Abstract
Objective:
This study investigated effects of S-allylmercaptocysteine (SAMC), diallyl disulfide (DADS), and vitamin B12 on inner ear functions and morphology after long-period high-level broadband noise exposure.
Materials and Methods:
Twenty-four healthy rats were randomly divided into four groups. First group was chosen as the control group. Vitamin B12, SAMC, and DADS were applied to other groups for 4 weeks. On the 14th day, each group was exposed to broadband noise. Auditory brainstem response test was performed before and immediately after noise exposure and repeated on the 2nd and 14th day.
Results:
Permanent threshold shifts were significantly lower in groups treated with vitamin B12, SAMC, and DADS. Histologically, cochleae of SAMC and DADS groups were found to be better preserved than the cochleae of vitamin B12 and control groups.
Conclusion:
Physiologically and histologically, SAMC and DADS reduced the long-term effects of noise. However, physiological recovery was not consistent with the morphological findings in vitamin B12 group.
Keywords: Diallyl disulfide, garlic extracts, noise-induced hearing loss, oxidation, S-allylmercaptocysteine, vitamin B12
INTRODUCTION
Noise can be defined as sounds over a certain level that can cause damage on hearing.[1] Noise-induced hearing loss (NIHL) is a health problem that has been known for many years. The US National Institute of Occupational Safety and Health has defined harmful noise as the exposure to loud sounds over 85 dB for 8 h in a day.[2] Sounds over this level cause irreversible damage in hair cells. Exposure to loud sounds, for a long time, can result in prominent changes in the inner ear, and especially in the organ of Corti. The most significant damage caused by noise is the damage on the cilia of outer and inner hair cells. However, not only hair cells, but also lateral and medial walls of cochlea, cochlear neurons, and basilar membrane can be damaged especially with high-level and long-lasting noise exposure.[3] As the major mechanism of NIHL is oxidative damage,[4] the efficacy of different antioxidants and oxidative stress-reducing agents have been tested on experimental animals. Ascorbic acid, resveratrol, and coenzyme Q10 showed beneficial effects on treatment and prevention of NIHL in previous studies.[5,6,7] S-allylmercaptocysteine (SAMC) and diallyl disulfide (DADS) were shown to have antioxidants properties.[8] In this present study, we aimed to evaluate the protective effects of SAMC, DADS, and vitamin B12 on inner ear function and morphology after being exposed to long-period and high-level broadband noise.
MATERIALS AND METHODS
Formation of treatment groups
Twenty-four young adult, healthy, male Wistar rats weighing 200 to 250 g were investigated. Animals were divided into four groups, each consisting of six rats. The groups were divided as group A control (receiving saline, 100 mg/kg/day, orally), group B (receiving vitamin B12, 1 mg/kg/day, intraperitoneally), group C (receiving SAMC, 100 mg/kg/day, orally), and group D (receiving DADS, 50 mg/kg/day, orally).
Basal auditory brainstem response (ABR) measurements were performed on all animals before drug treatment. Treatments were administered for 28 days, and all groups were exposed to noise simultaneously on the 14th day. ABR tests were performed before noise exposure and immediately after noise exposure. The ABR tests were also repeated at 2nd and 14th day. Hearing threshold shift (HTS) of the 14th day after noise exposure was accepted as permanent threshold shift (PTS). SAMC and DADS molecules were supplied from Wakunaga Pharmaceutical Co. Ltd. (Osaka, Japan) to be used in this study. Animals were supplied by the Experimental Research Center of Gazi University after approval of the local ethics committee for research on experimental animals.
Formation of noise model
Broadband noise was applied at 110-dB intensity for an 8-h period for NIHL model. Noise was continuously created in an isolated room with an audiometry device with two free-field loudspeakers located 50 cm away from each other. Acoustic stimulus was intensified by “Power Amplifier AP 70” model amplifier and presented with “JBL Northridge E Series EC25” model speaker. Noise level was checked by a sound level meter for calibration at 110-dB SPL. During the exposure of the noise, animals were allowed free access to water and food.
Anesthesia
Before audiological examinations, anesthesia was administered to rats by intramuscular injection of 5 mg/kg xylazine and 35 mg/kg ketamine HCl mixture solution. Before sacrificing rats, high-dose xylazine and ketamine HCl mixture was administered via intramuscular route and rats were sacrificed by blood collection via intracardiac puncture.
Audiological examination
Unilateral (right) ABRs of all rats were controlled before the study. Ear examination of all subjects was performed with an otoscope. Normal anatomy of external auditory canal and eardrum was controlled for all animals. ABR measurements of rats were performed with Bio-Logic Systems Corp. Brand Navigator Pro Model (version 2.2.0). “Click” and “tone-burst” stimuli were used in ABR assessment. The tests were performed in a room, where the noise level did not exceed 50 dBA. Three-needle electrodes were placed at right mastoid (reference), vertex (active), and left mastoid (ground) subdermally to record ABR responses. Rarefaction polarity click stimuli were presented through insert headphone. The stimulus was recorded with 100 to 1500-Hz filtering, a repetition rate of 20/s, and an analyze time of 10 msn, and 1000 responses were averaged. The impedances of the electrodes were verified to be between 0 and 5 kΩ before the measurement. Tone-burst stimulus was applied at 4, 6, and 8-kHz alternating polarity, and by 50 to 1500-Hz band-pass filtering. The stimulus was initially delivered at 80-dB SPL, and the intensity level was reduced by 10-dB intervals to the threshold. Closer to threshold levels, the threshold was detected with 5-dB intensity changes. At least two traces were created for each measurement to ensure the reliability of the wave. The ABR threshold was defined as the lowest intensity level in which wave II was observable.
Surgical procedure
Cochleae were immediately removed after sacrification of the animals by performing right temporal bone resection and bony section harboring. The cochleae were placed in glutaraldehyde solutions. Acquired materials were evaluated by histologists.
Histological examination
After decalcification process and fixation, 1-µm thick sections were taken from prepared blocks with LKB Leica ultramicrotome and stained with toluidine blue. Images were taken from thick sections examined in Leica DM 4000 (Germany) computer-enhanced imaging system; these sections were marked and 0.2 to 0.5-µm thin sections were taken on formvar-coated copper grids. To provide contrast, sections were stained with uranyl acetate and lead citrate, and photographed by assessing in Carl Zeiss EVO LS 10+ED transmission electron microscope (TEM). A table of “Degeneration Criteria” was developed for the stained samples, and numerical data for this table were defined by histologists as follows: 0 = No, 1 = Weak, 2 = From weak to medium, 3 = Medium, 4 = From medium to intensive, and 5 = Intensive. These numerical values referred to seven parameters (basilar membrane, stereociliary and kinociliary loss in inner hair cells, necrosis in inner hair cells, losses in outer hair cells and stria vascularis cells, and myelin loss in auditory nerve axons).
Statistical assessment
Continuous variables were examined by the Shapiro–Wilk test to check for normality of distribution. Continuous variables are presented as mean ± standard deviation and standard error, and categorical data are presented as percentages or frequencies. Student t-test/one-way analysis of variance and Mann–Whitney U-test/Kruskal–Wallis H-test were used to compare parametric and nonparametric continuous variables, respectively. Categorical variables were compared by Chi-square test. A two-tailed P value of less than 0.05 was considered as statistically significant. All data were analyzed using SPSS version 23.0.
RESULTS
Auditory brainstem response audiometry results
HTS levels of 4, 6, and 8 kHz measured by tonal ABR and click ABR levels were presented for all groups in Figure 1. We observed a borderline significant difference among groups after immediate application of noise in 8-kHz HTS levels [Figure 2]. In addition, post hoc tests revealed that 8-kHz PTS (14th day HTS after noise) level was found to be significantly higher in control group compared to treatment groups. However, no difference was observed among the treatment groups [Figure 3]. The level of recuperation between 2nd and 14th day in 8-kHz HTS level was statistically significant in treatment groups but not in control group [Figure 3].
Figure 1.
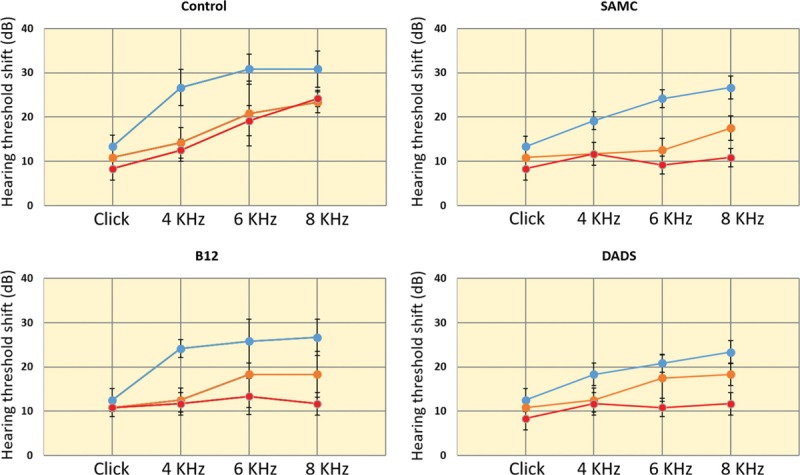
Hearing threshold shifts obtained from Click and tonal (4, 6, and 8 kHz) auditory brainstem response tests were presented for all groups. Colors represent the timing of the auditory brainstem response tests. Blue: HTS of immediately after noise exposure; Orange: HTS of 2nd day after noise exposure; Red: HTS of 14th day after noise exposure. dB = decibel, kHz = kilohertz
Figure 2.
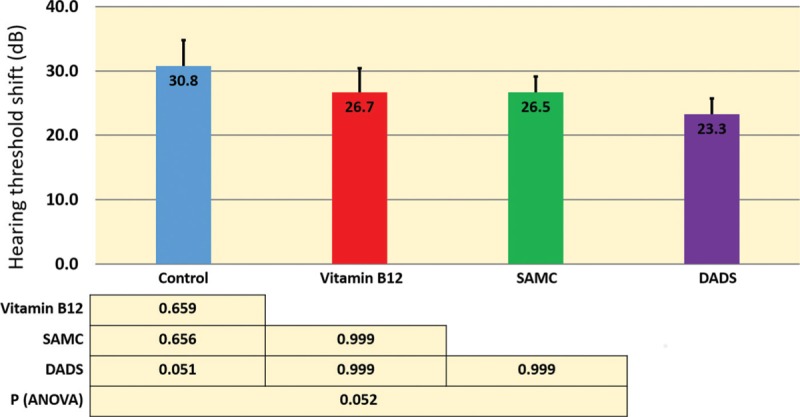
HTS obtained from tonal auditory brainstem response tests (8 kHz) immediately after noise exposure are shown. One-way analysis of variance test among all groups and post hoc Bonferroni test results are presented in tabular form within the image. dB = decibel
Figure 3.
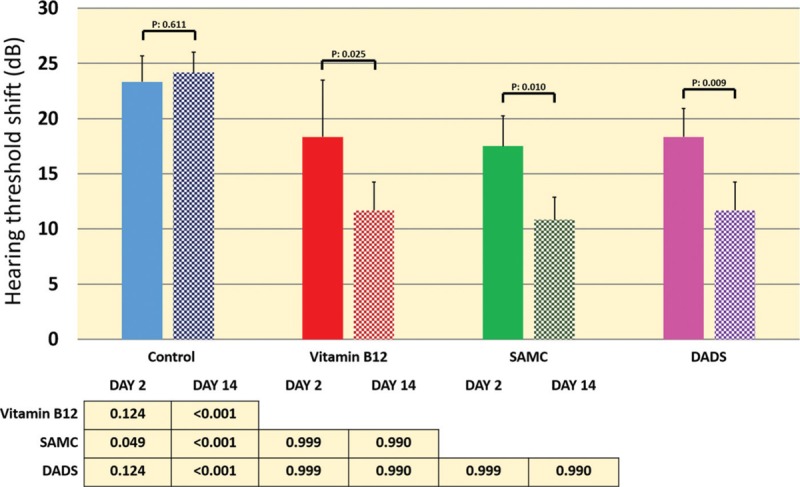
HTS obtained from tonal auditory brainstem response tests (8 kHz) on the 2nd and 14th day after noise exposure are presented. The table in the figure indicates the results of one-way analysis of variance test and post hoc Bonferroni test results for the measurements of the 2nd and 14th day. P values represented above the bars are indicating the comparison between the 2nd and 14th day (paired t-test) (recuperation level) within each of the study group. dB = decibel
Light microscopy results
No statistically significant difference was determined between control and B12 groups with regard to doubling in basilar membrane, stereociliary loss in inner hair cells, kinociliary loss in inner hair cells, necrosis in inner hair cells, losses in outer hair cells, losses in stria vascularis cells, and myelin loss in auditory nerve axons (P > 0.05). The difference by the means of these parameters was statistically significant in subjects both in SAMC (P < 0.05) and DADS (P < 0.05) groups compared to control group [Figure 4].
Figure 4.
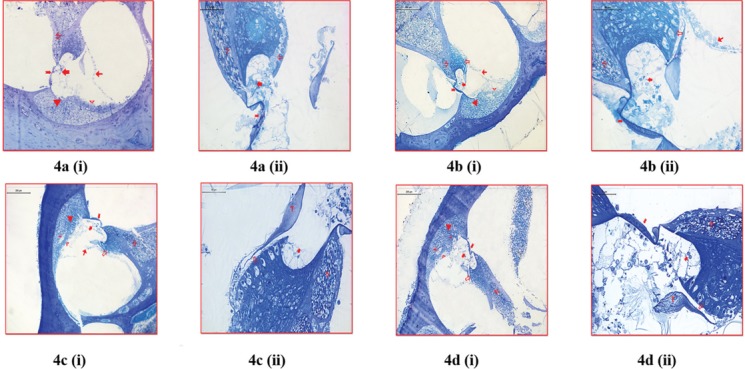
Semithin sections of groups. a(i) In semithin sections of control group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; →: Reissner membrane; ▸: stria vascularis;  : auditory nerve axons; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). a(ii) In semithin sections of control group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane;
: auditory nerve axons; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). a(ii) In semithin sections of control group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane;  : auditory nerve axons; and
: auditory nerve axons; and  : inner hair cell observed (toluidine blue ×400). b(i) In semithin sections of vitamin B12 administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; →: Reissner membrane; ▸: stria vascularis;
: inner hair cell observed (toluidine blue ×400). b(i) In semithin sections of vitamin B12 administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; →: Reissner membrane; ▸: stria vascularis;  ;
;  : inner hair cell; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). b(ii) In semithin sections of vitamin B12 administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; →: Reissner membrane;
: inner hair cell; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). b(ii) In semithin sections of vitamin B12 administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; →: Reissner membrane;  : auditory nerve axons; and
: auditory nerve axons; and  : inner hair cell observed (toluidine blue ×400). c(i) In semithin sections of SAMC administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; →: Reissner membrane; ▸: stria vascularis;
: inner hair cell observed (toluidine blue ×400). c(i) In semithin sections of SAMC administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; →: Reissner membrane; ▸: stria vascularis;  : auditory nerve axons;
: auditory nerve axons;  : inner hair cell;
: inner hair cell;  : edema on veins; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). c(ii) In semithin sections of SAMC administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells,
: edema on veins; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). c(ii) In semithin sections of SAMC administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells,  : auditory nerve axons;
: auditory nerve axons;  : inner hair cell; and T: tectorial membrane observed (toluidine blue ×400). d(i) In semithin sections of DADS administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; ▸: stria vascularis;
: inner hair cell; and T: tectorial membrane observed (toluidine blue ×400). d(i) In semithin sections of DADS administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane; ▸: stria vascularis;  : auditory nerve axons;
: auditory nerve axons;  : inner hair cell;
: inner hair cell;  : edema on veins; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). d(ii) In semithin sections of DADS administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane;
: edema on veins; and ▾: basal, intermediary, and marginal cells observed (toluidine blue ×100). d(ii) In semithin sections of DADS administration group, •: cytoplasm and nucleus remains of outer hair; Hensen and Claudius cells, •: basilar membrane;  : auditory nerve axons;
: auditory nerve axons;  : inner hair cell; and T: tectorial membrane observed (toluidine blue ×400).
: inner hair cell; and T: tectorial membrane observed (toluidine blue ×400).
Electron microscopy results
Prominent degenerative histological findings were observed in control group in the axons of auditory nerve in the organ of Corti right below inner hair cells. Myelin figures in axoplasm, myelin loss around the axon, serious losses in actin filaments located in axoplasm, significant degenerative changes in the nucleus and cytoplasm of Schwann cell, and significant losses in the number of axons were observed. Cell boundaries and organelle structures were barely distinguished in electron microscope examinations of inner hair cell, and cells were determined to have necrotic structure and disintegrated cytoplasmic contents. Although cell types and cytoplasmic boundaries could not be distinguished, degenerative formations were observed in terminals of the auditory nerve in basal areas of the cells [Figure 5a].
Figure 5.
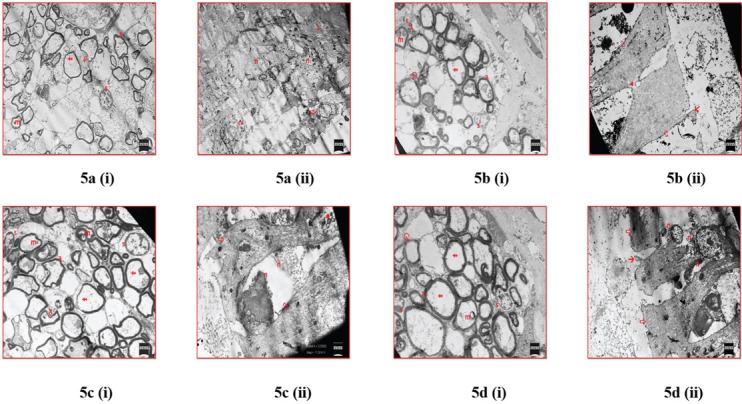
Electron microscopy examinations of groups. a(i) In electron microscopy examinations of control group, a: axon; m: myelin figure;  : myelin loss;
: myelin loss;  : loss in actin filaments; s: Schwann cell observed (uranyl acetate–lead citrate ×7.45K). a(ii) In electron microscopy, examinations of control group n: necrotic cell,
: loss in actin filaments; s: Schwann cell observed (uranyl acetate–lead citrate ×7.45K). a(ii) In electron microscopy, examinations of control group n: necrotic cell,  : terminals of the auditory nerve; and
: terminals of the auditory nerve; and  : >inner hair cell observed (uranyl acetate–lead citrate ×3.78K). b(i) In electron microscopy examinations of B12 administration group, a: axon; m: myelin figure;
: >inner hair cell observed (uranyl acetate–lead citrate ×3.78K). b(i) In electron microscopy examinations of B12 administration group, a: axon; m: myelin figure;  : loss in actin filaments; s: Schwann cell;
: loss in actin filaments; s: Schwann cell;  : separation in myelin; and L: lipid droplets observed in Schwann cell (uranyl acetate–lead citrate ×7.49K). b(ii) In electron microscopy examinations of vitamin B12 administration group, ▸: side connections; ↘: basal areas of kinocilia; and
: separation in myelin; and L: lipid droplets observed in Schwann cell (uranyl acetate–lead citrate ×7.49K). b(ii) In electron microscopy examinations of vitamin B12 administration group, ▸: side connections; ↘: basal areas of kinocilia; and  : inner hair cell observed (uranyl acetate–lead citrate ×9.88K). c(i) In electron microscopy, examinations of SAMC administration group, a: axon; m: myelin figure;
: inner hair cell observed (uranyl acetate–lead citrate ×9.88K). c(i) In electron microscopy, examinations of SAMC administration group, a: axon; m: myelin figure;  : myelin loss;
: myelin loss;  : loss in actin filaments; and s: Schwann cell observed (uranyl acetate–lead citrate ×9.43K). c(ii) In electron microscopy, examinations of SAMC administration group n: necrotic cell, ?: mitochondria and
: loss in actin filaments; and s: Schwann cell observed (uranyl acetate–lead citrate ×9.43K). c(ii) In electron microscopy, examinations of SAMC administration group n: necrotic cell, ?: mitochondria and  : inner hair cell observed (uranyl acetate–lead citrate ×11.20K). d(i) In electron microscopy examinations of DADS administration group, a: axon; m: myelin figure;
: inner hair cell observed (uranyl acetate–lead citrate ×11.20K). d(i) In electron microscopy examinations of DADS administration group, a: axon; m: myelin figure;  : loss in actin filaments; s: Schwann cell; and
: loss in actin filaments; s: Schwann cell; and  : myelin separation observed (uranyl acetate–lead citrate ×9.35K). d(ii) In electron microscopy examinations of DADS administration group, n: necrotic cell; ↘: basal areas of kinocilia; and
: myelin separation observed (uranyl acetate–lead citrate ×9.35K). d(ii) In electron microscopy examinations of DADS administration group, n: necrotic cell; ↘: basal areas of kinocilia; and  : inner hair cell observed (uranyl acetate–lead citrate ×9.09K).
: inner hair cell observed (uranyl acetate–lead citrate ×9.09K).
Although fewer degenerative results were detected in electron microscope examinations of B12 administration group compared to control group, separations in the myelin of some axons, nucleus structure of some Schwann cells, and perinuclear chromatins were observed to have normal structure. As for many Schwann cells, they were apparent between axons with degenerative cytoplasms filled with lipid droplets. Although myelin formations were determined in some axoplasm structures, the most degenerative findings were determined to be missing in cytoskeleton elements in the axoplasm. In electron microscopy assessments performed on inner hair cells of this group, it was observed that cytoplasmic boundaries of cells were distinguished more clearly, side connections of the cells were protected, but stereocilia had completely disappeared on apical cell membranes, and only basal areas of kinocilia were protected. Due to the complete removal of polarization and depolarization structures in the cell membrane, this group was determined to be a group that will experience serious hearing loss such as the control group [Figure 5b].
It was determined that axon loss in SAMC administration group was lower than the other two groups and that myelin loss in this group was lower compared to other groups. However, it was apparent that other degenerative findings observed in other groups were also present in this group. In examinations of this group performed on inner hair cells, it was observed that cell cytoplasm, cytoskeleton structure, and particularly mitochondria can be distinguished; however, crista loss in many of the mitochondria and presence of necrotic areas in cytoplasms were determined. Compared to control group and vitamin B12 administration group, this group was determined to be more efficient than the other two groups with regard to the protection of cellular structures. However, as in other groups, stereociliary and kinociliary structures have completely disappeared in this group [Figure 5b].
In DADS administration group, findings in thin structure level were determined to be very similar to the findings in SAMC administration group; however, the presence of necrosis was determined in some inner hair cells [Figure 5b].
When all groups were compared with each other at electron microscopy level, it was determined that SAMC administration presented the best histological findings, similar to findings obtained in semithin section staining.
DISCUSSION
In this study, we investigated the protective impact of vitamin B12, SAMC, and DADS on NIHL. The main findings can be summarized as follows: (1) SAMC and DADS have a potential beneficial effect on NIHL by improving physiological and morphological damage, and (2) vitamin B12 recuperates NIHL, but this impact is limited to improvement of function by showing no effect on cochlear morphology.
Noise-induced damage does not only consist of the mechanical damage in hair cells but also occurs due to contemporarily release of free oxygen radicals, which result in molecular damage.[9] Free oxygen radicals that are identified in NIHL development are superoxide, hydrogen peroxide and hydroxide, and nitrogen derivatives such as nitrogen oxide and peroxynitrite.[10] Although there are studies performed with numerous antioxidant molecules on NIHL, there is no proven and established treatment modality yet.
We used TEM to investigate cellular oxidative damage in the components of the cochlea after NIHL. Thus, we investigated the doubling in basilar membrane, stereociliary and kinociliary loss in inner hair cells, necrosis in inner hair cells, losses in outer hair cells and stria vascularis cells, and myelin loss in auditory nerve axons. TEM or scanning electron microscopy (SEM) can also be used for histological examinations. SEM is often used for surface inspection of hair-cell damage.[11]
In formation of NIHL model, broadband or pure tone sound can be used.[7] We used broadband noise in this study because we planned to create extensive oxidative damage on the entire cochlea.
Actin and filament nuclei in hair cells have been shown to renew themselves every 48 h, even in the absence of damage.[12] In this present study, recovery in hearing functions was observed in all groups within 48 h after noise exposure. However, recuperation in hearing function stopped in the control group, whereas it also proceeded after first 48 h for all the treatment groups. The ABR tests revealed better results for treatment groups compared to control group between 2nd and 14th day. Therefore, it can be claimed that the antioxidative agents may also have an impact on treatment of NIHL in addition to its preventive effects.
Yamashita et al. have revealed that oxidative damage caused by noise exposure continues 1 week after the first application. They also stated that antioxidant agents decrease the degree of cochlear damage.[13] In general, the agents were given before the noise exposure in varying protocols of treatment strategies.[5,6,7,8,9,10,11] However, as the oxidative stress is shown to be still present for a week after noise exposure, we decided to administer the agents 14 days before and 14 days after noise exposure in our study.
Vitamin B12 is used in the body as a coenzyme or cofactor in many important events such as DNA synthesis. Its deficiency affects folic acid metabolism and causes elevated homocysteine levels in the body.[14] It is an important risk factor for hyperhomocysteinemia, atherosclerosis, and cardiovascular diseases.[15] Oxidative stress balance of tissues is also disrupted in hyperhomocysteinemia and it increases oxidative damage.[16] B12 is also known to have significant effects on myelinization and nerve regeneration. Its deficiency causes various neurological deficits that have been shown both in human and animal studies.[17] Moreover, there are studies showing that neural regeneration is more significant upon reaching supraphysiological levels with high-dose vitamin B12 administration.[18] Studies performed about vitamin B12 effects on NIHL are generally retrospective studies.[19] In a study that investigated impact of vitamin B12 on NIHL, no preventive effect of vitamin B12 was reported by suggesting that longer treatment durations would have shown different results.[20] However, we found similar results on histological findings despite administering vitamin B12 for 28 days.
Garlic extracts are the molecules that have been kept in special environment, with various organosulfur contents responsible for antioxidant effect. We have used SAMC and DADS molecules in our study, which are active components with demonstrated antioxidant properties. We have determined the doses to be used from studies performed on gentamicin nephrotoxicity.[21] Among many studies performed by using SAMC, DADS, and other garlic extracts, only one study was performed on ototoxicity by investigating the protective efficacy on gentamicin ototoxicity.[8] Besides antioxidant effects, major effects of these molecules are neuroprotection[22,23] and antiaging.[24] In many studies, protective effects were demonstrated in chronic neurodegenerative diseases such as Alzheimer’s, Huntington’s, and Parkinson’s by acting as a neuron-protective and antioxidant agent.[25] Thus, in the present study, we have investigated SAMC and DADS, which have antioxidant properties and also affect neuronal recovery and circulation positively, but never used before for the treatment of NIHL.ABR tests performed immediately after noise exposure showed borderline significant differences among groups; on contrary, PTS levels showed significant differences between control group and treatment groups (B12, SAMC, and DADS) by having lower values for the latter. Morphologic examination revealed that SAMC and DADS groups showed better recovery compared to control and vitamin B12 group. No significant difference was found in morphological examination between control and vitamin B12 group. We claim that entire hearing physiology could not be elucidated by only considering cochlear morphological findings as functional outcomes of treatment groups were found to be similar despite having varying levels of morphological damage on cochlear levels; although cochlear degeneration levels of the control group and vitamin B12 group were similar, the functional hearing test results differed significantly. The conflicting findings between morphology of cochlea and auditory functions have been stated before.[26] Moreover, noise exposure damages entire auditory system, and ABR results reflect the response from whole auditory system. Focusing on the morphological cochlear damage is not adequate to evaluate the complex molecular auditory physiology.
CONCLUSION
In the present study, we found that vitamin B12, SAMC, and DADS improved PTS evaluated by ABR tests on NIHL model. However, only SAMC and DADS molecules showed an impact on morphological recuperation.
Financial support and sponsorship
This study was funded by a dedicated grant of the Gazi University Scientific Research Projects Department (grant number 01/2015-14).
Ethical standards
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed and approval of the local ethics committee for research on experimental animals was obtained.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to Prof. Dr. Nebil Göksu for his support and guidance with preparation of the manuscript.
REFERENCES
- 1.Proctor TB, Velde TM, Dayal VS, Bhattacharyya TK, Artwohl J, Towle VL. Auditory brain stem response in young and old guinea pigs. Am J Otol. 1998;19:226–9. [PubMed] [Google Scholar]
- 2.Rabinowitz PM. The public health significance of noise-induced hearing loss. Noise-induced hearing loss. :13–25. [Google Scholar]
- 3.Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC. Cellular mechanisms of noise-induced hearing loss. Hear Res. 2017;349:129–37. doi: 10.1016/j.heares.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–9. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich UR, Fischer I, Brieger J, Rümelin A, Schmidtmann I, Li H, et al. Ascorbic acid reduces noise-induced nitric oxide production in guinea pig ear. Laryngoscope. 2008;118:837–42. doi: 10.1097/MLG.0b013e31816381ae. [DOI] [PubMed] [Google Scholar]
- 6.Seidman M, Babu S, Tang W, Naem E, Quirk WS. Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg. 2003;129:463–70. doi: 10.1016/S0194-59980301586-9. [DOI] [PubMed] [Google Scholar]
- 7.Fetoni AR, Piacentini R, Fiorita A, Paludetti G, Troiani D. Water-soluble co-enzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–16. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Uzun L, Uzun L, Kokten N, Cam OH, Kalcioglu MT, Ugur MB, et al. The effect of garlic derivatives (S-allylmercaptocysteine, diallyl disulfide, and S-allylcysteine) on gentamicin induced ototoxicity: An experimental study. Clin Exp Otorhinolaryngol. 2016;9:309–13. doi: 10.21053/ceo.2015.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan H, Wang X, Hill K, Chen J, Lemasters J, Yang SM, et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal. 2015;22:1308–24. doi: 10.1089/ars.2014.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svere A, Vedul T. Diffusion-based model for noise-induced hearing loss. Norwegian University of Science and Technology Department of Electronics and Telecommunications, Master of Science in Electronics. 2007. [Google Scholar]
- 11.Lim DJ, Melnick W. Acoustic damage of the cochlea. A scanning and transmission electron microscopic observation. Arch Otolaryngol. 1971;94:294–305. doi: 10.1001/archotol.1971.00770070486002. [DOI] [PubMed] [Google Scholar]
- 12.Raphael Y. Cochlear pathology, sensory cell death and regeneration. Br Med Bull. 2002;63:25–38. doi: 10.1093/bmb/63.1.25. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–9. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 14.[No authors listed] Lowering blood homocysteine with folic acid based supplements: Meta-analysis of randomized trials. BMJ. 1998;316:894–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Wilcken DE, Wilcken B. The natural history of vascular disease in homocystinuria and the effects of treatment. J Inherit Metab Dis. 1997;20:295–300. doi: 10.1023/a:1005373209964. [DOI] [PubMed] [Google Scholar]
- 16.Moat SJ, Bonham JR, Cragg RA, Powers HJ. Elevated plasma homocysteine elicits an increase in antioxidant enzyme activity. Free Radic Res. 2000;32:171–9. doi: 10.1080/10715760000300171. [DOI] [PubMed] [Google Scholar]
- 17.Scalabrino G, Corsi MM, Veber D, Buccellato FR, Pravettoni G, Manfridi A, et al. Cobalamin (vitamin B(12)) positively regulates interleukin-6 levels in rat cerebrospinal fluid. J Neuroimmunol. 2002;127:37–43. doi: 10.1016/s0165-5728(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 18.Hobbenaghi R, Javanbakht J, Hosseini E, Mohammadi S, Rajabian M, Moayeri P, et al. Neuropathological and neuroprotective features of vitamin B12 on the dorsal spinal ganglion of rats after the experimental crush of sciatic nerve: An experimental study. Diagn Pathol. 2013;8:123. doi: 10.1186/1746-1596-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Gok U, Halifeoglu I, Canatan H, Yildiz M, Gursu MF, Gur B. Comparative analysis of serum homocysteine, folic acid and vitamin B12 levels in patients with noise-induced hearing loss. Auris Nasus Larynx. 2004;31:19–22. doi: 10.1016/j.anl.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Kibar S, Aydin S, Sanli A, Paksoy M, Yilmaz H, Sirvanci S. Evaluation of effect of vitamin B12 on noise-induced hearing loss by distortion product otoacoustic emission (DPOAE) and scanning electron microscopy. J Int Adv Otol. 2013;9:167–74. doi: 10.5152/iao.2015.368. [DOI] [PubMed] [Google Scholar]
- 21.Pedraza-Chaverri J, Gonzalez-Orozco AE, Maldonado PD, Barrera D, Medina-Campos ON, Hernandez-Pando R. Diallyl disulfide ameliorates gentamicin-induced oxidative stress and nephropathy in rats. Eur J Pharmacol. 2003;473:71–8. doi: 10.1016/s0014-2999(03)01948-4. [DOI] [PubMed] [Google Scholar]
- 22.Moriguchi T, Nishiyama N, Saito H, Katsuki H. Trophic effects of aged garlic extract (AGE) and its fractions on primary cultured hippocampal neurons from fetal rat brain. Phytother Res. 1996;10:468–72. [Google Scholar]
- 23.Aguilera P, Chánez-Cárdenas ME, Ortiz-Plata A, León-Aparicio D, Barrera D, Espinoza-Rojo M, et al. Aged garlic extract delays the appearance of infarct area in a cerebral ischemia model, an effect likely conditioned by the cellular antioxidant systems. Phytomedicine. 2010;17:241–7. doi: 10.1016/j.phymed.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Moriguchi T, Takashina K, Chu PJ, Saito H, Nishiyama N. Prolongation of life span and improved learning in the senescence-accelerated mouse produced by aged garlic extract. Biol Pharm Bull. 1994;17:1589–94. doi: 10.1248/bpb.17.1589. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-De La Cruz V, González-Cortés C, Pedraza-Chaverrí J, Maldonado PD, Andrés-Martínez L, Santamaría A. Protective effect of S-allylcysteine on 3-nitropropionic acid induced lipid peroxidation and mitochondrial dysfunction in rat brain synaptosomes. Brain Res Bull. 2006;68:379–83. doi: 10.1016/j.brainresbull.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise induced hair cell loss. Hear Res. 2000;146:134–42. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]


