Abstract
The BRAF-V600E mutation is the most common and specific oncogenic event known in papillary thyroid carcinoma (PTC). However, it remains controversial whether there is an association between the BRAF-V600E mutation and clinicopathologically aggressive characteristics of PTC. The purpose of the present retrospective study was to investigate the significance of the BRAF-V600E mutation in predicting prognostic and aggressive clinicopathological characteristics according to a new age-based stratification. Clinical data and the BRAF-V600E mutation status of 475 patients with PTC were downloaded from The Cancer Genome Atlas database. The association between BRAF-V600E status and clinicopathological characteristics was analyzed by χ2 test or Fisher's exact test. Recurrence-free survival rate (RFS) was analyzed using the Kaplan-Meier method. Aggressive clinicopathological factors associated with recurrence were analyzed by Cox multivariate regression. This study was conducted on 219 cases of patients with PTC with a known BRAF-V600E mutational status. In the ≥55 years age group, BRAF-V600E was found to be significantly associated with aggressive PTC characteristics, including tumor size, PTC subtype, radioactive iodine (RAI) dose, follow-up time, recurrence, recurrence risk stage, advanced T stage, advanced N stage and American Joint Committee on Cancer (III/IV) stage (all P<0.05). RFS was analyzed by the log-rank test and exhibited statistically significant differences in the ≥55 years group (P=0.041), but there was no significant difference in the <55 group (P=0.757), according to the BRAF-V600E mutation status. The BRAF-V600E gene was excluded from the recurrence Cox multivariate regression model. The BRAF-V600E mutation was found to better predict aggressive and recurrent PTC based on age stratification with the cut-off age of 55 years. The synergistic interaction between BRAF-V600E mutation and the new age stratification may help clinicians reach the optimal decision in terms of surgical approach and the extent of surgery.
Keywords: papillary thyroid carcinoma, BRAF mutation, prognosis, recurrence
Introduction
Thyroid cancer is the most common endocrine malignancy, and its incidence has rapidly increased globally over the past few decades. The incidence rate of thyroid cancer tripled from 4.9 to 14.3 per 100,000 individuals between 1975 and 2009 (1). Papillary thyroid carcinoma (PTC) is the most frequent type of thyroid cancer, accounting for 80–90% of all thyroid malignancies (2), and conventional PTC is the main histological variant (3).
The BRAF-V600E mutation gene is the most frequent oncogenic genetic alteration in PTC, accounting for approximately 45% of PTC cases, and has been shown to be associated with a poor prognosis of PTC (4). This mutation is associated with aggressive tumor behavior, disease recurrence and disease-specific mortality in PTC (5–7). Numerous studies have documented the oncogenic molecular mechanisms of BRAF-V600E driving the aggressiveness of PTC (8,9). The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control reconvened in 2016 to update the tumor node metastasis (TNM) staging system and published its 8th edition, in which significant changes were introduced regarding thyroid cancer (10). The age factor is a major change in the revised 8th edition of AJCC, as the age of 55 years has been introduced as the cut-off point demarcating the age-related prognostic risk of thyroid cancer, and patients aged >55 years appear to have a worse prognosis compared with patients younger than 55 years (10). A large multicenter study by Xing et al (5) demonstrated that the BRAF-V600E mutation was significantly associated with the risk of recurrence in PTC. Based on these data, it appears that the BRAF-V600E status in isolation is not sufficient to substantially contribute to risk stratification in the majority of the patients. In addition, an incremental improvement in risk stratification may be achieved if the BRAF-V600E mutational status is considered in the context of other standard clinicopathological risk factors (11).
Therefore, synergistic interaction between BRAF-V600E mutation and age stratification may better predict the prognosis of PTC. However, it was previously reported that the BRAF-V600E mutational status was not an independent prognostic factor when patients were grouped by age into two categories (<45 and ≥45 years) and into three categories (<35, 35–60 and ≥60 years) (12). The aim of the present study was to investigate whether the synergistic interaction between BRAF-V600E mutational status and the new age stratification with a cut-off age of 55 years is more efficient in predicting the prognosis of PTC.
Materials and methods
Data source
The Cancer Genome Atlas (TCGA) Data Portal (https://tcga-data.nci.nih.gov) is the result of a collaboration between the National Cancer Institute and the National Human Genome Research Institute (13). A comprehensive multi-dimensional map of the key genomic changes in 33 types of cancer has been generated. High-quality tumor and matched normal samples from 507 patients with thyroid cancer were collected and identified via the TCGA database, of which 475 patients had complete clinicopathological data.
Patient grouping and definition
A total of 475 patients were divided into two groups according to the new cut-off age, namely the <55 years and the ≥55 years age groups. Lymph node positivity was defined as a count of positive lymph nodes >5. Tumors were staged according to the TNM staging system recommended by the 8th edition of AJCC (10). Based on the 2015 American Thyroid Association (ATA) Management Guidelines (11), patients with gross extrathyroidal extension (ETE), incomplete tumor resection, distant metastases or lymph nodes >3 cm were classified into the high risk of recurrence group; those with aggressive histological characteristics, minor ETE, vascular invasion, or >5 involved lymph nodes (0.2–3 cm) were classified into the intermediate risk of recurrence group; and those with intrathyroidal differentiated thyroid cancer (DTC) (11) and ≤5 lymph node micrometastases (<0.2 cm) were considered as the low risk of recurrence group.
General information
In the present study, gene-level gene expression data from mRNA-seq, BRAF-V600E mutation data and clinicopathological information of 475 patients with PTC were extracted from TCGA up until September 26, 2018. A total of 475 patients diagnosed with PTC, including 352 women (74.1%) and 123 men (25.9%), were investigated. The median age of the patients was 46 years [interquartile range (IQR), 35–58 years]. Based on the cut-off age of 55 years, the patients were divided into the <55 years and the ≥55 years age groups. Patient age, sex, ethnicity, tumor size, multifocality, lymphocytic thyroiditis, histology, lymph node positivity, ETE, residual tumor, radioactive iodine (RAI) therapy, RAI dose, recurrence follow-up time, mortality follow-up time, TNM stage and AJCC stage (Tables I and II) were compared between the two groups. The overall BRFA-V600E mutation prevalence was 50.3% and the overall PTC recurrence rate was 6.4%.
Table I.
Univariate analyses of association between BRAF-V600E mutation status and clinicopathological parameters in <55-age group.
| BRAF-V600E | |||||
|---|---|---|---|---|---|
| Patients' parameters | Total | Mutation | Wild-type | Odds ratio (95% CI) | P-value |
| Age, years (mean ± standard deviation) | 38±10 | 39±10 | 37±10 | NA | 0.083 |
| Sex, n | |||||
| Female | 249 | 128 | 121 | 1 | 0.910 |
| Male | 69 | 36 | 33 | 1.031 (0.605–1.759) | |
| Ethnicity category, n | |||||
| Caucasian | 207 | 116 | 91 | 1 | 0.742 |
| Asian | 38 | 20 | 18 | 0.872 (0.436–1.744) | |
| Black | 15 | 7 | 8 | 0.686 (0.240–1.963) | |
| Tumor size, cm (mean ± standard deviation) | 1.7±0.8 | 1.6±0.9 | 1.7±0.8 | NA | <0.001a |
| Tumor foci, n | |||||
| Unifocality | 285 | 36 | 249 | 1 | 0.036a |
| Multifocality | 33 | 0 | 33 | 1.133 (1.085–1.182) | |
| Lymphocytic thyroiditis, n | |||||
| No | 240 | 119 | 120 | 1 | 0.099 |
| Yes | 45 | 29 | 17 | 1.720 (0.898–3.296) | |
| Histology, n | |||||
| CPTC | 237 | 142 | 95 | 1 | <0.001a |
| FVPTC | 63 | 9 | 54 | 0.112 (0.053–0.237) | |
| TCPTC | 18 | 13 | 5 | 1.739 (0.600–5.039) | |
| Lymph nodes positivity, n (>5) | |||||
| No | 190 | 102 | 88 | 1 | 0.618 |
| Yes | 60 | 30 | 30 | 0.863 (0.483–1.542) | |
| ETE, n (gross) | |||||
| No | 307 | 160 | 147 | 1 | 1.000b |
| Yes | 2 | 1 | 1 | 0.919 (0.057–14.822) | |
| Residual tumor, n | |||||
| No | 253 | 137 | 116 | 1 | 0.098 |
| Yes | 29 | 11 | 18 | 0.517 (0.235–1.140) | |
| RAI therapy, n | |||||
| No | 123 | 62 | 61 | 1 | 0.771 |
| Yes | 163 | 85 | 78 | 1.072 (0.671–1.713) | |
| RAI dose, mCi (mean ± standard deviation) | 121±59 | 120±50 | 122±68 | NA | <0.001a |
| Recurrence follow-up, months | 21 (14–44) | 26 (16–50) | 18 (12–39) | NA | 0.001a |
| Recurrence, n | |||||
| No | 278 | 143 | 135 | 1 | 0.865 |
| Yes | 13 | 7 | 6 | 1.101 (0.361–3.360) | |
| Mortality follow-up, months | 21 (13–44) | 25 (15-51.25) | 18 (12-39.5) | NA | 0.001a |
| Mortality, n | |||||
| No | 318 | 164 | 154 | 1 | NA |
| Yes | 0 | 0 | 0 | NA | |
| Recurrence risk stage, n | |||||
| Low | 108 | 26 | 82 | 1 | <0.001a |
| Intermediate | 163 | 124 | 39 | 10.028 (5.675–17.719) | |
| High | 33 | 13 | 20 | 2.050 (0.898–4.682) | |
| T stage, nc | |||||
| 1 | 221 | 114 | 107 | 1 | 0.981 |
| 2 | 69 | 36 | 33 | 1.024 (0.596–1.759) | |
| 3 | 28 | 14 | 14 | 0.939 (0.428–2.061) | |
| 4 | 0 | NA | NA | NA | |
| N stage nc | |||||
| 0 | 133 | 62 | 71 | 1 | 0.105 |
| 1a | 68 | 42 | 26 | 1.850 (1.019–3.357) | |
| 1b | 45 | 21 | 24 | 1.002 (0.509–1.973) | |
| M stage, nc | |||||
| 0 | 178 | 96 | 82 | 1 | 1.000b |
| 1 | 4 | 2 | 2 | 0.854 (0.118–6.199) | |
| AJCC stage, nc | |||||
| I | 314 | 162 | 152 | 1 | 1.000b |
| II | 4 | 2 | 2 | 0.938 (0.131–6.744) | |
Recurrence/mortality follow-up (months) were described by median or interquartile range. Low recurrence risk, intrathyroidal differentiated thyroid cancer and ≤5 LN micrometastases (<0.2 cm); intermediate recurrence risk, aggressive histology, minor ETE, vascular invasion, or >5 involved lymph nodes (0.2–3 cm); high recurrence risk, gross ETE, incomplete tumor resection, distant metastases or lymph node >3 cm. CI, confidence intervals; CPTC, conventional papillary thyroid cancer; FVPTC, follicular variant papillary thyroid cancer; TCPTC, tall cell variant papillary thyroid cancer; ETE, extrathyroidal extension; RAI, radioactive iodine; T, tumor size; N, lymph node; M, metastasis; AJCC staging, 8th edition American Joint Committee on Cancer staging; aP<0.05; bFisher's exact test; c(11).
Table II.
Univariate analyses of association between BRAF-V600E mutation status and clinicopathological parameters in ≥55-age group.
| BRAF-V600E | |||||
|---|---|---|---|---|---|
| Patients' parameters | Total | Mutation | Wild-type | Odds ratio (95% CI) | P-value |
| Age, years (mean ± standard deviation) | 65±9 | 65±8 | 65±9 | NA | 0.976 |
| Sex, n | |||||
| Female | 103 | 51 | 52 | 1 | 0.546 |
| Male | 54 | 24 | 30 | 0.816 (0.421–1.580) | |
| Ethnicity category, n | |||||
| Caucasian | 107 | 57 | 50 | 1 | 0.428b |
| Asian | 9 | 5 | 4 | 1.096 (0.279–4.309) | |
| Black | 11 | 3 | 8 | 0.329 (0.083–1.308) | |
| Tumor size, cm (mean ± standard deviation) | 1.9±1.0 | 1.7±0.9 | 2.1±1.1 | NA | 0.036a |
| Tumor foci, n | |||||
| Unifocality | 85 | 46 | 39 | 1 | 0.136 |
| Multifocality | 69 | 29 | 40 | 0.615 (0.324–1.167) | |
| Lymphocytic thyroiditis, n | |||||
| No | 116 | 59 | 57 | 1 | 0.784 |
| Yes | 21 | 10 | 11 | 0.878 (0.346–2.227) | |
| Histology, n | |||||
| CPTC | 106 | 53 | 53 | 1 | <0.001a |
| FVPTC | 33 | 7 | 26 | 0.269 (0.108–0.674) | |
| TCPTC | 18 | 15 | 3 | 5.000 (1.367–18.287) | |
| Lymph nodes positivity, n (>5) | |||||
| No | 93 | 46 | 47 | 1 | 0.231 |
| Yes | 22 | 14 | 8 | 1.788 (0.685–4.665) | |
| ETE, n (gross) | |||||
| No | 134 | 63 | 71 | 1 | 0.169 |
| Yes | 17 | 11 | 6 | 2.066 (0.722–5.910) | |
| Residual tumor, n | |||||
| No | 110 | 51 | 59 | 1 | 0.111 |
| Yes | 25 | 16 | 9 | 2.057 (0.837–5.051) | |
| RAI therapy, n | |||||
| No | 61 | 35 | 26 | 1 | 0.051 |
| Yes | 83 | 34 | 49 | 0.515 (0.264–1.007) | |
| RAI dose, mCi (mean ± standard deviation) | 122±52 | 125±20 | 102±62 | NA | 0.032a |
| Recurrence follow-up, months | 19 (12–34) | 21 (12–47) | 18 (12-28.3) | NA | 0.018a |
| Recurrence, n | |||||
| No | 132 | 58 | 74 | 1 | 0.031a |
| Yes | 15 | 11 | 4 | 3.509 (1.062–11.590) | |
| Mortality follow-up, months | 20 (13–35) | 25 (14–48) | 18 (12.5–27.5) | NA | 0.006a |
| Mortality, n | |||||
| No | 143 | 66 | 77 | 1 | 0.236 |
| Yes | 14 | 9 | 5 | 1.968 (0.631–6.136) | |
| Recurrence risk stage, n | |||||
| Low | 54 | 9 | 45 | 1 | <0.001a |
| Intermediate | 58 | 42 | 16 | 13.125 (5.238–32.887) | |
| High | 39 | 24 | 15 | 8.000 (3.052–20.967) | |
| T stage, nc | |||||
| 1 | 85 | 32 | 53 | 1 | 0.036a,b |
| 2 | 37 | 22 | 15 | 2.429 (1.103–5.349) | |
| 3 | 27 | 15 | 12 | 2.070 (0.861–4.975) | |
| 4 | 8 | 6 | 2 | 4.969 (0.945–26.116) | |
| N stage, nc | |||||
| 0 | 84 | 36 | 48 | 1 | 0.036a |
| 1a | 18 | 13 | 5 | 3.467 (1.133–10.606) | |
| 1b | 26 | 16 | 10 | 2.133 (0.867–5.250) | |
| M stage, nc | |||||
| 0 | 88 | 44 | 44 | 1 | 1.000b |
| 1 | 5 | 3 | 2 | 1.500 (0.239–9.420) | |
| AJCC stage, nc | |||||
| I/II | 143 | 64 | 79 | 1 | 0.016a |
| III/IV | 14 | 11 | 3 | 0.938 (0.131–6.744) | |
Recurrence/mortality follow-up (months) were described by median or interquartile range. Low recurrence risk, intrathyroidal differentiated thyroid cancer and ≤5 LN micrometastases (<0.2 cm); intermediate recurrence risk, aggressive histology, minor ETE, vascular invasion, or >5 involved lymph nodes (0.2–3 cm); high recurrence risk, gross ETE, incomplete tumor resection, distant metastases or lymph node>3 cm. CPTC, conventional papillary thyroid cancer; CI, confidence intervals; FVPTC, follicular variant papillary thyroid cancer; TCPTC, tall cell variant papillary thyroid cancer; ETE; extrathyroidal extension; RAI, radioactive iodine; T, tumor size; N, lymph node; M, metastasis; AJCC staging, 8th edition American Joint Committee on Cancer staging. aP<0.05; bFisher's exact test; c(11).
Clustering analysis
Genes that were differentially expressed between positive and negative for BRAF-V600E mutation status in the two age groups were assessed using RStudio software program (http://www.r-project.org). The heat map was generated using ‘pheatmap’ package in ‘R’ software (RStudio version 1.1.463) (14) to visualize the gene expression pattern, with red and green color representing highly expressed and lowly expressed genes, respectively.
Statistical analysis
Age, tumor size, and RAI dose are presented as mean ± SD while recurrence and mortality follow-up times are presented as median and quartile ranges Sex, ethnicity, tumor foci, lymphocytic thyroiditis, histology, lymph nodes positivity (>5), ETE, residual tumor, RAI therapy, recurrence, recurrence risk stage, T stage, N stage, M stage and AJCC stage are presented as the frequency. The statistical analysis was performed using SPSS, version 19 (IBM Corp.). The association of BRAF-V600E mutation status and each clinicopathological variable was assessed using the Pearson's χ2 test and Fisher's exact test when the number of patient cases was <5. The Kaplan-Meier analysis and log-rank test were used to analyze recurrence-free survival (RFS) distribution and to compare the differences between Kaplan-Meier curves for BRAF-V600E status. The odds ratio (OR) was determined by univariate analysis, and ORs with 95% confidence intervals (CIs) were calculated. Multivariate analyses were conducted with the Cox regression analysis method on disease recurrence, and hazard ratios (HRs) with 95% CIs were calculated. All P-values were two-sided, and P<0.05 was considered to indicate statistically significant differences.
Results
Patient demographics
The overall median follow-up time for all patients was 20 months (IQR, 14–38.3 months). The median follow-up time was 21 months (IQR, 14–44 months) in the <55 years group and 19 months (IQR, 12–34 months) in the ≥55 years group.
Association between BRAF-V600E mutation status and clinicopathological parameters in the <55 years age group
In this group, of the 318 patients who were diagnosed with PTC [including 249 women (78.3%) and 69 men (21.7%), with a mean age at diagnosis of 38±10 years (range, 15–54 years) and a mean tumor size of 1.7±0.8 cm], 232 patients (51.6%) were positive for BRAF-V600E mutation.
The univariate analyses demonstrated a significant association between the presence of BRAF-V600E and tumor size (P<0.001), multifocality (P=0.036), histology (P<0.001), RAI dose (P<0.001), follow-up time (P=0.001) and recurrence risk stage (P<0.001), whereas there was no significant association with age, sex, ethnicity, lymphocytic thyroiditis, lymph node positivity, ETE, residual tumor, RAI treatment, recurrence, TNM stage and AJCC stage (Table I).
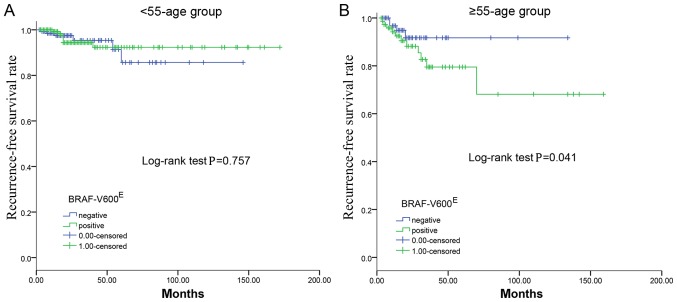
The median follow-up time was 21 months (IQR, 14–44 months), during which time 13 patients (4.5%) developed recurrence; there were no reported fatalities. The univariate analyses revealed no significant association between the BRAF-V600E mutation and PTC recurrence (P=0.865). Furthermore, the Kaplan-Meier plot for RFS did not reveal a statistically significant difference between the presence and absence of BRAF-V600E (P=0.757; Fig. 1A).
Figure 1.
Kaplan-Meier survival analysis of RFS in patients according to BRAF-V600E status. (A) There was no significant difference in survival between the patients with or without the BRAF-V600E mutation in the <55-age group. (B) Patients with the BRAF-V600E mutation had worse RFS compared with patients without the BRAF-V600E mutation in the ≥55-age group. RFS, recurrence-free survival. The 0.00-censored indicates patients who are negative for the BRAF-V600E mutation and the 1.00-censored indicates patients who are positive for the BRAF-V600E mutation.
Association between BRAF-V600E mutation status and clinicopathological parameters in the ≥55 years age group
The group included a total of 157 patients diagnosed with PTC, including 103 women (65.6%) and 54 men (34.4%). The mean age of the patients was 65±9 years and the mean tumor size was 1.9±1.0 cm. The prevalence rate of the BRAF-V600E mutation was 47.8% (75 cases).
In the ≥55 years age group, the results on univariate analysis demonstrated a significant association between BRAF-V600E mutation and tumor size (P=0.036), histology (P<0.001), RAI therapy dose (P=0.032), recurrence follow-up time (P=0.018), recurrence (P=0.031), mortality follow-up time (P=0.006), recurrence risk stage (P<0.001), advanced T stage (P=0.036), advanced N stage (P=0.036) and advanced AJCC stage (P=0.016) (Table II). There was no significant association between BRAF-V600E and age, sex, ethnicity, multifocality, lymphocytic thyroiditis, lymph node positivity, ETE, residual tumor, RAI therapy, mortality or M stage (all P≥0.05).
During a mean follow-up of 28 months (range, 3–157 months), 15 cases (10.2%) of recurrence were recorded. The presence of BRAF-V600E mutation was associated with lower RFS in the ≥55 year age group (P=0.041; Fig. 1B).
Recurrence risk factors
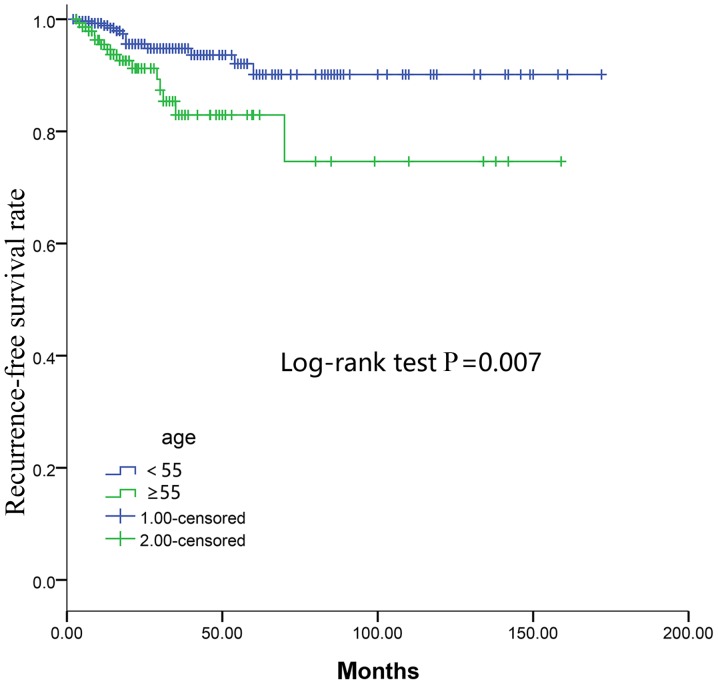
Multivariate regression analysis controlling for BRAF-V600E, male sex, multifocality, histology, ETE, residual tumor, T stage, N stage, M stage and AJCC stage found that only advanced N stage (P=0.038) and advanced M stage (P=0.028) were independent predictors of recurrence in the <55 age group (Table III). Notably, multivariate analysis demonstrated that male sex (P=0.026), multifocality (P=0.034), N stage (P=0.001) and M stage (P=0.005) were independent predictors of recurrence in the ≥55 age group (Table III). However, the Cox multivariate regression demonstrated that BRAF-V600E was not an independent predictor of recurrence in either of the two groups (P=0.758 and 0.993, respectively; Table III). In addition, the Kaplan-Meier curves of RFS revealed that the ≥55 age group had a lower RFS rate compared with the <55 age group as determined by the log-rank test (P=0.007; Fig. 2).
Table III.
Cox multivariate regression analyses of factors associated with recurrence.
| <55-age group | ≥55-age group | |||
|---|---|---|---|---|
| Clinicopathologic features | HR (95% CI) | P-value | HR (95% CI) | P-value |
| BRAF | 0.842 (0.282–2.513) | 0.758 | 0.995 (0.356–2.781) | 0.993 |
| Male sex | 1.423 (0.315–6.422) | 0.647 | 2.345 (1.108–4.963) | 0.026a |
| Multifocality | 1.753 (0.583–5.272) | 0.318 | 3.180 (2.932–5.850) | 0.034a |
| Histology | 0.716 (0.190–2.693) | 0.621 | 1.333 (0.677–2.623) | 0.405 |
| ETE | 0.716 (0.190–2.693) | 0.621 | 0.422 (0.055–3.256) | 0.408 |
| Residual tumor | 2.484 (0.682–9.050) | 0.168 | 0.999 (0.219–4.559) | 0.999 |
| T stage | 1.363 (0.641–2.902) | 0.421 | 1.268 (0.779–2.065) | 0.340 |
| N stage | 2.453 (1.051–5.722) | 0.038a | 7.873 (5.121–14.781) | 0.001a |
| M stage | 16.010 (1.358–188.707) | 0.028a | 9.043 (1.966–41.602) | 0.005a |
| AJCC stage | 4.215 (0.541–32.855) | 0.170 | 1.093 (0.240–4.968) | 0.908 |
HR, hazard ratios; CI, confidence intervals; ETE, extrathyroidal extension; T, tumor; N, lymph node; M, metastasis; AJCC, the 8th edition American Join Committee on Cancer staging. aP<0.05.
Figure 2.
Kaplan-Meier survival analysis of RFS. Patients in the ≥55-age group had worse RFS rates compared with patients in the <55-age group. RFS, recurrence-free survival. The 1.00-censored indicates patients in the <55 age group and the 2.00-censored indicates patients in the ≥55 age group.
Heat map result by clustering analysis
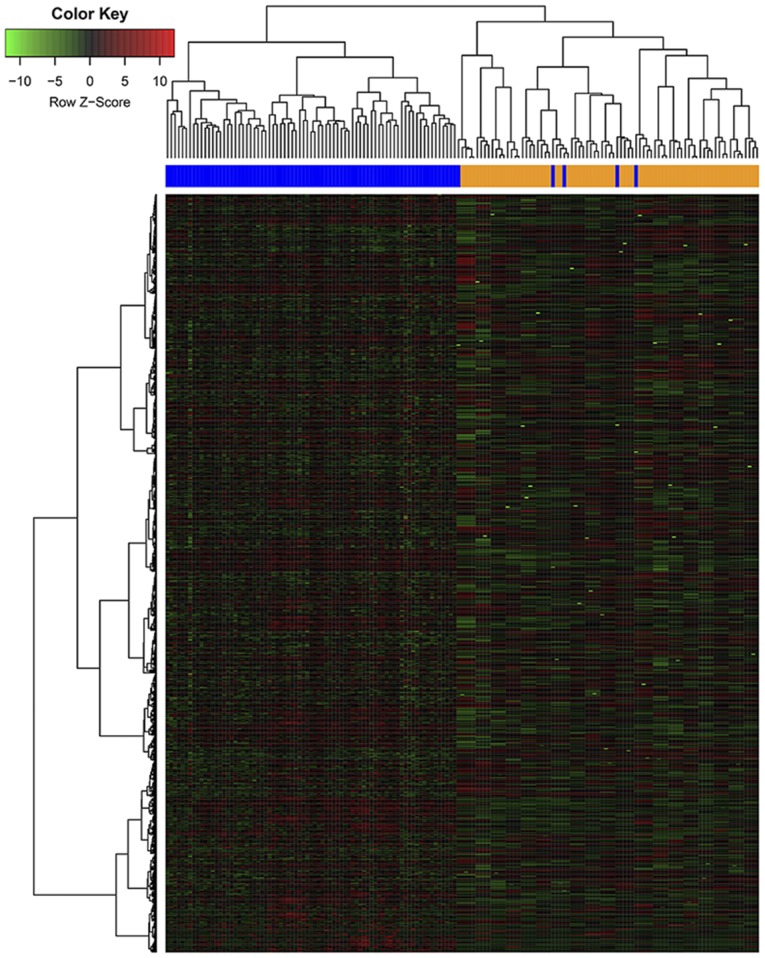
Gene variability was then computed using the median absolute deviation. The 3,120 most variable genes were selected. The heat map generated from the gene expression data revealed that there was a significant clustering effect between the positivity and negativity for BRAF-V600E mutation in the ≥55 year age group (Fig. 3). By contrast, the gene expression data demonstrated that there was no significant difference between the two groups in the <55 year age group.
Figure 3.
Heat map results of the of BRAF-V600E mutation-positive and -negative cases in the ≥55 years age group. Gene expression pattern according to the BRAF-V600E status. Supervised clustering of PTCs did exhibit a significant clustering effect between BRAF-V600E mutation-positive and -negative status. Each cell in the matrix represents the expression level of a gene feature in an individual pattern. Red or green, high or low expression, respectively, as indicated in the scale bar. PTC, papillary thyroid carcinoma.
Discussion
PTC is a well-differentiated papillary carcinoma with a relatively low mortality rate among thyroid cancers (14). However, the rate of disease recurrence or persistence is high, up to 30% (15,16). The BRAF-V600E mutation has been reported as a prognostic molecular marker in PTC (8,17,18). However, the prevalence of the BRAF-V600E mutation in PTC ranges between 30 and 80% (19). Therefore, this molecular marker is of limited value for clinical decision-making in the majority of PTC cases. For thyroid cancer, the recent 8th edition of the AJCC staging system strongly emphasizes the overall age-related risk, with a cut-off age at 55 years (10). In addition, several studies have demonstrated an association of BRAF-V600E with older age and poor clinical outcomes, including PTC recurrence and PTC-specific mortality (5,7). The present study investigated the hypothesis that BRAF-V600E may better predict PTC aggressiveness and recurrence based on this age stratification.
This study demonstrated no significant difference between the presence of BRAF-V600E mutation and ethnicity, in the ≥55-age group and the <55-age group. Therefore, we believe that the BRAF-V600E mutation is not significantly heterogeneous in different ethnicities. By categorizing patients in the aforementioned two age groups the effectiveness of BRAF-V600E in predicting aggressiveness and recurrence of PTC in terms of the cut-off age at 55 years improved. Furthermore, the heat map revealed significant clustering between the positive and negative BRAF-V600E cases in the ≥55 age group. Similar to the results reported by Xing et al (5), in the ≥55 group, the presence of the BRAF-V600E mutation was significantly associated with tumor size, histology, RAI dose, lymph node metastasis (LNM), advanced AJCC stage (III/IV) and tumor recurrence, which are the major factors associated with a worse prognosis of PTC (11). By contrast, in the <55 age group, the prognostic implications of the BRAF-V600E mutation in PTC were limited.
In the present study, the RFS distribution suggested that the ≥55 group exhibited a lower survival rate compared with the <55 group, and the latter group had a better prognosis. In addition, it was demonstrated that BRAF-V600E is useful for predicting prognosis based on age stratification with the cut-off at 55 years. The molecular mechanism underlying the age-dependent effect of the BRAF-V600E mutation on the prognosis of patients with PTC remains to be defined (20). It is possible that certain age-related genes, such as immune response-associated genes (21), may cooperate with BRAF-V600E in conferring poor prognosis, as BRAF-V600E was shown to be associated with abnormal immune response in human cancers, including PTC (22,23). The present study further demonstrated that the presence of the BRAF-V600E mutation was associated with a lower survival rate on RFS analysis in the ≥55 age group. However, in the <55 age group, there was no significant difference in survival between the presence and absence of the BRAF-V600E mutation. Therefore, BRAF-V600E may better predict the prognosis and recurrence of PTC based on the 55 year age stratification.
In addition, this study demonstrated that the synergistic interaction of BRAF-V600E and age stratification was of greater assistance for clinicians in terms of optimal decision-making regarding surgical approach and the extent of surgery. It has been reported that central LNM is the most common cause of disease recurrence in PTC (24,25). However, prophylactic central lymph node dissection is not routinely recommended based on the currently published guideline (11). The results of the present study demonstrated that BRAF-V600E was significantly associated with LNM in the ≥55 age group, and positive BRAF-V600E mutation status was associated with a higher risk of LNM compared with the negative mutation status. This study confirmed the association between the BRAF-V600E mutation and a higher risk of LNM in the ≥55 age group. By contrast, in the <55 age group, there was no significant association between BRAF-V600E and LNM. Whether the presence of BRAF-V600E with regional LNM exert a synergistic effect on increasing the rate of locoregional disease recurrence remains elusive. Further RFS analysis demonstrated that there was no significant difference between positive and negative BRAF-V600E mutation status in the LNM subgroup in the two age groups. Therefore, prophylactic central neck dissection may be performed in positive BRAF-V600E mutation cases, particularly in the ≥55 years age group. However, the synergistic interaction between BRAF-V600E and LNM is unclear, and patients with PTC who are BRAF-V600E-positive with LNM may not require more aggressive treatment compared with patients with PTC who are BRAF-V600E-negative with LNM.
Although RAI is routinely recommended by the 2015 ATA guidelines for patients with intermediate- to high-risk DTC, there is currently no consensus regarding the dose required for ablation (11). There may be an association between the BRAF-V600E mutation and the expression of certain genes, including sodium/iodide symporter, thyroid-stimulating hormone receptor, thyroperoxidase, thyroglobulin, and pendrin (26). The sodium/iodide symporter gene is involved in RAI metabolism, therefore mutations could result in impaired sodium/iodide symporter expression, and also a decrease in iodide-metabolizing gene expression of thyrotropin receptor, thyroglobulin and thyroperoxidase (27). This may be a possible explanation for RAI therapy failure and recurrence of PTC (28). Although tumors with the BRAF-V600E mutation tend to be RAI-resistant, that knowledge, to the best of our knowledge, has yet to affect decision-making regarding RAI therapy. The results of the present study revealed no significant association between BRAF-V600E and RAI therapy in the <55 and ≥55 age groups. However, the presence of BRAF-V600E was associated with higher RAI therapy dose in the ≥55 age group. A large multicenter study reported that the association between BRAF-V600E-positive mutation and high-dose RAI therapy is controversial (5). Further research conducted by the present study revealed no significant difference between high- and low-dose RAI on RFS analysis in a low- to intermediate-risk group (based on the 2015 ATA risk stratification system). Therefore, it is uncertain whether higher-dose RAI therapy is required for patients with PTC, who harbor the BRAF-V600E mutation. However, larger samples and cohort studies are required to confirm whether older patients with the BRAF-V600E mutation require high-dose RAI therapy to improve prognosis.
In addition, the presence of BRAF-V600E was not found to be an independent prognostic factor for predicting recurrent disease on univariate and multivariate analyses, when patients were grouped into <45 and ≥45 years age groups (12). Although BRAF-V600E had no independent impact on RFS, the BRAF-V600E mutation better predicted aggressive clinicopathological characteristics and PTC recurrence based on age stratification using 55 years as the cut-off.
There were several limitations to the present study. First, the sample size was relatively small, with 475 cases downloaded from TCGA. Second, not all of the clinicopathological data was complete therefore some data is missing. Third, this study was a retrospective analysis using TCGA data, and prospective clinical trials are required to provide more reliable results. Fourth, information associated with the extent of surgery and thyrotropin suppression is not recorded by TCGA. Finally, the sex ratio of women to men is 352 to 123, consistent with the ratio of epidemiology (3.1:1) (1). However, in the <55 age group, gender distribution was unequal at 249 women and 69 male, which may cause bias.
In summary, the BRAF-V600E mutation in PTC better predicts aggressive and recurrent disease based on stratification by 55 years of age. The synergistic interaction between the BRAF-V600E mutation and age stratification with a cut-off of 55 years may be more helpful for clinicians and facilitate optimal decision-making regarding surgical approach and the extent of surgery.
Acknowledgements
The authors would like to thank Ms. Yiming Tan (Southwestern University of Finance and Economics, Chengdu) for editing the language within the manuscript.
Funding
This study was supported by Guangzhou Medicine and Health Care Technology projects (grant nos., 20161A011008 and 20171A011243), Guangdong Medical Research fund project (grant no., A2017415), Guangdong Traditional Chinese Medicine Scientific research subject (grant no., 20152039).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in The Cancer Genome Atlas repository, (https://tcga-data.nci.nih.gov).
Authors' contributions
XG and BX conceived the present study. XG wrote the manuscript. XG, LP and JL designed and revised the manuscript. FS, JF, MG, ZC and WW analyzed and interpreted the data. WC, PH, XD, WZ assisted in data analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 2.Alzahrani AS, Xing M. Impact of Iymph node metastases identified on central neck dissection (CND) on the recurrence of papillary thyroid cancer: Potential role of BRAFV600E mutation in defining CND. Endocr Relat Cancer. 2013;20:13–22. doi: 10.1530/ERC-12-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen X, Liu R, Xing M. A Six-genotype genetic prognostic model for papillary thyroid cancer. Endocr Relat Cancer. 2017;24:41–52. doi: 10.1530/ERC-16-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 5.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. doi: 10.1200/JCO.2015.61.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: A meta-analysis. J Clin Endocrinol Metab. 2012;97:4559–4570. doi: 10.1210/jc.2012-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. BRAF (V600E) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 8.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, Kim KW, Hahn SK, Youn YK, Kim KH, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A meta-analysis. Cancer. 2012;118:1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 10.Amin MB. AJCC Cancer Staging Manual. 8th. Springer; New York, NY: 2017. [DOI] [Google Scholar]
- 11.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takacsova E, Kralik R, Waczulikova I, Zavodna K, Kausitz J. A different prognostic value of BRAFV600E mutation positivity in different age groups of patients with papillary thyroid cancer. Neoplasma. 2017;64:156–164. doi: 10.4149/neo_2017_120. [DOI] [PubMed] [Google Scholar]
- 13.Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): An immeasurable source of knowledge. Contemp Oncol (Pozn) 2015;19:A68–A77. doi: 10.5114/wo.2014.47136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omry-Orbach G. Risk stratification in differentiated thyroid cancer: An ongoing process. Rambam Maimonides Med. 2016:7. doi: 10.5041/RMMJ.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, Tennvall J, Bombardieri E, European Association of Nuclear Medicine (EANM) Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–1959. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
- 17.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 18.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 19.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 20.Shen X, Zhu G, Liu R, Viola D, Elisei R, Puxeddu E, Fugazzola L, Colombo C, Jarzab B, Czarniecka A, et al. Patient age-associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol. 2018;36:438–445. doi: 10.1200/JCO.2017.74.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist. 2009;14:216–221. doi: 10.1634/theoncologist.2008-0194. [DOI] [PubMed] [Google Scholar]
- 22.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid. 2014;24:1385–1393. doi: 10.1089/thy.2014.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H, Kim TH, Choe JH, Kim JH, Kim JS, Oh YL, Hahn SY, Shin JH, Chi SA, Jung SH, et al. Patterns of initial recurrence in completely resected papillary thyroid carcinoma. Thyroid. 2017;27:908–914. doi: 10.1089/thy.2016.0648. [DOI] [PubMed] [Google Scholar]
- 25.Suh YJ, Kwon H, Kim SJ, Choi JY, Lee KE, Park YJ, Park DJ, Youn YK. Factors affecting the locoregional recurrence of conventional papillary thyroid carcinoma after surgery: A retrospective analysis of 3381 patients. Ann Surg Oncol. 2015;22:3543–3549. doi: 10.1245/s10434-015-4448-9. [DOI] [PubMed] [Google Scholar]
- 26.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840–2843. doi: 10.1210/jc.2006-2707. [DOI] [PubMed] [Google Scholar]
- 28.Zoghlami A, Roussel F, Sabourin JC, Kuhn JM, Marie JP, Dehesdin D, Choussy O. BRAF mutation in papillary thyroid carcinoma: Predictive value for long-term prognosis and radioiodine sensitivity. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131:7–13. doi: 10.1016/j.anorl.2013.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in The Cancer Genome Atlas repository, (https://tcga-data.nci.nih.gov).