Abstract
Background
Variome may be used for designating complex system of interplay between genomic variations specific for an individual or a disease. Despite the recognized complexity of genomic basis for phenotypic traits and diseases, studies of genetic causes of a disease are usually dedicated to the identification of single causative genomic changes (mutations). When such an artificially simplified model is employed, genomic basis of phenotypic outcomes remains elusive in the overwhelming majority of human diseases. Moreover, it is repeatedly demonstrated that multiple genomic changes within an individual genome are likely to underlie the phenome. Probably the best example of cumulative effect of variome on the phenotype is CNV (copy number variation) burden. Accordingly, we have proposed a variome concept based on CNV studies providing the evidence for the existence of a CNVariome (the set of CNV affecting an individual genome), a target for genomic analyses useful for unraveling genetic mechanisms of diseases and phenotypic traits.
Conclusion
Variome (CNVariome) concept suggests that a genomic milieu is determined by the whole set of genomic variations (CNV) within an individual genome. The genomic milieu is likely to result from interplay between these variations. Furthermore, such kind of variome may be either individual or disease-specific. Additionally, such variome may be pathway-specific. The latter is able to affect molecular/cellular pathways of genome stability maintenance leading to occurrence of genomic/chromosome instability and/or somatic mosaicism resulting in somatic variome. This variome type seems to be important for unraveling disease mechanisms, as well. Finally, it appears that bioinformatic analysis of both individual and somatic variomes in the context of diseases- and pathway-specific variomes is the most promising way to determine genomic basis of the phenome and to unravel disease mechanisms for the management and treatment of currently incurable diseases.
Keywords: Copy number variations, Genome variations, Pathways, Somatic mosaicism, Variome
— You should always count everything, because everything counts.
Raymond M. Smullyan
(Alice in Puzzle-Land —
A Carrollian Tale for Children under Eighty)
Variome is a term, which is generally used in the context of The Human Variome Project dedicated to collecting and integrating genomic data for the identification of disease-causing genome variations [1]. On the other hand, variome may be defined as a complete (near-complete) set of genomic variations in an individual (individual variome or Vi). Alternatively, a set of genomic variations associated with specific phenotypic traits or disease/condition may be described as a trait-specific or disease-specific variome (Vds). Actually, almost all relevant studies of disease-causing genetic changes in the whole genomic context might be designated as “variome analysis”, inasmuch as these studies are targeted at uncovering the whole set of genomic variations associated with a specific phenotype. For instance, genome-wide association studies (GWAS) is a picturesque, albeit not always successful, example of establishing Vds or the variome specific for a phenotypic trait/condition at the sequence level. Roughly, one can subdivide a general variome (Vi or Vds) into two large distinct “variomic subtypes”: sequence variome (all the genomic variations detectable at the sequence level) and variome encompassing all the copy number variations (CNVs) or “CNVariome” adding balanced/imbalanced chromosome rearrangements and chromosomal heteromorphisms. Here, we focus on CNVariome to present the variome concept, which is able to become a useful system for high-throughput analysis of the functional consequences of individual and disease-specific genomic variability.
CNVs have long been observed to contribute to genetic diversity in health and disease [2–5]. However, despite significant advances in CNV biology, difficulties in describing phenotypic outcome of both rare and common CNVs require an extensive bioinformatic analysis for identifying the pathogenic value (if any) of a genomic change from an individual CNVariome [6, 7]. An average individual genome exhibits myriads of sequence variants and hundreds of CNVs; bioinformatic analysis seems to be indispensable for evaluating each variant for defining the contribution to the phenotype [8, 9]. To succeed in gene/CNV prioritization or systems biology (OMICS/pathway-based) analysis, a big data analysis of genomic variations performed at genomic, epigenetic, proteomic and metabolome levels is applied [7, 10–12]. Moreover, recent studies have shown that CNVs are able to interact between each other within Vi in a complex and multilateral fashion [13]. Therefore, systems biology analyses of genomic variations suggest that causative variants are not isolated. Indeed, since genome variations are able to either increase or decrease the effect of each other, Vi may be considered as a kind of “genomic milieu” for disease-causing (trait-associated) variations.
The assumption that CNVariome is a genomic milieu modulating functional effects of CNVs may be further supported by the observation on the so-called “CNV burden” (a fraction of CNVs in an individual genome with appreciable functional consequences) [14–16], which perfectly fits the variome concept or, more exactly, the CNVariome concept. Furthermore, experimental testing of two- and multiple-hit hypothesis has formed a firm basis for the CNVariome concept. According to the hypothesis, CNVs are able to increase or decrease gene dosage creating a genomic background or milieu (the first hit). This milieu is highly sensible to subsequent “second hit” or “multiple hits” (i.e. sporadic mutations, somatic mosaicism or chromosome/genome instability), which produces specific phenotypes [17–20]. Therefore, to define the genomic milieu created by CNVariome, it is to address all detectable CNVs in an individual genome. These data would be important for the definition of Vds, as well.
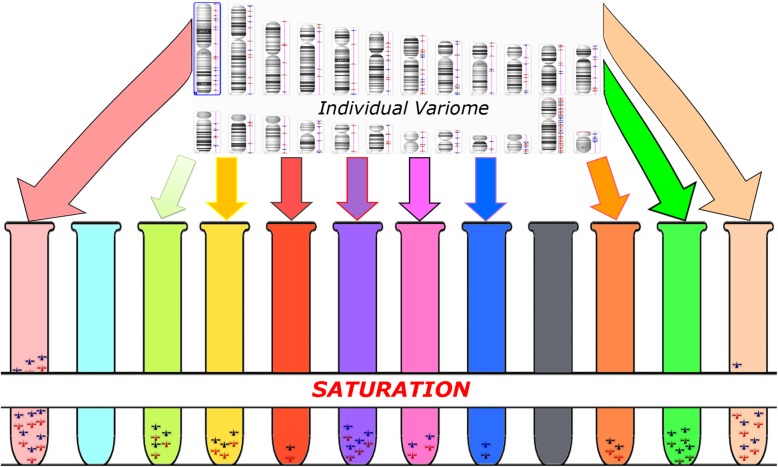
The phenotypic effect of genomic variations is achieved through the impact on specific molecular and cellular pathways. Although the spectrum of functional consequences of genomic variations seems to be extremely broad, it is generally acknowledged that an effect (even if it is low) on molecular pathways/processes does exist [12, 21–23]. Additionally, common variants are able to possess functional effects at the protein level [22]. Therefore, to address genomic variations’ impact on molecular pathways, one has to take into account the whole set of variants detected in an individual genome or, in other words, one has to analyze the variome. As to CNVs (CNVariome), our previous studies have shown that both common CNVs with a slight functional effect on a pathway (e.g. CNVs affecting genes involved in the cell cycle pathway) are able to cause chromosome instability detectable by cytogenetic analysis [20, 24, 25]. Similar results have been reported in studies of variome at the sequence level in health and disease highlighting new genomic mechanisms for interindividual diversity achieved through the functional variation in molecular pathways [26, 27]. More importantly, it is suggested that such genomic variations contribute to intercellular functional diversity, as well [28–30]. Genome variability/instability in cancer is probably the best example of how multiple chromosome abnormalities, CNVs and/or gene mutations may lead to dramatic functional changes of molecular and cellular pathways [31–33]. Actually, single-cell systems biology analyses have shown that such pathway changes mediated by genomic variations do occur [34, 35]. Therefore, it is useful to introduce another “variomic subtype”, i.e. pathway-specific variome(s) (Vps) covering the set of genomic variations affecting specific pathway. Thus, Vi or Vds appears to be composed of a number of Vps. Taking into account how CNVs functionally affect molecular and cellular pathways mediating the phenotypic outcome [12–14, 16–19, 35–37], one can suggest that appreciable effects of Vps are likely to be the result of a number of variants that affects the pathway. In other words, the effect results from a kind of saturation in genomic variations or in CNVs. Figure 1 schematically depicts the variome (CNVariome) concept in the light of functional effects of genomic variations on molecular and cellular pathways.
Fig. 1.
Schematic representation of the variome (CNVariome) concept; a set of genomic variations (e.g. CNV) may contribute to functional variability of molecular/cellular pathways or create several Vps symbolically depicted as colored laboratory tubes; when the number of genomic variations achieves a critical level (i.e. saturation in genomic variations), an alteration to the pathway occurs (e.g. left-most and right-most tubes). A screenshot of CNV analysis by Chromosome Analysis Suite (ChAS) 3.10.15© 2015 Affymetrix Inc. was used to depict individual variome (CNVariome)
Molecular pathways are the intermediate link between the genome and the phenome [13, 23]. Moreover, the pathways are promising targets for molecular-oriented therapy in diseases associated with genomic pathology (i.e. chromosomal abnormalities and CNVs) [29, 38–40]. Therefore, the identification of Vps or decomposition of Vi/Vds into a set of Vps would be useful for unraveling disease mechanisms to develop effective management and treatment of currently incurable genetic diseases.
An important aspect of genomic variability is referred to somatic genome variations or somatic mosaicism. Indeed, this phenomenon reflect an important, albeit commonly overlooked, ability of genome (cellular genomes) to vary through ontogeny [37, 41]. Somatic mutations cause cancer [31–34, 42]. Somatic mosaicism has been consistently associated with interindividual/intercellular genomic heterogeneity in health and disease [43–45]. More precisely, neurodevelopmental diseases have been repeatedly associated with chromosomal mosaicism and mosaic CNVs/gene mutations [37, 46–49]. Chromosomal mosaicism and instability has been demonstrated to mediate neurodegeneration [48–57]. Further studies have indicated that chromosome instability is an important element of a pathogenic cascade of neurodegenerative diseases [52, 54–57]. Therefore, to address genomic (genetic) mechanisms for human diseases in their complexity, it is unavoidable to consider somatic genome variations. In the light of the variome concept, we introduce the somatic variome (Vs), which may be defined as a set of somatic genome variations (somatic mosaicism + genomic and chromosomal instability) detected in an individual. Somatic mosaicism and genomic/chromosomal instability are likely to occur due to altered molecular/cellular pathways controlling genome stability maintenance, cell cycle and programmed cell death [18, 24, 31, 42, 45, 57]. We hypothesize that a set of Vps of these pathways is able to produce a kind of susceptibility of cellular genomes to genomic/chromosomal instability and/or somatic mosaicism. Therefore, systems biology analyses of Vi and molecular cytogenetic survey of chromosome/genome instability may identify the link between non-mosaic (germline inherited/sporadic) and somatic genome variations. Figure 2 shows the interplay between Vi and Vs according to the concept. The result of addressing the interplay between Vi and Vs might be a “constitutional variome”, which might be basic genomic background of all the somatic cells evolved/developed from single zygote encompassing acquired somatic mutations in different tissues produced by genetic-environmental interactions and ontogenetic genome variations. The “constitutional variome” might represent a complex system of interactions between the whole set of individual and intercellular genome variations uniquely describing the intrinsic genomic milieu of an individual. The genomic milieu should reflect the whole burden of genomic variations including individual combinations of inherited variants, which are able to modulate the phenotype (i.e. reduced penetrance or increased severity of disease manifestations and phenotypic traits) or, in other words, to form an “inheritance pattern” applicable not only to monogenic diseases, but also to multifactorial disorders in a threshold manner as shown in Fig. 1.
Fig. 2.
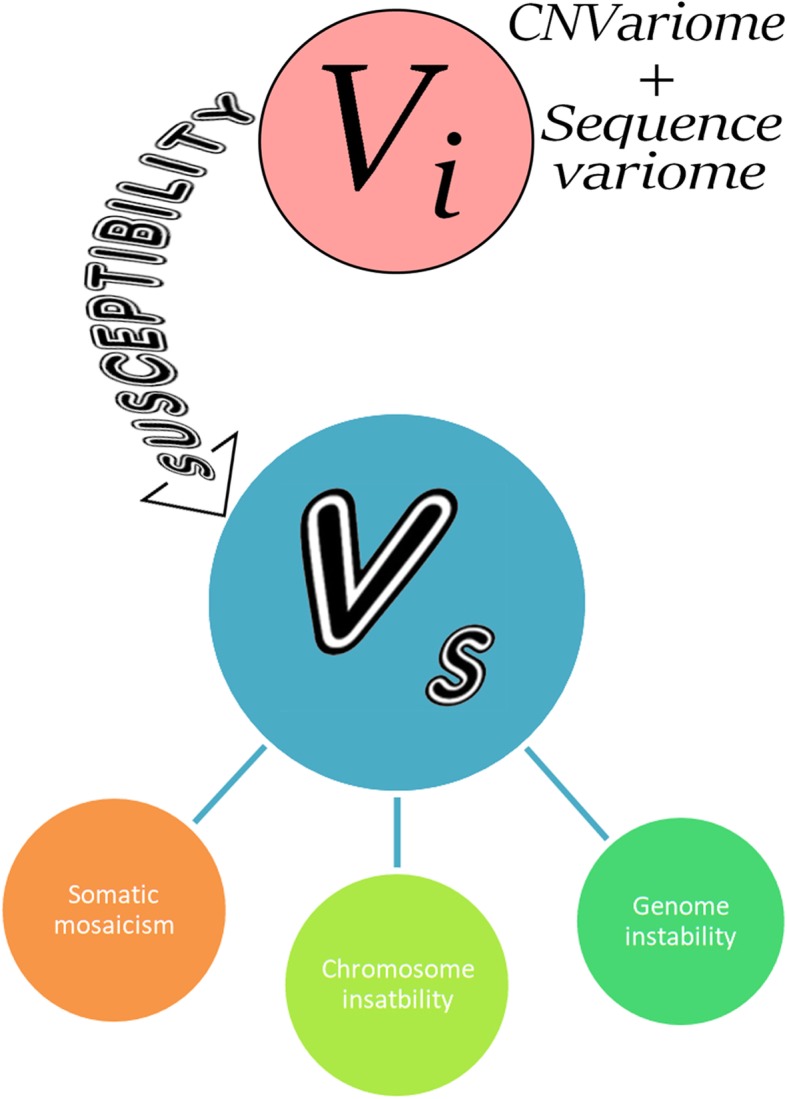
Interplay between Vi and Vs; CNVariome and sequence variome forming Vi may alter pathways critical for maintaining genome stability, cell cycle, and/or programmed cell death; the result of such alterations is likely to be a susceptibility for specific Vs, which encompass somatic mosacism as well as chromosome and genome instability
The variome concept proposes that functional variability of molecular/cellular pathways determining the phenome is the result of the effect of all the variants of an individual genome (variome). The effects can be cumulative, interchangeable, mutually exclusive or neutral. However, one should bear in mind that the whole Vi/Vds should be addressed to determine the intrinsic effect. In the genomic context, Vi and Vds are composed of two variome subtypes: sequence variome and CNVariome. In the functional genomic context, Vi /Vds may be decomposed into a set of Vps. In the (molecular) cytogenetic context, the CNVariome concept suggests that the individual uniqueness at molecular and cellular levels may result from an individual set of CNVs (in addition to sequence variome). If Vi/Vds is enriched in Vps of genome stability maintenance, cell cycle, and programmed cell death, a susceptibility to genomic/chromosomal instability and/or somatic mosaicism may occur. Accordingly, Vs should be addressed in addition to Vi/Vds for comprehensive evaluation of the whole spectrum of genomic changes contributing to the phenome. Since the famous quote “Ontogeny recapitulates phylogeny” from Ernst Haeckel’s classic works remains more-or-less actual [58–60], variome concept encompassing Vi/Vds, Vps and Vs seems to applicable in the evolutionary context, as well, allowing us to finalize description of the concept by another famous quotation “Nothing in biology makes sense except in the light of evolution” from Theodosius Dobzhansky [61]. To this end, we believe that the concept is able to provide a valuable system for determining the genomic basis of the phenome and for unravel disease mechanisms to succeed in the management and treatment of currently incurable genetic diseases.
Acknowledgments
Not applicable.
Abbreviations
- CNVs
Copy number variations
- GWAS
Genome-wide association studies
- Vds
Disease-specific variome
- Vi
Individual variome
- Vps
Pathway-specific variome
- Vs
Somatic variome
Authors’ contributions
IYI conceived the core idea of the concept and wrote the manuscript. SGV and YBY made significant theoretic contribution. All authors have read and approved the final manuscript.
Funding
Professors SG Vorsanova and IY Iourov are partially supported by RFBR and CITMA according to the research project No. 18–515-34005. Prof. IY Iourov is supported by the Government Assignment of the Russian Ministry of Science and Higher Education, Assignment no. AAAA-A19–119040490101-6. Prof. SG Vorsanova is supported by the Government Assignment of the Russian Ministry of Health, Assignment no. AAAA-A18–118051590122-7.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ivan Y. Iourov, Email: ivan.iourov@gmail.com
Svetlana G. Vorsanova, Email: svorsanova@mail.ru
Yuri B. Yurov, Email: y_yurov@yahoo.com
References
- 1.Burn J, Watson M. The human Variome project. Hum Mutat. 2016;37(6):505–507. doi: 10.1002/humu.22986. [DOI] [PubMed] [Google Scholar]
- 2.Lee C, Iafrate AJ, Brothman AR. Copy number variations and clinical cytogenetic diagnosis of constitutional disorders. Nat Genet. 2007;39(7 Suppl):S48–S54. doi: 10.1038/ng2092. [DOI] [PubMed] [Google Scholar]
- 3.Iourov IY, Vorsanova SG, Yurov YB. Molecular cytogenetics and cytogenomics of brain diseases. Curr Genomics. 2008;9(7):452–465. doi: 10.2174/138920208786241216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochstenbach R, Buizer-Voskamp JE, Vorstman JA, Ophoff RA. Genome arrays for the detection of copy number variations in idiopathic mental retardation, idiopathic generalized epilepsy and neuropsychiatric disorders: lessons for diagnostic workflow and research. Cytogenet Genome Res. 2011;135(3–4):174–202. doi: 10.1159/000332928. [DOI] [PubMed] [Google Scholar]
- 5.Zarrei M, MacDonald JR, Merico D, Scherer SW. A copy number variation map of the human genome. Nat Rev Genet. 2015;16(3):172–183. doi: 10.1038/nrg3871. [DOI] [PubMed] [Google Scholar]
- 6.MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, Adams DR, Altman RB, Antonarakis SE, Ashley EA, Barrett JC, Biesecker LG, Conrad DF, Cooper GM, Cox NJ, Daly MJ, Gerstein MB, Goldstein DB, Hirschhorn JN, Leal SM, Pennacchio LA, Stamatoyannopoulos JA, Sunyaev SR, Valle D, Voight BF, Winckler W, Gunter C. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508(7497):469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iourov IY, Vorsanova SG, Yurov YB. In silico molecular cytogenetics: a bioinformatic approach to prioritization of candidate genes and copy number variations for basic and clinical genome research. Mol Cytogenet. 2014;7(1):98. doi: 10.1186/s13039-014-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MJ, Sham PC, Wang J. Genetic variant representation, annotation and prioritization in the post-GWAS era. Cell Res. 2012;22(10):1505–1508. doi: 10.1038/cr.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suwinski P, Ong C, Ling MHT, Poh YM, Khan AM, Ong HS. Advancing personalized medicine through the application of whole exome sequencing and big data analytics. Front Genet. 2019;10:49. doi: 10.3389/fgene.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linghu B, Snitkin ES, Hu Z, Xia Y, Delisi C. Genome-wide prioritization of disease genes and identification of disease-disease associations from an integrated human functional linkage network. Genome Biol. 2009;10(9):R91. doi: 10.1186/gb-2009-10-9-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu C, Kim GB, Kim WJ, Kim HU, Lee SY. Current status and applications of genome-scale metabolic models. Genome Biol. 2019;20(1):121. doi: 10.1186/s13059-019-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iourov IY, Vorsanova SG, Yurov YB. Pathway-based classification of genetic diseases. Mol Cytogenet. 2019;12:4. doi: 10.1186/s13039-019-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen M, Girirajan S. An interaction-based model for neuropsychiatric features of copy-number variants. PLoS Genet. 2019;15(1):e1007879. doi: 10.1371/journal.pgen.1007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girirajan S, Brkanac Z, Coe BP, Baker C, Vives L, Vu TH, Shafer N, Bernier R, Ferrero GB, Silengo M, Warren ST, Moreno CS, Fichera M, Romano C, Raskind WH, Eichler EE. Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet. 2011;7(11):e1002334. doi: 10.1371/journal.pgen.1002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desachy G, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, Yoshida CK, Weiss LA. Increased female autosomal burden of rare copy number variants in human populations and in autism families. Mol Psychiatry. 2015;20(2):170–175. doi: 10.1038/mp.2014.179. [DOI] [PubMed] [Google Scholar]
- 16.Aguirre M, Rivas MA, Priest J. Phenome-wide burden of copy-number variation in the UK biobank. Am J Hum Genet. 2019;105(2):373–383. doi: 10.1016/j.ajhg.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girirajan S, Eichler EE. Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet. 2010;19(R2):R176–R187. doi: 10.1093/hmg/ddq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iourov IY, Vorsanova SG, Yurov YB. Somatic cell genomics of brain disorders: a new opportunity to clarify genetic-environmental interactions. Cytogenet Genome Res. 2013;139(3):181–188. doi: 10.1159/000347053. [DOI] [PubMed] [Google Scholar]
- 19.Andrews T, Honti F, Pfundt R, de Leeuw N, Hehir-Kwa J, Vulto-van Silfhout A, de Vries B, Webber C. The clustering of functionally related genes contributes to CNV-mediated disease. Genome Res. 2015;25(6):802–813. doi: 10.1101/gr.184325.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vorsanova SG, Yurov YB, Iourov IY. Neurogenomic pathway of autism spectrum disorders: linking germline and somatic mutations to genetic-environmental interactions. Curr Bioinformatics. 2017;12(1):19–26.
- 21.Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature. 2009;461(7261):218–223. doi: 10.1038/nature08454. [DOI] [PubMed] [Google Scholar]
- 22.Mahlich Y, Reeb J, Hecht M, Schelling M, De Beer TAP, Bromberg Y, Rost B. Common sequence variants affect molecular function more than rare variants? Sci Rep. 2017;7(1):1608. doi: 10.1038/s41598-017-01054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, McGrail DJ, Latysheva N, Yi S, Babu MM, Sahni N. Pathway perturbations in signaling networks: linking genotype to phenotype. Semin Cell Dev Biol. 2018. 10.1016/j.semcdb.2018.05.001. [DOI] [PMC free article] [PubMed]
- 24.Iourov IY, Vorsanova SG, Zelenova MA, Korostelev SA, Yurov YB. Genomic copy number variation affecting genes involved in the cell cycle pathway: implications for somatic mosaicism. Int J Genomics. 2015;2015:757680. doi: 10.1155/2015/757680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yurov YB, Iourov IY, Vorsanova SG. Network-based classification of molecular cytogenetic data. Curr Bioinformatics. 2017;12(1):27–33.
- 26.Bromberg Y, Kahn PC, Rost B. Neutral and weakly nonneutral sequence variants may define individuality. Proc Natl Acad Sci U S A. 2013;110(35):14255–14260. doi: 10.1073/pnas.1216613110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Miller M, Astrakhan Y, Petersen BS, Schreiber S, Franke A, Bromberg Y. Identifying Crohn’s disease signal from variome analysis. Genome Med. 2019;11(1):59. doi: 10.1186/s13073-019-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iourov IY, Vorsanova SG, Yurov YB. Single cell genomics of the brain: focus on neuronal diversity and neuropsychiatric diseases. Curr Genomics. 2012;13(6):477–488. doi: 10.2174/138920212802510439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heng HH, Horne SD, Chaudhry S, Regan SM, Liu G, Abdallah BY, Ye CJ. A postgenomic perspective on molecular cytogenetics. Curr Genomics. 2018;19(3):227–239. doi: 10.2174/1389202918666170717145716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawe JS, Theis FJ, Heinig M. Inferring interaction networks from multi-omics data. Front Genet. 2019;10:535. doi: 10.3389/fgene.2019.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heng HH. Debating cancer: the paradox in cancer research. New Jersey: World Scientific Publishing Company; 2015. [Google Scholar]
- 32.Loeb LA. Human cancers express mutator phenotypes: origin, consequences and targeting. Nat Rev Cancer. 2011;11(6):450–457. doi: 10.1038/nrc3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox EJ, Prindle MJ, Loeb LA. Do mutator mutations fuel tumorigenesis? Cancer Metastasis Rev. 2013;32(3–4):353–361. doi: 10.1007/s10555-013-9426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frost HR, Amos CI. A multi-omics approach for identifying important pathways and genes in human cancer. BMC Bioinformatics. 2018;19(1):479. doi: 10.1186/s12859-018-2476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patange S, Girvan M, Larson DR. Single-cell systems biology: probing the basic unit of information flow. Curr Opin Syst Biol. 2018;8:7–15. doi: 10.1016/j.coisb.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girirajan S, Rosenfeld JA, Coe BP, Parikh S, Friedman N, Goldstein A, Filipink RA, JS MC, Angle B, Meschino WS, Nezarati MM, Asamoah A, Jackson KE, Gowans GC, Martin JA, Carmany EP, Stockton DW, Schnur RE, Penney LS, Martin DM, Raskin S, Leppig K, Thiese H, Smith R, Aberg E, Niyazov DM, Escobar LF, El-Khechen D, Johnson KD, Lebel RR, Siefkas K, Ball S, Shur N, McGuire M, Brasington CK, Spence JE, Martin LS, Clericuzio C, Ballif BC, Shaffer LG, Eichler EE. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med. 2012;367(14):1321–1331. doi: 10.1056/NEJMoa1200395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iourov IY, Vorsanova SG, Yurov YB. Somatic genome variations in health and disease. Curr Genomics. 2010;11(6):387–396. doi: 10.2174/138920210793176065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heng HH, Regan S. A systems biology perspective on molecular cytogenetics. Curr Bioinforma. 2017;12(1):4–10. doi: 10.2174/1574893611666160606163419. [DOI] [Google Scholar]
- 39.Iourov IY, Vorsanova SG, Voinova VY, Yurov YB. 3p22.1p21.31 microdeletion identifies CCK as Asperger syndrome candidate gene and shows the way for therapeutic strategies in chromosome imbalances. Mol Cytogenet. 2015;8:82. doi: 10.1186/s13039-015-0185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iourov IY. Cytopostgenomics: what is it and how does it work? Curr Genomics. 2019;20(2):77–78. doi: 10.2174/138920292002190422120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yurov YB, Vorsanova SG, Iourov IY. Ontogenetic variation of the human genome. Curr Genomics. 2010;11(6):420–425. doi: 10.2174/138920210793175958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye CJ, Stilgenbauer L, Moy A, Liu G, Heng HH. What is karyotype coding and why is genomic topology important for cancer and evolution? Front Genet. 2019;10:1082. doi: 10.3389/fgene.2019.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iourov IY, Vorsanova SG, Yurov YB. Chromosomal variation in mammalian neuronal cells: known facts and attractive hypotheses. Int Rev Cytol. 2006;249:143–191. doi: 10.1016/S0074-7696(06)49003-3. [DOI] [PubMed] [Google Scholar]
- 44.Rohrback S, Siddoway B, Liu CS, Chun J. Genomic mosaicism in the developing and adult brain. Dev Neurobiol. 2018;78(11):1026–1048. doi: 10.1002/dneu.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iourov Ivan Y., Vorsanova Svetlana G., Yurov Yuri B., Kutsev Sergei I. Ontogenetic and Pathogenetic Views on Somatic Chromosomal Mosaicism. Genes. 2019;10(5):379. doi: 10.3390/genes10050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yurov YB, Vorsanova SG, Iourov IY, Demidova IA, Beresheva AK, Kravetz VS, Monakhov VV, Kolotii AD, Voinova-Ulas VY, Gorbachevskaya NL. Unexplained autism is frequently associated with low-level mosaic aneuploidy. J Med Genet. 2007;44(8):521–525. doi: 10.1136/jmg.2007.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D'Gama AM, Walsh CA. Somatic mosaicism and neurodevelopmental disease. Nat Neurosci. 2018;21(11):1504–1514. doi: 10.1038/s41593-018-0257-3. [DOI] [PubMed] [Google Scholar]
- 48.Iourov IY, Liehr T, Vorsanova SG, Mendez-Rosado LA, Yurov YB. The applicability of interphase chromosome-specific multicolor banding (ICS-MCB) for studying neurodevelopmental and neurodegenerative disorders. Res Results Biomedicine. 2019;5(3):4–9. doi: 10.18413/2658-6533-2019-5-3-0-1. [DOI] [Google Scholar]
- 49.Potter H, Chial HJ, Caneus J, Elos M, Elder N, Borysov S, Granic A. Chromosome instability and mosaic aneuploidy in neurodegenerative and neurodevelopmental disorders. Front Genet. 2019;10:1092. doi: 10.3389/fgene.2019.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Yurov YB. Increased chromosome instability dramatically disrupts neural genome integrity and mediates cerebellar degeneration in the ataxia-telangiectasia brain. Hum Mol Genet. 2009;18(14):2656–2669. doi: 10.1093/hmg/ddp207. [DOI] [PubMed] [Google Scholar]
- 51.Iourov IY, Vorsanova SG, Liehr T, Yurov YB. Aneuploidy in the normal, Alzheimer’s disease and ataxia-telangiectasia brain: differential expression and pathological meaning. Neurobiol Dis. 2009;34(2):212–220. doi: 10.1016/j.nbd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Arendt T, Brückner MK, Mosch B, Lösche A. Selective cell death of hyperploid neurons in Alzheimer's disease. Am J Pathol. 2010;177(1):15–20. doi: 10.2353/ajpath.2010.090955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iourov IY, Vorsanova SG, Yurov YB. Genomic landscape of the Alzheimer’s disease brain: chromosome instability — aneuploidy, but not tetraploidy — mediates neurodegeneration. Neurodegener Dis. 2011;8(1–2):35–37. doi: 10.1159/000315398. [DOI] [PubMed] [Google Scholar]
- 54.Yurov YB, Vorsanova SG, Iourov IY. The DNA replication stress hypothesis of Alzheimer's disease. Sci World J. 2011;11:2602–2612. doi: 10.1100/2011/625690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Granic A, Potter H. Mitotic spindle defects and chromosome mis-segregation induced by LDL/cholesterol-implications for Niemann-pick C1, Alzheimer’s disease, and atherosclerosis. PLoS One. 2013;8(4):e60718. doi: 10.1371/journal.pone.0060718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajic V, Spremo-Potparevic B, Zivkovic L, Isenovic ER, Arendt T. Cohesion and the aneuploid phenotype in Alzheimer's disease: a tale of genome instability. Neurosci Biobehav Rev. 2015;55:365–374. doi: 10.1016/j.neubiorev.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Yurov YB, Vorsanova SG, Iourov IY. Chromosome instability in the neurodegenerating brain. Front Genet. 2019;10:892. doi: 10.3389/fgene.2019.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haeckel E. Generelle Morphologie der Organismen, 2 Bde. Berlin: Georg Reimer; 1866. [Google Scholar]
- 59.Ohno S. Why ontogeny recapitulates phylogeny. Electrophoresis. 1995;16(9):1782–1786. doi: 10.1002/elps.11501601295. [DOI] [PubMed] [Google Scholar]
- 60.Olsson L, Levit GS, Hoßfeld U. The “biogenetic law” in zoology: from Ernst Haeckel’s formulation to current approaches. Theory Biosci. 2017;136(1–2):19–29. doi: 10.1007/s12064-017-0243-4. [DOI] [PubMed] [Google Scholar]
- 61.Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am Biol Teach. 2013;75(2):87–92. doi: 10.1525/abt.2013.75.2.reprint. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.