Abstract
Gastric cancer is one of the most common gastrointestinal tumor types, and the incidence and mortality rates are higher in men compared with women. Various studies have revealed that gastric cancer is a spectrum of tumor types, which have biological and genetic diversity. It has proven to be difficult to improve the overall survival and disease-free survival of patients with gastric cancer through the use of traditional surgery and chemoradiation, as gastric cancer is usually identified at an advanced stage. In consequence, the outcome is frequently poor. Thus, novel biomarkers and anticancer targets are required to improve the outcome. As the identification of biomarkers has increased due to advances in research and the greater availability of bioinformatics and functional genomics, the potential therapeutic regimens available have also increased concurrently. These advances have also improved the ability to predict responses to chemotherapy, targeted therapy and immunotherapy, whilst other biomarkers predict post-treatment survival and recurrence based on their expression. This review focuses closely on the important functions of biomarkers in the timely diagnosis and treatment of gastric cancer, in addition to the advances in the study of certain novel markers in gastric cancer.
Keywords: gastric cancer, biomarker, diagnosis, prognosis, targeted therapy, immunotherapy
1. Introduction
Gastric cancer is the third leading cause of cancer-associated mortality and the fifth most common malignant tumor type globally, with ~50% of all cases emerging from Eastern Asia, where China has the highest incidence rate, and where novel cases of gastric cancer and mortalities account for 42.6–45.0% of all cases globally (1,2). The majority of cases of gastric cancer are usually not diagnosed until an advanced stage, and therefore the outcome is often poor, with a 5-year survival rate of no more than 30%, including patients who have undergone surgery (3). However, the 5-year survival likelihood following early gastric cancer treatment may be >90%, and even may result in being cured (4). The rate of diagnosis and treatment of early-stage gastric cancer in China is <10%, considerably lower than Japan (70%) and South Korea (50%) (5). Therefore, it is worthwhile investigating methods for the early diagnosis and management of gastric cancer, including biomarkers associated with pathogenesis and pathological type. Understanding these biomarkers may assist in providing the ideal therapeutic option for an individuals' specific case of gastric cancer. Detecting the presence of biomarkers may have promising potential to detect cancer at earlier stages and thus improve the monitoring of the progression of a cancer. Furthermore, by obtaining information on the specific biomarker expression profile of a patient, treatment options may be better tailored for each patient, thus improving prognosis. The aim of the review was to focus on providing a comprehensive overview of the biomarkers in patients who suffer from stomach cancer, their diagnostic, prognostic and clinical value and therapeutic application for future prospects.
Gastric cancer is a type of epithelial malignant neoplasm. A number of various factors contribute to the pathogenesis of gastric cancer, including environmental factors and genetic factors (6). Environmental factors and lifestyle choices include obesity, smoking (7), bile reflux and chronic infections, particularly with Helicobacter pylori (H. pylori), which contribute to the development of stomach cancer (8). Globally, ~50% of all patients with gastric cancer present with evidence of a H. pylori infection (9), and H. pylori was considered to be the first carcinogen by the World Health Organization (WHO) and International Agency for Research on Cancer (IACR) in 1994 (10). There are hereditary factors, in addition to environmental factors, including a germline mutation in the cadherin-1 (CDH1) gene, which results in hereditary diffuse gastric cancer (11). Patients with inherited conditions, including Lynch syndrome, familial adenomatous polyps and Peutz-Jeghers syndrome result in a substantially higher risk of developing gastric carcinoma (12).
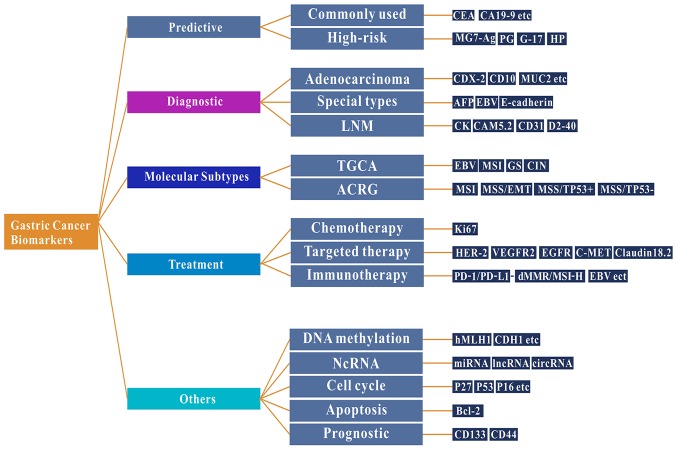
The treatment of gastric cancer is dependent on the morphology of the cancer tissue at the earliest stage. The pathological classification of gastric cancer is based on the histological structure and cell biological characteristics. Different classifications of gastric cancer types have different morphological structures, biological behaviors and underlying molecular mechanisms (8). At present, gastric cancer is primarily classified using the Borrmann, Lauren or WHO classification systems, although there are numerous pathological classification systems for gastric cancer (13,14). Advanced cancer types may be classified into four macroscopic types on the basis of the criteria proposed by Borrmann: Polypoid, fungating, ulcerated and infiltrative (13). The Lauren classification is the most widely used histological classification, for either early or advanced cancer types (14), which classifies gastric cancer as two major subtypes: Intestinal and diffuse. The diffuse variant may affect the majority of the stomach and is frequently called linitis plastica or leather bottle stomach. Intestinal-type gastric cancer occurs more frequently in elderly male patients and is thought to be associated with better survival rates (15). In 2010, WHO published an additional histological classification system for stomach cancer, which is divided into five categories: Tubular, papillary, mucinous, poorly cohesive (signet ring cell carcinoma belongs to this group) and mixed (8). Histological classification has no substantial impact on the treatment options available for patients with gastric cancer, therefore, novel biomarkers to aid in the early diagnosis and treatment of gastric cancer are required. In the present review, the following topics are discussed: i) Well-known and emerging biomarkers of gastric cancer; ii) the impact that high-throughput technologies have had on identifying biomarkers; and iii) biomarkers associated with the immunotherapy of gastric cancer and their value as predictors of prognosis (Fig. 1).
Figure 1.
Function and research findings of biomarkers in gastric cancer. Common and emerging biomarkers used in gastric cancer, including biomarkers associated with the molecular subtypes, chemotherapy, targeted therapy and immunotherapy of gastric cancer in addition to their direct potential function in improving the diagnosis and treatment options in patients with gastric cancer. CEA, carcinoembryonic antigen; CA, cancer antigen; CD, cluster of differentiation; MUC2, mucin 2, oligomeric mucus/gel forming; AFP, α-fetoprotein; EBV, Epstein Barr virus; HER-2, erb-b2 receptor tyrosine kinase 2; VEGFR2, vascular endothelial growth factor receptor 2; EGFR, epidermal growth factor receptor; PD-1, programmed cell death 1; dMMR, deficient mismatch repair; MSI-H, high levels of microsatellite instability; hMLH1, human mutL homolog 1; CDH1, cadherin-1; miRNA, microRNA; lncRNA, long non-coding RNA; circRNA, circular RNA; Bcl-2, BCL2 apoptosis regulator; ncRNA, non-coding RNA; TCGA, The Cancer Genome Atlas; ACRG, Asian Gastric Cancer Research Group; MG7-Ag, monoclonal gastric cancer 7 antigen; PG, pepsinogen; G-17, gastrin-17.
2. Definition of a biomarker
With the advancement of medicine, the definition of a biomarker has also changed accordingly. In 1998, the National Institutes of Health Biomarker Definition Working Group defined biomarker as ‘a feature of objective measurement and assessment of pharmacological responses to normal biological processes, pathogenic processes or therapeutic interventions’ (16). Then, Becking and Chen (17) defined a biomarker as ‘any material structure or process that can be measured in the body or its products and effect or predict the incidence of prognosis disease’. Each of these definitions are similar. Common biomarkers include carbohydrates, proteins, nucleic acids, small metabolite lipids and cytogenetics, in addition to all tumor cells identified in body fluids that are involved in the regulation of physiological and pharmacological processes (18). However, Strimbu and Tavel (19) summarized the importance of biomarkers by stating ‘the foremost issue at present is determined by the link between any given measurable biomarker and relevant clinical endpoints’. With the development of molecular biology, an increasing number of tumor markers have been discovered. Tumor markers, not only with high sensitivity but also specificity, are still being discovered. Similarly, numerous molecular biomarkers have demonstrated their potential efficacy as diagnostic and prognostic tools in gastric cancer, yet they still require further confirmation of their use in day-to-day clinical practice (20).
3. Commonly used biomarkers
Serological biomarkers of gastric mucosa
South Korea and Japan have the most complete prevention and screening gastric cancer program globally, which result in a high detection rate of early gastric cancer in these countries (21). At present, carcinoembryonic antigens including CEA (22), CA19-9 (23) and CA72-4 (24) are the most widely used markers for detecting gastric cancer in the clinical practice. These markers lack the sensitivity and specificity required for evaluating the diagnosis and prognosis of gastric cancer, making their efficacy questionable. Therefore, the screening value for early gastric cancer is limited. However, the positive expression of monoclonal gastric cancer 7 antigen (MG7-Ag) indicates a high risk of gastric cancer (25). Nevertheless, the sensitivity and specificity of MG7-Ag as a single marker in the diagnosis of gastric cancer may not be sufficient, and further clinical research is required to evaluate its value in diagnosis for the early screening of gastric cancer.
Pepsinogen (PG) may be divided into two subtypes, PGI and PGII, according to its biochemical and immunological activity characteristics (26). PG is a good indicator of the exocrine function of the gastric antral mucosa and may be called ‘serological biopsy’ (26). When gastric mucosal atrophy occurs, serum PGI and/or the PGI/II ratio (PGR) level are decreased (27). Furthermore, H. pylori infection is a necessary condition for the occurrence of intestinal subtype gastric cancer which accounts for the majority of cases of gastric cancer, but it is not a sufficient condition (28,29). Thus, previously combining serum PG levels with H. pylori antibodies (including the ‘ABC method’) were used to assess the risk of gastric cancer and screened for patients with a high-risk of developing gastric cancer. Konturek et al (30) reported that elevated levels of serum gastrin-17 (G-17) may indicate the risk of gastric cancer. Additionally, Shiotani et al (31) reported that serum G-17 combined with PG may enhance the diagnostic value of gastric cancer. Previously, when five serological markers markers were combined with PGI, PGII, PGR, (H. pylori) antibody and G-17 as a screening strategy for gastric cancer, it was revealed that a decrease in the levels of PGI and PGR were associated with a high risk of gastric cancer, whereas low (<0.5 pmol/l) and high (>4.7 pmol/l) G-17 levels were associated with a higher risk of suffering from gastric cancer (32). Therefore, it has been indicated that screening strategies which combine these serological markers may assist in identifying high-risk individuals, in addition to guiding targeted screening and precision prevention (32).
Diagnosis-associated tumor biomarkers of gastric cancer
Common types of adenocarcinoma, including tubular, papillary, mucinous, low adhesion carcinoma or mixed adenocarcinoma, often present with phenotypic features with intestinal epithelium [expressing mucin 2, CDX-2 and cluster of differentiation (CD)10] or gastric epithelium (expressing mucin 1, cell surface associated, mucin 5AC, olugomeric mucus/gel-forming and mucin 6, oligomeric mucus/gel forming) (33). In fact, it is frequently unnecessary to diagnose these common types of adenocarcinoma using immunohistochemistry (IHC). However, certain unique types of gastric cancer, including poorly differentiated neuroendocrine carcinoma, hepatoid adenocarcinoma, adenocarcinoma producing α-fetoprotein, gastric cancer with lymphoid stroma [typically associated with Epstein Barr virus (EBV) infection] and choriocarcinoma require specific biomarkers to confirm the diagnosis (34). Prior to a case of hereditary diffuse gastric cancer being diagnosed, IHC detection of E-cadherin and detection of mutations in the CDH1 gene mutations are required for screening or confirmation (11).
The specific pathological features of a patients' unique case of gastric cancer will have a substantial effect on the therapeutic regimen used and the patients' outcome (15). Lymph node micrometastasis (LNM) is one of the most important prognostic factors in patients with gastric cancer, including in patients who do present with evidence of lymph node metastasis (35). Comparatively speaking, IHC may be satisfactorily accurate for the detection of LNM compared with that of haemotoxylin and eosin staining. Cytokeratin AE1/AE3 and CAM5.2 are reliable biomarkers for the detection of epithelial tissues or cells in lymph nodes (36). If it is suspected that the cancer cells are present in the vasculature, the biomarkers CD31 and D2-40 are available (37). Additionally, the markers NF or S-100 may be used for the detection of nerve invasion (38).
Gastric cancer chemotherapy interrelated markers
Cell proliferation is closely associated with tumor progression, reflecting the invasiveness and final prognosis of various malignant tumor types including gastric cancer (39). Ki67 is a nuclear antigen that may be expressed at all stages of the cell proliferation cycle except in G0 cells. Cytotoxic chemotherapeutic agents are effective against tumor cells that have entered the cell division cycle (G1, S, G2 and M phases). A high level of Ki67 indicates that a greater number of cancer cells are entering the cell division cycle and may be the most effective indicator for chemotherapeutic drug therapies. Therefore, the Ki-67 antibody is widely used to evaluate the proliferative activity of tumor cells and to identify the presence of circulating cells to measure tissue growth (40). Although there is no consensus on the prognosis and predictive value of Ki-67 in malignant tumor types, studies have revealed that the Ki-67 index has notable implications for the prognosis of cancer (41). Therefore, the high expression of Ki-67 may be used as a marker for predicting poor prognosis in patients with gastric cancer. The levels of Ki-67 expression should thus be considered when selecting a suitable treatment option and comprehensive treatment. Thus, it is recommended to perform routine Ki67 detection on gastric cancer tissues to evaluate the proliferative status of the cancer cells and provide a reference for determining the efficacy of chemotherapy.
Markers associated with gastric cancer molecular targeted therapy
Human epidermal growth factor receptor-2 (HER-2) is a proto-oncogene located on chromosome 17, encoding a 185-kDa tyrosine kinase receptor which belongs to the epidermal growth factor receptor family. The phosphorylation of HER-2 initiates a signaling pathway resulting in cell division, proliferation, differentiation and anti-apoptotic signaling (42,43). Previous research has predicted that between 7–38% of gastric cancer types exhibit the amplification and/or overexpression of HER-2 (44–46). In 2010, the trastuzumab for gastric cancer study, a phase III, open-label, randomized controlled clinical trial, revealed that patients with gastric cancer who exhibited of HER-2 upregulation and treated with trastuzumab had significantly improved overall survival (OS) and disease-free survival (DFS) times, and a significantly increased objective response proportion, compared with chemotherapy alone (cisplatin + 5-fluoropyricil or capecitabine) (44). Therefore, on October 20, 2010, the U.S. Food and Drug Administration (FDA) granted approval for trastuzumab (Herceptin) in conjunction with cisplatin and a fluoropyrimidine (cisplatin or 5-fluoropyricil), for treating patients with HER-2 amplification or upregulation in metastatic gastric or gastroesophageal junction adenocarcinoma, who were untreated for metastatic tumor (44). The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for gastric cancer suggest the assessment of HER-2 overexpression by IHC and/or gene amplification through fluorescence in situ hybridization or another in situ hybridization method in tumor samples for patients with an unresectable locally advanced, recurrent or metastatic stomach cancer for whom trastuzumab may be beneficial (47). A consensus for HER-2 detection in patients with gastric cancer is thus required to improve individualized treatment for patients (48). Additionally, it is recommended that there is routine detection of HER-2 expression in patients with gastric cancer, so that a greater number of patients do not forego the opportunity for targeted therapy with the aim of improving the outcome of these patients. The vascular endothelial growth factor (VEGF) family is an important regulator of angiogenesis and lymphangiogenesis, which specifically binds to VEGFR receptor (VEGFR), promotes vascular and lymphangiogenesis and participates in the development and progression of a tumor (22). Therefore, VEGF antibody and VEGFR antagonists are used to block the blood supply to gastric cancer tissues when treating patients with gastric cancer. The anti-VEGF drug, bevacizumab, is a recombinant humanized monoclonal antibody against VEGF-A. Phase III clinical trials in the AVAGAST study revealed that bevacizumab, combined with capecitabine and cisplatin, as a first-line treatment for patients with advanced gastric cancer did not improve overall survival time. However, the results of the study revealed that there was a significant difference in survival benefits in the US, but not in Asia (49). Therefore, it is necessary to consider individual differences and other factors in future research to improved individualized treatment.
VEGFR belongs to the tyrosine kinase receptors family, which includes VEGFR-1, VEGFR-2 and VEGFR-3, of which VEGFR-2 is the primary receptor mediating increased vascular permeability (50). Ramucirumab is a completely humanized immunoglobulin G (IgG1) monoclonal antibody which targets the extracellular domain of VEGFR2, blocks the interaction with VEGFR ligands and inhibits receptor activation. It was demonstrated that in two global, randomized, double-blind phase III clinical trials, using ramucirumab combined with platinum and/or fluorouracil (REGARD trial) or ramucirumab combined with paclitaxel (RAINBOW trial), that the OS and DFS times in patients with gastric cancer whose cancer had deteriorated following the initial treatment, were significantly increased in the treatment group compared with the placebo, who were treated with the corresponding chemotherapeutics alone (51,52). In 2014, the FDA approved ramucirumab as a second-line therapy for the treatment of patients with advanced gastric or gastroesophageal junction adenocarcinoma with evidence of disease progression during or following treatment with fluoropyrimidine- or platinum-containing chemotherapeutics (53). The RAINBOW study revealed that Asian patients did not benefit significantly from anti-angiogenic therapy compared with patients treated with ramucirumab or ramucirumab combined with paclitaxel. However, there remain certain issues which need to be addressed. For example, anti-angiogenic drugs result in alterations to physiological vasoactive activity. Additionally, the mechanisms underlying the resistance to drugs targeting angiogenesis require further study which may partly be achieved through the identification of more reliable biomarkers (54). Epidermal growth factor receptor (EGFR) (also known as HER1) is a member of the human epidermal growth factor receptor family, which is a multifunctional glycoprotein on the human cell membrane with a relative molecular mass of 1.70×104 (55). The mutation and overexpression of EGFR is closely associated with malignant tumor types including gastric cancer (56). EGFR-targeting drugs may exhibit anti-tumor effects by inhibiting the binding of ligands to EGFR or exerting effects on the intracellular regions of EGFR and interfering with tyrosine kinase phosphorylation. Cetuximab is a human-mouse chimeric IgG1 monoclonal antibody against EGFR (57). In EXPAND, a phase III clinical trial, it was revealed that the addition of cetuximab to capecitabine-cisplatin provided no additional benefits to progression-free survival (PFS) time compared with chemotherapy alone as a first-line treatment of advanced gastric cancer (57). Panitumumab is a humanized IgG2 monoclonal antibody against EGFR. In a large-scale clinical phase III trial (REAL-3), it was demonstrated that the addition of panitumumab to epirubicin, oxaliplatin and capecitabine (EOC) chemotherapy significantly worsened the OS time from 11.3 to 8.8 months (58). Therefore, it was not recommended for patients with advanced esophageal adenocarcinoma, who were not selected. However, a large number of studies (59–61) have revealed inconsistent results, highlighting the need for a large-scale clinical study to validate whether patients with gastric cancer with evidence of EGFR expression benefit from targeted drugs. However, at present, there are no guidelines recommending the routine use of EGFR for the detection of gastric cancer.
Mesenchymal-epithelial transition factor gene (c-MET) is located on chromosome 7q21-31, which encodes for a protein tyrosine kinase belonging to the hepatocyte growth factor receptor family, and participates in regulating important cellular processes, including proliferation, differentiation, motility, cell cycle and apoptosis (62). Additionally, numerous studies have demonstrated that c-MET overexpression is associated with a poorer survival prognosis and predicted shorter PFS time in various tumor types, including gastric cancer (63–65). Multi-center retrospective studies in Japan have revealed that there is a significant difference in the OS time of patients who had high c-Met expression compared with those with no/low levels of c-Met expression (66). Catenacci et al (67) observed that when the overexpression of the MET protein in patients with chemotherapy-insensitive gastric cancer was present, these patients were able to remain in remission in a two year follow-up period when treated with a monovalent MET antibody therapy. Therefore, it is expected that examining the expression of MET in gastric carcinoma tissues using IHC may have potential clinical application value for gastric cancer c-Met targeted therapy. However, a phase III, randomized, double-blind, multicenter trial (the RILOMET-1 study), 609 patients with advanced MET-positive gastric or gastroesophageal junction tumor types were divided into a rilotumumab group (rilotumumab in combination with epirubicin, cisplatin and capecitabine) or a placebo group (placebo + epirubicin, cisplatin and capecitabine). It was demonstrated that the OS time of the rilotumumab group was worse compared with the placebo group, and the incidence of negative effects was increased. The study was aborted early due to an imbalance in mortalities between the groups (62). In conclusion, the exact efficacy of a targeted MET monoclonal antibody in patients with gastric cancer requires additional study to determine the reason for the less favorable results observed in the RILOMET-1 study. Identifying suitable molecular markers and investigating the optimum combinatorial solutions may ultimately improve patient outcomes. Mammalian target of rapamycin (mTOR) is a member of the phosphoinositide-3-kinase-associated kinase family, which is highly expressed in gastric cancer tissues. Hence, blocking the mTOR signaling pathway may inhibit the proliferation and metastasis of tumor cells (68). Everolimus is an oral rapamycin derivative that blocks the phosphorylation of mTOR and functions as an antitumor agent. A phase III clinical trial of Granite-1 revealed that everolimus did not improve the prognosis of patients with advanced gastric cancer compared with matching placebo (69). Additionally, one previous study revealed that the efficacy of everolimus was associated with the level of p-S6 (70). At present, the potential efficacy of anti-mTOR targeted therapy and biomarkers are still being determined.
The newly reported research regarding Claudin18.2 (CLDN18.2), a member of the Claudin family, has revealed that CLDN18.2 is associated with tumor development and progression and is located on the outer cell membrane. It is expressed in a variety of tumor types, in particular gastric cancer cells. These biological characteristics suggest that CLDN18.2 may be a potential therapeutic target. A monoclonal antibody against CLDN 18.2. Claudiximab (previously IMAB362), was the first targeted therapy for CLDN 18.2 expression (71). The antibody exerted an anti-tumor effect primarily through activating antibody-dependent cytotoxicity, complement-dependent cytotoxicity and regulation of the tumor microenvironment, in a recent phase II clinical study (FAST) that revealed that Claudiximab combined with EOX (epirubicin 50 mg/m2, oxaliplatin 130 mg/m2 d1 and capecitabine 625 mg/m2 bid, d1-21, every 21 days) significantly improved the PFS and OS times compared with EOX in patients with gastric cancer (72). Treatment with IMAB362 plus EOX in patients with advanced or metastatic gastroesophageal cancer was considered safe and effective. Therefore, this may be a promising target therapeutic option for patients with typically difficult malignancies to treat. However, it is still in phase II trials and will undergo further research.
4. High-throughput technology allows for novel molecular typing of gastric cancer
As the identification of novel biomarkers has resulted in an increase in potential therapeutic regimens, it is no longer sufficient to design treatment options that are not unique for a patients unique expression profile. For example, Herceptin may be used for human epidermal growth factor receptor 2 (HER2)-positive breast cancer, but not for the negative type. However, this may not be completely accurate, as patients who are positive for this marker may exhibit additional mutations. Interestingly, high-throughput technologies have brought tremendous changes, including next generation sequencing and gene array chips, which have allowed for the detection of a large number of markers at the same time, thus making it substantially easier to tailor a specific treatment regimen to an individuals' profile (73). It is well known that there are general classifications by Borrmann and histological classifications by Lauren and WHO in gastric cancer. In previous years, developments in the field of cancer genomics have been revolutionized by the molecular characterization of the different varieties of carcinomas including gastric cancer. Initially, there were only two molecular subtypes identified (74). Subsequently, Lei et al (75) at the Duke National University of Singapore identified three subtypes of gastric adenocarcinoma: Proliferative, metabolic and mesenchymal. In 2014, The Cancer Genome Atlas (TCGA) integration analysis based on somatic cell copy number array analysis, full exon sequence analysis, DNA methylation degree array analysis, mRNA sequence analysis, microRNA sequence analysis and anti-phase protein array analysis resulted in the identification of four different subtypes of gastric cancer: EBV-positive, microsatellite instability (MSI) type, stable genome (GS) and chromosomal instability (CIN) (76). Tumor types expressing EBV frequently underwent recurrent extreme DNA hypermethylation, and exhibit phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-α mutations, Janus kinase 2 amplification, CD274 [programmed death receptor ligand-1 antibody (PD-L1)] and programmed cell death 1 ligand 2 (PD-L2). The MSI subtype exhibited mutations in its DNA sequences, including the mutations of genes which encode targetable oncogenic signaling proteins. The GS tumor types were more common in the diffuse histological variant and mutations of ras homolog family member A or fusions involving RHO-family GTPase-activating proteins. Tumor types with CIN usually exhibited substantial aneuploidy and focal amplification of receptor tyrosine kinases. Each subtype could be distinguished by different biomarkers, as different markers represent different gene mutations. Meanwhile, they are caused by different factors, which should corresponding with different treatments (77). The Asian Gastric Cancer Research Group also divided gastric cancer into four molecular subtypes, including MSI and microsatellite stable (MSS) tumor types with either MSS/epithelial-mesenchymal transition, TP53 activity (MSS/TP53+) or TP53 inactivity (MSS/TP53−) (77) based on the analysis of major components, in 2015. These subtypes are similar to the TCGA subtypes, as the two studies identified MSI immunotherapy. As gastric cancer is a multifaceted and highly heterogeneous disease, these molecular classifications allow researchers to further understand gastric cancer and provide a good basis for innovative targeted therapies for treating patients with gastric cancer.
5. Markers associated with immunotherapy in gastric cancer
Immunotherapy has received growing attention as potential mechanism for treating patients with gastric cancer, due to its favorable curative effect and improved survival time. Discussion of immunotherapy always involves a discussion of biomarkers. The programmed death receptor-1 (PD-1)/PD-L1 was considered a breakthrough for the treatment of numerous tumor types. PD-1 is a negative co-stimulatory receptor, which is primarily expressed on activated T cells (78), and suppresses an excessive immune response through binding to its ligands, PD-L1 and PD-L2 (79,80). In addition to gastric cancer, PD-L1 is expressed in a range of tissues and is also expressed in certain other malignant tumor types (78,80–84). In tumor tissues, effector T-cell function may be inhibited by the binding of PD-1 to PD-L1, which results in the inhibition of the antitumor immune response and even accelerates neoplastic growth (78,79).
Nivolumab is a human IgG4 monoclonal antibody inhibitor of PD-1. The ATTRACTION-2 (ONO-4538-12) study was a randomized, double-blind, placebo-controlled, phase III clinical trial, which compared the efficacy and safety of nivolumab and a placebo in patients with advanced gastric cancer who underwent second line systemic treatment (85). Nivolumab treatment had previously been shown to significantly improve survival (69). In the clinical trial, nivolumab significantly reduced the risk of mortality by 37% compared with the placebo group. In addition, the 12-month OS rate was significantly higher in the nivolumab group compared with the placebo group. The OS rates were 26.2 and 10.9%, respectively (69). Nivolumab is now approved for the treatment of unresectable advanced or recurrent gastric cancer, based on the results of the ATTRACTION-2 study, and nivolumab has become an important treatment option for a variety of tumor types including gastric cancer. Pembrolizumab is an IgG4-κ humanized monoclonal antibody, which is selective and has a high-affinity, and binds to PD-1, preventing PD-1 from binding to PD-L1. Pembrolizumab is relatively safe and has exhibited potential anti-tumor activity in certain types of advanced solid tumor types and hematological malignancies (86). A number of countries have approved pembrolizumab for the treatment of advanced melanoma, and in the USA it is used for the treatment of metastatic non-small-cell lung cancer, which expresses PD-L1 and non-small-cell lung cancer with failed platinum-containing chemotherapeutics (86). Multiple studies have revealed that tumor types with an upregulated expression of PD-L1 have a higher rate of response when treated with pembrolizumab (87,88). In addition, nivolumab and atezolizumab also produced similar results (89,90). Furthermore, the higher the expression rate was of PD-L1, the higher the remission rate of the tumor was, and even resulted in complete cure in certain cases (88). Biomarkers which will help predict the response to inhibitors of PD-1 or PD-L1 are required. The detection of PD-L1 protein expression by IHC is a currently available method.
In a number of studies, PD-L1 overexpression was observed in >40% of human gastric cancer samples (81,82,91,92). The KEYNOTE-021 and KEYNOTE-059 clinical trials revealed an improved outcome in patients with advanced gastric cancer treated with pembrolizumab+5-FU+cisplatin compared with 5-FU+cisplatin and thus highlighting the potential use of immunotherapy for the treatment of patients with gastric cancer (93,94). PD-L1 Combined Positive Score (CPS) ≥1% in tumor types or mesenchymal cells was considered to be the cut-off value for treatment with pembrolizumab. Unfortunately, in the majority of solid tumor types, the effectiveness of a PD-1 inhibitor alone is 10–30%. One exception is the classic Hodgkin's lymphoma, where the efficiency is >60%. Although PD-1 inhibitors are not so efficient, they have a long-lasting effect (94). As the immune system has a functional ‘memory’, once the PD-1 inhibitor has exerted its effects, patients with certain early tumor types, including patients with malignant melanoma, kidney cancer and non-small cell lung cancer, are able to achieve clinical cure, that is, no recurrence, no progression and long-term survival (95). Whether this effect may be achieved in gastric cancer requires further study. In addition, certain patients may also achieve improved results with a combination of PD-1 inhibitors with other forms of therapy. At present, PD-1 inhibitors combined with chemotherapy have been approved for treating gastric cancer (96); however, additional studies are required to determine its efficacy.
Based on these studies, in September 2017, pembrolizumab was approved by the FDA as a third-line treatment for metastatic carcinoma or patients with recurrent locally advanced, gastric or gastroesophageal junction adenocarcinoma whose tumor types expressed PD-L1 as determined by an FDA-approved test in addition to use for any cancer with MSI-high (H) (97). A meta-analysis and systematic review of the literature have demonstrated that MSI-H and EBV-positive gastric cancer are often associated with improved prognosis and longer survival times (98). Derks et al (99) additionally reported that interferon-driven genes are abundant in MSI-H and EBV-positive gastric cancer types, suggesting that these patients are sensitive for PD-1 or PD-L1 inhibitors. Researchers have revealed that MSI-H and EBV positive cancer types have upregulated expression levels of PD-L1 (100). Therefore, the NCCN clinical practice guidelines for gastric cancer 2017 version 5 and 2018 version 1 have recommended that mismatch repair (MMR)/MSI-H, PD-L1 and tumor EBV status should be considered in patients with locally advanced, recurrent or distant metastases who are candidates for treatment with PD-1 inhibitors (101). The expression of the MMR proteins [mutL homolog 1 (MLH1), PMS1 homolog 2, mismatch repair system component, mutS homolog 2 and mutS homolog 6] and PD-L1 expression are assessed using IHC in clinical practice.
In addition, tumor mutation burden (TMB) is also an important biomarker for predicting the effect of PD-1 inhibitors (102). The definition of TMB is the number of somatic mutations in the whole genome subsequent to counting germline DNA variants. For efficacy analysis, patients may be divided into three groups: TMB high burden (≥248), medium burden (143–247) and low burden (<143) groups (103). Studies have revealed that the efficacy of PD-1/PD-L1 inhibitors in a tumor are significantly associated with TMB (102,104). Hence, patients with a high TMB may be more likely to benefit from immunotherapy. However, there are some difficulties in assessing TMB in clinical practice, including a long detection period, poor platform accessibility, high costs and inconsistent standards. TMB testing requires strict experimental conditions and has a low success rate at present. Furthermore, there is insufficient data at present to support the notion that TMB benefits OS (104). Therefore, additional research is required to fully understand the implications and clinical applications of TMB.
6. Other markers with potential clinical application value
DNA methylation
DNA methylation is a frequent epigenetic event that serves an important function in cancer development (105). Hypermethylation of gastric cancer in CPG islands are interrelated with the gene silencing of numerous tumor suppressor genes, including human MLH1, p16, CDH1 (E-cadherin) and RUNX family transcription factor 3 (RUNX3) genes. Cancer-derived DNA methylation may be detected easily in the serum of patients with gastric cancer (106). A number of studies have reported that methylation of the p16 gene and the CDH1 gene were detectable in the serum of 20–50% of patients with gastric cancer, but not in the cancer-free and control patients (107,108). Thirty percent of patients with gastric cancer had RUNX3 methylation, detected in the peripheral circulation, and it was associated with tumor stage, vascular invasion and lymph node metastasis (109). However, serum RUNX3 methylation levels were significantly reduced in patients with gastric cancer following surgery (110). Therefore, the detection of abnormal DNA methylation in serum is an effective tool for cancer screening, disease monitoring and prognosis determination.
Among the epigenetic changes involved, DNA methylation is associated with anticancer drug resistance (111). A number of methylation alterations to apoptotic genes have been identified and used as epigenetic biomarkers for determining either chemoresistance or chemosensitivity to anticancer drugs (111). According to Choi et al (112), the hypermethylation of cyclin dependent kinase inhibitor 2A (p16INK4a) may be a useful biomarker for 5-florouracil-sensitive and resistant gastric cancer, respectively. Furthermore, the hypomethylations of adhesion G protein-couples receptor L2 and G2 and S-phase expressed 1 are potential biomarkers for cisplatin-sensitive gastric cancer. Additionally, the hypomethylation of ATP binding cassette subfamily B member 1 may be a useful biomarker, regardless of the drug type, for chemotherapy-resistant gastric cancer.
Non-coding RNA (ncRNA)
In previous years, ncRNA, including microRNA (miR/miRNA), long ncRNA (lncRNA) and circular RNA (circRNA), have been identified as important regulators of protein-coding genes, and are involved in the regulation of cell development, differentiation and the occurrence of a tumor. They have also been demonstrated to serve key functions in the evolution and development of gastric cancer (113–115).
miRNAs are small ncRNA molecules that regulate gene expression at the post-transcriptional level (116). miRNAs have a large range of gene regulatory functions and are involved in a series of biological processes including regulating apoptosis, cell proliferation, cell differentiation and development, epigenetic genetic regulation and serve a notable function in the development and progression of cancer (117,118). Numerous miRNAs have been revealed to be expressed at varying degrees in gastric cancer, regulating the signaling pathways, and certain specific miRNAs are associated with the occurrence, progression and prognosis of gastric cancer (119,120). Using the microarray analysis of miRNAs, 22 types of miRNAs were demonstrated to be upregulated and 13 species were downregulated, compared with non-tumor gastric tissue (119,120). miR125b, miR199a and miR-433 are miRNAs that serve an important function in the progression of cancer. Furthermore, the low expression of miR-433 and high expression of miR214 are independent predictors of poor prognosis (121). In gastric cancer, the downregulation of miR-148a results in reduced tumor metastasis and causes lymph node metastasis and disease progression when miR-148a is downregulated (122). miR-335 inhibits metastasis by regulating BCL2 like 2 and specificity protein-1 in gastric cancer (123). Furthermore, miRNA-421 is a promising tumor biomarker with diagnostic potential to monitor a number of different types of cancer. Gastric juice levels of miR421 in patients with gastric cancer were significantly different compared with patients with a benign gastric disease (124). Therefore, miRNAs may function as potential biomarker targets for the molecular diagnosis of gastric cancer.
For the past few years, the dysregulated expression of numerous lncRNAs (ncRNAs that are >200 nucleotides long) have been demonstrated to be associated with tumorigenesis using next-generation sequencing; and these lncRNAs perform basic regulatory functions by modulating tumor cell proliferation, apoptosis, invasion and metastasis (125). Differential expression of lncRNAs serve critical functions in the carcinogenesis of gastric cancer. A number of studies have revealed that lncRNA H19 is upregulated and highly expressed in gastric cancer and is involved in the complex molecular regulation of gastric cancer, and thus may serve as a potential diagnostic marker and molecular therapeutic target, particularly for early tumor screening (126,127). Pang et al (128) illuminated that the expression levels of LINC00152 in gastric cancer tissues was significantly higher compared with normal tissues and normal healthy controls, and was associated with invasion. Additionally, this previous study demonstrated that the lncRNA LINC00152 may be a novel biomarker for predicting gastric cancer (99). Furthermore, high urothelial cancer associated 1 (UCA1) expression is associated with tumor size, reduced differentiation, adavanced Tumor-Node-Metastasis stages and increased invasion depth in gastric cancer. Therefore UCA1 may also serve as a potential marker for the early detection and prognostic prediction of gastric cancer (129). It was demonstrated that a three-lncRNA signature, including UCA1, long stress-induced non-coding transcript 5 and phosphatase and tensin homolog pseudogene 1, was confirmed to be a potential diagnostic biomarker for detecting gastric cancer (130). Hence, it is clear that the study of the pathophysiology of lncRNA expression in gastric cancer is a novel research trend which may improve the diagnosis and treatment of patients with gastric cancer.
CircRNA, a novel type of ncRNA which is dissimilar to the well-known linear RNA, forms a covalently closed continuous loop. In circular RNA, the 3′ end and 5′ end are usually joined together (131). At present, research on the function of circRNA in cancer remains in its infancy. Although a large number of circRNAs have been demonstrated to be downregulated by using next-generation RNA sequencing and bioinformatics analysis, there is emerging evidence that numerous circRNAs are abnormally expressed in various diseases, including certain types of cancer, which may function as oncogenes or tumor suppressor genes (132,133). Certain circRNAs serve a function in various aspects of biology and disease, particularly in cancer (134,135). Notable, studies have revealed that select circRNAs are upregulated or downregulated in patients with gastric cancer. Chen et al (136) revealed that circRNA Pvt1 oncogene was upregulated in gastric cancer and functioned as an miRNA sponge, regulating the expression of the target genes of the miRNA in gastric cancer, and ultimately demonstrated a proliferative effect and potential as a prognostic marker. In addition, three independent studies demonstrated that hsa_circ_0000190 (137), hsa_circ_0000096 (138) and hsa_circ_002059 (139) were downregulated in gastric cancer tissues compared with the adjacent noncancerous tissues. Due to the tissue, timing and disease specificity of circRNA expression, circRNAs have attracted substantial interest in cancer research. At present, circRNAs present high specificity in gastric cancer, which indicates that they may have promise as prospective novel biomarkers. However, their function and mechanism remain yet to be elucidated.
Cell cycle regulators
The normal progression of the cell cycle is regulated by positive regulators of cyclins, cyclin-dependent kinase (CDK) complexes, and negative regulators of cell cycle-dependent kinase inhibitors (CKI). The overexpression of cell cycle positive regulators, abnormal activity and lack of expression of CKI result in disorders of cell proliferation (140). Hence, any defects in cell cycle regulators affect the outcome of patients with gastric cancer, as cell-cycle regulators are involved in a number of processes, including the proliferation of cancer. An important regulator is cyclin E, which is also a useful prognostic factor in gastric cancer. The activity of CDK is inhibited by CKIs, including P16, P21 and P27 (141). The downregulation of P27 serves as a negative predictor of cyclin E-positive tumor types (142–144). In addition, the function of P53, a tumor suppressor gene, is pivotal to cell cycle regulation, DNA repair and apoptosis. Multivariate analysis has revealed that the upregulation of P53 was an independent prognostic factor in patients with advanced gastric cancer and it was associated with poor prognosis (145). However, P53 has no substantial prognostic value in the initial phase of gastric cancer in patients (146).
Apoptosis-associated factors
The growth of a tumor depends on the ratio of cell proliferation to apoptosis. In addition to the function of oncogenes and tumor suppressor genes, factors that regulate cell apoptosis also serve a crucial function in the development of a number of tumor types, including gastric cancer. Certain programmed cell death factors are good prognostic indicators for gastric cancer. Apoptosis is achieved through the interaction between pro-apoptotic and anti-apoptotic molecules (147). The BCL2 apoptosis regulator (Bcl-2) gene, which is a crucial antiapoptotic gene, influences the intrinsic apoptosis pathway (147). At the same time, Bcl-2 may facilitate tumor invasion and metastasis (148). It has been suggested that the inhibition of Bcl-2 expression is an important strategy for the treatment of various cancer types, including gastric cancer (149,150). The sensitivity of tumor cells to apoptosis may be detected and evaluated by assessing the levels of the proto-oncogene Bcl-2, which is a useful prognostic factor of survival in patients with advanced gastric cancer (151). The newest inhibitory apoptotic family, survivin, may suppress apoptosis via pathways other than those associated with the Bcl-2 family (152). One study hypothesized that survivin may be expressed in gastric cancer, although the nuclear localization of survivin is thought to delay physiological development (153). Thus, survivin may be used as a prognostic factor for poor outcome in patients with gastric cancer (154).
Prognostic markers of gastric cancer
In previous years, the association between certain potentially enriched markers of gastric cancer stem cells including CD133, CD44 and leucine rich repeat containing G protein-coupled receptor 5 and the biological behavior and prognosis of gastric cancer in patients has attracted increasing attention. If CD133 is highly expressed in gastric cancer cells, the cells display increased malignant biological behaviors, as its upregulation is associated with tumor progression, chemotherapy resistance, relapse and poor prognosis (155). Therefore, the positive expression of CD133 in gastric cancer cells may contribute to the prognosis of patients (156).
The CDH1 gene is a tumor suppressor gene which encodes E-cadherin, a transmembrane glycoprotein involved in cell adhesion and epithelial differentiation (157). Mutation of this gene are associated with the occurrence and progression of hereditary diffuse gastric cancer and sporadic gastric cancer. A lack of expression of E-cadherin is an independent prognostic factor for gastric cancer, and predicts a worse prognosis in patients (158). E-cadherin-negative and nuclear β-catenin-positive gastric cancer are often associated with the poor differentiation of gastric cancer, loss of adhesion, increased infiltration ability, increased tumor progression and a poorer prognosis (159). Consequently, the expression of CD133 or E-cadherin may be used to predict the prognosis of patients with gastric cancer.
7. Conclusion
Gastric cancer is a malignant tumor type with high rates of incidence and mortality globally (1). Gastric cancer is a group of tumor types which are diverse in their biology and genetics; and furthermore, there are various factors at work in the etiology of this cancer, including environmental and genetic factors (6). Although endoscopy and imaging technology have improved the detection of early-stage gastric carcinoma-associated lesions, gastric cancer has a wide range of morphological heterogeneity. It is easy to overlook certain cases of this disease if only the morphology is relied upon, and advances in the field of understanding biomarkers have helped to improve the early diagnosis and accuracy of diagnosis of gastric cancer early as well as the prognosis and treatment of various diseases including cancer.
The majority of cases of gastric cancer are either moderately or severely advanced at first diagnosis; however, the application of biomarkers may improve the early detection of gastric cancer screening and diagnostic accuracy. As the correct treatment may improve the prognosis of patients, it is important to tailor treatments according the unique biomarker expression profile of each patient. In particular, certain markers may guide the individual and precise therapy of gastric cancer in order to maximize a patient's survival time, instead of or in addition to relying on histological classification and chemotherapy completely. So far, targeted therapies for a small number of patients with gastric cancer have been performed instead of or in addition to using trastuzumab and ramucirumab, which are the second-line treatments for advanced gastric cancer when either HER2 or VEGFR2 expression are upregulated, respectively. The PD-1 inhibitor pembrolizumab has been approved by the FDA as a third-line (or higher) treatment for patients with recurrent locally advanced or metastatic gastric adenocarcinoma. The NCCN guidelines recommend the detection of PD-L1 or MSI/MMR, as PD-1 inhibitors are effective in patients with MSI-H/deficient MMR and a high expression of PD-L1 (76). Targeted therapy and immunotherapy have presented clinicians with a novel method for treating patients with advanced gastric cancer.
Numerous markers have now been identified for targeted therapy and immunotherapy, and each marker exhibits advantages and disadvantages (44,50,56). With the development of high-throughput technologies, various markers will be identified in the future, not only at the organ level but also at the genetic level. All tumor types that express the same markers may be treated using similar therapeutic regimens, despite the location of the tumor. Conversely, tumor types which are located in the same organ but exhibit different biomarker profiles may be treated with using different therapeutic regimens. In summary, the identification of novel and effective biomarkers is required to improve the diagnosis of gastric cancer, in order to strengthen the accuracy of gastric cancer diagnosis, determine the prognosis and predict the pathogenesis, and establish a novel and effective treatment option for patients with gastric cancer.
Acknowledgements
Not applicable.
Funding
The present review was supported by the Hunan Provincial Groundbreaking Platform Open Fund of University of South China (grant no. 18K076), the Doctoral Research Fund of the University of South China (grant no. 2016XQD21), the Student Research Learning and Innovative Experimental Project of University of South China (grant nos. 2016NH055XJXZ and 2017XJXZ030), the Hunan Provincial Key Subject Fund of Basic Medical Sciences and the University of South China and Horizontal Cooperation Project of Yueyang Maternal and Child Health Hospital (grant no. 2018KHX43).
Availability of data and materials
Not applicable.
Authors' contributions
ZZ and DMY drafted the manuscript. DMY, GX and YX acquired the data. WM, YXL, WL and YL interpreted the data. DY, ZZ and YL edited the manuscript and revised it. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007) Gastric Cancer. 2018;21:144–154. doi: 10.1007/s10120-017-0716-7. [DOI] [PubMed] [Google Scholar]
- 4.Sumiyama K. Erratum to: Past and current trends in endoscopic diagnosis for early stage gastric cancer in Japan. Gastric Cancer. 2017;20:562. doi: 10.1007/s10120-016-0659-4. [DOI] [PubMed] [Google Scholar]
- 5.Ren W, Yu J, Zhang ZM, Song YK, Li YH, Wang L. Missed diagnosis of early gastric cancer or high-grade intraepithelial neoplasia. World J Gastroenterol. 2013;19:2092–2096. doi: 10.3748/wjg.v19.i13.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nie Y, Wu K, Yu J, Liang Q, Cai X, Shang Y, Zhou J, Pan K, Sun L, Fang J, et al. A global burden of gastric cancer: The major impact of China. Expert Rev Gastroenterol Hepatol. 2017;11:651–661. doi: 10.1080/17474124.2017.1312342. [DOI] [PubMed] [Google Scholar]
- 7.Fock KM. Review article: The epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–260. doi: 10.1111/apt.12814. [DOI] [PubMed] [Google Scholar]
- 8.Li ZS, Li Q. The latest 2010 WHO classification of tumors of digestive system. Zhonghua Bing Li Xue Za Zhi. 2011;40:351–354. (In Chinese) [PubMed] [Google Scholar]
- 9.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 10.Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 11.van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE, Hardwick RH, Ausems MG, et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361–374. doi: 10.1136/jmedgenet-2015-103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saigusa S, Tanaka K, Mohri Y, Ohi M, Shimura T, Kitajima T, Kondo S, Okugawa Y, Toiyama Y, Inoue Y, Kusunoki M. Clinical significance of RacGAP1 expression at the invasive front of gastric cancer. Gastric Cancer. 2015;18:84–92. doi: 10.1007/s10120-014-0355-1. [DOI] [PubMed] [Google Scholar]
- 13.Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 15.Luu C, Thapa R, Woo K, Coppola D, Almhanna K, Pimiento JM, Chen DT, Marquez DD, Hodul PJ. Does histology really influence gastric cancer prognosis? J Gastrointest Oncol. 2017;8:1026–1036. doi: 10.21037/jgo.2017.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NIH-FDA conference. Biomarkers and surrogate endpoints: Advancing clinical research and applications. Abstracts. Dis Markers. 1998;14:187–334. doi: 10.1155/1998/698239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becking GC, Chen BH. International programme on chemical safety (IPCS) environmental health criteria on boron human health risk assessment. Biol Trace Elem Res. 1998;66:439–452. doi: 10.1007/BF02783154. [DOI] [PubMed] [Google Scholar]
- 18.Abbas M, Habib M, Naveed M, Karthik K, Dhama K, Shi M, Dingding C. The relevance of gastric cancer biomarkers in prognosis and pre- and post-chemotherapy in clinical practice. Biomed Pharmacother. 2017;95:1082–1090. doi: 10.1016/j.biopha.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5:463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng FP, Ding J, Yu ZC, Han QL, Guo CC, Liu N, Fan DM. Oral attenuated Salmonella typhimurium vaccine against MG7-Ag mimotope of gastric cancer. World J Gastroenterol. 2005;11:1833–1836. doi: 10.3748/wjg.v11.i12.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsu A, Yoshida S, Saijo N. Disparities in gastric cancer chemotherapy between the East and West. J Clin Oncol. 2006;24:2188–2196. doi: 10.1200/JCO.2006.05.9758. [DOI] [PubMed] [Google Scholar]
- 22.Tatsuta M, Itoh T, Okuda S, Yamamura H, Baba M, Tamura H. Carcinoembryonic antigen in gastric juice as an aid in diagnosis of early gastric cancer. Cancer. 1980;46:2686–2692. doi: 10.1002/1097-0142(19801215)46:12<2686::AID-CNCR2820461225>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Ishigami S, Natsugoe S, Hokita S, Che X, Tokuda K, Nakajo A, Iwashige H, Tokushige M, Watanabe T, Takao S, Aikou T. Clinical importance of preoperative carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer. J Clin Gastroenterol. 2001;32:41–44. doi: 10.1097/00004836-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Yin LK, Sun XQ, Mou DZ. Value of combined detection of serum CEA, CA72-4, CA19-9 and TSGF in the diagnosis of gastric cancer. Asian Pac J Cancer Prev. 2015;16:3867–3870. doi: 10.7314/APJCP.2015.16.9.3867. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, Chen Z, Juan SJ, Yong XY, Pan BR, Fan DM. Detection of circulating gastric carcinoma-associated antigen MG7-Ag in human sera using an established single determinant immuno-polymerase chain reaction technique. Cancer. 2000;88:280–285. doi: 10.1002/(SICI)1097-0142(20000115)88:2<280::AID-CNCR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133–141. doi: 10.1007/BF02774209. [DOI] [PubMed] [Google Scholar]
- 27.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–253. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 28.Rugge M, Genta RM, Di Mario F, El-Omar EM, El-Serag HB, Fassan M, Hunt RH, Kuipers EJ, Malfertheiner P, Sugano K, Graham DY. Gastric cancer as preventable disease. Clin Gastroenterol Hepatol. 2017;15:1833–1843. doi: 10.1016/j.cgh.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Graham DY. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719–731.e3. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konturek SJ, Starzynska T, Konturek PC, Karczewska E, Marlicz K, Lawniczak M, Jaroszewicz-Heigelman H, Bielanski W, Hartwich A, Ziemniak A, Hahn EG. Helicobacter pylori and CagA status, serum gastrin, interleukin-8 and gastric acid secretion in gastric cancer. Scand J Gastroenterol. 2002;37:891–898. doi: 10.1080/003655202760230838. [DOI] [PubMed] [Google Scholar]
- 31.Shiotani A, Iishi H, Uedo N, Kumamoto M, Nakae Y, Ishiguro S, Tatsuta M, Graham DY. Histologic and serum risk markers for noncardia early gastric cancer. Int J Cancer. 2005;115:463–469. doi: 10.1002/ijc.20852. [DOI] [PubMed] [Google Scholar]
- 32.Tu H, Sun L, Dong X, Gong Y, Xu Q, Jing J, Bostick RM, Wu X, Yuan Y. A serological biopsy using five stomach-specific circulating biomarkers for gastric cancer risk assessment: A multi-phase study. Am J Gastroenterol. 2017;112:704–715. doi: 10.1038/ajg.2017.55. [DOI] [PubMed] [Google Scholar]
- 33.Shiroshita H, Watanabe H, Ajioka Y, Watanabe G, Nishikura K, Kitano S. Re-evaluation of mucin phenotypes of gastric minute well-differentiated-type adenocarcinomas using a series of HGM, MUC5AC, MUC6, M-GGMC, MUC2 and CD10 stains. Pathol Int. 2004;54:311–321. doi: 10.1111/j.1440-1827.2004.01625.x. [DOI] [PubMed] [Google Scholar]
- 34.Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R, Corvalan AH, Carrascal E, Abdirad A, Anwar M, et al. Determinants of Epstein-Barr virus-positive gastric cancer: An international pooled analysis. Br J Cancer. 2011;105:38–43. doi: 10.1038/bjc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Zhang GJ, Wang J, Zheng KY, Fu W. Current status of lymph node micrometastasis in gastric cancer. Oncotarget. 2017;8:51963–51969. doi: 10.18632/oncotarget.17495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hata M, Machi J, Mamou J, Yanagihara ET, Saegusa-Beecroft E, Kobayashi GK, Wong CC, Fung C, Feleppa EJ, Sakamoto K. Entire-volume serial histological examination for detection of micrometastases in lymph nodes of colorectal cancers. Pathol Oncol Res. 2011;17:835–841. doi: 10.1007/s12253-011-9390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giorgadze TA, Baloch ZW, Pasha T, Zhang PJ, Livolsi VA. Lymphatic and blood vessel density in the follicular patterned lesions of thyroid. Mod Pathol. 2005;18:1424–1431. doi: 10.1038/modpathol.3800452. [DOI] [PubMed] [Google Scholar]
- 38.Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291X(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Aziz A, Ahmed RA, Ibrahiem AT. Expression of pRb, Ki67 and HER 2/neu in gastric carcinomas: Relation to different histopathological grades and stages. Ann Diagn Pathol. 2017;30:1–7. doi: 10.1016/j.anndiagpath.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 41.Genc CG, Falconi M, Partelli S, Muffatti F, van Eeden S, Doglioni C, Klumpen HJ, van Eijck C, Nieveen VDE. Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann Surg Oncol. 2018;25:2467–2474. doi: 10.1245/s10434-018-6518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: A 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Wu N, Li J. Novel targeted agents for gastric cancer. J Hematol Oncol. 2012;5:31. doi: 10.1186/1756-8722-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 45.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, et al. Amplification of HER-2 in gastric carcinoma: Association with topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 46.Gravalos C, Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 47.Wood DE, Kazerooni E, Baum SL, Dransfield MT, Eapen GA, Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar R, et al. Lung cancer screening, version 1.2015: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2015;13:23–34. doi: 10.6004/jnccn.2015.0006. [DOI] [PubMed] [Google Scholar]
- 48.Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: A practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 49.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 50.Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L, Tong Z, Wang S, Li J, Wang Z, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. Bmc Cancer. 2014;14:820. doi: 10.1186/1471-2407-14-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derde LPG, Cooper BS, Goossens H, Malhotra-Kumar S, Willems RJL, Gniadkowski M, Hryniewicz W, Empel J, Dautzenberg M, Annane D, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: An interrupted time series study and cluster randomised trial. Lancet Infect Dis. 2014;14:31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Ou SH. Towards the goal of personalized medicine in gastric cancer-time to move beyond HER2 inhibition. Part II: Targeting gene mutations and gene amplifications and the angiogenesis pathway. Discov Med. 2013;16:7–14. [PubMed] [Google Scholar]
- 53.Javle M, Li Y, Tan D, Dong X, Chang P, Kar S, Li D. Biomarkers of TGF-β signaling pathway and prognosis of pancreatic cancer. PLoS One. 2014;9:e85942. doi: 10.1371/journal.pone.0085942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elice F, Rodeghiero F, Falanga A, Rickles FR. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol. 2009;22:115–128. doi: 10.1016/j.beha.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29(Suppl 1):i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, Fejzo MS, Hecht JR, Slamon DJ, Finn RS. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res. 2010;16:1509–1519. doi: 10.1158/1078-0432.CCR-09-1112. [DOI] [PubMed] [Google Scholar]
- 57.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 58.Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Guo W, Zhang W, Yin J, Zhang J, Zhu X, Liu T, Chen Z, Wang B, Chang J, et al. A multi-center phase II study and biomarker analysis of combined cetuximab and modified FOLFIRI as second-line treatment in patients with metastatic gastric cancer. BMC Cancer. 2017;17:188. doi: 10.1186/s12885-017-3174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tebbutt NC, Price TJ, Ferraro DA, Wong N, Veillard AS, Hall M, Sjoquist KM, Pavlakis N, Strickland A, Varma SC, et al. Panitumumab added to docetaxel, cisplatin and fluoropyrimidine in oesophagogastric cancer: ATTAX3 phase II trial. Br J Cancer. 2016;114:505–509. doi: 10.1038/bjc.2015.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: Prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738–746. doi: 10.1111/j.1365-2559.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 62.Catenacci D, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467–1482. doi: 10.1016/S1470-2045(17)30566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha SY, Lee J, Kang SY, Do IG, Ahn S, Park JO, Kang WK, Choi MG, Sohn TS, Bae JM. MET overexpression assessed by new interpretation method predicts gene amplification and poor survival in advanced gastric carcinomas. Mod Pathol. 2013;26:1632–1641. doi: 10.1038/modpathol.2013.108. [DOI] [PubMed] [Google Scholar]
- 64.Lee SJ, Lee J, Park SH, Park JO, Lim HY, Kang WK, Park YS, Kim ST. c-MET overexpression in colorectal cancer: A poor prognostic factor for survival. Clin Colorectal Cancer. 2018;17:165–169. doi: 10.1016/j.clcc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Pasquini G, Giaccone G. C-MET inhibitors for advanced non-small cell lung cancer. Expert Opin Investig Drugs. 2018;27:363–375. doi: 10.1080/13543784.2018.1462336. [DOI] [PubMed] [Google Scholar]
- 66.Kuboki Y, Matsusaka S, Minowa S, Shibata H, Suenaga M, Shinozaki E, Mizunuma N, Ueno M, Yamaguchi T, Hatake K. Circulating tumor cell (CTC) count and epithelial growth factor receptor expression on CTCs as biomarkers for cetuximab efficacy in advanced colorectal cancer. Anticancer Res. 2013;33:3905–3910. [PubMed] [Google Scholar]
- 67.Catenacci DV, Henderson L, Xiao SY, Patel P, Yauch RL, Hegde P, Zha J, Pandita A, Peterson A, Salgia R. Durable complete response of metastatic gastric cancer with anti-Met therapy followed by resistance at recurrence. Cancer Discov. 2011;1:573–579. doi: 10.1158/2159-8290.CD-11-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Batran SE, Ducreux M, Ohtsu A. mTOR as a therapeutic target in patients with gastric cancer. Int J Cancer. 2012;130:491–496. doi: 10.1002/ijc.26396. [DOI] [PubMed] [Google Scholar]
- 69.Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, et al. Everolimus for previously treated advanced gastric cancer: Results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935–3943. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wainberg ZA, Soares HP, Patel R, DiCarlo B, Park DJ, Liem A, Wang HJ, Yonemoto L, Martinez D, Laux I, et al. Phase II trial of everolimus in patients with refractory metastatic adenocarcinoma of the esophagus, gastroesophageal junction and stomach: Possible role for predictive biomarkers. Cancer Chemother Pharmacol. 2015;76:61–67. doi: 10.1007/s00280-015-2744-5. [DOI] [PubMed] [Google Scholar]
- 71.Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
- 72.Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol. 2017;10:105. doi: 10.1186/s13045-017-0473-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen X, Wang M, Yang X, Wang Y, Yu L, Sun J, Ding J. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+ breast cancer after breast-conserving surgery. Theranostics. 2019;9:6080–6098. doi: 10.7150/thno.36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476–485, 485.e1-e11. doi: 10.1053/j.gastro.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lei Z, Tan IB, Das K, Deng N, Zouridis H, Pattison S, Chua C, Feng Z, Guan YK, Ooi CH, et al. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013;145:554–565. doi: 10.1053/j.gastro.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Cancer Genome Atlas Research Network, corp-author. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–456. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 78.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42–52. doi: 10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 82.Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu GL, Luo H, Yang YX, Dai XY, Zhou SF, Wang D. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther. 2015;9:901–909. doi: 10.2147/DDDT.S75152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y, Chen Y, Shi Q, Yu G, Zhang X. PD-L1 expression analysis in gastric carcinoma tissue and blocking of tumor-associated PD-L1 signaling by two functional monoclonal antibodies. Tissue Antigens. 2007;69:19–27. doi: 10.1111/j.1399-0039.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 85.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 86.Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L, Espino G, et al. Phase I study of pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286–4293. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 87.Giuliani J, Bonetti A. Immune-checkpoint inhibitors in head and neck squamous cell carcinoma: Cost-efficacy in second-line treatment based on programmed death-ligand 1 (PD-L1) level. Oral Oncol. 2019;97:143–145. doi: 10.1016/j.oraloncology.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 88.Kerr KM, Hirsch FR. Programmed death ligand-1 immunohistochemistry: Friend or foe? Arch Pathol Lab Med. 2016;140:326–331. doi: 10.5858/arpa.2015-0522-SA. [DOI] [PubMed] [Google Scholar]
- 89.Boyiadzis M, Bishop MR, Abonour R, Anderson KC, Ansell SM, Avigan D, Barbarotta L, Barrett AJ, Van Besien K, Bergsagel PL, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of hematologic malignancies: Multiple myeloma, lymphoma, and acute leukemia. J Immunother Cancer. 2016;4:90. doi: 10.1186/s40425-016-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. J Clin Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 91.Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, Li Q, Chen M, Wang L. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol. 2014;96:284–291. doi: 10.1016/j.yexmp.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J, Wu C. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol. 2015;20:273–281. doi: 10.1007/s10147-014-0701-7. [DOI] [PubMed] [Google Scholar]
- 93.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 94.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, Blank C, Cranmer LD, Robert C, Pavlick AC, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 96.Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, Keegan P, McKee AE, Pazdur R. FDA approval summary: Pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24:103–109. doi: 10.1634/theoncologist.2018-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joshi SS, Maron SB, Catenacci DV. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future Oncol. 2018;14:417–430. doi: 10.2217/fon-2017-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin Cancer Res. 2017 Jul 26; doi: 10.1158/1078-0432.CCR-16-2211. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, et al. Abundant PD-L1 expression in epstein-barr virus-infected gastric cancers. Oncotarget. 2016;7:32925–32932. doi: 10.18632/oncotarget.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma C, Patel K, Singhi AD, Ren B, Zhu B, Shaikh F, Sun W. Programmed death-ligand 1 expression is common in gastric cancer associated with epstein-barr virus or microsatellite instability. Am J Surg Pathol. 2016;40:1496–1506. doi: 10.1097/PAS.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 101.Qiu H, Zhou Z. Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:160–164. (In Chinese) [PubMed] [Google Scholar]
- 102.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, Rizvi NA, Hirsch FR, Selvaggi G, Szustakowski JD, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33:853–861.e4. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 106.Mitsuno M, Kitajima Y, Ide T, Ohtaka K, Tanaka M, Satoh S, Miyazaki K. Aberrant methylation of p16 predicts candidates for 5-fluorouracil-based adjuvant therapy in gastric cancer patients. J Gastroenterol. 2007;42:866–873. doi: 10.1007/s00535-007-2113-1. [DOI] [PubMed] [Google Scholar]
- 107.Leung WK, To KF, Chu ES, Chan MW, Bai AH, Ng EK, Chan FK, Sung JJ. Potential diagnostic and prognostic values of detecting promoter hypermethylation in the serum of patients with gastric cancer. Br J Cancer. 2005;92:2190–2194. doi: 10.1038/sj.bjc.6602636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ikoma H, Ichikawa D, Daito I, Nobuyuki T, Koike H, Okamoto K, Ochiai T, Ueda Y, Yamagishi H, Otsuji E. Clinical application of methylation specific-polymerase chain reaction in serum of patients with gastric cancer. Hepatogastroenterology. 2007;54:946–950. [PubMed] [Google Scholar]
- 109.Sakakura C, Hamada T, Miyagawa K, Nishio M, Miyashita A, Nagata H, Ida H, Yazumi S, Otsuji E, Chiba T. Quantitative analysis of tumor-derived methylated RUNX3 sequences in the serum of gastric cancer patients. Anticancer Res. 2009;29:2619–2625. [PubMed] [Google Scholar]
- 110.Homma N, Tamura G, Honda T, Matsumoto Y, Nishizuka S, Kawata S, Motoyama T. Spreading of methylation within RUNX3 CpG island in gastric cancer. Cancer Sci. 2006;97:51–56. doi: 10.1111/j.1349-7006.2005.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen CC, Lee KD, Pai MY, Chu PY, Hsu CC, Chiu CC, Chen LT, Chang JY, Hsiao SH, Leu YW. Changes in DNA methylation are associated with the development of drug resistance in cervical cancer cells. Cancer Cell Int. 2015;15:98. doi: 10.1186/s12935-015-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi SJ, Jung SW, Huh S, Chung YS, Cho H, Kang H. Alteration of DNA methylation in gastric cancer with chemotherapy. J Microbiol Biotechnol. 2017;27:1367–1378. doi: 10.4014/jmb.1704.04035. [DOI] [PubMed] [Google Scholar]
- 113.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kwok ZH, Tay Y. Long noncoding RNAs: Lincs between human health and disease. Biochem Soc Trans. 2017;45:805–812. doi: 10.1042/BST20160376. [DOI] [PubMed] [Google Scholar]
- 116.Bartel DP, Chen CZ. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 117.Liu N, Chen NY, Cui RX, Li WF, Li Y, Wei RR, Zhang MY, Sun Y, Huang BJ, Chen M, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: A microRNA expression analysis. Lancet Oncol. 2012;13:633–641. doi: 10.1016/S1470-2045(12)70102-X. [DOI] [PubMed] [Google Scholar]
- 118.Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: Research and practice. Pathol Res Pract. 2011;207:608–612. doi: 10.1016/j.prp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ. MicroRNA dysregulation in gastric cancer: A new player enters the game. Oncogene. 2010;29:5761–5771. doi: 10.1038/onc.2010.352. [DOI] [PubMed] [Google Scholar]
- 120.Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: A microRNA expression analysis. Lancet Oncol. 2010;11:136–146. doi: 10.1016/S1470-2045(09)70343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang TS, Yang XH, Wang XD, Wang YL, Zhou B, Song ZS. MiR-214 regulate gastric cancer cell proliferation, migration and invasion by targeting PTEN. Cancer Cell Int. 2013;13:68. doi: 10.1186/1475-2867-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Tian Q, Wu J, et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 123.Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang X, Jiang L, Sun Z, Miao Z, Xu H. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene. 2012;31:1398–1407. doi: 10.1038/onc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang X, Cui L, Ye G, Zheng T, Song H, Xia T, Yu X, Xiao B, Le Y, Guo J. Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumour Biol. 2012;33:2349–2355. doi: 10.1007/s13277-012-0497-x. [DOI] [PubMed] [Google Scholar]
- 125.Bhan A, Mandal SS. Long noncoding RNAs: Emerging stars in gene regulation, epigenetics and human disease. ChemMedChem. 2014;9:1932–1956. doi: 10.1002/cmdc.201300534. [DOI] [PubMed] [Google Scholar]
- 126.Zhou X, Yin C, Dang Y, Ye F, Zhang G. Identification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancer. Sci Rep. 2015;5:11516. doi: 10.1038/srep11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang F, Bi J, Xue X, Zheng L, Zhi K, Hua J, Fang G. Up-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cells. FEBS J. 2012;279:3159–3165. doi: 10.1111/j.1742-4658.2012.08694.x. [DOI] [PubMed] [Google Scholar]
- 128.Pang Q, Ge J, Shao Y, Sun W, Song H, Xia T, Xiao B, Guo J. Increased expression of long intergenic non-coding RNA LINC00152 in gastric cancer and its clinical significance. Tumour Biol. 2014;35:5441–5447. doi: 10.1007/s13277-014-1709-3. [DOI] [PubMed] [Google Scholar]
- 129.Zheng Q, Wu F, Dai WY, Zheng DC, Zheng C, Ye H, Zhou B, Chen JJ, Chen P. Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin Transl Oncol. 2015;17:640–646. doi: 10.1007/s12094-015-1290-2. [DOI] [PubMed] [Google Scholar]
- 130.Dong L, Qi P, Xu MD, Ni SJ, Huang D, Xu QH, Weng WW, Tan C, Sheng WQ, Zhou XY, et al. Circulating CUDR, LSINCT-5 and PTENP1 long noncoding RNAs in sera distinguish patients with gastric cancer from healthy controls. Int J Cancer. 2015;137:1128–1135. doi: 10.1002/ijc.29484. [DOI] [PubMed] [Google Scholar]
- 131.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 132.Hentze MW, Preiss T. Circular RNAs: Splicing's enigma variations. EMBO J. 2013;32:923–925. doi: 10.1038/emboj.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu H, Liu Y, Bian Z, Zhang J, Zhang R, Chen X, Huang Y, Wang Y, Zhu J. Circular RNA YAP1 inhibits the proliferation and invasion of gastric cancer cells by regulating the miR-367-5p/p27 Kip1 axis. Mol Cancer. 2018;17:151. doi: 10.1186/s12943-018-0902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lyu D, Huang S. The emerging role and clinical implication of human exonic circular RNA. Rna Biol. 2017;14:1000–1006. doi: 10.1080/15476286.2016.1227904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qu S, Zhong Y, Shang R, Zhang X, Song W, Kjems J, Li H. The emerging landscape of circular RNA in life processes. Rna Biol. 2017;14:992–999. doi: 10.1080/15476286.2016.1220473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen J, Li Y, Zheng Q, Bao C, He J, Chen B, Lyu D, Zheng B, Xu Y, Long Z, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208–219. doi: 10.1016/j.canlet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 137.Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. doi: 10.1016/j.cca.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 138.Li P, Chen H, Chen S, Mo X, Li T, Xiao B, Yu R, Guo J. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer. 2017;116:626–633. doi: 10.1038/bjc.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 140.Kawakami M, Mustachio LM, Rodriguez-Canales J, Mino B, Roszik J, Tong P, Wang J, Lee JJ, Myung JH, Heymach JV, et al. Next-generation CDK2/9 inhibitors and anaphase catastrophe in lung cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zohny SF, Baothman OA, El-Shinawi M, Al-Malki AL, Zamzami MA, Choudhry H. The KIP/CIP family members p21^{Waf1/Cip1} and p57^{Kip2} as diagnostic markers for breast cancer. Cancer Biomark. 2017;18:413–423. doi: 10.3233/CBM-160308. [DOI] [PubMed] [Google Scholar]
- 142.Tenderenda M. A study on the prognostic value of cyclins D1 and E expression levels in resectable gastric cancer and on some correlations between cyclins expression, histoclinical parameters and selected protein products of cell-cycle regulatory genes. J Exp Clin Cancer Res. 2005;24:405–414. [PubMed] [Google Scholar]
- 143.Nitti D, Belluco C, Mammano E, Marchet A, Ambrosi A, Mencarelli R, Segato P, Lise M. Low level of p27(Kip1) protein expression in gastric adenocarcinoma is associated with disease progression and poor outcome. J Surg Oncol. 2002;81:167–176. doi: 10.1002/jso.10172. [DOI] [PubMed] [Google Scholar]
- 144.Xiangming C, Natsugoe S, Takao S, Hokita S, Tanabe G, Baba M, Kuroshima K, Aikou T. The cooperative role of p27 with cyclin E in the prognosis of advanced gastric carcinoma. Cancer. 2000;89:1214–1219. doi: 10.1002/1097-0142(20000915)89:6<1214::AID-CNCR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 145.Sgambato A, Migaldi M, Leocata P, Ventura L, Criscuolo M, Di Giacomo C, Capelli G, Cittadini A, De Gaetani C. Loss of p27Kip1 expression is a strong independent prognostic factor of reduced survival in N0 gastric carcinomas. Cancer. 2000;89:2247–2257. doi: 10.1002/1097-0142(20001201)89:11<2247::AID-CNCR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 146.Ichiyoshi Y, Oiwa H, Tomisaki S, Sakaguchi Y, Ohno S, Maehara Y, Sugimachi K. Overexpression of p53 is associated with growth pattern and prognosis in advanced gastric cancer. Hepatogastroenterology. 1997;44:546–553. [PubMed] [Google Scholar]
- 147.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 148.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 149.Long J, Menggen Q, Wuren Q, Shi Q, Pi X. MiR-219-5p inhibits the growth and metastasis of malignant melanoma by targeting BCL-2. Biomed Res Int. 2017;2017:9032502. doi: 10.1155/2017/9032502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kang MH, Reynolds CP. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Compare D, Rocco A, Liguori E, D'Armiento FP, Persico G, Masone S, Coppola-Bottazzi E, Suriani R, Romano M, Nardone G. Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis. J Clin Pathol. 2011;64:677–682. doi: 10.1136/jcp.2010.087858. [DOI] [PubMed] [Google Scholar]
- 152.Kim MA, Lee HE, Lee HS, Yang HK, Kim WH. Expression of apoptosis-related proteins and its clinical implication in surgically resected gastric carcinoma. Virchows Arch. 2011;459:503–510. doi: 10.1007/s00428-011-1150-6. [DOI] [PubMed] [Google Scholar]
- 153.Komatsu S, Ichikawa D, Tsujiura M, Konishi H, Takeshita H, Nagata H, Kawaguchi T, Hirajima S, Arita T, Shiozaki A, et al. Prognostic impact of circulating miR-21 in the plasma of patients with gastric carcinoma. Anticancer Res. 2013;33:271–276. [PubMed] [Google Scholar]
- 154.Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, Takano Y. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 2001;163:109–116. doi: 10.1016/S0304-3835(00)00677-7. [DOI] [PubMed] [Google Scholar]
- 155.Ishigami S, Ueno S, Arigami T, Uchikado Y, Setoyama T, Arima H, Kita Y, Kurahara H, Okumura H, Matsumoto M, et al. Prognostic impact of CD133 expression in gastric carcinoma. Anticancer Res. 2010;30:2453–2457. [PubMed] [Google Scholar]
- 156.Hashimoto K, Aoyagi K, Isobe T, Kouhuji K, Shirouzu K. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014;17:97–106. doi: 10.1007/s10120-013-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Uemura T. The cadherin superfamily at the synapse: More members, more missions. Cell. 1998;93:1095–1098. doi: 10.1016/S0092-8674(00)81452-X. [DOI] [PubMed] [Google Scholar]
- 158.Vleminckx K, Vakaet LJ, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-M. [DOI] [PubMed] [Google Scholar]
- 159.Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987–993. doi: 10.3748/wjg.v8.i6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.