Abstract
Acute lymphoblastic leukaemia (ALL) is one of the most common and curable types of cancer in paediatric patients. However, chemotherapeutic resistance is a difficult but common obstacle when treating leukaemia in the clinical setting. Studies have demonstrated that drug resistance is partly attributable to autophagy induced by multiple chemotherapeutic agents. As an evolutionarily conserved non-histone chromatin-binding protein, high mobility group box protein 1 (HMGB1) is considered to be an important factor in autophagy, and regulates autophagy at multiple levels via different subcellular localisations. In the present study, it was revealed that chemotherapeutic drugs induced autophagy in leukaemia cells and that translocation of HMGB1 from the nucleus to the cytoplasm is an important molecular event in this process. It was further demonstrated that poly (ADP-ribosylation) of HMGB1 facilitates its acetylation, thereby inducing HMGB1 translocation and ultimately promoting chemotherapy-induced autophagy in leukaemic cells. Targeted HMGB1 translocation may overcome chemotherapy-induced autophagy in leukaemia.
Keywords: acute lymphoblastic leukaemia, autophagy, high mobility group box protein 1, post-translational modification
Introduction
Leukaemia is the most common cancer among paediatric patients with a worldwide incidence rate of ~40–50 per million (1,2). However, even with comprehensive treatment strategies, including chemotherapy, radiotherapy and stem cell transplantation, the prognoses of patients with primary resistant T-cell acute lymphoblastic leukaemia (T-ALL) who fail to achieve complete haematological remission or who relapse following a transient initial response remain poor (1,2). Chemotherapeutic resistance is considered to be the primary cause of this problem, and understanding how cancer cells acquire resistance is the primary challenge for chemotherapy.
A number of molecular mechanisms have been suggested to be the underlying reason for drug resistance, including enhanced DNA damage repair ability, apoptosis inactivation, target mutation or deletion, angiogenesis, transporter-mediated drug efflux and autophagy (3,4). Autophagy, which plays an intricate role in cell death and survival, has recently been considered a potential mechanism underlying chemotherapeutic resistance in cancer cells (5). Autophagy has dual functions in tumour resistance: Chemotherapeutic drugs may either induce autophagic cell death, or induce protective autophagy to promote cell survival by recovering metabolites, saving energy and avoiding oxidative damage (5,6). The role of autophagy in chemotherapy is complex and depends on the tumour and drug type as well as the basic autophagy process (6–8).
High mobility group box 1 (HMGB1), a member of the HMGB superfamily, has a tripartite structure composed of an A box, a B box and a C-terminal acidic tail (9). As a chromatin-associated protein, HMGB1 is widely present in eukaryotic nuclei, and it plays an important role in the cytoplasm and extracellular space (10,11). HMGB1 relocation is crucial for cell survival and death (12–16). A number of studies have demonstrated that upregulated HMGB1 expression or increased release of HMGB1 promotes drug resistance in numerous different types of cancer, such as leukaemia, lung cancer and osteosarcoma (10,17–19). These findings suggest that HMGB1 is a potential target for chemotherapy.
Unlike other secretory proteins, HMGB1 induces atypical lysosome-mediated vesicle transport via lysophosphatidylcholine due to the lack of signal peptides (20,21). HMGB1 migration from the nucleus to the cytoplasm is the most important step in this process. Among the mechanisms regulating HMGB1 translocation, such as post-translational modifications and the pathways for calcium signalling, reactive oxygen species signalling, janus kinase (JAK)-signal transducer and activator of transcription (STAT) signalling, p53 and inflammasomes, the association between HMGB1 post-transcriptional modification and translocation is currently the most well-known (21–25). Poly (ADP-ribose) polymerase (PARP1) is the most important ADP-ribosomal polymerase, and it catalyses the transfer of ADP-ribose moieties from NAD+ to itself and other acceptor proteins (26). PARP1 plays a critical role in the cytoplasmic translation of HMGB1 via poly (ADP-ribosylation) (16,26). Preliminary studies have demonstrated that poly (ADP-ribosylation) of HMGB1 promotes its acetylation and, thus, facilitates HMGB1 translocation (27,28). Furthermore, acetylation of the lysine residues in HMGB1 is believed to be a precondition for HMGB1 translocation into the cytoplasm (29); however, the specific molecular mechanism underlying this requires further study.
In the present study, it was demonstrated that chemotherapeutic drugs could induce leukaemic cells to undergo cytoprotective autophagy, thus inducing drug resistance. HMGB1 translocation represents a decisive step in chemotherapy-induced autophagy. In addition, the association between poly (ADP-ribosylation) and HMGB1 acetylation was investigated in the present study, and it was revealed that poly (ADP-ribosylation) of HMGB1 affects its acetylation and promotes HMGB1 translocation-associated chemotherapy-induced autophagy in leukaemia cells. These results suggest that inhibiting HMGB1 translocation may increase chemotherapeutic efficacy and aid in overcoming drug resistance.
Materials and methods
Antibodies and reagents
The antibody specific for acetylated lysine (catalogue no. 441S) and the rabbit monoclonal antibody IgG XPTM isotype control (catalogue no. 3900S) were obtained from Cell Signaling Technology, Inc. The antibody specific for PAR (catalogue no. 4336-BPC-100) was obtained from Trevigen. Antibodies specific for HMGB1 (catalogue no. H9539), p62 (catalogue no. P0067), LC3 (catalogue no. L8918) and anti-Flag antibodies (catalogue no. F1804) were obtained from Sigma-Aldrich; Merck KGaA. Acetylated-lysine antibody (catalogue no. 9441S) was obtained from Cell Signaling Technology, Inc. PAR antibody (catalogue no. 4336-BPC-100) was obtained from Trevigen. Antibodies specific for β-actin (catalogue no. 7D2C10), laminB (catalogue no. 12987-1-AP), and tubulin (catalogue no. 11224-1-AP) were obtained from ProteinTech Group, Inc. All aforementioned antibodies were diluted to 1:1,000 to detect the target protein. The secondary antibodies, including sheep anti-mouse IgG-HRP (catalogue no. RM3001), sheep anti-rabbit IgG-HRP (catalogue no. RM3002) were obtained from Beijing Ray Antibody Biotech. Cy3-conjugated secondary antibody (dilution, 1:50; catalogue no. ZF-0134) were obtained from Zsbio Commerce Store (http://www.zsbio.com). IPKine horseradish peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG Light Chain (1:10,000; catalogue no. A25012; Abbkine Scientific Co., Ltd.) was used as a secondary antibody to detect target proteins without interference from denatured IgG in the western blot. Daunorubicin (DNR) was purchased from MedChemExpress.
Cell culture
The Jurkat and RS4:11 human acute leukaemic cell lines were purchased from the Type Culture Collection of the Chinese Academy of Sciences. Jurkat and RS4:11 cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) with 10% foetal bovine serum (HyClone; GE Healthcare Life Sciences), 2 mM L-glutamine, and 1% penicillin/streptomycin (HyClone; GE Healthcare Life Sciences) at 37°C in an atmosphere of 5% CO2.
Drug treatment
Jurkat cells were treated with DNR (0, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 or 6.4 µM/ml) or DNR (0.4 µM/ml) for 24 h. In the pre-experiment, Jurkat and RS4:11 cells were treated with DNR for 24 h, however it was revealed that RS4:11 cells were in a very poor state and almost all cells died, which affected the stability of the experimental results (data not shown). Therefore, in the subsequent experiments, RS4:11 cells were treated with DNR (0, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 or 6.4 µM/ml) or DNR (0.4 µM/ml) for 12 h. Jurkat cells were transfected with lentivirus, and HMGB1NC, HMGB1MT1, HMGB1MT2 and HMGB1WT cells were treated with or without DNR (0.4 µM) for 24 h.
Cell viability analysis
Cells were plated in 96-well plates at a density of 5×104/ml. Cell viability was measured using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc.) following chemotherapeutic drug treatment according to the manufacturer's protocol.
Western blot analysis
The two cell lines were subjected to the aforementioned different DNR concentrations, collected and lysed with RIPA buffer solution (Beyotime Institute of Biotechnology). The protein concentration was determined by BCA method (Beyotime Institute of Biotechnology). The samples (30 µg) were separated via SDS-PAGE (10 or 12% gel) and transferred onto a polyvinylidene fluoride (PVDF) membrane (EMD Millipore). After blocking with 5% non-fat dried milk for 1 h at room temperature, the membranes were incubated with primary antibodies, including HMGB1, p62, LC3II/I, Acetylated-lysine antibody and PAR antibody, diluted to 1:1,000 overnight at 4°C. Secondary antibodies, including sheep anti-mouse IgG-HRP and sheep anti-rabbit IgG-HRP were applied at a 1:5,000 dilution and IPKine horseradish peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG Light Chain were applied at a 10,000 dilution for 1 h at room temperature. β-actin and tubulin were used as loading controls to detect the expression of whole protein. β-actin was also used as an internal reference to detect the expression of cytoplasmic protein, while laminB was used to detect the expression of nuclear protein. The target protein expressions were detected with an enhanced chemiluminescence reagent (EMD Millipore) using a G:BOX XT4 system (Syngene).
Lentivirus infection
Experiments were performed in 6-well plates at a density of 5×106 cells/well. Lentiviruses were purchased from Obio Technology. In the present study, the lysine residues were mutated at amino acids 28, 29, 30, 180, 182, 183, 184 and 185 in HMGB1 to alanine in order to generate mutant type-1 cells (HMGB1MT1), and the glutamate residues were mutated at amino acids 40, 47 and 179 to alanine to generate mutant type-2 cells (HMGB1MT2), however not all the lysine residues and glutamate residues in HMGB1. Jurkat cells were transformed with lentiviruses: Normal control (NC), pLenti-EF1a-EGFP-F2A-Puro-CMV-MCS; wild type (WT), pLenti-EF1a-EGFp-P2A-Puro-CMV-HMGB1-3Flag; mutant type 1 (MT1), pLenti-EF1a-EGFp-P2A-Puro-CMV-HMGB1 mut1-3Flag; mutant type2 (MT2), pLenti-EF1a-EGFp-P2A-Puro-CMV-HMGB1 mut2-3Flag. According to the manufacturer's protocol provided by OBiO Technology Corp., Ltd., the transfection was performed using a lentivirus with 5 µg/ml polybrene (OBiO Technology Corp., Ltd.) and antibiotic selection was performed using 1 µg/ml puromycin (Beijing Solarbio Science & Technology Co., Ltd.). Proteins were subjected to immunoprecipitation with Protein G Magnetic Beads (Bimake; http://biotool.cn) anti-HMGB1 antibodies (1:50; cat. no. H9539; Sigma-Aldrich; Merck KGaA) at 4°C overnight and the lentivirus expression was detected with anti-Flag antibodies. Cells were cultured at 37°C in a humidified incubator containing 5% CO2 for 3–5 generations and then used for subsequent experiments.
Immunoprecipitation analysis
Cells subjected to the different treatments were collected and lysed with RIPA buffer solution. The whole-cell lysates (1,000 µg) were precleared with Protein G Magnetic Beads for 1 h at room temperature, then incubated with normal control IgG or anti-HMGB1 antibodies (cat. no. H9539; 1:50; Sigma-Aldrich; Merck KGaA) at 4°C overnight to form immune complexes. The samples were then added to a Protein G Magnetic Bead reaction to capture the immune complexes. After washing with wash buffer, the samples were removed under denaturing conditions in 50 µl of 2X SDS sample buffer and boiled at 100°C for 10 min. The substrate was collected and analysed via western blotting as described above. In this study, the cell lysates were subjected to a pull-down assay with Rabbit Control IgG (catalogue no. AC005; control group) or HMGB1 antibody (cat. no. H9539; 1:50; Sigma-Aldrich; Merck KGaA) at 4°C overnight and then the cell lysates were immunoblotted with anti-acetylated lysine (1:1,000; cat. no. 9441S; Cell Signaling Technology, Inc.), anti-poly (ADP-ribosylation) (1:1,000; cat. no. 4336-BPC-100; Trevigen) and anti-HMGB1 antibodies (cat. no. H9539; 1:1,000; Sigma-Aldrich; Merck KGaA) 4°C overnight.
Cytoplasmic and nuclear extract preparation
Cytoplasmic and nuclear extracts were prepared using a Nuclear and Cytoplasmic Protein Extraction kit (Beyotime Institute of Biotechnology). Cells subjected to the different treatments were collected. After washing 3 times with ice-cold PBS, the cells were resuspended in 200 µl of ice-cold cytoplasmic extraction buffer A for 10 min. The cell lysates were incubated with cytoplasmic extraction buffer B for 1 min in an ice bath, vortexed for 5 sec, and centrifuged at 12,000 × g for 5 min at 4°C. Supernatants were aliquoted and stored at −80°C. Nuclear pellets were then resuspended in 50 µl of nuclear extraction buffer. The lysates were vortexed four times for 15 sec at 7-min intervals, and then centrifuged at 12,000 × g for 5 min at 4°C. Nuclear extracts were aliquoted and stored at −80°C.
Immunofluorescence analysis
Cells were collected, fixed with 4% formaldehyde at 4°C for 15 min, permeabilised in 0.3% Triton X-100 in PBS for 10 min, and incubated in 5% BSA blocking buffer for 1 h at room temperature. The cells were then incubated with primary antibody in 1% bovine serum albumin [Boster Biological Technology Co. Ltd. (http://www.boster.com.cn/about/index.html)] overnight at 4°C. The cells were washed three times with 1% Tween in PBS, then incubated with a Cy3-conjugated secondary antibody [dilution, 1:50; catalogue no. ZF-0134; Zsbio Commerce Store (http://www.zsbio.com)] for 1 h at room temperature in the dark, then incubated with DAPI for 5 min. The cells were resuspended and added to a laser confocal petri dish. Images were captured with a confocal microscope (Carl Zeiss, Inc.) at a magnification of ×40.
The translocation of HMGB1
By examining the expression of HMGB1 in the nucleus and cytoplasm through western blot analysis, and using immunofluorescence technology, the translocation of HMGB1 could be detected. When the nucleus HMGB1 translocated to the cytoplasm, the HMGB1 protein increased in the cytoplasm, whereas it decreased in the nucleus. Similarly, through immunofluorescence, it was observed that when HMGB1 was transferred from nucleus to cytoplasm, the fluorescence intensity in the cytoplasm increased, while the fluorescence intensity decreased in the nucleus.
Transmission electron microscopy
The cells were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/l phosphate buffer (pH 7.4) at 4°C for 2 h, followed by 1% OsO4. After dehydration and Epon-812:100% acetone embedding at room temperature, thin sections (50–80 nm) were stained with uranyl acetate and lead citrate respectively at 4°C for 15 min, and viewed under a Tecnai G2 Spirit Twin election microscope (FEI; Thermo Fisher Scientific, Inc).
Statistical analysis
All data are expressed as the mean ± standard deviation. Paired Student's t-test was used for comparisons between two groups and one-way analysis of variance (ANOVA) was performed for comparisons between more than two groups. When the ANOVA was significant, a Tukey post-hoc test was performed. P<0.05 was considered to indicate a statistically significant result. All statistical analyses were conducted by SPSS version 18.0 software (SPSS, Inc.).
Results
Chemotherapeutic drugs induce autophagy in leukaemia cells
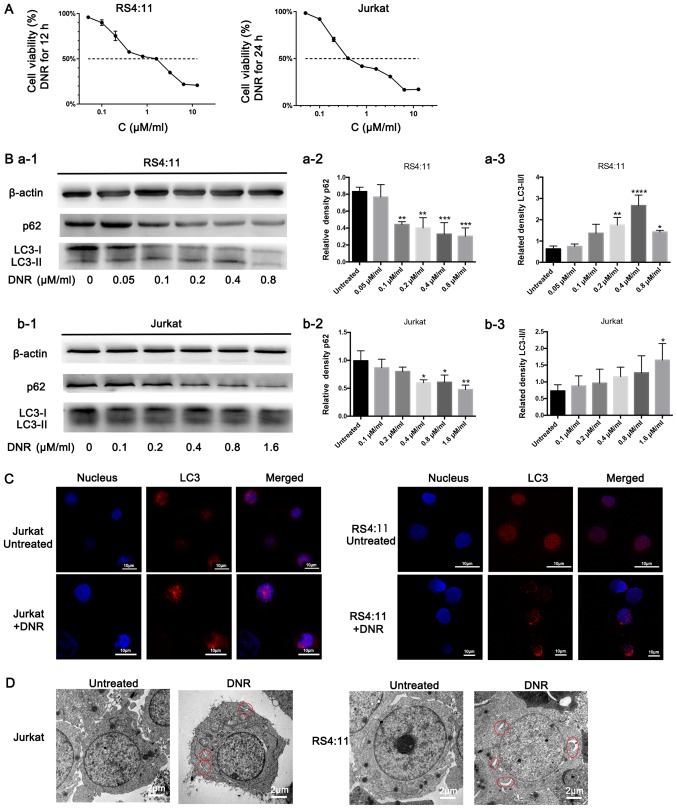
DNR is a major anti-tumour agent that is widely used to treat leukaemia. As presented in Fig. 1A, DNR significantly damaged the Jurkat and RS4:11 cells in a dose-dependent manner, particularly the RS4:11 cells, as RS4:11 cells were treated with DNR for 12 h only, while Jurkat cells were treated with DNR for 24 h. In order to investigate whether autophagy occurred when the leukaemia cells were treated with chemotherapeutic drugs, the present study conducted relevant experiments, and the results are presented below. Based on the western blot analysis, the chemotherapy-induced LC3-II/I ratio increased as the DNR concentration increased. The level of p62, an adaptor between the autophagy machinery and its substrates, gradually decreased as the drug concentrations increased (Fig. 1B). The immunofluorescence analysis revealed that the changes in endogenous LC3 puncta were consistent with the western blot analysis results in the two cell lines (Fig. 1C). Furthermore, the ultrastructural analysis revealed that chemotherapy-treated cells had more autophagosomes and autophagolysosomes during chemotherapy compared with the untreated cells in both cell lines (Fig. 1D). Fig. 1 demonstrates that the chemotherapeutic drugs induced autophagy in the leukaemia cells.
Figure 1.
Chemotherapeutic drugs induce autophagy in leukaemia cells. (A) Chemotherapeutic drugs damaged the leukaemia cells dose-dependently. Cell viability was measured using a Cell Counting Kit-8. (B) Cell lysates were subjected to western blotting to detect LC3-II/I and p62 expression. β-actin was used as the loading control. Quantified data are presented (p62 or LC3-II/I/β-actin). (a-1) Western blot diagram of RS4:11 cells. (a-2) p62 quantitative data of RS4:11 cells. (a-3) LC3II/I quantitative data of RS4:11 cells. (b-1) Western blot diagram of Jurkat cells. (b-2) p62 quantitative data of Jurkat cells. (b-3) LC3II/I quantitative data of Jurkat cells. (C) LC3 was stained via immunofluorescence and analysed under a confocal microscope to measure the LC3 puncta (LC3, Cy3 staining; nucleus, DAPI staining). (D) Cells were subjected to transmission electron microscopy to observe autophagosome-like structures (indicated by red circles). Data are the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001, compared with the untreated group. DNR, daunorubicin.
Translocation of HMGB1 is associated with chemotherapy-induced autophagy
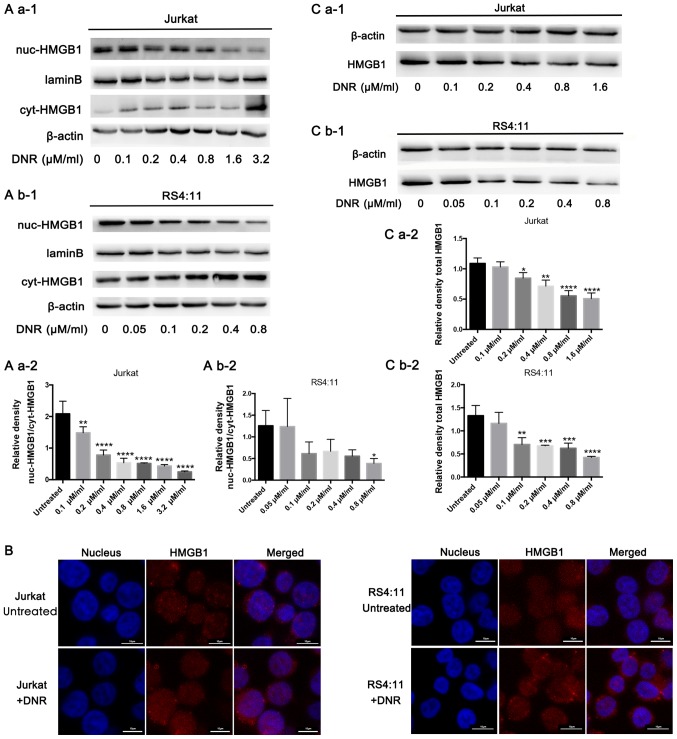
In order to investigate the potential role of HMGB1 in regulating chemotherapeutic drug anticancer activity, the present study focused on HMGB1 localisation. HMGB1 expression was detected in both the nucleus and cytoplasm separately via western blotting. In Jurkat cells, HMGB1 expression decreased in the nucleus but increased in the cytoplasm following DNR treatment. In the RS4:11 cells, HMGB1 expression decreased in the nucleus following DNR treatment. In addition, the expression of HMGB1 in the cytoplasm decreased at 0.05 and 0.2 µM/ml DNR concentration, but not significantly, and there was no significant change in HMGB1 expression in the cytoplasm at other DNR concentrations (Fig. 2A b-1 and b-2). This subcellular localisation of HMGB1 was detected indirectly using immunofluorescence techniques, through which red fluorescence was observed in the nucleus and cytoplasm following DNR treatment in the two cell lines, but the red fluorescence remained primarily in the nucleus without DNR treatment (Fig. 2B). After repeated experiments and statistical analyses, the results presented in Fig. 2A and B suggested that HMGB1 may be transferred from the nucleus to the cytoplasm in Jurkat and RS4:11 cells during chemotherapeutic treatment. In the present study, HMGB1 expression did not increase in Jurkat or RS4:11 cells following DNR stimulation. By contrast, Fig. 2C demonstrates that the HMGB1 protein levels in the Jurkat and RS4:11 cells decreased gradually as the DNR concentration increased (P<0.05). Therefore, it was speculated that the HMGB1 that had been released early was not a newly synthesised protein in the cytoplasm, but it was transferred from the nucleus to the cytoplasm and eventually released from the cell (10,30). These results suggest that HMGB1 translocation is necessary for chemotherapy-induced autophagy.
Figure 2.
HMGB1 translocation is associated with chemotherapeutic drug-induced autophagy. (A) Cell lysates were separated into cytosolic and nuclear fractions. Cytosolic and nuclear HMGB1 levels were assayed via western blotting. β-actin was used as a loading control to detect the expression of cytoplasmic protein, while laminB was used to detect the expression of nuclear protein. Quantified data are presented [(nuc-HMGB1/laminB)/(cyt-HMGB1/β-actin)]. (a-1) Western blot diagram of Jurkat cells. (a-2) nuc-HMGB1/cyt-HMGB1 quantitative data of Jurkat cells. (b-1) Western blot diagram of RS4:11 cells. (b-2) nuc-HMGB1/cyt-HMGB1 quantitative data of RS4:11 cells. (B) Intracellular HMGB1 was stained via indirect immunofluorescence and analysed under a confocal microscope to detect the location of HMGB1 (HMGB1, Cy3 staining; nucleus, DAPI staining). (C) Cell lysates were subjected to western blotting to detect HMGB1 expression. β-actin was used as a loading control. Quantified data are presented (HGMB1/β-actin). Data are the mean ± standard deviation of three independent experiments. (a-1) Western blot diagram of Jurkat cells. (a-2) Total HMGB1 quantitative data of Jurkat cells. (b-1) Western blot diagram of RS4:11 cells. (b-2) Total HMGB1 quantitative data of RS4:11 cells. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001, compared with the untreated group. HMGB1, high mobility group box protein 1; DNR, daunorubicin; Cyt, cytoplasm; Nuc, nucleus.
Poly (ADP-ribosylation) of HMGB1 facilitates its acetylation
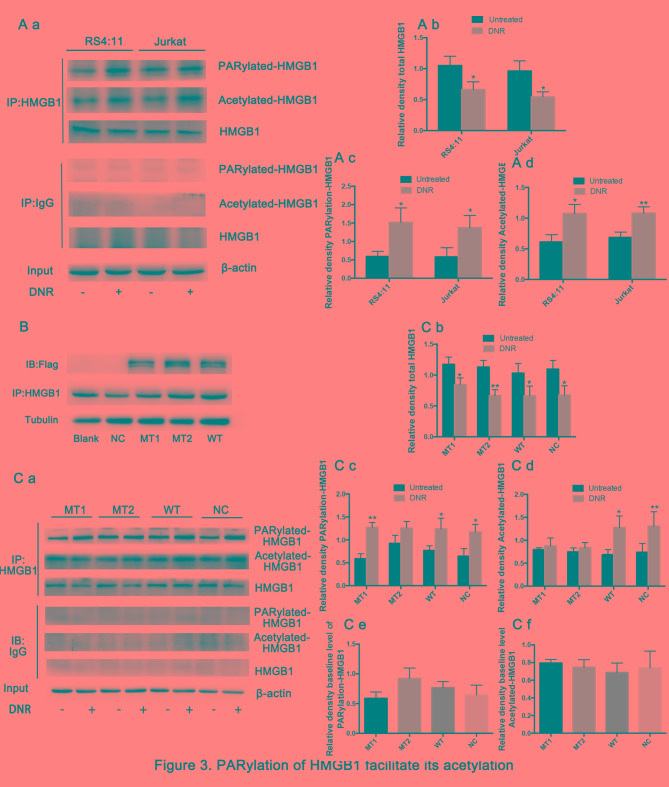
The present study then sought to further define the mechanisms by which HMGB1 translocation occurs. Previous studies have demonstrated that post-translational modifications, such as acetylation and ribosylation, are critical for HMGB1 translocation from the nucleus to the cytoplasm (31–33). Therefore, the present study immunoprecipitated the cell lysates with an HMGB1-specific antibody, and these immunoprecipitated proteins were immunoblotted with specific anti-poly (ADP-ribosylation) and anti-acetylation antibodies. Fig. 3A demonstrates that the total HMGB1 was decreased and the poly (ADP-ribosylation) and acetylation of HMGB1 increased significantly in both cell lines following DNR treatment. The aforementioned lysine and glutamate residues are primarily located near nuclear localisation sequences (NLS), which are frequently modified by acetylation and poly (ADP-ribosylation), respectively, and previous studies have demonstrated that they may be associated with the re-localization of HMGB1 (26,29,34). HMGB1 was enriched by immunoprecipitation, and specific anti-Flag antibodies were used to detect lentiviral expression via western blotting (Fig. 3B). HMGB1MT1, HMGB1MT2 and wild-type Jurkat cells (HMGB1WT) were successfully constructed. Theoretically, the expression level of HMGB1 protein in HMGB1MT1, HMGB1MT2 and HMGB1WT cells should be higher than that in normal control Jurkat cells (HMGB1NC) group, but in fact, this was not the case. Considering that HMGB1 is highly expressed in a number of different types of tumour cell, including leukaemia, these results indicated insignificant HMGB1 overexpression (10). Fig. 3C demonstrates that the total HMGB1 in different cells presented a decreasing trend following DNR treatment. However, HMGB1 acetylation and poly (ADP-ribosylation) were significantly increased following DNR treatment in HMGB1WT cells and normal control Jurkat cells (HMGB1NC). By contrast, following DNR treatment, the HMGB1 acetylation level remained low, while the HMGB1 poly (ADP-ribosylation) expression was increased in HMGB1MT1 cells, indicating that blocking HMGB1 acetylation did not affect its poly (ADP-ribosylation). In HMGB1MT2 cells, HMGB1 poly (ADP-ribosylation) did not increase following DNR treatment, and the degree of HMGB1 acetylation increased slightly, but there was no statistical significance. In addition, considering the data presented in Fig. 3A and C, the present study concluded that the poly (ADP-ribosylation) of HMGB1 may facilitate its acetylation.
Figure 3.
Poly (ADP-ribosylation) of HMGB1 facilitates its acetylation. (A) Cell lysates were immunoprecipitated with an HMGB1 antibody, followed by western blot analysis. The acetylation or poly (ADP-ribosylation) levels were measured using antibodies specific for acetylated lysine or poly (ADP-ribosylation). β-actin was used as a loading control. Quantified data are presented (HMGB1/β-actin, PARylation-HMGB1 or Acetylated-HMGB1/HMGB1/β-actin). (a Western blot diagram of RS4:11 and Jurkat cells. (b) Total HMGB1 quantitative data of RS4:11 and Jurkat cells. (c) PARylation-HMGB1 quantitative data of RS4:11 and Jurkat cells. (d) Aceytlation-HMGB1 quantitative data of RS4:11 and Jurkat cells. (B) HMGB1MT1, HMGB1MT2 and HMGB1WT cells were successfully constructed. The lysine residues at amino acids 28, 29, 30, 180, 182, 183, 184 and 185 of HMGB1 in HMGB1MT1 cells were mutated to alanine, and the glutamate residues at 40, 47 and 179 of HMGB1 in HMGB1MT2 cells were mutated to alanine. The whole protein of HMGB1MT1, HMGB1MT2, HMGB1WT, HMGB1NC and Jurkat cells were subjected to immunoprecipitation with anti-HMGB1 antibodies and then subjected to western blotting to detect the expression of lentivirus with anti-Flag antibodies. Tubulin was used as a loading control. (C) Cell lysates were subjected to a pull-down assay with an HMGB1 antibody and immunoblotted with anti-acetylated lysine, anti-poly (ADP-ribosylation) and anti-HMGB1 antibodies. β-actin was used as a loading control. Quantified data are presented (HMGB1/β-actin, PARylation-HMGB1 or Acetylated-HMGB1/HMGB1/β-actin). Data are the mean ± standard deviation of three independent experiments. (a) Western blot diagram of MT1, MT2, WT and NC cells. (b) Total HMGB1 quantitative data of MT1, MT2, WT and NC cells. (c) PARylation-HMGB1 quantitative data of MT1, MT2, WT and NC cells. (d) Aceytlation-HMGB1 quantitative data of MT1, MT2, WT and NC cells. *P<0.05, **P<0.01, Fig. 3Cb-d compared with the untreated group and Fig. 3Ce and f compared with the NC group. HMGB1, high mobility group box protein 1; DNR, daunorubicin; MT, mutant type; WT, wild type; NC, normal control; UT, untreated group.
Poly (ADP-ribosylation) and acetylation play important roles in HMGB1 translocation, which is required for chemotherapeutic drug-induced autophagy
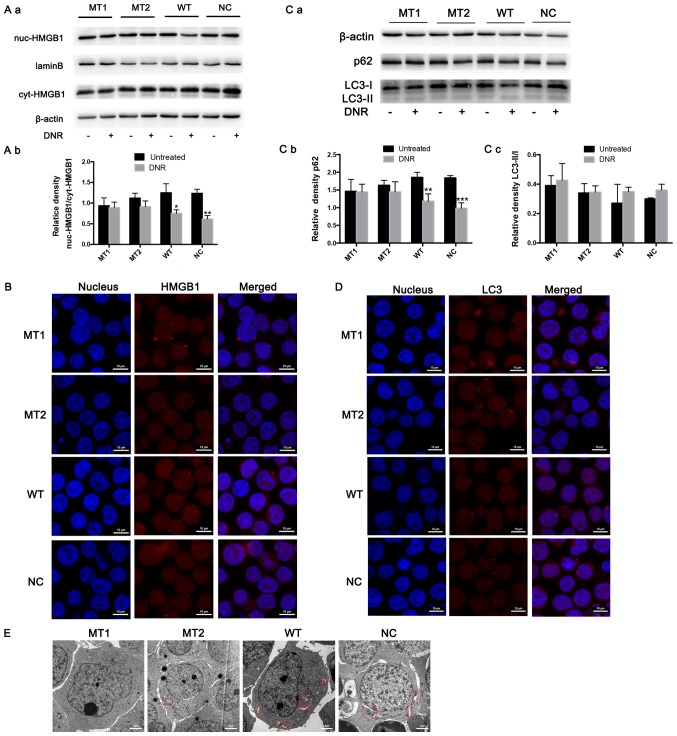
HMGB1 expression in the nucleus and cytoplasm was detected via western blotting. The results revealed that HMGB1 expression was significantly increased in the cytoplasm of HMGB1WT and HMGB1NC cells following DNR treatment; however, the HMGB1 expression in the nucleus and cytoplasm of HMGB1MT1 and HMGB1MT2 cells did not change significantly (Fig. 4A). In addition, following immunofluorescence labelling of HMGB1, red fluorescence was observed in the nucleus and cytoplasm of the HMGB1WT and HMGB1NC cells following DNR treatment. However, in the HMGB1MT1 and HMGB1MT2 cells, the red fluorescence in the cytoplasm was weaker than that in the HMGB1WT and HMGB1NC cells following DNR treatment (Fig. 4B). To further demonstrate that HMGB1 translocation is associated with chemotherapy-induced autophagy, the present study conducted relevant experiments. The western blot analysis revealed that the LC3-II/I ratio increased (but not significantly) and that p62 levels decreased following DNR treatment in the HMGB1WT and HMGB1NC cells, while the LC3-II/I ratio and p62 level did not change significantly in the HMGB1MT1 or HMGB1MT2 cells (Fig. 4C). Immunofluorescence revealed that the changes in endogenous LC3 puncta indicated increased autophagy levels in the HMGB1WT and HMGB1NC cells, while the autophagy levels of the HMGB1MT1 and HMGB1MT2 cells remained low (Fig. 4D). Compared with the HMGB1WT and HMGB1NC cells, fewer autophagosomes and autophagolysosomes were observed in the HMGB1MT1 and HMGB1MT2 cells following chemotherapy, which was the same as the trend observed in the immunofluorescence analysis (Fig. 4E). Blocking the acetylation and poly (ADP-ribosylation) of HMGB1 significantly decreased the translocation of HMGB1 from the nucleus to the cytoplasm and inhibited the induction of autophagy during chemotherapy in Jurkat cells (Fig. 4). Combined with the data in Figs. 3 and 4, these results demonstrate that poly (ADP-ribosylation) of HMGB1 facilitated its acetylation, thereby inducing HMGB1 translocation and ultimately promoting chemotherapy-induced autophagy in leukaemia cells.
Figure 4.
HMGB1 translocation is associated with chemotherapeutic drug-induced autophagy. (A) Cell lysates were separated into cytosolic and nuclear fractions. Cytosolic and nuclear HMGB1 were assayed via western blotting. β-actin was used as a loading control to detect the expression of cytoplasmic protein, while laminB was used to detect the expression of nuclear protein. Quantified data are presented [(nuc-HMGB1/laminB)/(cyt-HMGB1/β-actin)]. (a) Western blot diagram of MT1, MT2, WT and NC cells. (b) nuc-HMGB1/cyt-HMGB1 quantitative data of J MT1, MT2, WT and NC cells. (B) Intracellular HMGB1 was stained via indirect immunofluorescence and analysed under a confocal microscope to detect the location of HMGB1 (HMGB1, Cy3 staining; nucleus, DAPI staining). (C) The cell lysates were subjected to western blotting to detect LC3-II/I and p62 expression. β-actin was used as a loading control. Quantified data are presented (p62 or LC3-II/I/β-actin). (a) Western blot diagram of MT1, MT2, WT and NC cells. (b) p62 quantitative data of MT1, MT2, WT and NC cells. (c) LC3II/I quantitative data of MT1, MT2, WT and NC cells. (D) LC3 was stained via indirect immunofluorescence and analysed under a confocal microscope to measure the LC3 puncta (LC3, staining with Cy3; nucleus, staining with DAPI). (E) Cells were subjected to transmission electron microscopy to observe autophagosome-like structures (indicated by red circles). Data are the mean ± standard deviation of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared with the untreated group. HMGB1, high mobility group box protein 1; MT, mutant type; WT, wild type; NC, normal control; UT, untreated group; DNR, daunorubicin.
Discussion
Drug resistance in leukaemia cells is a primary factor leading to the development of refractory and recurrent leukaemia. A number of studies have demonstrated that drug resistance in leukaemia is partly attributable to autophagy induced by multiple chemotherapeutic drugs. For example, inhibiting autophagy by pharmacological inhibitors or genetic knockdown of critical autophagy-associated genes, such as Atg5 and Atg7, could enhance the anticancer effects of chemotherapeutic drugs (35,36). DNR is one of the most widely used chemotherapy drugs for leukaemia, and the cytotoxicity mediated by DNR is thought to result from drug-induced DNA damage (37). Chen et al (38) and Kudoh et al (39) suggested that DNR also triggers a pathway that negatively regulates apoptosis, and the phospholipase C-dependent diacylglycerol (DAG)/raf-1/mitogen-activated protein kinase (MEK) cascade and the DAG independent phosphoinositide 3-kinase (PI3K)/protein kinase C ζ type cascade play significant roles in this process. raf-1/MEK and PI3K are believed to be involved in autophagy signalling (40,41). Han et al (35) demonstrated for the first time that DNR can induce cytoprotective autophagy in K562 cells by activating the MEK/extracellular signal-regulated kinase-1 signalling pathway.
In the present study, it was difficult to detect changes in LC3-II protein levels in leukaemia cells via western blotting (Figs. 1B and 4C). Cytoplasmic LC3 forms LC3-I by enzymatic hydrolysis of a small segment of polypeptide, which then binds to Phosphatidylethanolamine (PE) and converts to membrane LC3-II (36). Therefore, it was speculated that the LC3-II protein was difficult to detect for the following reasons: i) The cytoplasm of leukaemia cells is small and the membrane protein is difficult to dissolve in the conventional RIPA list; ii) LC3-II, as a part of autophagy, fuses with lysosome to form autophagic lysosome and degrades due to the autophagy (42). Autophagy is a highly dynamic, multi-step process. Although it is difficult to obtain a satisfactory and convincing result regarding the increase in the chemotherapy-induced LC3-II/I using western blotting alone as indicated in Fig. 1B, by combining the results of the p62 level via western blotting (Fig. 1B), immunofluorescence (Fig. 1C) and transmission electron microscopy (Fig. 1D), conclusions could be drawn that indicated that the level of chemotherapeutic-induced autophagy was increased in leukaemia cells. Consistent with these results, the present study revealed that DNR triggered both apoptosis and autophagy in leukaemia cells (Fig. 1).
HMGB1 acts as both a tumour suppressor and an oncogenic factor in tumourigenesis and cancer therapy (43). Bell et al (44) demonstrated that HMGB1 appears in the medium of Jurkat and U937 cells time-dependently following chemotherapeutic drug treatment. In addition, high HMGB1 expression is suggested to be closely associated with tumour occurrence, and plays an important role in regulating tumour cell autophagy and apoptosis (10,45). Tang et al (46) demonstrated that in human pancreatic and colon cancer cells, anticancer drugs such as melphalan and paclitaxel could enhance the autophagy production of tumour cells by increasing the release of HMGB1 and its binding to the receptor for advance glycation endproducts. Zhan et al (47) demonstrated that the chemotherapeutic drug vincristine can promote the release of HMGB1 in gastric cancer cells and upregulate the expression of Mcl-1 protein in the Bcl-2 protein family, thereby producing anti-apoptotic effects. HMGB1 in breast cancer cells can promote cell tolerance in chemotherapy and chemoradiotherapy. Luo et al (48) demonstrated that miR-129-5p can enhance the efficacy of radiotherapy by targeting HMGB1 to decrease autophagy caused by breast cancer radiotherapy. Liu et al (13) and Hu et al (49) demonstrated that treatment with an HMGB1-neutralising antibody improved the sensitivity of leukaemia cells to chemotherapy, while exogenous HMGB1 made the cells more resistant to drug-induced cytotoxicity. In the present study, there was no significant upregulation of HMGB1 observed in the whole-cell protein samples following DNR treatment, but the HMGB1 protein may have transferred from the nucleus to the cytoplasm (Figs. 2, 3A and C, and 4A and B).
Previous studies have demonstrated that HMGB1 is highly expressed in a number of different types of tumour, including leukaemia, and it is more abundant on the surface of metastatic tumour cell membranes (10) and is closely associated with chemotherapy-induced drug resistance (12). HMGB1 is believed to regulate autophagy at multiple levels via different subcellular localisations (17,46,50). Although the function of HMGB1 in the cytoplasm remains unclear, evidence suggests that the primary function of HMGB1 in the cytoplasm is to provide positive regulatory factors for autophagy, as was first reported in 2010 (45). A previous study demonstrated that HMGB1 is translocated from the nucleus to the cytoplasm following chemotherapeutic treatment in leukaemia cells, and cytoplasmic HMGB1 then promotes the dissociation of Beclin1-Bcl-2 complexes and modifies Beclin1 binding to PI3k catalytic subunit 3, thus initiating autophagosome formation and upregulating autophagy (17,25). Figs. 1, 2 and 4 suggest that HMGB1 is an important regulator of chemotherapy-induced autophagy and that HMGB1 translocation is a key step in this mechanism.
HMGB1 contains two NLS and two nuclear export sequences (NES), and post-translational modification of amino acids on NLS and NES can result in the relocation of HMGB1 (20,32). In the present study, acetylation and poly (ADP-ribosylation) of HMGB1 were increased during chemotherapy-induced autophagy (Fig. 3A). This drew focus towards the post-transcriptional modifications of HMGB1 in the present study, including methylation, acetylation, phosphorylation and poly (ADP-ribosylation), which are key steps in HMGB1 translocation from the nucleus to the cytoplasm and its eventual release into the extracellular space (31). Different post-transcriptional modifications play important but varied roles in localising HMGB1 in the cytoplasm, but it is currently unclear which modification is dominant. Acetylation is regarded as a prerequisite for HMGB1 migration to the cytoplasm. Sterner et al (51) and Bonaldi et al (29) observed that the acetylation of certain lysine residues in NLS promotes HMGB1 migration from the nucleus to the cytoplasm and active secretion of HMGB1 to the extracellular space. Lu et al (24) demonstrated that the JAK/STAT1 signalling pathway played a key role in HMGB1 hyperacetylation and cytoplasmic accumulation. Pan et al (52) and Dhupar et al (53) revealed that interferon regulatory factor 1 promoted HMGB1 acetylation through histone acetyltransferases, which was required for lipopolysaccharide (LPS)-induced HMGB1 cytoplasmic accumulation and release. Consistent with these results, the present study revealed that in HMGB1MT1 cells, HMGB1 translocation was inhibited (Fig. 4A and B).
Poly (ADP-ribosylation) is one of the most important methods of protein posttranslational modification (54). Ditsworth et al (34) suggested that in a DNA-alkylating damage model, PARP1 activity played a role in the nuclear-to-cytosolic translocation of HMGB1. Davis et al (26) reported that LPS and alkylating agents could induce PARP1 activation and ADP-ribosylation to ultimately release HMGB1. Consistent with these results, the present study revealed that the level of HMGB1 poly (ADP-ribosylation) was not upregulated in HMGB1MT2 cells, and HMGB1 expression in the cytoplasm did not change significantly compared with the untreated group. Furthermore, immunofluorescence in the HMGB1 sublocalisation did not change. Therefore, it can be concluded that HMGB1 translocation from the nucleus to the cytoplasm depends on the poly (ADP-ribosylation) of HMGB1 (Figs. 3C and 4A and B).
An increasing number of studies have demonstrated that acetylation or poly (ADP-ribosylation) can promote HMGB1 translocation, but few studies have addressed the interaction between these modifications. Recently, Yang et al (27) reported for the first time that PARP1 can promote HMGB1 acetylation through poly (ADP-ribosylation). However, the association between poly (ADP-ribosylation) and acetylation and the role of these modifications in HMGB1 translocation in leukaemia remain unclear. Figs. 3B and 4A and B demonstrate that in HMGB1MT1 cells, although the level of poly (ADP ribosylation) modification increased, HMGB1 expression in the cytoplasm did not increase compared with that in the untreated group, and no changes occurred in the sublocalisation of HMGB1 via immunofluorescence. This suggests that poly (ADP ribosylation) modification of HMGB1 facilitates its acetylation but is insufficient to induce HMGB1 translocation. The present study hypothesised that the possible mechanism for this poly (ADP-ribosylation)/acetylation-controlled distribution was that the poly ADP-ribosylation of the glutamic acid residues altered the conformation of the A box, thereby exposing the acetylation site and promoting acetylation of the lysine residues in HMGB1. These HMGB1 modifications decrease its ability to bind to DNA and promote its translocation.
Combining the results of previous studies with those of the present, the process of chemotherapy-induced autophagy in leukaemic cells can be elucidated; revealing that poly (ADP-ribosylation) of HMGB1 enhanced HMGB1 acetylation and ultimately promoted chemotherapy-induced autophagy of leukaemic cells mediated by the HMGB1 translocation. Chemotherapeutic drugs are thought to induce apoptosis that is dependent on nuclear HMGB1 induction (50).
The association between HMGB1 and chemotherapeutic drug-induced autophagy has been extensively studied and understood, but the role of HMGB1 in the cytoplasm remains to be poorly understood (17,45). In addition, studies focussing on the underlying molecular mechanism of influencing HMGB1 translocation primarily concentrated on inflammation, sepsis and other diseases, and rarely focussed on leukaemia (16,26). As a specific type of tumour, leukaemia is different from solid tumours. Particularly in T-ALL, despite targeted gene therapy and chimeric antigen receptor T-cell therapy, the prognosis of children with drug-resistant or relapsed leukaemia remains poor (55,56). In the present study, Jurkat cells, an acute T-lymphoblastic leukaemia cell line, were mutated to verify that poly (ADP-ribosylation) of HMGB1 facilitated its acetylation and promoted HMGB1 translocation-associated chemotherapy-induced autophagy, which will ultimately provide a theoretical basis and new therapeutic targets for drug resistance in T-ALL. Therefore, the present study is of great significance in the clinical treatment of T-ALL and the therapeutic strategies that inhibit HMGB1 transfer to decrease HMGB1 expression in the cytoplasm and extracellular space are promising and may improve the effectiveness of chemotherapy.
The present study demonstrated that poly (ADP-ribosylation) of HMGB1 facilitates its acetylation, thereby inducing HMGB1 translocation and ultimately promoting chemotherapy-induced autophagy in leukaemic cells.
Acknowledgements
The authors would like to thank Dr Sarah Conte, for her assistance in revising an earlier version of this manuscript.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81570140) and the Doctoral Start-up Fund of Natural Science Foundation of Guangdong Province (grant no. 2016A030310161).
Availability of data and materials
The datasets used and/or analysed during the present are available from the corresponding author on reasonable request.
Authors' contributions
YL and JF designed the study. YL and JX collated and analysed the data. YL wrote the manuscript. XL and JF participated in revising manuscript. XL also performed the study and collected background information. All authors read and approved the final submitted manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2016;16:494–507. doi: 10.1038/nrc.2016.63. [DOI] [PubMed] [Google Scholar]
- 2.Richter-Pechańska P, Kunz JB, Hof J, Zimmermann M, Rausch T, Bandapalli OR, Orlova E, Scapinello G, Sagi JC, Stanulla M, et al. Identification of a genetically defined ultra-high-risk group in relapsed pediatric T-lymphoblastic leukemia. Blood Cancer J. 2017;7:e523. doi: 10.1038/bcj.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 4.Khamisipour G, Jadidi-Niaragh F, Jahromi AS, Zandi K, Hojjat-Farsangi M. Mechanisms of tumor cell resistance to the current targeted-therapy agents. Tumour Biol. 2016;37:10021–10039. doi: 10.1007/s13277-016-5059-1. [DOI] [PubMed] [Google Scholar]
- 5.Nencioni A, Cea M, Montecucco F, Longo VD, Patrone F, Carella AM, Holyoake TL, Helgason GV. Autophagy in blood cancers: Biological role and therapeutic implications. Haematologica. 2013;98:1335–1343. doi: 10.3324/haematol.2012.079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evangelisti C, Evangelisti C, Chiarini F, Lonetti A, Buontempo F, Neri LM, McCubrey JA, Martelli AM. Autophagy in acute leukemias: A double-edged sword with important therapeutic implications. Biochim Biophys Acta. 2015;1853:14–26. doi: 10.1016/j.bbamcr.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 7.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gump JM, Staskiewicz L, Morgan MJ, Bamberg A, Riches DW, Thorburn A. Autophagy variation within a cell population determines cell fate through selective degradation of Fap-1. Nat Cell Biol. 2014;16:47–54. doi: 10.1038/ncb2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange SS, Vasquez KM. HMGB1: The jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog. 2009;48:571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, Yu Y, Fan XG, Yan Z, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi ME, Agresti A. HMG proteins: Dynamic players in gene regulation and differentiation. Curr Opin Genet Dev. 2005;15:496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, Xie M, Yin X, Livesey KM, Lotze MT, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25:23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- 14.Stevens NE, Chapman MJ, Fraser CK, Kuchel TR, Hayball JD, Diener KR. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci Rep. 2017;7:5850. doi: 10.1038/s41598-017-06205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee W, Kwon OK, Han MS, Lee YM, Kim SW, Kim KM, Lee T, Lee S, Bae JS. Role of moesin in HMGB1-stimulated severe inflammatory responses. Thromb Haemost. 2015;114:350–363. doi: 10.1160/TH14-11-0969. [DOI] [PubMed] [Google Scholar]
- 16.Lu B, Wang H, Andersson U, Tracey KJ. Regulation of HMGB1 release by inflammasomes. Protein Cell. 2013;4:163–167. doi: 10.1007/s13238-012-2118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong Q, Xu LH, Xu W, Fang JP, Xu HG. HMGB1 translocation is involved in the transformation of autophagy complexes and promotes chemoresistance in leukaemia. Int J Oncol. 2015;47:161–170. doi: 10.3892/ijo.2015.2985. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Yang L. The function and mechanism of HMGB1 in lung cancer and its potential therapeutic implications. Oncol Lett. 2018;15:6799–6805. doi: 10.3892/ol.2018.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingston JA, Wang WL, Tsai JW, Lazar AJ, Leung CH, Lin H, Advani S, Daw N, Santiago-O'Farrill J, Hollomon M, et al. Analysis of HSP27 and the autophagy marker LC3B+ puncta following preoperative chemotherapy identifies high-risk osteosarcoma patients. Mol Cancer Ther. 2018;17:1315–1323. doi: 10.1158/1535-7163.MCT-17-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito I, Fukazawa J, Yoshida M. Post-translational methylation of high mobility group box 1 (HMGB1) causes its cytoplasmic localization in neutrophils. J Biol Chem. 2007;282:16336–16344. doi: 10.1074/jbc.M608467200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008;181:5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Antoine DJ, Kwan K, Lundbäck P, Wähämaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci USA. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livesey KM, Kang R, Vernon P, Buchser W, Loughran P, Watkins SC, Zhang L, Manfredi JJ, Zeh HJ, III, Li L, et al. p53/HMGB1 complexes regulate autophagy and apoptosis. Cancer Res. 2012;72:1996–2005. doi: 10.1158/0008-5472.CAN-11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis K, Banerjee S, Friggeri A, Bell C, Abraham E, Zerfaoui M. Poly(ADP-ribosyl)ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol Med. 2012;18:359–369. doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z, Li L, Chen L, Yuan W, Dong L, Zhang Y, Wu H, Wang C. PARP-1 mediates LPS-induced HMGB1 release by macrophages through regulation of HMGB1 acetylation. J Immunol. 2014;193:6114–6123. doi: 10.4049/jimmunol.1400359. [DOI] [PubMed] [Google Scholar]
- 28.Yang M, Liu L, Xie M, Sun X, Yu Y, Kang R, Yang L, Zhu S, Cao L, Tang D. Poly-ADP-ribosylation of HMGB1 regulates TNFSF10/TRAIL resistance through autophagy. Autophagy. 2015;11:214–224. doi: 10.4161/15548627.2014.994400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ugrinova I, Pasheva EA, Armengaud J, Pashev IG. In vivo acetylation of HMG1 protein enhances its binding affinity to distorted DNA structures. Biochemistry. 2001;40:14655–14660. doi: 10.1021/bi0113364. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Wang Y. HMG modifications and nuclear function. Biochim Biophys Acta. 2010;1799:28–36. doi: 10.1016/j.bbagrm.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard SA, Jiang Y, Xiang LH, Zhou S, Wang J, Su Z, Xu H. Post-translational modifications of high mobility group box 1 and cancer. Am J Transl Res. 2017;9:5181–5196. [PMC free article] [PubMed] [Google Scholar]
- 33.VanPatten S, Al-Abed Y. High mobility group box-1 (HMGb1): Current wisdom and advancement as a potential drug target. J Med Chem. 2018;61:5093–5107. doi: 10.1021/acs.jmedchem.7b01136. [DOI] [PubMed] [Google Scholar]
- 34.Ditsworth D, Zong WX, Thompson CB. Activation of poly(ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem. 2007;282:17845–17854. doi: 10.1074/jbc.M701465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han W, Sun J, Feng L, Wang K, Li D, Pan Q, Chen Y, Jin W, Wang X, Pan H, Jin H. Autophagy inhibition enhances daunorubicin-induced apoptosis in K562 cells. PLoS One. 2011;6:e28491. doi: 10.1371/journal.pone.0028491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livesey KM, Tang D, Zeh HJ, Lotze MT. Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs. 2009;10:1269–1279. [PubMed] [Google Scholar]
- 37.Laurent G, Jaffrézou JP. Signaling pathways activated by daunorubicin. Blood. 2001;98:913–924. doi: 10.1182/blood.V98.4.913. [DOI] [PubMed] [Google Scholar]
- 38.Chen JJ, Wu R, Yang PC, Huang JY, Sher YP, Han MH, Kao WC, Lee PJ, Chiu TF, Chang F, et al. Profiling expression patterns and isolating differentially expressed genes by cDNA microarray system with colorimetry detection. Genomics. 1998;51:313–324. doi: 10.1006/geno.1998.5354. [DOI] [PubMed] [Google Scholar]
- 39.Kudoh K, Ramanna M, Ravatn R, Elkahloun AG, Bittner ML, Meltzer PS, Trent JM, Dalton WS, Chin KV. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000;60:4161–4166. [PubMed] [Google Scholar]
- 40.Klionsky DJ. Autophagy: From phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacquin E, Leclerc-Mercier S, Judon C, Blanchard E, Fraitag S, Florey O. Pharmacological modulators of autophagy activate a parallel noncanonical pathway driving unconventional LC3 lipidation. Autophagy. 2017;13:854–867. doi: 10.1080/15548627.2017.1287653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang R, Zhang Q, Zeh HJ, III, Lotze MT, Tang D. HMGB1 in cancer: Good, bad, or both? Clin Cancer Res. 2013;19:4046–4057. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell CW, Jiang W, Reich CF, III, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–C1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 45.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, III, Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhan Z, Li Q, Wu P, Ye Y, Tseng HY, Zhang L, Zhang XD. Autophagy-mediated HMGB1 release antagonizes apoptosis of gastric cancer cells induced by vincristine via transcriptional regulation of Mcl-1. Autophagy. 2012;8:109–121. doi: 10.4161/auto.8.1.18319. [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Chen J, He L. mir-129-5p attenuates irradiation-induced autophagy and decreases radioresistance of breast cancer cells by targeting HMGB1. Med Sci Monit. 2015;21:4122–4129. doi: 10.12659/MSM.896661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu YH, Yang L, Zhang CG. HMGB1-a as potential target for therapy of hematological malignancies. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:560–564. doi: 10.7534/j.issn.1009-2137.2014.02.055. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 50.Tang D, Kang R, Livesey KM, Kroemer G, Billiar TR, Van Houten B, Zeh HJ, III, Lotze MT. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sterner R, Vidali G, Allfrey VG. Studies of acetylation and deacetylation in high mobility group proteins. Identification of the sites of acetylation in HMG-1. J Biol Chem. 1979;254:11577–11583. [PubMed] [Google Scholar]
- 52.Pan PH, Cardinal J, Li ML, Hu CP, Tsung A. Interferon regulatory factor-1 mediates the release of high mobility group box-1 in endotoxemia in mice. Chin Med J (Engl) 2013;126:918–924. [PubMed] [Google Scholar]
- 53.Dhupar R, Klune JR, Evankovich J, Cardinal J, Zhang M, Ross M, Murase N, Geller DA, Billiar TR, Tsung A. Interferon regulatory factor 1 mediates acetylation and release of high mobility group box 1 from hepatocytes during murine liver ischemia-reperfusion injury. Shock. 2011;35:293–301. doi: 10.1097/SHK.0b013e3181f6aab0. [DOI] [PubMed] [Google Scholar]
- 54.Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: Where are we today and where are we going? Microbiol Mol Biol Rev. 2006;70:789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vadillo E, Dorantes-Acosta E, Pelayo R, Schnoor M. T cell acute lymphoblastic leukemia (T-ALL): New insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018;32:36–51. doi: 10.1016/j.blre.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Yuan T, Yang Y, Chen J, Li W, Li W, Zhang Q, Mi Y, Goswami RS, You JQ, Lin D, et al. Regulation of PI3K signaling in T cell acute lymphoblastic leukemia: A novel PTEN/Ikaros/miR-26b mechanism reveals a critical targetable role for PIK3CD. Leukemia. 2017;31:2355–2364. doi: 10.1038/leu.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present are available from the corresponding author on reasonable request.