pathways, we undertook gene network depiction and analysis using the GeneMANIA (http://www.genemania.org) web tool. We selected seven genes (ARHGAP9, epidermal growth factor receptor (EGFR), mitogen-activated protein kinase 1 (MAPK1, also known as ERK2), mitogen-activated protein kinase 14 (MAPK14, also known as p38α), mitogen-activated protein kinase kinase 3 (MKK3), mitogen-activated protein kinase kinase 6 (MKK6), and glioma-associated oncogene homolog 1 (Gli1)) showing potential inter-correlations (Supplementary Fig. S1). Non-parametric Spearman's correlation coefficients (based on microarray data) identified interactions among ARHGAP9, EGFR, MAPK1 (ERK2), MAPK14 (p38α), MKK3, MKK6, and Gli1. Table VI shows that expression of ARHGAP9 correlated positively with that of Gli1, which regulates the Hedgehog signaling pathway. In addition, ARHGAP9 interacted with MKK6 and MAPK1 (ERK2), both of which are essential components of the MAPK signal transduction pathway (P<0.05 for both).
Table VI.
Spearman correlation coefficients of Gli1, ARHGAP9, EGFR, MKK3, MKK6, MAPK1 (ERK2) and MAPK14 (p38α) in BC.
| Gli1 | ARHGAP9 | EGFR | MKK3 | MKK6 | MAPK1 (ERK2) | MAPK14 (p38α) | |
|---|---|---|---|---|---|---|---|
| Gli1 | |||||||
| Spearman's Rho | 1.000 | 0.518b | −0.009 | 0.099 | −0.042 | 0.178a | −0.202b |
| P-value | . | 0.000 | 0.911 | 0.205 | 0.589 | 0.022 | 0.009 |
| ARHGAP9 | |||||||
| Spearman's Rho | 0.518b | 1.000 | 0.084 | 0.125 | −0.168a | 0.233b | −0.138 |
| P-value | 0.000 | . | 0.283 | 0.109 | 0.031 | 0.003 | 0.076 |
| EGFR | |||||||
| Spearman's Rho | −0.009 | 0.084 | 1.000 | 0.194a | −0.118 | 0.301b | 0.192b |
| P-value | 0.911 | 0.283 | . | 0.012 | 0.130 | 0.000 | 0.013 |
| MKK3 | |||||||
| Spearman's Rho | 0.099 | 0.125 | 0.194a | 1.000 | 0.101 | 0.327b | 0.315b |
| P-value | 0.205 | 0.109 | 0.012 | . | 0.195 | 0.000 | 0.000 |
| MKK6 | |||||||
| Spearman's Rho | −0.042 | −0.168a | −0.118 | 0.101 | 1.000 | −0.093 | −0.056 |
| P-value | 0.589 | 0.031 | 0.130 | 0.195 | . | 0.233 | 0.472 |
| MAPK1(ERK2) | |||||||
| Spearman's Rho | 0.178a | 0.233b | 0.301b | 0.327b | −0.093 | 1.000 | 0.167a |
| P-value | 0.022 | 0.003 | 0.000 | 0.000 | 0.233 | . | 0.032 |
| MAPK14 (p38α) | |||||||
| Spearman's Rho | −0.202b | −0.138 | 0.192a | 0.315b | −0.056 | 0.167a | 1.000 |
| P-value | 0.009 | 0.076 | 0.013 | 0.000 | 0.472 | 0.032 | . |
P<0.05.
P<0.01. BC, bladder cancer.
Discussion
ARHGAP9 sits adjacent to Gli1 on human chromosome 12q13.3; two genes have overlapping 16 bases in their 3′-ends (13), suggesting that Gli1 and ARHGAP9 may regulate each other. Studies suggest that Gli1 is down-regulated in BC (25); indeed, Gli1 is considered to be the most reliable biomarker of Hedgehog pathway activity (25–27). The microarray data presented herein shows that mRNA expression of Gli1 and ARHGAP9 were down-regulated in BC tissues, and that there was a positive correlation between the two (Table VI); this indicates that ARHGAP9, which lies adjacent to Gli1, might be a novel regulator of Gli1.
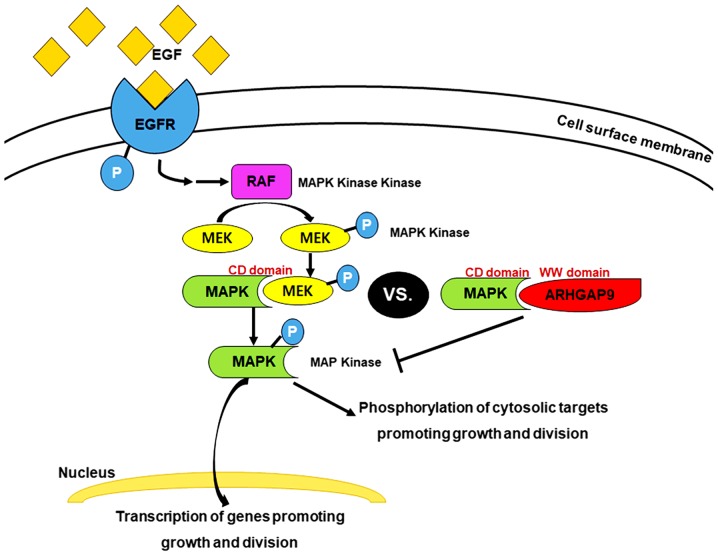
As a novel MAP kinase docking protein, ARHGAP9 associates specifically with ERK2 and p38α via complementarily charged residues within the WW domain of ARHGAP9 and the CD domains of ERK2 and p38α. This interaction suppresses MAP kinase activation; but does not affect that of RhoGAP (9). MAPK activation is a common event in tumor progression and metastasis. Inhibition of ERK1/2 and p38 MAP kinase pathways in BC could inhibit proliferation and growth (28). The key target in this signal transduction pathway is EGFR, a receptor tyrosine kinase (29). Binding of EGF to EGFR in BC activates EGFR, which is already overexpressed; furthermore, the Ras-MAPK pathway is activated through the MAPK/ERK pathway. This continuous ‘ON’ status of MAPK signaling results in overexpression of MEK2 and MKK3, 4, and 6, which lie upstream of MAP kinase (i.e., ERK2 and p38α) and activate ERK2 and p38α, leading to reduced interaction between ARHGAP9 and ERK2 or p38α in BC (this is probably attributable to competitive displacement by overexpressed docking proteins) (Fig. 4). The microarray data revealed a competitive correlation between expression of ARHGAP9 mRNA and that of MKK6, and a positive correlation between ARHGAP9 and ERK2 (Table VI). These findings suggest that ARHGAP9 acts as a tumor suppressor gene in BC. EGFR acts as a receptor molecule in the MAPK signaling pathway, and is a prognostic marker for many cancer types, including BC (30). Our previous study showed that EGFR is a progression-related gene in MIBC; increased expression of EGFR is associated with a poor prognosis (31). Here, we found that lower expression of ARHGAP9 was related to poor PFS and CSS (Fig. 3A and B), which is consistent with previous results. However, no definitive evidence has been demonstrated on the recurrence rate of MIBC after radical cystectomy, and the definition of local and distant recurrence is not standardized (32). In our preliminary study, twenty-six MIBC patients received radical cystectomy and only three of them were manifested recurrence, such result should be examined in further study with more samples for the statistically significant validation of the survival analysis.
Figure 4.
ARHGAP9-mediated regulation of the MAPK signaling pathway in BC. ARHGAP9 associates specifically with ERK2 and p38α via complementarily charged residues within the WW domain of ARHGAP9 and the CD domains of ERK2 and p38α. The binding of EGF to EGFR activates EGFR, which is already overexpressed in BC. Furthermore, the Ras-MAPK pathway is activated through the MAPK/ERK pathway. The increased expression of various upstream kinases (including MEK2 and MKK3, 4 and 6, which interact with ERK2 and p38α, respectively) reduces interaction between ARHGAP9 and ERK2 and p38α in BC. ARHGAP9, Rho GTPase-activating protein 9; BC, bladder cancer.
Furthermore, the ARHGAP9 mRNA expression could predict the recurrence of NMIBC, that is, lower expression of ARHGAP9 was related to poor RFS (Fig. 2A). In particular, T1HG BC patients with higher expression of ARHGAP9 experienced less recurrence and progression (Fig. 2B and C). A more careful monitoring and optimal treatment recommendation should be implemented for T1HG BCs because of their highly recurrent nature and risk of progression to MIBC (33), which highlights the strategy for predicting prognosis. This study indicates that ARHGAP9 gene has a good performance in predicting prognosis of T1HG BC patients.
In addition, TCGA data from the Human Pathology Atlas (https://www.proteinatlas.org/ENSG00000123329-ARHGAP9/pathology/tissue/urothelial+cancer) show that BC patients with higher expression of ARHGAP9 mRNA tend to survive longer, though it is not statistically significant (P=0.069). On the basis of the results of this study, we can conclude that ARHGAP9 regulates growth and proliferation of BC by regulating the MAPK signaling pathway. Future studies should use real-time PCR assays to validate the results of microarray tests to confirm reliability of the data. For a better understanding of ARHGAP9, its protein levels in BC should be evaluated and the experimental samples should be increased to reduce the statistical limitations in the future. Moreover, the function of miR-3620, which interacted with ARHGAP9 mRNA, could be clarified by validating the function of ARHGAP9 in the future.
In conclusion, our findings provide a novel tumor suppressor gene in BC, which could be served as an independent prognostic marker for stratification of NMIBC and MIBC patients into favorable and poor prognosis. Moreover, a new paradigm in BC tumorigenesis and pathogenesis is estimated, since this novel gene seems to involve in the crucial tumorigenesis signaling pathways.
Supplementary Material
Acknowledgements
The biospecimens used in the present study were provided by the Chungbuk National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare, and Family Affairs. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols. The authors would like to thank Ms. Eun-Ju Shim from the National Biobank of Korea at Chungbuk National University Hospital for preparing samples and her excellent technical assistance.
Glossary
Abbreviations
- ARHGAP9
Rho GTPase-activating protein 9
- BC
Bladder cancer
- CI
confidence interval
- CIS
carcinoma in situ
- CSS
cancer-specific survival
- EGFR
epidermal growth factor receptor
- Gli1
glioma-associated oncogene homolog 1
- HR
hazard ratio
- MAPK1
mitogen-activated protein kinase 1 (also known as ERK2)
- MAPK14
mitogen-activated protein kinase 14 (also known as p38α)
- MIBC
muscle invasive BC
- MKK3
mitogen-activated protein kinase kinase 3
- MKK6
mitogen-activated protein kinase kinase 6
- NGS
next generation sequencing
- NMIBC
non-muscle invasive BC
- PFS
progression-free survival
- Real-time PCR
real-time polymerase chain reaction
- RFS
recurrence-free survival
- T1HG
T1 high grade
Funding
The present study was supported by the International Science and Business Belt Program of the Ministry of Science, ICT and Future Planning (grant no. 2015-DD-RD-0070); the National Research Foundation of Korea funded by the Korean government (grant no. 2018R1A2B2005473); and the Basic Science Research Program of the National Research Foundation of Korea, funded by the Ministry of Education (grant no. 2017R1D1A1B03033629).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XMP, PJ, SJY and WJK designed the study and all experiments. XMP performed the experiments. YHK, YJB, YX, SPS and SKM collected patient samples. XMP, CY, HWK and WTK assisted with data collection. XMP, JYL, IYK, YHC, EJC and SJY analyzed the data. WJK provided funding. XMP, SJY and WJK wrote the manuscript.
Ethics approval and consent to participate
The collection and analysis of all samples were approved by the Institutional Review Board at Chungbuk National University (approval no. GR2010-12-010). The study methodologies conformed with the standards set by the Declaration of Helsinki. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lotan Y, Black PC, Caba L, Chang SS, Cookson MS, Daneshmand S, Kamat AM, McKiernan JM, Pruthi RS, Ritch CR, et al. Optimal trial design for studying urinary markers in bladder cancer: A collaborative review. Eur Urol Oncol. 2018;1:223–230. doi: 10.1016/j.euo.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Soukup V, Čapoun O, Cohen D, Hernandez V, Babjuk M, Burger M, Compérat E, Gontero P, Lam T, MacLennan S, et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 World Health Organization grading classification systems in non-muscle-invasive bladder cancer: A European Association of Urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur Urol Suppl. 2017;72:801–813. doi: 10.1016/j.eururo.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Westhoff E, Witjes JA, Fleshner NE, Lerner SP, Shariat SF, Steineck G, Kampman E, Kiemeney LA, Vrieling A. Body mass index, diet-related factors, and bladder cancer prognosis: A systematic review and meta-analysis. Bladder Cancer. 2018;4:91–112. doi: 10.3233/BLC-170147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Kong D, Land S, Dyson G, Sakr WA, Sarkar FH. Comprehensive molecular oncogenomic profiling and miRNA analysis of prostate cancer. Am J Transl Res. 2013;5:200–211. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JY, Yun SJ, Jeong P, Piao XM, Kim YH, Kim J, Subramaniyam S, Byun YJ, Kang HW, Seo SP, et al. Identification of differentially expressed miRNAs and miRNA-targeted genes in bladder cancer. Oncotarget. 2018;9:27656–27666. doi: 10.18632/oncotarget.24441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa Y, Kawasoe T, Daigo Y, Nishiwaki T, Ishiguro H, Takahashi M, Kitayama J, Nakamura Y. Isolation of a novel human gene, ARHGAP9, encoding a rho-GTPase activating protein. Biochem Bioph Res Commun. 2001;284:643–649. doi: 10.1006/bbrc.2001.5022. [DOI] [PubMed] [Google Scholar]
- 7.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST0330891. [DOI] [PubMed] [Google Scholar]
- 8.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 9.Ang BK, Lim CY, Koh SS, Sivakumar N, Taib S, Lim KB, Ahmed S, Rajagopal G, Ong SH. ArhGAP9, a novel MAP kinase docking protein, inhibits Erk and p38 activation through WW domain binding. J Mol Signal. 2007;2:1. doi: 10.1186/1750-2187-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe AB, Hall A. Rho GTPases in transformation and metastasis. Adv Cancer Res. 2002;84:57–80. doi: 10.1016/S0065-230X(02)84003-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Ha M. Silencing ARHGAP9 correlates with the risk of breast cancer and inhibits the proliferation, migration, and invasion of breast cancer. J Cell Biochem. 2018;119:7747–7756. doi: 10.1002/jcb.27127. [DOI] [PubMed] [Google Scholar]
- 13.Katoh Y, Katoh M. Integrative genomic analyses on GLI1: Positive regulation of GLI1 by Hedgehog-GLI, TGFβ-Smads, and RTK-PI3K-AKT signals, and negative regulation of GLI1 by Notch-CSL-HES/HEY, and GPCR-Gs-PKA signals. Int J Oncol. 2009;35:187–192. doi: 10.3892/ijo_00000328. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Ksheersagar P, Sharma P. Diagnosis and treatment of bladder cancer. Am Fam Physician. 2009;80:717–723. [PubMed] [Google Scholar]
- 15.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS, Jr, Schellhammer PF. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urology. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2016. Eur Urol. 2017;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A, European Association of Urology EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Kim WT, Kim J, Yan C, Jeong P, Choi SY, Lee OJ, Chae YB, Yun SJ, Lee SC, Kim WJ. S100A9 and EGFR gene signatures predict disease progression in muscle invasive bladder cancer patients after chemotherapy. Ann Oncol. 2014;25:974–979. doi: 10.1093/annonc/mdu037. [DOI] [PubMed] [Google Scholar]
- 19.Bolstad BM, Irizarry RA, Åstrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 20.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 21.Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS, Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010;9:3. doi: 10.1186/1476-4598-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Nishiyama T, Shimizu K, Kadota K. TCC: An R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics. 2013;14:219. doi: 10.1186/1471-2105-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Pignot G, Vieillefond A, Vacher S, Zerbib M, Debre B, Lidereau R, Amsellem-Ouazana D, Bieche I. Hedgehog pathway activation in human transitional cell carcinoma of the bladder. Br J Cancer. 2012;106:1177–1186. doi: 10.1038/bjc.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 27.Kimura H, Stephen D, Joyner A, Curran T. Gli1 is important for medulloblastoma formation in Ptc1+/- mice. Oncogene. 2005;24:4026–4036. doi: 10.1038/sj.onc.1208567. [DOI] [PubMed] [Google Scholar]
- 28.Kumar B, Sinclair J, Khandrika L, Koul S, Wilson S, Koul HK. Differential effects of MAPKs signaling on the growth of invasive bladder cancer cells. Int J Oncol. 2009;34:1557–1564. doi: 10.3892/ijo_00000285. [DOI] [PubMed] [Google Scholar]
- 29.Spiess PE, Czerniak B. Dual-track pathway of bladder carcinogenesis: Practical implications. Arch Pathol Lab Med. 2006;130:844–852. doi: 10.5858/2006-130-844-DPOBCP. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–S15. doi: 10.1016/S0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 31.Kim WJ, Kim SK, Jeong P, Yun SJ, Cho IC, Kim IY, Moon SK, Um HD, Choi YH. A four-gene signature predicts disease progression in muscle invasive bladder cancer. Mol Med. 2011;17:478–485. doi: 10.2119/molmed.2010.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mari A, Campi R, Tellini R, Gandaglia G, Albisinni S, Abufaraj M, Hatzichristodoulou G, Montorsi F, van Velthoven R, Carini M, et al. Patterns and predictors of recurrence after open radical cystectomy for bladder cancer: A comprehensive review of the literature. World J Urol. 2018;36:157–170. doi: 10.1007/s00345-017-2115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun SJ, Kim SK, Kim WJ. How do we manage high-grade T1 bladder cancer? Conservative or aggressive therapy? Investig Clin Urol. 2016;57(Suppl 1):S44–S51. doi: 10.4111/icu.2016.57.S1.S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.