Abstract
Zinc finger protein 703 (ZNF703) is a new member of the zinc finger protein family of transcription factors that plays an important role during embryogenesis in metazoans. The overexpression of ZNF703 contributes to tumorigenesis and progression of a number of malignancies by activating the Akt/mammalian target of rapamycin (mTOR) signaling pathway. This pathway is activated in medullary thyroid cancer (MTC), but its mechanism of action is not yet fully understood. The aim of the present study was to examine the role of ZNF703 and its association with Akt/mTOR activation in MTC. The present study used the phosphorylation of Akt1 protein at serine 473 (pAkt473) as an indicator of signaling activation. Immunohistochemistry (IHC) staining and western blot analyses were performed in order to examine the expression of ZNF703 in 34 cases of MTC and 12 cases of corresponding normal thyroid tissues. ZNF703 expression in MTC was significantly higher compared with the corresponding normal thyroid tissues (P<0.05). Furthermore, expression of ZNF703 was associated with tumor size, lymph node metastasis and advanced stage of disease. IHC also demonstrated that the level of ZNF703 was positively correlated with p-Akt473 in the 34 cases of MTC. The human MTC cell line TT was selected for further investigation as TT cells exhibit Akt/mTOR activation. The biological effects of silencing ZNF703 in TT cells on proliferation and apoptosis, both in vitro and in vivo were investigated in the present study. ZNF703 silencing inhibited the proliferation of TT cells in vitro and inhibited xenograft tumor growth in vivo. These effects were accompanied by the substantial decrease of pAkt473 and the induction of p53 protein. These results demonstrate that ZNF703 may play a relevant role in MTC due to its association with the Akt/mTOR signaling pathway.
Keywords: zinc finger protein 703, medullary thyroid cancer, proliferation, Akt/mammalian target of rapamycin signaling
Introduction
Thyroid carcinoma represents the most common endocrine malignancy in humans, with an increasing incidence worldwide (1). For example, the incidence of thyroid cancer in the USA has increased from 4.6 cases per 100,000 individuals between 1974 and 1977 to 14.4 cases per 100,000 individuals between 2010 and 2013 (2). It is expected to be the fourth most common cancer by 2030 (3). There are four different types of thyroid cancer according to the derivation of thyroid carcinomas (4). Medullary thyroid cancer (MTC) stems from calcitonin-producing parafollicular C cells of the thyroid and constitutes 5–8% of all thyroid cancers (5,6). A major challenge of the treatment of MTC, compared with differentiated thyroid carcinoma, is its early metastasis and failure to respond to thyroid stimulating hormone suppression and radioiodine (7–10). Therefore, additional studies of the molecular mechanisms responsible for the development of MTC and novel targeted therapies for MTC are urgently required.
The Akt/mammalian target of rapamycin (mTOR) signaling pathway is critical in the tumorigenesis and the progression of tumors, including MTC (11–14). The activation of Akt/mTOR signaling occurs in 96–100% of MTCs (13,15), thus targeting this pathway has become a potentially novel therapeutic intervention for MTC (16–18). Although the receptor tyrosine kinase (RET) mutation is the most common cause of Akt/mTOR activation in MTC, a significant portion of patients are resistant to RET inhibitors (16,19). Therefore, other factors involved in the activation of Akt/mTOR signaling in MTC require further investigation in order to determine methods to inhibit signaling at multiple levels.
Zinc finger protein 703 (ZNF703), which was first described in zebrafish (20), is expressed in almost all human adult tissues and localizes to the nucleus to regulate gene expression primarily as transcription repressors (21). Previously, ZNF703 was identified as an oncogene from an amplicon on chromosome 8p11.23 in luminal B breast cancers (22,23). Additionally, high mRNA and protein levels of ZNF703 were detected in non-small cell lung cancer (NSCLC), gastric cancer, cholangiocarcinoma and oral squamous cell carcinoma (24–26). Furthermore, it has been reported that ZNF703 contributes to tumor progression in a number of malignancies by activating the Akt/mTOR signaling pathway (24,27,28); however, whether ZNF703 participates in the activation of Akt/mTOR in MTC remains unclear.
In the present study, the expression of ZNF703 was detected and the association between its expression and the clinicopathological features of patients with MTC was evaluated. The present study used immunohistochemistry (IHC) to detect the phosphorylation of Akt1 protein at serine 473 (pAkt473), as an indicator of Akt/mTOR signaling activation in patients with MTC, and analyzed the association between the level of ZNF703 and pAkt473 in MTC. Subsequently, the biological consequence of ZNF703 silencing in cultured MTC-derived TT cells was analyzed in order to characterize the role of ZNF703 in MTC. In order to further determine whether ZNF703 is associated with Akt/mTOR activation, the present study detected pAkt473 levels after ZNF703 knockdown in TT cells. The results of the present study provide a molecular basis for the function of ZNF703 in MTC as well as a potential novel target for therapeutic intervention.
Materials and methods
Tissue specimens and cell culture
A total of 34 patients with histologically confirmed MTC at Tangshan Gongren Hospital and Tangshan Renmin Hospital (Tangshan, China) were recruited between January 2018 and June 2018 for the present study. A total of 34 fresh tumor tissues and 12 corresponding normal thyroid tissues (adjacent normal tissues were 2 cm away from the tumors) were obtained during surgery. The samples were stored in liquid nitrogen (−196°C) for western blot assays or were fixed in 4% paraformaldehyde solution for 48 h at room temperature and then embedded in paraffin for IHC staining. Patients' characteristics are presented in Table I. The present study was approved by the Ethical Committee of Tangshan Gongren Hospital and all patients provided written informed consent prior to their participation in the present study.
Table I.
Association between ZNF703 and pAkt473 protein expression and clinicopathological features of patients with MTC.
| ZNF703, n | pAkt473, n | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Total no., n | Negative | Positive | P-value | Negative | Positive | P-value |
| All cases | 46 | ||||||
| Normal | 12 | 9 | 3 | 0.028 | 10 | 2 | <0.001 |
| MTC | 34 | 13 | 21 | 8 | 26 | ||
| Age | |||||||
| ≤45 years | 11 | 5 | 6 | 0.709 | 2 | 9 | >0.999 |
| >45 years | 23 | 8 | 15 | 6 | 17 | ||
| Sex | |||||||
| Male | 11 | 6 | 5 | 0.262 | 1 | 10 | 0.227 |
| Female | 23 | 7 | 16 | 7 | 16 | ||
| Tumor size | |||||||
| ≤4 cm | 17 | 10 | 7 | 0.013 | 8 | 9 | 0.003 |
| >4 cm | 17 | 3 | 14 | 0 | 17 | ||
| Lymph node metastasis | |||||||
| − | 13 | 9 | 4 | 0.009 | 7 | 6 | 0.002 |
| + | 21 | 4 | 17 | 1 | 20 | ||
| Disease stage (37) | |||||||
| I, II | 13 | 9 | 4 | 0.009 | 7 | 6 | 0.002 |
| III, IV | 21 | 4 | 17 | 1 | 20 | ||
ZNF703, zinc finger protein 703; pAkt473, Akt1 protein at serine 473; MTC, medullary thyroid cancer.
Human MTC TT cells were obtained from the American Type Culture Collection and cultured in RPMI 1640 containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C, in 5% CO2 humidified atmosphere. The culture medium was changed every 3 days, and the cells were passaged at a 1:3 dilution every 5–6 days using 0.25% pancreatic enzyme-EDTA (Gibco; Thermo Fisher Scientific, Inc.).
Immunohistochemical staining
MTC tissues were fixed in 4% paraformaldehyde for 24 h at room temperature and embedded in paraffin. Paraffin-embedded tissue samples were cut into 4-µm thick sections and dewaxed using the standard xylene dewaxing method (29). Fixed tissues were dehydrated with xylene and ethanol: i) 50% ethanol for 4 h; ii) 75% ethanol for 4 h; iii) 85% ethanol for 3 h; iv) 95% ethanol for 2 h; v) 100% ethanol for 1 h; vi) 100% ethanol for 1 h; vii) 1:1 ethanol-xylene for 1 h; viii) xylene for 1 h and ix) xylene for 30 min at room temperature. Antigen retrieval was performed in 0.01 M sodium citrate buffer (pH 6.0) (Beijing Solarbio Science & Technology Co., Ltd.) at 95°C in a water bath for 10 min. Deparaffinized sections were blocked with 20% normal goat serum (Yeasen Biotech Co., Ltd.). at 37°C for 20 min and incubated with 3% hydrogen peroxide at room temperature for 10 min to eliminate endogenous peroxidase. Tissue samples were incubated with rabbit anti-ZNF703 (1:50; cat. no. ab137054; Abcam) or anti-pAKT473 antibody (1:100; cat. no. ab81283; Abcam) overnight at 4°C. Subsequently, the sections were washed twice with PBS and incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (undiluted; cat. no. PV-9000; Jinqiao Biotech) for 30 min at 37°C. After washing three times with PBS at room temperature, for 5 min each time, the sections were incubated with diaminobenzidine solution (Jinqiao Biotech) to visualize the positive signal. The sections were counterstained with hematoxylin for 20 sec at room temperature, rehydrated in graded ethanol solutions (alcohol series of 75, 80, 90 and 100%), clarified with xylene and sealed with cover slips. The negative control sections were processed in the same protocol aforementioned, with the exception of using 20% normal rabbit serum (1:50; AmyJet Scientific Inc) instead of primary antibody. Breast cancer sections with known positive expression of ZNF703 were used as positive controls. ZNF703 and pAkt473 were located in the nuclei and cytoplasm of the tumor cells. The slides were blindly examined by two senior pathologists from Tangshan Gongren Hospital. Cells within five randomly selected fields were counted under a light microscope (magnification, ×20). The percentages of positive tumor cells in the respective fields were calculated, and a threshold >10% was used to define pAkt473 positivity (13). ZNF703 expression was determined based on the percentage of positive cells, combined with staining intensity. The percentage of positive cells was divided into four levels and assigned the following number of points: 0 point for <10% positive cells, 1 point for 10–25%, 2 points for 26–75% and 3 points for >75% positive cells. The intensity of staining was classified as the following: 0 point for no staining, 1 point for weak staining (light yellow), 2 points for moderate staining (yellowish-brown) and 3 points for strong staining (brown). The sum of the staining-extent score and intensity score was used as the final staining score for ZNF703 expression, with a final score of ≥5 considered positive (27).
Small interfering (si)RNA- and lentivirus short (sh)hairpin RNA-mediated inhibition of ZNF703
For in vitro studies, siRNA was used to knockdown expression of ZNF703. Human ZNF703 siRNA (sense strand, 5′-AGGACAAGUCCAGCUUCAAGCCCUATT-3′; antisense strand, 5′-UAGGGCUUGAAGCUGGACUUGUCCUTT-3′) and negative control siRNA (non-silencing siRNA) were purchased from Hanheng RNAi Company. Non-transfected cells were used as the blank control group. TT cells were seeded in 6-well culture plates at a density of 3×105 cells. Following incubation overnight, the cells were transiently transfected with ZNF703-siRNA (2.5 µg/well) or negative control siRNA (2.5 µg/well) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The ability of ZNF703-siRNA to decrease ZNF703 mRNA and protein expression was analyzed by reverse transcription (RT) PCR and western blotting 72 h after transfection. For the in vivo studies, the present study used lentivirus interference to specifically and stably knockdown the expression of ZNF703 in TT cells. Lentiviruses carrying shRNA, targeting ZNF703 (LV sh-ZNF703) were generated using the pHBLV-U6-ZsGreen-Puro vector (Hanheng Biotechnology Co. Ltd.). Cells that were not infected by lentivirus were sorted by puromycin. The multiplicity of infection was 10 (3×105 cells transfected per well and 3×106 TU lentivirus transfected per well in a 6-well plate) in the presence of polybrene (Sigma-Alrich; Merck KGaA). The sequence of ZNF703-specific shRNA was 5′-AGGACAAGTCCAGCTTCAAGCCCTA-3′. Lentiviruses carrying non-silencing shRNA were used as the negative control group. The sequence of non-silencing shRNA was 5′-GAAAGCCTGCCGGTGACTAA-3′. Non-transfected cells were used as the blank control group. The ability of LV sh-ZNF703 to decrease ZNF703 expression was confirmed by RT-PCR 72 h after transfection.
RT-quantitative (RT-q) PCR analysis
The steps were performed according to the manufacturer's protocol of the cDNA Synthesis kit and the SYBR Green PCR Supermix kit (both from Invitrogen; Thermo Fisher Scientific, Inc.). RNA was isolated from TT cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The purity and concentration of RNA were determined. RNA was reverse transcribed into cDNA using the cDNA Synthesis kit. qPCR was subsequently performed using the SYBR Green PCR Supermix kit, with the Rotor Gene-3000 instrument (Corbett Life Science; Qiagen GmbH). Reactions were performed in 20-µl reactions with 1 µl cDNA. The following primer pairs were used for the RT-qPCR: ZNF703: Forward, 5′-AACGGCCCACATGAGTCAAT-3′ and reverse, 5′-GGCGGGGATCATGTCGTTAT-3′; and GAPDH: Forward, 5′-GAAAGCCTGCCGGTGACTAA-3′ and reverse, 5′-AGGAAAAGCATCACCCGGAG-3′. The following thermocycling conditions were used for the RT-PCR: 95°C for 2 min, 45 cycles of 95°C for 15 sec and 60°C for 30 sec. Relative expression was quantified using the 2−ΔΔCq method (30) and normalized to the internal reference gene GAPDH.
MTT analysis
The MTT assay was used to assess the proliferation of cells transfected with ZNF703-siRNA. A total of 1×104 TT cells were seeded in 96-well plates and incubated with 10 ml MTT solution (5 mg/ml; Sigma Aldrich; Merck KGaA), at 37°C for 4 h, in order to determine cell viability at 0, 24, 48, 72 and 96 h post-siRNA transfection. Following incubation, the cell culture medium was removed and 150 µl of DMSO (Sigma Aldrich; Merck KGaA) was added to dissolve the MTT. Cell proliferation was subsequently analyzed at a wavelength of 490 nm, using a microplate reader (Bio-Rad Laboratories, Inc.).
Flow cytometry analysis
The effects of ZNF703-siRNA on the apoptosis of TT cells were determined by flow cytometry. Cells in each group at 72 h post-transfection were trypsinized using 0.25% trypsin (Beijing Solarbio Science & Technology Co., Ltd.) at room temperature for 1 min and collected by centrifugation at 100 × g for 5 min at room temperature. The cells were incubated with 0.5 ml of the binding buffer and 1 µl AnnexinV-FITC from the Annexin V-FITC Apoptosis Detection kit (Merck KGaA) at room temperature for 10 min, according to the manufacturer's protocol. Next, the cells were re-suspended in fresh 0.5 ml binding buffer containing 5 µl propidium iodide (PI) at room temperature for 5 min. Apoptotic TT cells were subsequently measured with the FACSCalibur flow cytometer and BD CellQuest Pro software version 6.0 (BD Biosciences).
In vivo tumor growth assay
For the tumor xenograft assay, 18 nude mice were purchased from the Chinese Academy of Sciences (Shanghai, China). This experiment was approved by the Committee on the Use of Live Animals in Teaching and Research of North China University of Science and Technology (Tangshan, China). Tumor xenografts were established in 4-week-old female nude mice [specific pathogen free (SPF), BALB/C] weighing between 15–18 g. The total number of mice were divided into three groups (six mice per sub-group), with mice in each group under similar conditions. Normal TT cells, TT cells stably expressing scrambled shRNA and ZNF703 shRNA suspended in culture medium without FCS, were each injected into the right armpit of each mouse. The mice were maintained in the laboratory animal center of North China University of Science and Technology in a specific pathogen free environment at room temperature (25±1)°C, 40–50% relative humidity, 12 h light/dark cycle, and fed an autoclaved diet. The mice had constant access to food and water. Tumor size was measured with a vernier caliper, and tumor volumes were calculated using the following formula: Long axis × minor axis2/2. The growth curve of the xenograft tumor was plotted. The humane endpoint used in the present study was tumor ulceration. Mice were euthanized by cervical dislocation after 30 days, and the tumor tissues were weighed and collected for protein isolation and western blot analyses.
Western blot analysis
Proteins were extracted from 12 paired MTCs and non-cancerous thyroid tissue samples, from TT cells 72 h after transfection, or from xenograft tumor tissues, using RIPA buffer (Beyotime Institute of Biotechnology) with protease inhibitors phenylmethylsulfonyl fluoride (1:200; Beyotime Institute of Biotechnology). Total protein concentration was determined using a BCA kit (Beyotime Institute of Biotechnology), according to the manufacturer's protocol. Aliquots of protein (25 µg/lane) was separated via SDS-PAGE (10% gel) and transferred onto PVDF membranes. Membranes were blocked in 5% non-fat milk in TBS containing 0.05% Tween-20 (TBST) for 2 h at room temperature. The membranes were incubated with primary antibodies against; ZNF703 (1:300; cat. no. ab137054; Abcam), Akt (1:500; cat. no. sc-517582; Santa Cruz Biotechnologies), pAkt473 (1:500; cat. no. sc-514032; Santa Cruz Biotechnologies), p53 (1:500; cat. no. sc-47698; Santa Cruz Biotechnologies) or β-actin (1:500; cat. no. sc-517582; Santa Cruz Biotechnologies), overnight at 4°C. Membranes were washed with PBS three times and subsequently probed with anti-mouse (1:500; cat. no. A0216; Beyotime Institute of Biotechnology) or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:500; cat. no. A0208; Beyotime Institute of Biotechnology). Protein bands were visualized using a DAB detection reagent (Beyotime Institute of Biotechnology) and expression was quantified using Quantity One software v4.6.6 (Bio-Rad Laboratories, Inc.) with β-actin as the loading control.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 software (SPSS, Inc.). The χ2 test was used to analyze the difference in IHC staining of ZNF703 and pAkt473 between MTC and normal thyroid tissues. The Spearman rank correlation test was used to assess the correlation between ZNF703 and pAKT473 expression. Other statistical data are presented as the mean ± standard deviation. The paired t-test was used to compare the expression of ZNF703 between the 12 paired thyroid tissues. Single-factor one-way ANOVA, followed by the Dunnett's test were used to compare multiple samples. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of ZNF703 and pAKT473 in MTC tissue samples
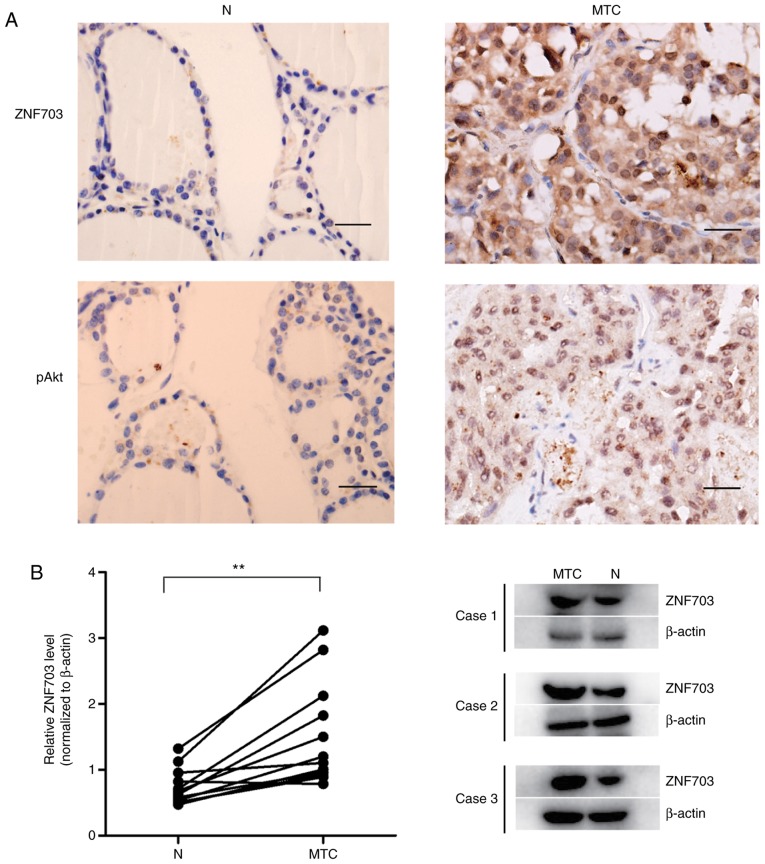
The present study assessed ZNF703 protein levels in 34 MTC patient tissues and 12 corresponding normal thyroid tissue samples using IHC, in order to determine whether ZNF703 is differentially expressed in MTC. Of the 34 MTC tissue samples, 21 (61.8%) had higher expression of ZNF703. In contrast, three normal thyroid tissues (25.0%) had higher staining of ZNF703 (Fig. 1A). The present study further detected the expression of ZNF703 in 12 paired MTCs and adjacent normal thyroid tissues within the same patient using western blotting. The results demonstrated a significantly higher expression of ZNF703 in MTC compared with adjacent normal tissue (P<0.05; Fig. 1B). These results suggest that ZNF703 may be upregulated in MTC.
Figure 1.
ZNF703 expression in MTC and normal thyroid tissues. (A) ZNF703 and pAKT473 were examined by IHC in 34 MTC tissues and 12 normal thyroid tissues. Scale bar, 25 µm. (B) Expression of ZNF703 was examined by western blotting in 12 paired thyroid tissues. Three representative western blots are shown. **P<0.01 vs. normal thyroid tissues. N, normal thyroid tissues; MTC, medullary thyroid cancer tissues; p, phosphorylated.
The present study subsequently evaluated the association between ZNF703 protein expression and clinicopathological characteristics among the 34 patients with MTC, and demonstrated that ZNF703 protein expression was associated with tumor size, lymph node metastasis and pathological stage of MTC (P<0.05; Table I), regardless of age and sex (P>0.05; Table I). These results suggest that ZNF703 upregulation may be associated with MTC progression.
In order to investigate whether ZNF703 was associated with the Akt/mTOR activation, the present study used IHC to detect pAKT473 as an indicator of Akt/mTOR signaling pathway activation in 34 tumor samples. Of the 34 MTC tissue samples, 26 (76.5%) stained positive for pAKT473 (Fig. 1A; Table I). Upregulation of ZNF703 was associated with pAKT473 expression (P<0.05; Table II).
Table II.
Association between ZNF703 and pAkt473 expression.
| Variables | pAkt473 | Total, n | Rs | P-value | |
|---|---|---|---|---|---|
| ZNF703 | Negative | Positive | 0.420 | 0.013 | |
| Negative | 6 | 7 | 13 | ||
| Positive | 2 | 19 | 21 | ||
| Total | 8 | 26 | 34 | ||
ZNF703, zinc finger protein 703; pAkt473, Akt1 protein at serine 473.
ZNF703-siRNA inhibits proliferation and promotes apoptosis of TT cells in vitro
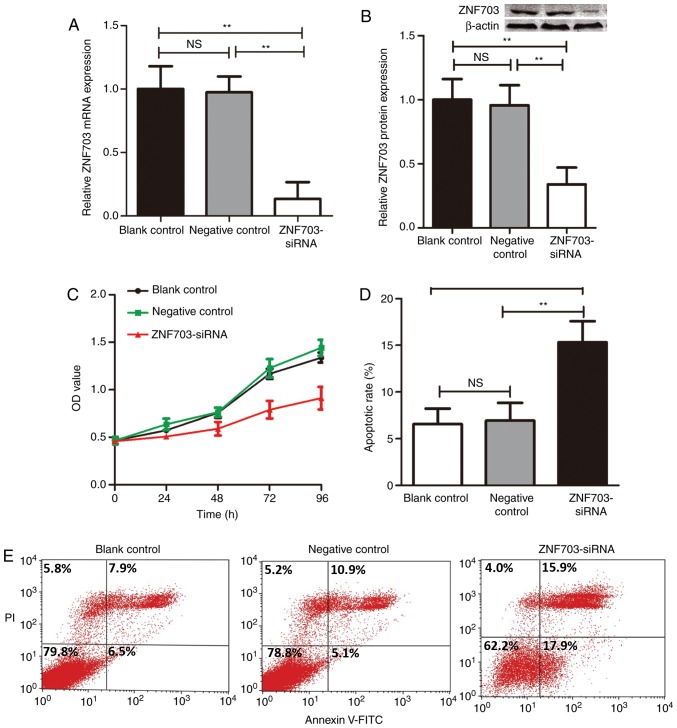
The present study knocked down the expression of ZNF703 using siRNA-mediated silencing in TT cells, a human MTC-derived cell line, in order to determine the function of ZNF703 in MTC. The ability of ZNF703-siRNA to decrease ZNF703 mRNA and protein expression was assessed by RT-qPCR and western blotting. Transfection of ZNF703-siRNA decreased the expression of ZNF703 mRNA and ZNF703 protein in TT cells compared with non-silencing siRNA-transfected cells (P<0.05; Fig. 2A and B), respectively. An MTT assay was utilized to assess cell proliferation following transfection of ZNF703-siRNA (Fig. 2C). The proliferation of TT cells was significantly decreased after inhibiting the expression of ZNF703 compared with non-silencing siRNA-transfected cells (P<0.01).
Figure 2.
ZNF703-siRNA suppresses proliferation and promotes the apoptosis of TT cells. (A) TT cells were transfected with ZNF703-siRNA. The mRNA level of ZNF703 was examined using reverse transcription-quantitative PCR. (B) The protein level of ZNF703 was assessed by western blotting. (C) Cell proliferation was evaluated using the MTT assay after ZNF703 silencing. (D and E) Cell apoptosis was evaluated by flow cytometry after ZNF703 silencing. **P<0.01, n=3. siRNA, small interfering RNA; NS, no significance.
Subsequently, the present study assessed the effects of ZNF703-siRNA on the apoptosis of TT cells using flow cytometry with Annexin V and PI staining. The results of the present study demonstrated that cells transfected with ZNF703-siRNA demonstrated significantly higher apoptosis rates compared with non-silencing siRNA-transfected cells (P<0.05; Fig. 2D and E).
ZNF703 silencing inhibits MTC tumor growth in vivo
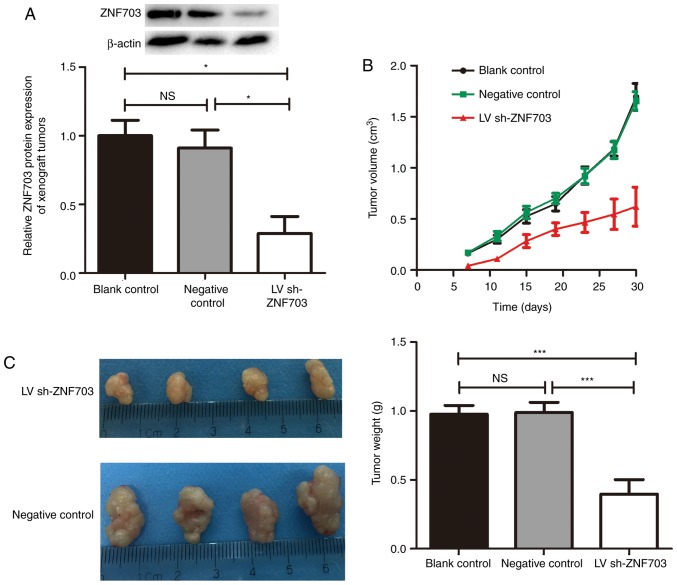
In order to further evaluate the effects of ZNF703 expression on MTC growth, the present study examined the effects of ZNF703-silencing on MTC nude mice xenografts. Western blotting of ZNF703 in xenograft tumors confirmed that ZNF703 expression had been inhibited and maintained throughout the experiment (Fig. 3A). The growth of xenograft tumors was significantly lower in the LV sh-ZNF703 group compared with the blank control and negative control groups (P<0.05; Fig. 3B). After 30 days, ZNF703-inhibited mice exhibited a significant decrease in tumor weight (P<0.05; Fig. 3C) compared with the blank control and negative control groups. There was no statistical difference between the blank control and negative control groups (P>0.05). These results suggest that the downregulation of ZNF703 inhibits proliferation of TT cells in vivo.
Figure 3.
ZNF703 silencing in TT cells suppresses xenograft tumor growth. (A) Expression of ZNF703 protein in xenograft tumors from individual mice carrying either TT cells transfected with control-shRNA or TT cells transfected with ZNF703-shRNA was examined by western blotting. Each data point represents the mean ± SD of six xenograft tumors. (B) Volumes of xenograft tumors derived from cells with control-shRNA or ZNF703-shRNA were measured over time. Each data point represents the mean ± SD of six xenograft tumors. (C) Weights of xenograft tumors derived from cells with control-shRNA or ZNF703-shRNA were measured at 30 days. Left, photographs of xenograft tumors. Right, weights of tumors. Each data point represents the mean ± SD of six xenograft tumors. *P<0.05; ***P<0.001. NS, no significance; SD, standard deviation; shRNA, short hairpin RNA; LV, lentiviruses.
ZNF703 modulates proliferation and apoptosis of TT cells through the Akt signaling pathway
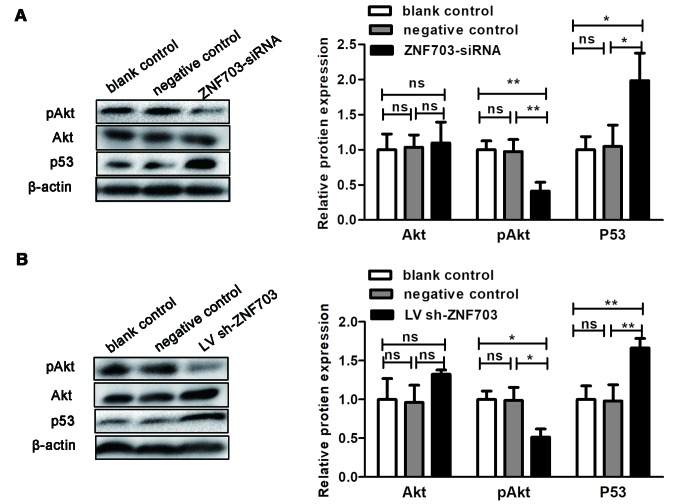
In order to further investigate whether ZNF703 expression was associated with activation of the Akt/mTOR signaling pathway, the present study detected levels of Akt and pAkt473 following ZNF703 knockdown in TT cells and xenograft tumors. ZNF703 inhibition significantly decreased pAkt473 protein expression both in vitro and in vivo (P<0.05; Fig. 4). Akt protein levels were not altered in vitro or in vitro following ZNF703 knockdown (P>0.05; Fig. 4). It has been reported that ZNF703 may facilitate p53 degradation (21). In order to verify whether the increased apoptosis rate in TT cells after ZNF703 silencing was associated with p53, the present study investigated the gene expression of p53 following ZNF703 silencing in TT cells. ZNF703 inhibition significantly induced p53 protein expression both in vitro and in vivo (P<0.05; Fig. 4). These results indicate that ZNF703 may promote cell proliferation through activation of the Akt/mTOR signaling pathway.
Figure 4.
ZNF703 silencing regulates the levels of pAKT473 and p53 protein in vitro and in vivo. (A) Akt, pAKT473 and p53 protein expression in TT cells, with or without ZNF703 silencing, were determined by western blotting. Each data point represents the mean ± SD of three independent experiments. (B) Akt, pAKT473 and p53 protein expression in xenograft tumors, with or without ZNF703 silencing, were determined by western blotting. Each data point represents the mean ± SD of six xenograft tumors. *P<0.05; **P<0.01. NS, no significance; SD, standard deviation; siRNA, small interfering RNA; shRNA, short hairpin RNA.
Discussion
Zinc finger protein is widely expressed in eukaryotes and is involved in a number of important biological processes such as cell differentiation, proliferation and apoptosis (21). ZNF703 is a newly identified member of the neutrophil extracellular trap (NET) zinc finger subfamily (21). NET proteins lack a nuclear localization signal, unlike other nuclear proteins (31). Therefore, ZNF703 needs to interact with other proteins that assist with its localization to the nucleus (21,31). Sircoulomb et al (23) identified ZNF703 as a co-factor comprising; i) DNA damage-binding protein 1 (DDB1); ii) cullin-4 (CUL4); iii) associated factor 7 (DCAF7); iv) prohibitin-2 (PHB2) and v) nuclear receptor corepressor 2 (NCOR2), which regulated self-renewal activity of breast cancer stem cells.
Recent studies have revealed that the deregulation of ZNF703 is associated with malignant tumors (22,23), but the role of ZNF703 in MTC remains unknown. In the present study, IHC staining of 34 MTC tissues and western blot analyses of 12 paired MTC and adjacent normal thyroid tissues revealed that the protein levels of ZNF703 were upregulated in MTC tissues compared with normal thyroid tissues. These results are consistent with previous reports, which have demonstrated the upregulation of ZNF703 in breast cancer (22,23,28), gastric cancer (25) and cholangiocarcinoma (26) compared with normal tissues.
Furthermore, ZNF703 protein expression was demonstrated to be associated with tumor size, nodal status and clinical stage. In addition, high expression of ZNF703 in MTC was observed, which is consistent with the possibility that ZNF703 has transforming and tumorigenic properties in MTC (26). The Akt/mTOR signaling pathway plays a critical role in tumorigenesis and progression of MTC (11–14), consistent with the present study where 26 (76.5%) of the MTCs exhibited pathway activation. There was a positive correlation between the level of ZNF703 and pAkt473 in MTC, which suggests that ZNF703 may play a role in MTC through the Akt/mTOR signaling pathway.
In order to investigate the role of ZNF703 in MTC cells, the present study used siRNA and lentivirus shRNA in order to determine whether inhibition of ZNF703 in TT cells affect processes associated with tumor progression. The results of the present study demonstrate that inhibition of ZNF703 expression results in inhibition of proliferation and induction of apoptosis of TT cells. These results are in accordance with previous reports in which gastric cancer (25), cholangiocarcinoma (26) and oral squamous cell carcinoma (27) cells were suppressed when the expression of ZNF703 was inhibited. These results support the clinical findings of the present study and suggest that ZNF703 may be a functional biomarker for MTC.
It has been demonstrated that ZNF703 enhances cell proliferation and metastasis in oral squamous cell carcinoma, breast cancer and NSCLC by activation of the Akt/mTOR signaling pathway (24,27,28). In order to further verify whether ZNF703 was involved in activation of the Akt/mTOR pathway in MTC, the present study detected pAkt473 levels following knockdown of ZNF703 in TT cells. The results of the present study demonstrated that pAkt473 decreased after inhibition of ZNF703 in vitro and in vivo, which is consistent with the IHC results. Given the inhibition of the Akt signaling pathway following knockdown of ZNF703 and the concomitant inhibition of TT cell proliferation in vitro and in vivo, the results of the present study suggest that ZNF703 gives rise to activation of the Akt/mTOR signaling pathway, resulting in the development and progression of MTC.
The present study also demonstrated an increase in the levels of p53 protein after inhibition of ZNF703. P53 is a noted tumor suppressor and is degraded by ubiquitin-mediated proteolysis (32). ZNF703 may facilitate the ubiquitination and destabilization of p53 through the E3 ubiquitin ligase complex CUL4-DDB1-DCAF7 (33) because of its interaction with DCAF (24). In addition, pAkt phosphorylates E3 ligase murine double minute 2 (MDM2) at Ser166 and Ser186 residues, increasing the ubiquitination activity of MDM2, thereby promoting p53 degradation (34–36). Thus, the induction of p53 may be caused directly or indirectly by ZNF703 knockdown.
Collectively, the results of the present study demonstrate that ZNF703 is upregulated in MTC and its expression is associated with tumor size, lymph node metastasis and pathological stage of MTC. Furthermore, ZNF703 expression is associated with pAkt473 levels, whereas silencing of ZNF703 inactivates the Akt/mTOR pathway and induces p53 expression to inhibit proliferation and induce apoptosis. Thus, ZNF703 may be a key regulator of MTC development and progression, and may serve as a valuable therapeutic target in MTC due to its association with the Akt/mTOR signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation of Hebei Province (grant no. H2018105066).
Availability of data and materials
All data generated or analyzed during the present study are included in the published article.
Authors' contributions
XY, GL, WL, FY and XX designed the present study. XY, LZ, DL and QW performed the experiments. XY and GL wrote the manuscript and analyzed the data. All authors approved the final published version of this article.
Ethics approval and consent to participate
The present study was approved by the Ethical Committee of Tangshan Gongren Hospital (approval no. GRYY-LL-2017-58), and their approval was also given for procedures performed in Tangshan Renmin Hospital. All patients provided informed consent prior to their inclusion. All experiments involving animals were approved by the Committee on the Use of Live Animals in Teaching and Research of North China University of Science and Technology (approval no. 2017168).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2019 Oct 15; doi: 10.1038/s41574-019-0263-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/S0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 5.Trimboli P, Ulisse S, Graziano FM, Marzullo A, Ruggieri M, Calvanese A, Piccirilli F, Cavaliere R, Fumarola A, D'Armiento M. Trend in thyroid carcinoma size, age at diagnosis, and histology in a retrospective study of 500 cases diagnosed over 20 years. Thyroid. 2006;16:1151–1155. doi: 10.1089/thy.2006.16.1151. [DOI] [PubMed] [Google Scholar]
- 6.Matias-Guiu X, De Lellis R. Medullary thyroid carcinoma: A 25-year perspective. Endocr Pathol. 2014;25:21–29. doi: 10.1007/s12022-013-9287-2. [DOI] [PubMed] [Google Scholar]
- 7.Rahmani N, Abbas Hashemi S, Fazli M, Raisian M. Clinical management and outcomes of papillary, follicular and medullary thyroid cancer, surgery. Med Glas (Zenica) 2013;10:164–167. [PubMed] [Google Scholar]
- 8.Maxwell JE, Sherman SK, O'Dorisio TM, Howe JR. Medical management of metastatic medullary thyroid cancer. Cancer. 2014;120:3287–3301. doi: 10.1002/cncr.28858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernani V, Kumar M, Chen AY, Owonikoko TK. Systemic treatment and management approaches for medullary thyroid cancer. Cancer Treat Rev. 2016;50:89–98. doi: 10.1016/j.ctrv.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q, Lin X, Ding L, Zeng Y, Pang D, Ouyang N, Xiang Y, Yao H. ARHGAP42 promotes cell migration and invasion involving PI3K/Akt signaling pathway in nasopharyngeal carcinoma. Cancer Med. 2018;7:3862–3874. doi: 10.1002/cam4.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagliano T, Bellio M, Gentilin E, Molè D, Tagliati F, Schiavon M, Cavallesco NG, Andriolo LG, Ambrosio MR, Rea F, et al. mTOR, p70S6K, AKT, and ERK1/2 levels predict sensitivity to mTOR and PI3K/mTOR inhibitors in human bronchial carcinoids. Endocr Relat Cancer. 2013;20:463–475. doi: 10.1530/ERC-13-0042. [DOI] [PubMed] [Google Scholar]
- 13.Tamburrino A, Molinolo AA, Salerno P, Chernock RD, Raffeld M, Xi L, Gutkind JS, Moley JF, Wells SA, Jr, Santoro M. Activation of the mTOR pathway in primary medullary thyroid carcinoma and lymph node metastases. Clin Cancer Res. 2012;18:3532–3540. doi: 10.1158/1078-0432.CCR-11-2700. [DOI] [PubMed] [Google Scholar]
- 14.Campos M, Kool MM, Daminet S, Ducatelle R, Rutteman G, Kooistra HS, Galac S, Mol JA. Upregulation of the PI3K/Akt pathway in the tumorigenesis of canine thyroid carcinoma. J Vet Intern Med. 2014;28:1814–1823. doi: 10.1111/jvim.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouvaraki MA, Liakou C, Paraschi A, Dimas K, Patsouris E, Tseleni-Balafouta S, Rassidakis GZ, Moraitis D. Activation of mTOR signaling in medullary and aggressive papillary thyroid carcinomas. Surgery. 2011;150:1258–1265. doi: 10.1016/j.surg.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Manfredi GI, Dicitore A, Gaudenzi G, Caraglia M, Persani L, Vitale G. PI3K/Akt/mTOR signaling in medullary thyroid cancer: A promising molecular target for cancer therapy. Endocrine. 2015;48:363–370. doi: 10.1007/s12020-014-0380-1. [DOI] [PubMed] [Google Scholar]
- 17.Giunti S, Antonelli A, Amorosi A, Santarpia L. Cellular signaling pathway alterations and potential targeted therapies for medullary thyroid carcinoma. Int J Endocrinol. 2013;2013:803171. doi: 10.1155/2013/803171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikas A, Vachhani S, Jensen K, Vasko V, Burman KD. Targeted therapies in thyroid cancer: An extensive review of the literature. Expert Rev Clin Pharmacol. 2016;9:1299–1313. doi: 10.1080/17512433.2016.1204230. [DOI] [PubMed] [Google Scholar]
- 19.Burke JF, Schlosser L, Harrison AD, Kunnimalaiyaan M, Chen H. MK-2206 causes growth suppression and reduces neuroendocrine tumor marker production in medullary thyroid cancer through akt inhibition. Ann Surg Oncol. 2013;20:3862–3868. doi: 10.1245/s10434-013-3168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreazzoli M, Broccoli V, Dawid IB. Cloning and expression of noz1, a zebrafish zinc finger gene related to Drosophila nocA. Mech Dev. 2001;104:117–120. doi: 10.1016/S0925-4773(01)00359-8. [DOI] [PubMed] [Google Scholar]
- 21.Pereira-Castro I, Costa AM, Oliveira MJ, Barbosa I, Rocha AS, Azevedo L, da Costa LT. Characterization of human NLZ1/ZNF703 identifies conserved domains essential for proper subcellular localization and transcriptional repression. J Cell Biochem. 2013;114:120–133. doi: 10.1002/jcb.24309. [DOI] [PubMed] [Google Scholar]
- 22.Slorach EM, Chou J, Werb Z. Zeppo1 is a novel metastasis promoter that represses E-cadherin expression and regulates p120-catenin isoform expression and localization. Gene Dev. 2011;25:471–484. doi: 10.1101/gad.1998111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sircoulomb F, Nicolas N, Ferrari A, Finetti P, Bekhouche I, Rousselet E, Lonigro A, Adélaïde J, Baudelet E, Esteyriès S, et al. ZNF703 gene amplification at 8p12 specifies luminal B breast cancer. EMBO Mol Med. 2011;3:153–166. doi: 10.1002/emmm.201100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baykara O, Dalay N, Kaynak K, Buyru N. ZNF703 overexpression may act as an oncogene in non-small cell lung cancer. Cancer Med. 2016;5:2873–2878. doi: 10.1002/cam4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang G, Ma F, Zhong M, Fang L, Peng Y, Xin X, Zhong J, Yuan F, Gu H, Zhu W, Zhang Y. ZNF703 acts as an oncogene that promotes progression in gastric cancer. Oncol Rep. 2014;31:1877–1882. doi: 10.3892/or.2014.2997. [DOI] [PubMed] [Google Scholar]
- 26.Li K, Wang J, Han J, Lan Y, Xie C, Pan S, Liu L. Overexpression of ZNF703 facilitates tumorigenesis and predicts unfavorable prognosis in patients with cholangiocarcinoma. Oncotarget. 2016;7:76108–76117. doi: 10.18632/oncotarget.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Deng X, Zhang J, Ou Z, Mai J, Ding S, Huo S. Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β signalling in oral squamous cell carcinoma. Cell Physiol Biochem. 2017;44:920–934. doi: 10.1159/000485360. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Mu X, Huang O, Xie Z, Jiang M, Geng M, Shen K. Luminal breast cancer cell lines overexpressing ZNF703 are resistant to tamoxifen through activation of Akt/mTOR signaling. PLoS One. 2013;8:e72053. doi: 10.1371/journal.pone.0072053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metgud R, Astekar MS, Soni A, Naik S, Vanishree M. Conventional xylene and xylene-free methods for routine histopathological preparation of tissue sections. Biotech Histochem. 2013;88:235–241. doi: 10.3109/10520295.2013.764015. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta CT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Pereira F, Duarte-Pereira S, Silva RM, da Costa LT, Pereira-Castro I. Evolution of the NET (NocA, Nlz, Elbow, TLP-1) protein family in metazoans: Insights from expression data and phylogenetic analysis. Sci Rep. 2016;6:38383. doi: 10.1038/srep38383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13:83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thirunavukarasou A, Singh P, Govindarajalu G, Bandi V, Baluchamy S. E3 ubiquitin ligase Cullin4B mediated polyubiquitination of p53 for its degradation. Mol Cell Biochem. 2014;390:93–100. doi: 10.1007/s11010-014-1960-3. [DOI] [PubMed] [Google Scholar]
- 34.Chang H, Li C, Huo K, Wang Q, Lu L, Zhang Q, Wang Y, Wang W. Luteolin prevents H2O2-induced apoptosis in H9C2 cells through modulating Akt-P53/Mdm2 signaling pathway. Biomed Res Int. 2016;2016:5125836. doi: 10.1155/2016/5125836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Dai B, Zhang H, Shi G, Shen Y, Ye D. Long non-coding RNA LOC572558 inhibits bladder cancer cell proliferation and tumor growth by regulating the AKT-MDM2-p53 signaling axis. Cancer Lett. 2016;380:369–374. doi: 10.1016/j.canlet.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Tu Y, Kim E, Gao Y, Rankin GO, Li B, Chen YC. Theaflavin-3, 3′-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells. Int J Oncol. 2016;48:2657–2665. doi: 10.3892/ijo.2016.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in the published article.