Abstract
Cisplatin-based chemotherapy is the standard regimen for patients with bladder cancer, but its effectiveness is limited by high toxicity and the development of drug resistance. β-elemene (β-ELE), a compound extracted from Rhizoma zedoariae, has antitumor activity in various malignancies and exhibits low toxicity. However, the effects and specific mechanism of β-ELE in bladder cancer remain unclear. The present study aimed to investigate the antitumor activity and possible mechanisms of β-ELE alone and in combination with cisplatin in bladder cancer cells. Cell viability was determined using Cell Counting Kit-8. Cell cycle and reactive oxygen species (ROS) analyses were performed by flow cytometry. Apoptosis was detected by Hoechst 33258 and Annexin-V/propidium iodide staining. Mitochondrial membrane potential was determined by staining with a JC-1 probe, flow cytometry and fluorescence microscopy. Protein expression was detected by western blotting. The results revealed that β-ELE significantly inhibited the proliferation of various bladder cancer cell lines and induced cell cycle arrest at G0/G1-phase in T24 and 5637 cells. Compared with cisplatin alone, co-treatment with β-ELE increased cisplatin-mediated cytotoxicity against T24 cells, which resulted in the loss of mitochondrial membrane potential and release of cytochrome c into the cytoplasm. Co-treatment with β-ELE and cisplatin enhanced ROS accumulation and activation of 5′AMP-activated protein kinase (AMPK), which induced apoptosis. The results of the present study suggested that β-ELE inhibited the proliferation of bladder cancer cells in vitro and enhanced cisplatin-induced mitochondria-dependent apoptosis via the ROS-AMPK signaling pathway. Combination therapy with β-ELE requires further investigation as a potential treatment of bladder cancer.
Keywords: β-elemene, cisplatin, apoptosis, 5′AMP-activated protein kinase, bladder cancer
Introduction
Urothelial bladder cancer is the 10th most commonly diagnosed carcinoma, with an estimated 549,000 new cases and 200,000 cancer-related deaths worldwide in 2018 (1). Among newly diagnosed cases, >70% are non-muscle-invasive bladder cancer, which exhibits a satisfactory 5-year survival rate, but is prone to relapse (2). Up to 10–15% of bladder cancers progress to muscle invasion, which has a poor prognosis despite treatment, including radical cystectomy, adjuvant chemotherapy, radiation therapy and immunotherapy (3). Cisplatin (CDDP)-based chemotherapy is the standard first-line treatment for patients with muscle-invasive bladder cancer. Cisplatin is a platinum-based compound used to treat various malignancies such as lung, ovarian, head and neck and bladder cancer (4). However, its therapeutic efficacy in the clinic is inevitably reduced by intrinsic or acquired chemoresistance that develops by poorly understood molecular mechanisms.
Elemene (ELE) (1-methyl-1-vinyl-2, 4-diisopropenyl-cyclohexane) is a novel broad-spectrum antitumor agent extracted from the traditional Chinese medical herb Rhizoma zedoariae (5). The antitumor activity of β-ELE, a class II antitumor drug, has been evaluated in human solid tumors; combined with other chemotherapy drugs, β-ELE can reduce the dose of cytotoxic agents and the occurrence of side effects (6). β-ELE was approved by the State Food and Drug Administration of China for the treatment of certain types of cancer, including malignant brain tumors, in clinical practice (7). β-ELE suppresses tumor activity by decreasing the mitochondrial membrane potential to promote apoptosis, causing cell cycle arrest, promoting necrosis and inhibiting angiogenesis (6,8). β-ELE has also been reported to inhibit cell proliferation and enhance cisplatin-induced cell death in bladder cancer, but the mechanisms have not been described (9,10).
Apoptosis, or programmed cell death, eliminates defective cells to maintain a balance between cell proliferation and differentiation (11). Dysfunction of apoptosis can result in tumor growth and distant metastasis and may be involved in drug resistance. Apoptosis occurs by either an extrinsic pathway (mediated by the death receptor) or an intrinsic pathway (mediated by loss of mitochondrial membrane potential and translocation of cytochrome c) (11). Generation of reactive oxygen species (ROS) results in a reduction in the mitochondrial membrane potential, imbalance of the anti-apoptotic and pro-apoptotic members of the Bcl-2 family, release of cytochrome c from the mitochondria to the cytoplasm and activation of the downstream caspase cascades (12). A previous study reported that β-ELE promoted platinum intake and oxaliplatin-induced intrinsic apoptosis in hepatocellular carcinoma cells (13). The effects of β-ELE and its mechanism of action with regards to bladder cancer have not been reported. Hence, the present study investigated the antitumor activity of β-ELE alone or in combination with cisplatin in cultured bladder cancer cells.
Materials and methods
Reagents and cell culture
Human bladder cancer cell lines SW780, 5637, J82, UM-UC-3, TCCSUP and T24 and human ureteral epithelium immortalized cell line SV-HUC-1 were purchased from the American Type Culture Collection; RT4 cells were purchased from Procell Life Science & Technology Co., Ltd. The cells were cultured in RPMI-1640 medium (Corning, Inc.) with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml penicillin and 100 µg/ml streptomycin (cat. no. C0222; Beyotime Institute of Biotechnology) at 37°C with 5% CO2. β-ELE was obtained from Dalian Holley Kingkong Pharmaceutical Co., Ltd. Monoclonal antibodies specific for p21 (cat. no. 2947), p27 (cat. no. 3686), cyclin D1 (cat. no. 2978), CDK4 (cat. no. 2906), CDK6 (cat. no. 3136), phospho-Akt (Thr308) (cat. no. 13038), total Akt (cat. no. 4685), phospho-STAT3 (cat. no. 9145), total STAT3 (cat. no. 12640), Bax (cat. no. 5023), Bcl-2 (cat. no. 4223), cytochrome c (cat. no. 11940), caspase-9 (cat. no. 9508), caspase-3 (cat. no. 9662), cleaved caspase-3 (cat. no. 9664), cleaved caspase-9 (cat. no. 7237), cleaved poly (ADP-ribose) polymerase (PARP; cat. no. 9548), PARP (cat. no. 9532), phospho-AMPKα (cat. no. 2535) and total AMPK (cat. no. 5832) were purchased from Cell Signaling Technology, Inc. N-acetyl-l-cysteine (NAC; ROS scavenger) and compound C (a specific inhibitor of AMPK) were purchased from Selleck Chemicals. Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
Cell viability
SV-HUC-1 and seven types of bladder cancer cells (T24, 5637, TCCSUP, J82, UM-UC-3, RT4 and SW780 cells) were seeded into 96-well plates at density of 5×103 cells/well and cultured at 37°C with 5% CO2 for 24 h. Following treatment with β-ELE at varying concentrations (0, 6.25, 12.5, 25, 50, 100, 150 and 200 µg/ml) for 24 h, 10 µl CCK-8 solution was added into each well. The cells were incubated for 2 h at 37°C. Absorbance at 450 nm was measured using a Tecan Infinite F200/M200 type multifunction microplate reader (Tecan Group, Ltd.). Cell viability was calculated as follows: Cell viability=[mean optical density (OD) of experimental group/mean OD of control group] ×100%.
Assessment of apoptosis by Hoechst 33258 staining
Hoechst 33258 staining (Beyotime Institute of Biotechnology) was performed according to the manufacturer's instructions. T24 cells were seeded into 6-well plates at a density of 5×104 cells/well and cultured at 37°C with 5% CO2 for 24 h. Cells were treated with cisplatin (20 µM) and/or β-ELE (50 µg/ml) at 37°C for 24 h, washed three times with PBS and incubated with Hoechst 33258 (10 µg/ml) for 10 min at room temperature (RT) in the dark. Morphological changes in nuclei for the evaluation of apoptosis were observed under a fluorescence microscope using a blue filter.
Cell cycle analysis
T24 and 5637 cells were seeded into 6-well plates at density of 5×104 cells/well and cultured at 37°C with 5% CO2 for 24 h. Cells were treated with different concentrations of β-ELE (0, 25, 50 and 75 µg/ml) at 37°C for 24 h, washed twice with PBS and fixed in cold 70% (v/v) ethanol for 48 h at 4°C. Prior to analysis, cells were washed with PBS, incubated with 100 µl RNase A (0.1 mg/ml) for 30 min at 37°C, stained with 2 µl propidium iodide (PI; Sigma-Aldrich; Merck KGaA) and incubated for another 30 min at RT in the dark according to the manufacturer's instructions. Samples were analyzed by flow cytometry using CellQuest™ Pro software (version 5.1 BD CellQuest Pro Software, BD Biosciences).
Flow cytometry for apoptosis detection
T24 cells were seeded into 6-well plates at density of 5×104 cells/well and cultured at 37°C with 5% CO2 for 24 h. Cells were incubated with cisplatin (20 µM) and/or β-ELE (50 µg/ml) for 24 h at 37°C, harvested and washed with PBS, re-suspended in binding buffer and stained with Annexin V-FITC and PI (cat. no. C1062M; all Beyotime Institute of Biotechnology) according to the manufacturer's instructions. The stained samples were analyzed by flow cytometry (BD Biosciences). Data were analyzed using CellQuest™ Pro software (version 5.1 BD CellQuest Pro Software, BD Biosciences).
Western blotting
T24 cells were seeded into 6-well plates at a density of 5×104 cells/well, and cultured at 37°C with 5% CO2 for 24 h. Cells were pretreated with Compound c (Selleck Chemicals) for 2 h, and treated with cisplatin (20 µM) and/or β-ELE (50 µg/ml) for a further 12 h at 37°C. Total protein of T24 cells were lysed in RIPA buffer (Beyotime Institute of Biotechnology). Mitochondrial and cytoplasmic components were isolated using the Cell Mitochondria Isolation kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. Protein concentration was measured using a BCA kit (Beyotime Institute of Biotechnology). The prepared protein samples (40 µg/ml) were loaded and subjected to 12% SDS-PAGE, transferred to PVDF membranes (EMD Millipore), blocked with 5% skimmed milk for 1.5 h at room temperature and incubated with primary antibodies overnight at 4°C. Following washing with TBST (10 mmol/l Tris, pH 7.5; 100 mmol/l NaCl; 0.1% Tween-20), the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (cat. no. 7074; Cell Signaling Technology, Inc.) or goat anti-mouse (cat. no. 7076; Cell Signaling Technology, Inc.) secondary antibody for 1.5 h at room temperature. Primary antibodies were diluted at 1:1,000 and secondary antibodies were diluted at 1:3,000. The bands were detected by enhanced chemiluminescence (EMD Millipore) and visualized using an ECL system (Pierce Chemical Co.). The results were quantified using Image J (version 2.0; National Institute of Health).
Detection of mitochondrial membrane potential by JC-1 staining
T24 cells were seeded into 6-well plates at a density of 5×104 cells/well and cultured at 37°C with 5% CO2 for 24 h. Cells were treated with cisplatin (20 µM) and/or β-ELE (50 µg/ml) for 12 h at 37°C and stained with a JC-1 probe (Beyotime Institute of Biotechnology) for 20 min at 37°C according to the manufacturer's instructions. The prepared cells were observed by fluorescence microscopy and detected by flow cytometry. Five visual fields were randomly selected for each sample by fluorescence microscopy (magnification, ×400). Flow cytometric data were analyzed using CellQuest™ Pro software (version 5.;1 BD CellQuest Pro Software; BD Biosciences).
Detection of ROS levels by dichloro-dihydro-fluorescein diacetate (DCFH-DA) staining
T24 cells were seeded into 6-well plates at a density of 5×104 cells/well and cultured at 37°C with 5% CO2 for 24 h. Cells were pretreated with N-acetyl-L-cysteine (NAC, Sigma-Aldrich; Merck KGaA) for 3 h, and treated with cisplatin (20 µM) and/or β-ELE (50 µg/ml) for another 12 h at 37°C, before being stained with DCFH-DA probe for 20 min at 37°C using the Reactive Oxygen Species Assay kit (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. Intracellular production of ROS was evaluated by flow cytometry using CellQuest™ Pro software (version 5.1; BD CellQuest Pro; BD Biosciences,).
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed using SPSS 22.0 software (IBM Corp.). One-way analysis of variance was used for comparisons among multiple groups, followed by the Newman-Keuls post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
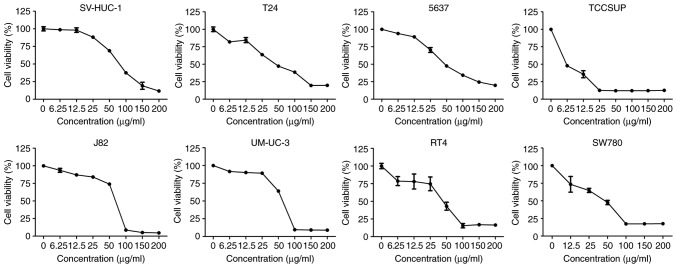
β-ELE inhibits the proliferation of human bladder cancer cells
Exposure to 0, 6.25, 12.5, 25, 50, 100, 150 and 200 µg/ml β-ELE for 24 h inhibited the proliferation of RT4, SW780, J82, UMUC-3, TCCSUP, 5637 and T24 human bladder cancer cells and SV-HUC-1 human urothelial cells. The results of the CCK-8 assay demonstrated that β-ELE effectively suppressed cell viability of all tested bladder cancer cell lines (Fig. 1). The half-maximal inhibitory concentrations (IC50) of β-ELE for each bladder cancer cell line and SV-HUC-1 cells are presented in Table I. The aforementioned result indicated that β-ELE had a notable anti-tumor effect on bladder cancer cells. As T24 and 5637 are the most commonly used and representative cell line in bladder cancer research, they were both selected for subsequent experiments.
Figure 1.
β-ELE reduces bladder cancer cell viability. Cell Counting Kit-8 assay results revealed that β-ELE treatment resulted in a strong inhibition of human bladder cancer cell proliferation compared with normal urothelial cells. The assays were performed in triplicate.
Table I.
IC50 of β-elemene in bladder cancer and normal urothelial cells.
| Cell line | IC50, µg/ml (mean ± SD) |
|---|---|
| SV-HUC-1 | 72.860±4.128 |
| T24 | 47.403±8.950 |
| 5637 | 61.553±4.070 |
| TCCSUP | 3.661±0.735 |
| J82 | 68.201±0.441 |
| UM-UC-3 | 72.129±1.121 |
| RT4 | 37.894±10.307 |
| SW780 | 37.703±7.323 |
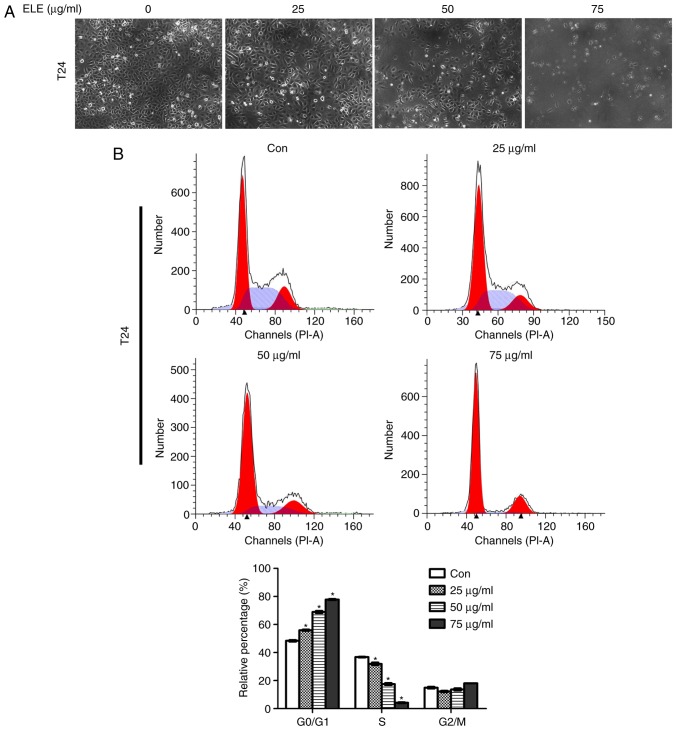
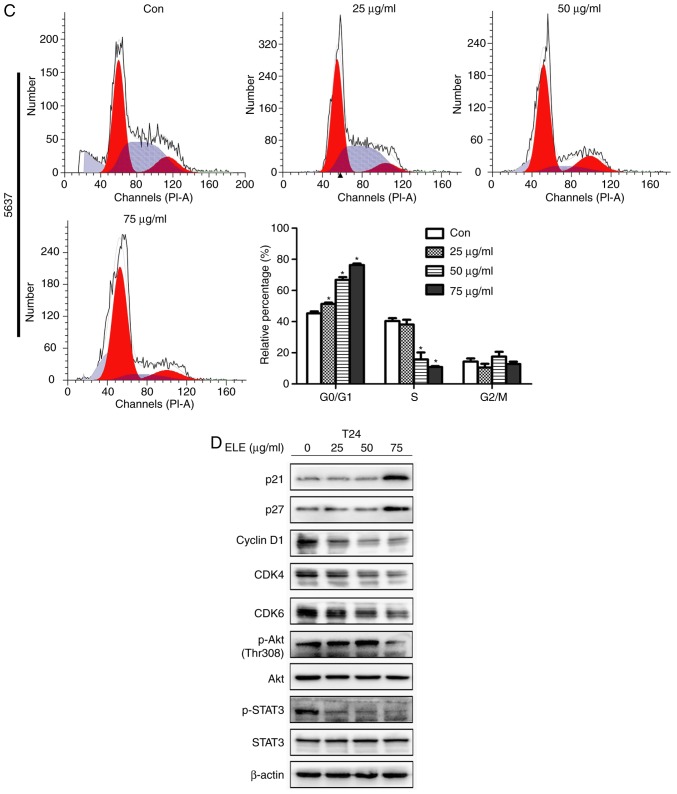
β-ELE induces cell cycle arrest in T24 cells
Regulation of the cell cycle was investigated by fluorescence microscopy and flow cytometry of T24 cells exposed to 0, 25, 50 or 75 µg/ml β-ELE. Cell numbers began to decrease at a dose of 50 µg/ml and were notably decreased at 75 µg/ml (Fig. 2A). Flow cytometry revealed that treatment with β-ELE induced dose-dependent G1-phase arrest in T24 and 5637 cells and significantly reduced the percentage of cells in the S-phase (Fig. 2B and C). Western blotting of proteins that modulate cell survival, cell cycle and cancer progression demonstrated that the expression levels of p-STAT3 and the cell cycle-related proteins cyclin D1, CDK4 and CDK6 were downregulated, and p21 and p27were upregulated by β-ELE. The phosphorylation of Akt (Thr 308) was reduced by 75 µg/ml β-ELE (Fig. 2D).
Figure 2.
β-ELE induces G0/G1 cell cycle arrest in T24 and 5637 cells. (A) T24 cells were counted using an inverted microscope following treatment with 0, 25, 50 or 75 µg/ml β-ELE for 24 h. (B and C) The percentages of (B) T24 and (C) 5637 cells in G0/G1, S and G2/M phase following treatment with 0, 25, 50 or 75 µg/ml β-ELE for 24 h were determined by flow cytometry. Data are presented as the mean ± SD, n=3. *P<0.05 vs. control. (D) Western blot analysis of the expression of cell cycle-related proteins and signaling molecules in T24 cells following treatment with 0, 25, 50 and 75 µg/ml β-ELE for 24 h. Con, control; β-ELE, β-elemene.
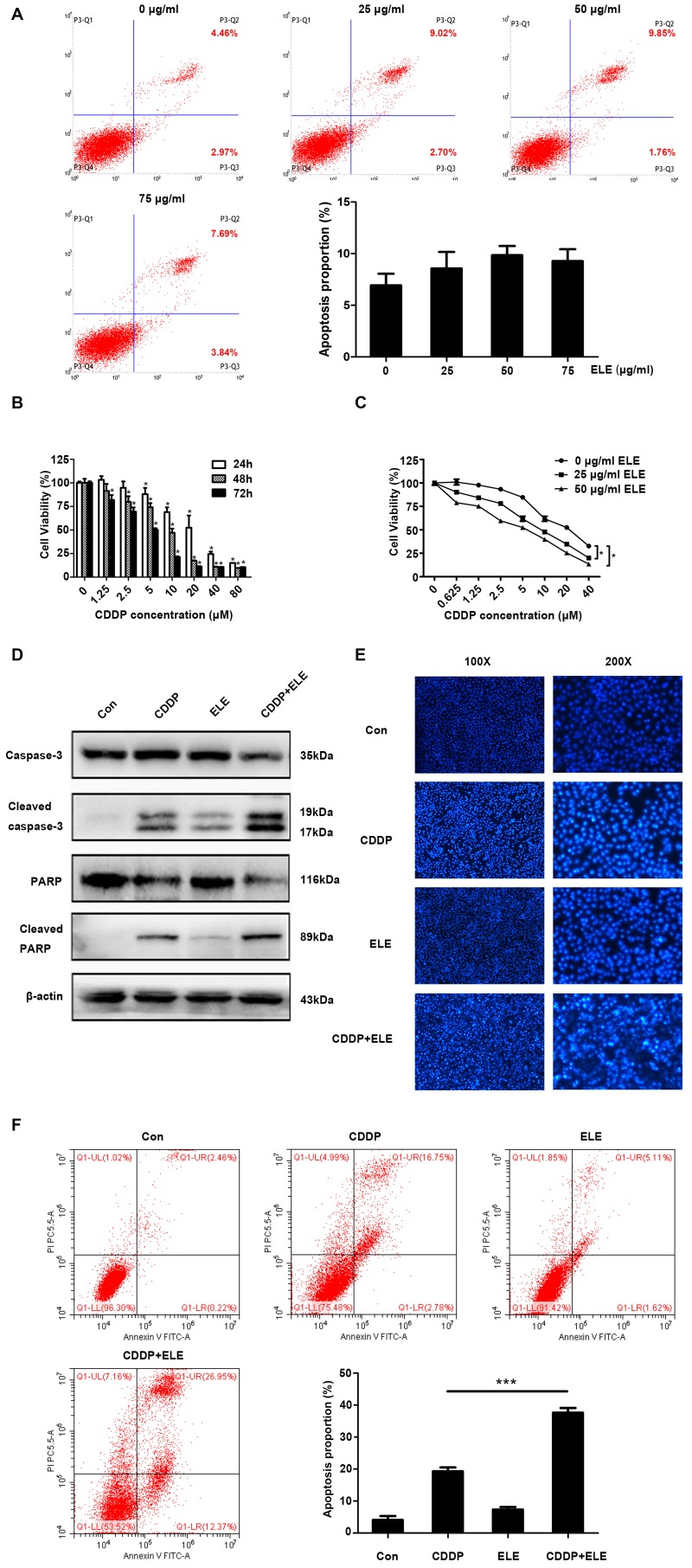
β-ELE potentiates cisplatin-induced apoptosis in T24 cells
β-ELE was previously demonstrated to promote apoptosis in T24 and 5637 human bladder carcinoma and primary bladder cancer cells (14). In the present study, β-ELE only induced a small increase in the apoptosis of T24 cells (Fig. 3A). Cisplatin significantly suppressed T24 cell viability in a time- and dose-dependent manner (Fig. 3B). The IC50 values were 20.07±2.18 µM at 24 h, 8.68±1.31 µM at 48 h and 4.63±0.40 µM at 72 h. The additive cytotoxicity of low (25 µg/ml) and moderate (50 µg/ml) dose β-ELE and cisplatin was stronger compared with that of cisplatin treatment alone at 24 h (Fig. 3C). Thus, 20 µM cisplatin and 50 µg/ml β-ELE were used in subsequent experiments. Western blotting results revealed that caspase-3 and PARP cleavage was increased by cisplatin combined with β-ELE compared with either β-ELE of cisplatin alone (Fig. 3D). Fluorescence microscopy of T24 cells stained with Hoechst 33258 (Fig. 3E) indicated the compounded effect of cotreatment with cisplatin and β-ELE, compared with either compound alone, which was consistent with the results of the flow cytometry apoptosis assay of Annexin-V stained cells (Fig. 3F).
Figure 3.
β-ELE potentiates cisplatin cytotoxicity. (A) Effect of different concentrations of β-ELE (0, 25, 50 and 75 µg/ml) on the apoptosis of T24 cells. (B) Cell Counting Kit-8 assay of the effects of cisplatin on the viability of T24 cells. The assays were performed in triplicate; data are presented as the mean ± SD. *P<0.05 vs. 0 µM CDDP. (C) Additive inhibitory effects of low (25 µg/ml) and moderate (50 µg/ml) doses of β-ELE and 20 µM cisplatin co-treatment on the proliferation of T24 cells. *P<0.05 vs. 0 µg/ml β-ELE. (D) Western blot assays demonstrated the effects of cisplatin and β-ELE co-treatment on cleaved caspase-3 and cleaved PARP expression. (E) Fluorescence microscopy of cells stained with Hoechst 33258. Original magnification, ×100 and ×200. (F) Annexin-V/propidium iodide staining revealed the effect of cisplatin and β-ELE co-treatment on the apoptotic rate of T24 cells. ***P<0.001 vs. cisplatin alone. CDDP, cisplatin; Con, control; PARP, poly (ADP-ribose) polymerase; β-ELE, β-elemene.
β-ELE promotes cisplatin-induced apoptosis via the mitochondrial pathway
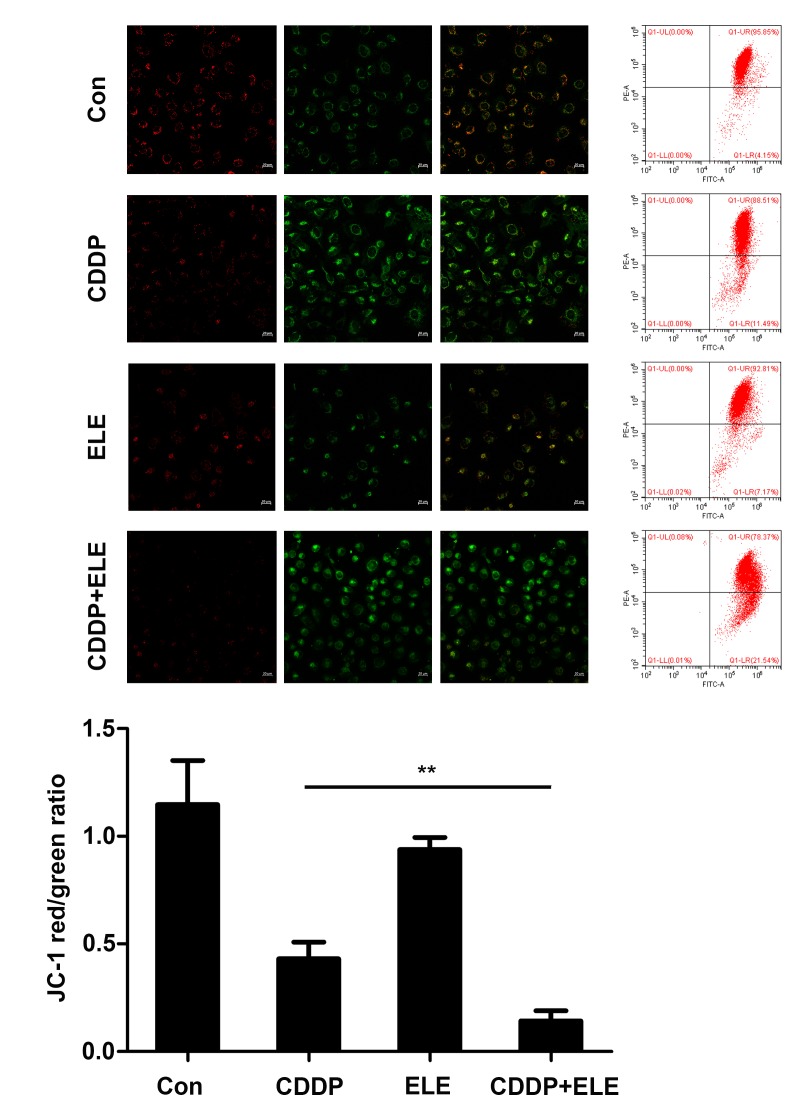
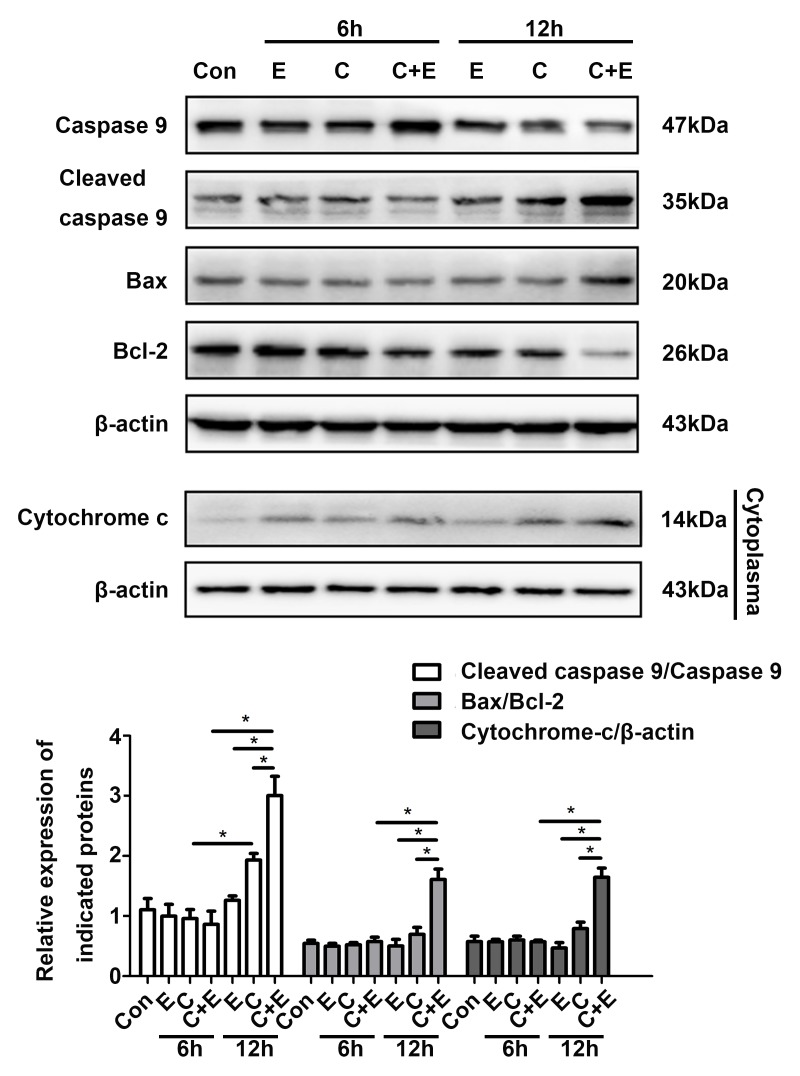
The augmentation of cisplatin cytotoxicity by β-ELE in cisplatin-resistant ovarian cancer cells has been demonstrated to be mediated in part by mitochondria-dependent apoptosis (15). In the present study, apoptotic T24 cell death induced by cisplatin and β-ELE was determined by the change in mitochondrial membrane potential (ΔΨm) indicated by the JC-1 fluorescent probe. The decrease in the red/green fluorescence ratio observed by fluorescence microscopy or flow cytometry was significantly larger in cells co-treated with cisplatin and β-ELE compared with those treated with cisplatin or β-ELE alone, and results of flow cytometry were statistically analyzed and shown in the bar graph (Fig. 4). Western blotting revealed upregulated expression of cleaved caspase-9, Bax and cytoplasmic cytochrome c, as well as downregulated expression of Bcl-2 following cisplatin and β-ELE co-treatment for 12 h compared with single drug treatment group (Fig. 5). Thus, the additive effect of β-ELE on cisplatin-induced apoptosis in T24 cells may be associated with the activation of the mitochondrial apoptosis pathway.
Figure 4.
β-ELE enhances cisplatin-induced reduction of mitochondrial membrane potential in T24 cells. T24 cells were treated for 12 h prior to determining the mitochondrial membrane potential by fluorescence microscopy and flow cytometry. Scale bar, 20 µm. mitochondrial membrane potential was calculated as the ratio of red-to-green fluorescence intensity. Data are presented as the mean ± SD of three independent experiments. **P<0.01 vs. cisplatin alone. CDDP, cisplatin; Con, control.
Figure 5.
Co-treatment of β-ELE and cisplatin promotes mitochondrial apoptosis-related proteins. Western blot assays of mitochondrial apoptosis-related proteins cleaved caspase-9, Bax and Bcl-2. Mitochondrial and cytosolic fractions were separated and cytochrome c was assayed in the cytosol fractions. Corresponding quantitative results were shown as bar graphs. *P<0.05. CDDP and C, cisplatin; E, β-ELE; Con, control; β-ELE, β-elemene.
β-ELE enhances cisplatin-induced apoptosis by activating the ROS-AMPK signaling pathway
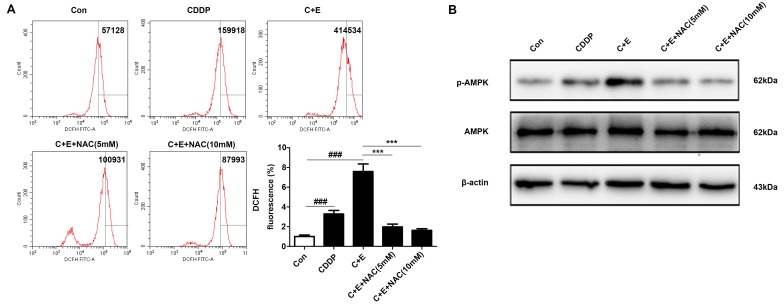
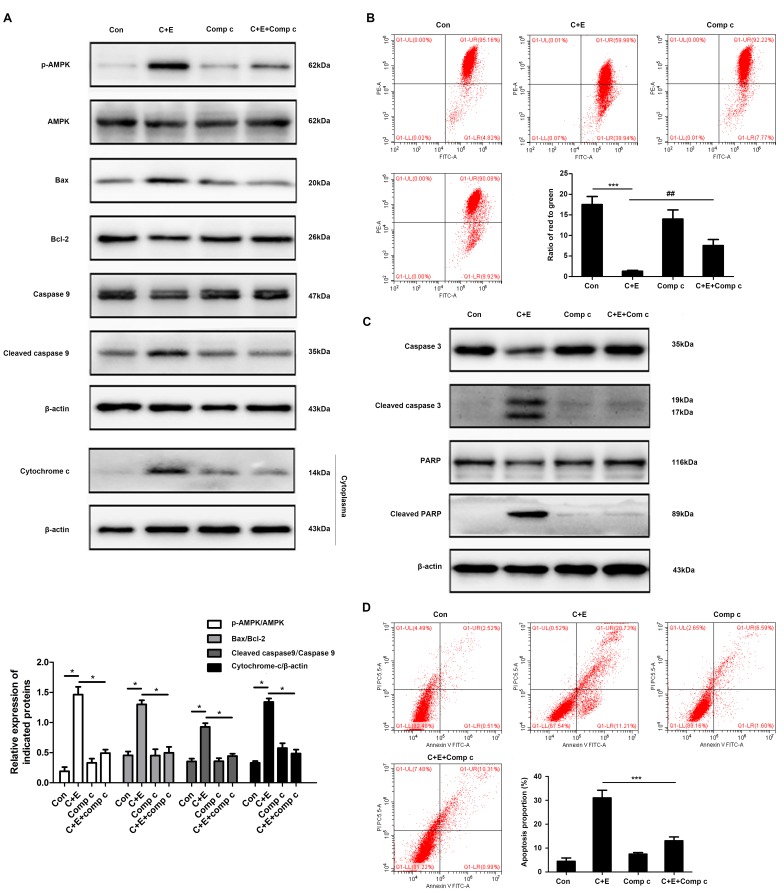
ROS generation and the activation of ROS-dependent pathways were associated with the induction of apoptosis in bladder cancer cells co-treated with cisplatin and curcumin (16). The involvement of ROS-dependent pathways in the regulation of apoptosis by co-treatment with cisplatin and β-ELE was evaluated by DCFH-DA assay of intracellular ROS in the present study. Flow cytometry results demonstrated that the intracellular ROS levels were significantly higher in cells co-treated with cisplatin and β-ELE compared with those in cells treated with cisplatin alone; pre-treatment with 5 or 10 mM NAC reversed ROS accumulation induced by the addition of cisplatin and β-ELE (Fig. 6A). Western blotting results demonstrated that AMPK phophorylation was increased by cisplatin and β-ELE co-treatment and reversed by NAC pretreatment (Fig. 6B). Considering the complicated roles of the ROS-AMPK signaling pathway in maintaining redox homeostasis and cell survival (17), it was speculated in the present study that the ROS-mediated AMPK activation may contribute to regulating apoptosis via the mitochondrial pathway. T24 cells were pretreated with 5 or 10 µM compound C, a specific inhibitor against AMPK, for 2 h and treated with cisplatin and β-ELE for 12 h. Pretreatment with compound C reversed the effects of cisplatin and β-ELE co-treatment on the expression of mitochondrial apoptosis-related proteins, including Bax, Bcl-2, cleaved caspase-9 and cytoplasmic cytochrome-c (Fig. 7A), as well as the loss of mitochondrial membrane potential (Fig. 7B). In addition, the inhibition of AMPK by compound C attenuated the levels of caspase-3 and PARP cleavage (Fig. 7C) and apoptotic rate (Fig. 7D) compared with cisplatin and β-ELE co-treatment. These results suggested that the ROS-AMPK signaling pathway may be involved in the intrinsic apoptosis induced by cisplatin and β-ELE co-treatment.
Figure 6.
β-ELE elevates cisplatin-induced AMPK signaling activation through ROS pathway. T24 cells were pretreated with 5 or 10 mM NAC for 1 h, then treated with cisplatin and β-ELE for 12 h. (A) ROS generation was determined by flow cytometry. ***P<0.001 vs. cisplatin and β-ELE; ###P<0.001 vs. control. (B) Western blot assay of phospho-AMPK expression. CDDP and C, cisplatin; E, β-ELE; Con, control; NAC, N-acetyl-l-cysteine; β-ELE, β-elemene.
Figure 7.
β-elemene (ELE) facilitates cisplatin-induced apoptosis by activating the AMPK signaling pathway. T24 cells were pretreated with 10 µM compound C for 2 h and treated with cisplatin and β-ELE for 12 h. (A) The expression of mitochondrial apoptosis-related proteins was assayed by western blotting. (B) Mitochondrial membrane potential was determined by flow cytometry. Representative images are shown. ***P<0.001 vs. control; ##P<0.01 vs. cisplatin plus β-ELE. T24 cells were pretreated with compound C and treated with cisplatin and β-ELE for an additional 24 h. (C) Cleaved caspase-3 and cleaved PARP expression were assayed by western blotting. (D) Apoptosis rates were measured by flow cytometry. Data are presented as the mean ± SD of three independent experiments. **P<0.01 vs. cisplatin plus β-ELE. C, cisplatin; E, β-ELE; Con, control; Com c, compound C; PARP, poly (ADP-ribose) polymerase; β-ELE, β-elemene.
Discussion
Cisplatin-based regimens are currently the standard treatment of advanced bladder cancer, but the 5-year survival rate of patients with muscle-invasive bladder cancer is <60% (2). The effectiveness of cisplatin is reduced by treatment-related side effects and the development of chemoresistance (18). Traditional Chinese medical herbs with anticancer activity, low toxicity and low rates of adverse effects can be combined with chemotherapeutic agents to improve clinical treatment response (6). β-ELE has previously been demonstrated to synergistically enhance cisplatin cytotoxicity in drug-resistant ovarian cancer cells by activating mitochondria-dependent signaling (15). β-ELE has also been reported to induce apoptosis in non-small cell lung cancer cells by triggering endoplasmic reticulum (ER) stress, activating the PRKR-like ER kinase/Ire1-α/activating transcription factor 6 pathway and downregulating Bcl-2 expression (19). In addition, β-ELE can induce different types of cell cycle arrest, including G0/G1, G2/M and S phase arrest, depending on the tumor type. For example, β-ELE reduced the expression of α-tubulin and interrupted microtubular polymerization to induce G2/M and S phase arrest in human liver cancer HepG2 cells. In addition, β-ELE can directly regulate the activity of CDK and cell cycle checkpoints to suppress tumor growth (20). β-ELE has been demonstrated to induce a persistent arrest at the G2/M phase in human platinum-sensitive and -resistant ovarian carcinoma cells by reducing the expression of CDK1, cyclin A and cyclin B1 and increasing that of p53 and p21, which are vital regulatory proteins that target cell cycle progression (21). By contrast, β-ELE increased the proportion of cells at the G0/G1 phase in a time- and dose-dependent manner in human nasopharyngeal carcinoma by inactivating phospho-STAT3, a transcription factor that participates in tumor cell growth and progression, and inhibiting the protein expression of DNA methyltransferase 1 (DNMT1) and enhancer of zeste homolog 2 (22). In the present study, β-ELE induced G0/G1 phase arrest in T24 and 5637 cells, which may have resulted from β-ELE-induced inactivation of phospho-AKT and phospho-STAT3, which are closely associated with tumor cell survival and cancer progression (23). A similar previous study demonstrated that the anticancer activity of β-ELE in T24 and 5637 bladder cancer cells was modulated by the PTEN and AKT pathway (14), which is in agreement with the results of the present study. The mechanisms of action of β-ELE used in combination with chemotherapeutic agents have not been clearly described. β-ELE has been demonstrated to enhance the radiosensitivity and temozolomide sensitivity of glioblastoma cells through the inhibition of the ataxia telangiectasia mutated-AKT and extracellular-signal-regulated kinase (ERK) signaling pathways (24). In addition, β-ELE has been reported to augment the cisplatin-induced inhibition of proliferation and promotion of apoptosis in gingival squamous cell carcinoma by blocking the activation of the JAK2-STAT3 signaling pathway (25). In MCF-7 breast cancer cells, β-ELE reversed Adriacin and docetaxel resistance by altering the expression of multidrug resistance-specific microRNAs, increasing PTEN and decreasing phosphoglycolate phosphatase gene expression in exosomes (26). Another study reported that β-ELE mediated an increase in cisplatin response of platinum-resistant ovarian cancer cells by inducing caspase-dependent apoptosis, decreasing mitochondrial transmembrane potential and releasing cytochrome c into the cytoplasm (15). In the present study, intrinsic apoptosis was triggered by the combined effects of cisplatin and β-ELE on mitochondrial membrane potential, increased cytosolic cytochrome c, increased Bax and cleaved caspase-9 expression and decreased Bcl-2 expression.
The effects of ROS on cell metabolism, homeostasis and survival, regulation of immune responses, induction of autophagy, apoptosis and the chemosensitivity of cancer cells are well-described (27,28). ROS is a positive regulator of AMPK, which activates glucose and fatty acid uptake and metabolism. Activation of AMPK phosphorylation by exogenous H2O2 is mediated by liver kinase B1 or calcium/calmodulin-dependent protein kinase β and an increase in the AMP/ATP ratio (29,30). An increase in ROS and AMPK phosphorylation in response to hypoxia can be abolished by antioxidants such as EUK-134. Hypoxia does not activate AMPK in cells deficient in mitochondrial DNA, with the exception of the presence of exogenous H2O2 (31). In addition, AMPK signaling is associated with increased cell survival. Activation of AMPKα and ERK1/2 by β-ELE is associated with the suppression of transcription factor Sp1 and DNMT1 protein expression, as well as the inhibition of non-small cell lung carcinoma cell proliferation in vitro (32). The blocking of AMPK activity by compound C or AMPK-targeting small interfering RNA inhibited hispidulin-induced ER stress and AMPK-mTOR signaling-promoted apoptosis in hepatocellular carcinoma cells (33). Metformin can induce apoptosis by activating the phosphorylation of AMPK at Thr172, and decreased phospho-AMPK expression has been demonstrated to reverse the inhibition of viability of gastric adenocarcinoma cells by metformin (34). In the present study, β-ELE promoted cisplatin-induced apoptosis accompanied by accumulation of ROS and upregulation of phospho-AMPKα. The apoptosis induced by co-treatment with β-ELE and cisplatin was reversed by an ROS scavenger and an AMPK inhibitor.
The present study mainly focused on the role of β-ELE in increasing the sensitivity of bladder cancer cells to cisplatin and it was revealed that the ROS-AMPK pathway-mediated mitochondrial dysfunction is considered to be involved in this process. Since β-ELE improved cell chemosensitivity to cisplatin, it may alleviate or reverse chemotherapy resistance by altering of the expression of multidrug resistance (MDR)-associated genes and proteins. In addition, a recent study revealed that β-ELE may repress the MDR process by inhibiting the expression of ATP-binding cassette transporters, such as P-glycoprotein (P-gp) and breast cancer resistance protein, which can pump chemotherapeutic drugs outside of cancer cells, leading to chemoresistance (35). β-ELE also regulates the expression of certain microRNAs (miRs; miR-34a, miR-222, miR-452 and miR-29a) that bind to the 3′-untranslated region of PTEN and P-gp to attenuate MDR (35). Drug-resistant bladder cancer cells exhibiting high expression levels of MDR-related genes were not available in the present study to test whether this process occurred in bladder cancer. Further specific studies on MDR in bladder cancer are needed in the future.
In conclusion, the results of the present study demonstrated that β-ELE suppressed the proliferation of bladder cancer cells in vitro and induced G0/G1 phase arrest in T24 and 5637 cells, which may have involved the AKT and STAT3 signaling pathways. β-ELE also enhanced cisplatin-induced mitochondrial apoptosis by activating the ROS-AMPK pathway. β-ELE activity may offer benefits that improve the response of bladder cancer to chemotherapeutic agents and reverse chemoresistance.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural Science Foundation of China (grant no. 81874092 and 81801482) and Chongqing Science & Technology Commission (grant no. cstc2019jcyi-msxmX0126).
Availability of data and materials
The datasets generated and analyzed during the study are available from the corresponding author on reasonable request.
Authors' contributions
DG designed and performed the experiments and wrote the manuscript. HY analyzed and organized the data and revised the manuscript. XG and WH assisted with the study design and obtained funding.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yin H, He W, Li Y, Xu N, Zhu X, Lin Y, Gou X. Loss of DUSP2 predicts a poor prognosis in patients with bladder cancer. Hum Pathol. 2019;85:152–161. doi: 10.1016/j.humpath.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, Böhle A, Kamat AM, Colombel M, Soloway M. Defining progression in nonmuscle invasive bladder cancer: It is time for a new, standard definition. J Urol. 2014;191:20–27. doi: 10.1016/j.juro.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 4.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai B, Zeng Y, Zeng Z, Zhang N, Li C, Zeng Y, You Y, Wang S, Chen X, Sui X, Xie T. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int J Nanomedicine. 2018;13:6279–6296. doi: 10.2147/IJN.S174527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang J, Qu X, Liu Y. β-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer. 2011;11:183. doi: 10.1186/1471-2407-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Z, Jacob JA, Loganathachetti DS, Nainangu P, Chen B. β-elemene: Mechanistic studies on cancer cell interaction and its chemosensitization effect. Front Pharmacol. 2017;8:105. doi: 10.3389/fphar.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, Wang Y, Luo H, Qiu W, Han H, Chen X, Yang L. β-elemene inhibits the proliferation of T24 bladder carcinoma cells through upregulation of the expression of Smad4. Mol Med Rep. 2013;7:513–518. doi: 10.3892/mmr.2012.1206. [DOI] [PubMed] [Google Scholar]
- 10.Li QQ, Wang G, Liang H, Li JM, Huang F, Agarwal PK, Zhong Y, Reed E. β-elemene promotes cisplatin-induced cell death in human bladder cancer and other carcinomas. Anticancer Res. 2013;33:1421–1428. [PMC free article] [PubMed] [Google Scholar]
- 11.Koosha S, Mohamed Z, Sinniah A, Ibrahim ZA, Seyedan A, Alshawsh MA. Antiproliferative and apoptotic activities of 8-prenylnaringenin against human colon cancer cells. Life Sci. 2019;232:116633. doi: 10.1016/j.lfs.2019.116633. [DOI] [PubMed] [Google Scholar]
- 12.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129–2144. doi: 10.7314/APJCP.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Lin Z, Zhang B, Guo L, Liu S, Li H, Zhang J, Ye Q. β-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci Rep. 2016;6:21010. doi: 10.1038/srep21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai B, Ma L, Nong S, Wu Y, Guo X, Pu J. β-elemene induced anticancer effect in bladder cancer through upregulation of PTEN and suppression of AKT phosphorylation. Oncol Lett. 2018;16:6019–6025. doi: 10.3892/ol.2018.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li QQ, Lee RX, Liang H, Zhong Y, Reed E. Enhancement of cisplatin-induced apoptosis by β-elemene in resistant human ovarian cancer cells. Med Oncol. 2013;30:424. doi: 10.1007/s12032-012-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park BH, Lim JE, Jeon HG, Seo SI, Lee HM, Choi HY, Jeon SS, Jeong BC. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget. 2016;7:63870–63886. doi: 10.18632/oncotarget.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren Y, Shen HM. Critical role of AMPK in redox regulation under glucose starvation. Redox Biol. 2019:101154. doi: 10.1016/j.redox.2019.101154. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Jiang ZY, Zhou YL, Qiu HH, Wang G, Luo Y, Liu JB, Liu XW, Bu WQ, Song J, et al. β-elemene regulates endoplasmic reticulum stress to induce the apoptosis of NSCLC cells through PERK/IRE1α/ATF6 pathway. Biomed Pharmacother. 2017;93:490–497. doi: 10.1016/j.biopha.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 20.Mao Y, Zhang J, Hou L, Cui X. The effect of beta-elemene on alpha-tubulin polymerization in human hepatoma HepG2 cells. Chin J Cancer Res. 2013;25:770–776. doi: 10.3978/j.issn.1000-9604.2013.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee RX, Li QQ, Reed E. β-elemene effectively suppresses the growth and survival of both platinum-sensitive and -resistant ovarian tumor cells. Anticancer Res. 2012;32:3103–3113. [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Tang Q, Yang L, Chen Y, Zheng F, Hann SS. Interplay of DNA methyltransferase 1 and EZH2 through inactivation of Stat3 contributes to β-elemene-inhibited growth of nasopharyngeal carcinoma cells. Sci Rep. 2017;7:509. doi: 10.1038/s41598-017-00626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao S, Xu J, Zhao K, Song P, Yan Q, Fan W, Li W, Lu C. Down-regulation of HPGD by miR-146b-3p promotes cervical cancer cell proliferation, migration and anchorage-independent growth through activation of STAT3 and AKT pathways. Cell Death Dis. 2018;9:1055. doi: 10.1038/s41419-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Zhou L, Zhao Y, Yuan Y. β-elemene enhances both radiosensitivity and chemosensitivity of glioblastoma cells through the inhibition of the ATM signaling pathway. Oncol Rep. 2015;34:943–951. doi: 10.3892/or.2015.4050. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Yu Y. Synergistic cytotoxicity of β-elemene and cisplatin in gingival squamous cell carcinoma by inhibition of STAT3 signaling pathway. Med Sci Monit. 2017;23:1507–1513. doi: 10.12659/MSM.903783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang HD, Yao YF, Zhong SL, Zhao JH, Tang JH. β-elemene reverses chemoresistance of breast cancer cells by reducing resistance transmission via exosomes. Cell Physiol Biochem. 2015;36:2274–2286. doi: 10.1159/000430191. [DOI] [PubMed] [Google Scholar]
- 27.Kruk J, Aboul-Enein HY, Kładna A, Bowser JE. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic Res. 2019;53:497–521. doi: 10.1080/10715762.2019.1612059. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Wang H, Wang J, Chen Y, Yin X, Shi G, Li H, Hu Z, Liang X. Emodin enhances cisplatin-induced cytotoxicity in human bladder cancer cells through ROS elevation and MRP1 downregulation. BMC Cancer. 2016;16:578. doi: 10.1186/s12885-016-2640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao S, Wu J, Zheng F, Tang Q, Yang L, Li L, Wu W, Hann SS. β-elemene inhibited expression of DNA methyltransferase 1 through activation of ERK1/2 and AMPKα signalling pathways in human lung cancer cells: The role of Sp1. J Cell Mol Med. 2015;19:630–641. doi: 10.1111/jcmm.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han M, Gao H, Xie J, Yuan YP, Yuan Q, Gao MQ, Liu KL, Chen XH, Han YT, Han ZW. Hispidulin induces ER stress-mediated apoptosis in human hepatocellular carcinoma cells in vitro and in vivo by activating AMPK signaling pathway. Acta Pharmacol Sin. 2019;40:666–676. doi: 10.1038/s41401-018-0159-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Lu CC, Chiang JH, Tsai FJ, Hsu YM, Juan YN, Yang JS, Chiu HY. Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling. Int J Oncol. 2019;54:1271–1281. doi: 10.3892/ijo.2019.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhai B, Zhang N, Han X, Li Q, Zhang M, Chen X, Li G, Zhang R, Chen P, Wang W, et al. Molecular targets of β-elemene, a herbal extract used in traditional Chinese medicine, and its potential role in cancer therapy: A review. Biomed Pharmacother. 2019;114:108812. doi: 10.1016/j.biopha.2019.108812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the study are available from the corresponding author on reasonable request.