Abstract
The purpose of the present study was to review the recurrence and prognostic factors of squamous cell carcinoma (SCC) associated with inverted papilloma (IP). A retrospective chart review was conducted on 21 patients with SCC associated with IP, in the nasal cavity and paranasal sinuses, between March 2007 to March 2017. All patients underwent surgical treatment: Surgery prior to or following adjuvant therapy was performed in 17 patients (81.0%). During a mean follow-up time of 47.4 months (range, 3–123 months), 9 patients (42.9%) experienced local recurrence, and the risk factors of T4 stage and invasive orbital cavity had a significant influence on recurrence. The 1-, 3-, and 5-year overall survival rates were 90.5, 75.4 and 68.5%, and the 1-, 3-, and 5-year disease-specific survival (DSS) rates were 90.5, 80.4 and 80.4%, respectively. The prognosis of patients with stage T4 was not satisfactory compared with those with stage T3 or less, and a positive surgical margin was also significantly associated with poor survival. Overall, SCC associated with IP has a favorable DSS, early diagnosis and complete resection of lesions is required for a good prognosis. Furthermore, aggressive surgical approaches combined with postoperative adjuvant therapy seem to be effective in tumors at stage T4.
Keywords: inverted papilloma, squamous cell carcinoma, sinonasal, recurrence, prognostic
Introduction
Inverted papilloma (IP) is a benign epithelial neoplasm in the sinonasal tract, accounting for 0.5–4.0% of all nasal tumors (1). IP is embryonically derived from the Schneiderian membrane, an ectodermal sinonasal-derived respiratory mucosa. There are three characteristic attributes of IP: Destructive or bone remodeling capacity, high rates of recurrence, and possible association with malignancy (1–3). Although the pathogenesis of IP is yet to be elucidated, certain studies have indicated human papillomavirus as a potential pathogenic factor, but its role is still unclear (4,5). Histologically, IP may be associated with a varying degree of dysplasia, atypia, carcinoma in situ, and frank squamous cell carcinoma (SCC) (2). The incidence of sinonasal SCC associated with IP ranges from 2–27% in the literature (6). SCC in IP frequently occurs metachronously, arising in the same site where the IP had previously been resected, or synchronously, diagnosed in the same initial lesion (7). In a meta-analysis of 63 case series representing >2,000 patients, Mirza et al (8) reported a 7.1% incidence of synchronous cancer and a 3.6% incidence of asynchronous SCC (8). Certain symptoms, including nasal obstruction, epitaxis and rhinorrhea are associated with the occurrence of IP and IP-related SCC (9); however, the lack of specificity of these symptoms makes the identification of IP and SCC-associated IP is often problematic. Therefore, complete surgical excision and long-term follow-up are recommended treatment options for these patients.
Due to the rarity of carcinomas associated with IP, there are few reports in the literature regarding its characteristics and subsequent survival rate (7,10). Hence, the purpose of the present study is to review the clinical characteristics, treatment outcomes, overall survival (OS), and disease-specific survival (DSS). In addition, recurrence and prognostic factors associated with this rare malignancy were also analyzed.
Materials and methods
Patient population
A retrospective chart review was performed on 408 patients, who were diagnosed with IP or carcinoma associated with IP in the nasal cavity and paranasal sinuses. Out of 408 patients, 21 cases (5.1%) of SCC associated with IP were treated at the Department of Otorhinolaryngology of the Affiliated Eye Ear Nose and Throat Hospital (AEENTH), Fudan University, between March 2007 and March 2017. The present study was approved by the institutional review board of AEENTH, Fudan University (China). Informed consent was obtained from all the patients.
Treatments and follow-up
All patients underwent surgical intervention, which was performed by Dr Dehui Wang, the surgical interventions included transnasal endoscopic resection and open surgical resection. Patient demographics, the distribution of the sex, the mean age and age range of the patients, Tumor-Node-Metastasis (TNM) staging (11), surgical approach, the need for an adjunct method, and relapse were analyzed. The time of follow-up was from the initial diagnosis at the AEENTH to the date of death or last contact.
Statistical analysis
The DSS and OS rates were calculated by the Kaplan-Meier method. The significance of differences in prognostic factors was analyzed by log-rank tests. The recurrence factors were analyzed by Fisher's exact probability. P<0.05 were considered to indicate a statistically significant difference. The SPSS 19.0 statistical software (SPSS, Inc.) was used for all statistical analyses.
Results
Demographic data
The characteristics of patients included in this series are shown in Table I. A total of 21 patients were identified, comprising of 18 (85.7%) males and 3 (14.3%) females; the mean age was 59.2 years (range, 35–81 years). There were 7 cases of right-side lesions and 14 cases of left-side lesions. The origin site was the maxillary sinus in 11 cases, and the nasal cavity and other sinuses in 10 cases. The invading sites outside the nasal cavity included the orbital cavity (orbital wall, 9 cases; intraorbit, 2 cases), infratemporal fossa (n=4), pterygopalatine fossa (n=3), alveolar bone (n=2), and facial subcutaneous tissue (n=1). The main symptoms of SCC associated with IP presented nasal obstruction and epistaxis; other symptoms included cheek pain, decreased vision and epiphora. According to the American Joint Committee on Cancer (AJCC) TNM Classification system (7th edition, 2010) (11), the tumor grades were as follows: T1, 2 cases (9.5%); T2, 1 case (4.8%); T3, 10 cases (47.6%); and T4, 8 cases (38.1%). The malignancy was synchronous in 13 patients (61.9%) and metachronous in 7 patients (38.1%).
Table I.
Features of squamous cell carcinoma associated with inverted papilloma.
| Patients/sex/age | Lateral/origin site | Extent site | Main symptom | TNM stage | Surgical procedure | Surgical margin | Type | Adjuvant therapy | Recurrence | Follow up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/M/58 | R/MS | NC, ES, IF, AB | Cheek pain | 4 | ETR + CLO | Positive | Synchronous | IT+CRT | 1 | 20 | AWD/brain metastasis |
| 2/M/59 | L/NC | / | Nasal obstruction | 1 | ETR | Free | Synchronous | No | No | 20 | NED |
| 3/M/49 | L/NC | MS, ES, OW | Nasal obstruction | 3 | ETR + CLO | Free | Metachronous | No | 1 | 22 | NED |
| 4/M/70 | L/NC | ES | Nasal obstruction | 2 | ETR | Free | Synchronous | CRT | No | 19 | DOC/cerebral infarction |
| 5/M/64 | R/MS | NC, ES, SS, PF, IF, AB | Nasal obstruction | 4 | ETR | Positive | Synchronous | RT + CRT | 2 | 12 | DOD |
| 6/F/68 | L/NS, ES | OW | Epistaxis | 3 | LR | Free | Synchronous | RT + CRT | 4 | 123 | DOD/liver metastasis |
| 7/M/67 | L/MS | PF, IF, ES, OW | Nasal obstruction | 4 | ETR | Free | Metachronous | RT | No | 61 | NED |
| 8/M/68 | L/MS | NC, ABFST | Cheek pain | 3 | ETR | Positive | Metachronous | RT | No | 59 | NED |
| 9/F/49 | R/MS | NC, ES | Nasal obstruction | 3 | ETR + PME | Free | Synchronous | No | No | 28 | Unknown |
| 10/M/66 | R/MS | / | Nasal obstruction | 1 | ETR | Free | Metachronous | No | No | 103 | NED |
| 11/M/35 | L/MS | ES, OW | Nasal obstruction | 3 | ETR | Free | Synchronous | RT + CRT | 2 | 75 | AWD |
| 12/M/53 | L/NC | MS, ES, SS, PF, IO | Decreased vision | 4 | ETR | Positive | Synchronous | RT + CRT | 1 | 3 | DOD |
| 13/M/55 | L/MS | NC, ES, IFOW | Epiphora | 4 | ETR | Free | Metachronous | RT + CRT | 1 | 17 | DOD |
| 14/M/45 | R/ES | FS, IO ACB | Epistaxis | 4 | ETR + EI | Positive | Metachronous | RT + CRT | 1 | 14 | DOD |
| 15/M/52 | L/MS | OW | Nasal obstruction | 3 | ETR | Free | Metachronous | RT | No | 60 | NED |
| 16/F/57 | R/NC | MS | Nasal obstruction | 3 | ETR | Free | Synchronous | RT | No | 87 | Unknown |
| 17/M/61 | L/NC | ES, MS | Nasal obstruction | 3 | ETR | Free | Synchronous | CRT | No | 54 | NED |
| 18/M/68 | R/NC | ES, MS | Nasal obstruction | 3 | ETR | Free | Synchronous | RT + CRT | No | 43 | DOC/Cerebral infarction |
| 19/M56 | L/MS | NC, ES, SS, OW, LS | Epiphora | 4 | ETR | Positive | Synchronous | RT | No | 12 | Unknown |
| 20/M/63 | L/NC, ES | FS, OW | Epistaxis | 4 | ETR | Free | Metachronous | RT | 4 | 60 | NED |
| 21/M/81 | L/MS | NC, ES | Epistaxis | 3 | ETR | Free | Synchronous | RT | No | 103 | NED |
MS, maxillary sinus; NC, nasal cavity; ES, ethmoid sinus; SS, sphenoid sinus; FS, frontal sinus PF; pterygopalatine fossa; IF, infratemporal fossa; AB, alveolar bone; OW, orbital wall; FST, facial subcutaneous tissue; IO, intra-orbit; LS, Lacrimal sac; ACB, anterior cranial base; ETR, endoscopic transnasal resection; CLO, Caldwell-Luc operation; EI, external incision; PME, partial maxillary excision; LR, lateral rhinotomy; IT, immunotherapy; RT, radiotherapy; CRT, chemoradiotherapy; NED, no evidence of disease; DOD, die of disease; AWD, alive with disease; DOC, die of another cause.
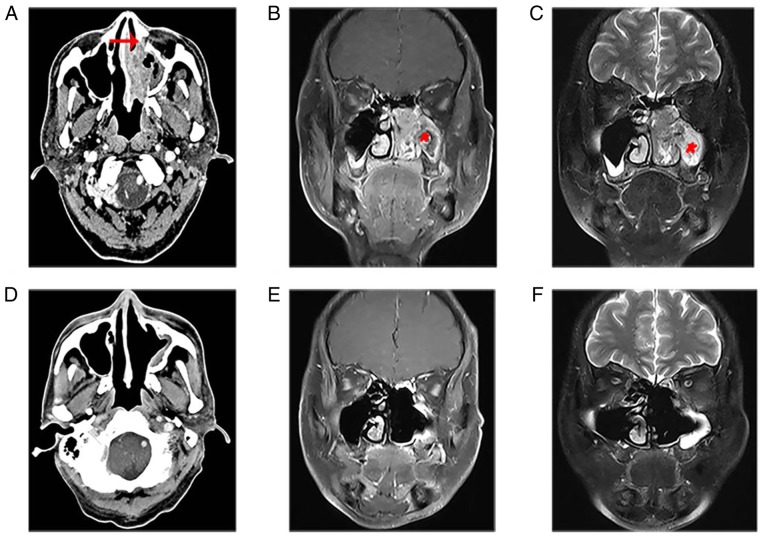
In the present study, patient 17 with stage T3 presented a large diffuse soft tissue mass in the left nasal cavity, which invaded the middle and inferior nasal meatus, maxillary sinus and ethmoid sinus. Enhanced CT showed partially destroyed bone of the maxillary sinus (Fig. 1A). The MRI showed that the nasal mass displayed characteristic CCP, which was lost in most of the mass of the maxillary sinus (Fig. 1B and C). The patients were treated with complete resection of the tumors under nasal endoscopy. Following 54 months of follow-up, there was no tendency of recurrence based on imaging examinations (Fig. 1D-F).
Figure 1.
The imaging characteristics of squamous cell carcinoma associated with inverted papilloma. (A) Enhanced computerized tomography showed a large, diffuse soft tissue mass in the left nasal cavity, which invaded the middle and inferior nasal meatus, maxillary sinus and ethmoid sinus, and partly destroyed the bone (red arrow) of the sinus cavity. (B) MRI showed the nasal mass displayed the characteristic convoluted cerebriform pattern, which was lost in most of the mass of the maxillary sinus (asterisk). (C) Coronal contrast-enhanced T1-weighted image showed a moderate signal. Coronal T2-weighted MRI showed a slightly high signal. (D) Enhanced CT demonstrated no obvious mass in the left nasal cavity, and soft tissue around the maxillary sinus was thickened without obvious enhancement. (E) Coronal T1-weighted and (F) T2-weighted MRI revealed no mass in the nasal cavity and slight mucosal enhancement in the perioperative wall. CT, computerized tomography; MRI, magnetic resonance imaging.
Treatment and Outcomes
All patients underwent surgical treatment, 15 patients (66.7%) received endoscopic transnasal resection, endoscopy combined with Caldwell-Luc surgery was administered in 4 patients (19.0%), and other surgical procedures included lateral rhinotomy (n=1) and endoscopy combined with external incision (n=1). In addition, patient 9 simultaneously underwent partial right maxillary excision. Surgery prior to or after adjuvant radiochemotherapy was performed in 7 patients (33.3%). Exclusive radiation therapy was carried out in 7 patients (33.3%), exclusive chemotherapy was performed in 2 patients (9.5%), and patient 1 underwent immunotherapy with programmed cell death-1 (PD-1) antibody drugs and chemotherapy following surgical resection of the tumor.
During a mean follow-up time of 47.4 months (range, 3–123 months), the 1-, 3-, and 5-year OS rates were 90.5, 75.4 and 68.5% (Fig. 2), and the 1-, 3-, and 5-year DSS was 90.5, 80.4 and 80.4%, respectively (Fig. 3). The tumors in 15 patients (71.4%) were completely resected at the AEENTH as the initial surgery, and residual tumors were found in 6 patients (28.6%). Nine patients (42.9%) experienced a local recurrence, and the risk factors of T4 stage and invasive orbital cavity had a significant influence on recurrence. Tumors with a positive surgical margin demonstrated a higher rate of recurrence than those with a negative margin, but there was no significant difference (P=0.33). In addition, the recurrence comparison did not reveal significant differences regarding the factors of sex, age ≥65 years or origin site (Table II). Five patients (23.8%) died due to tumor progression, and 2 patients (9.5%) died due to cerebral infarction accident. Remission was confirmed upon physical exam, and/or disappearance of the tumor was confirmed by imaging studies in 9 patients (42.9%), whereas 2 patients (9.5%) remained alive with the disease. Three patients were lost during follow-up.
Figure 2.
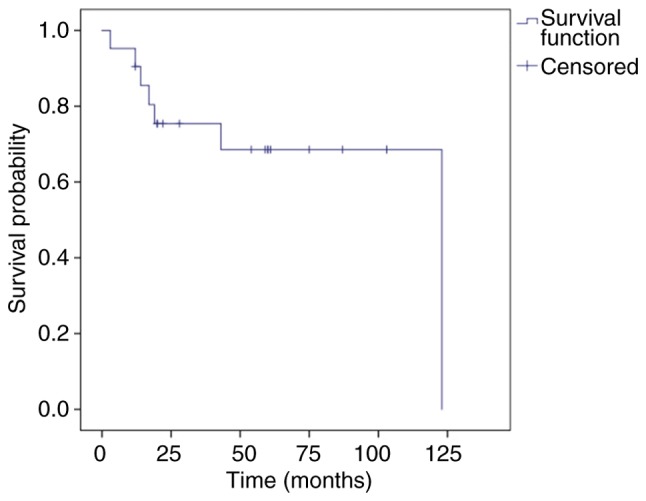
Kaplan-Meier curve displaying overall survival. The 1-, 3-, and 5-year overall survival were 90.5, 75.4 and 68.5%, respectively.
Figure 3.
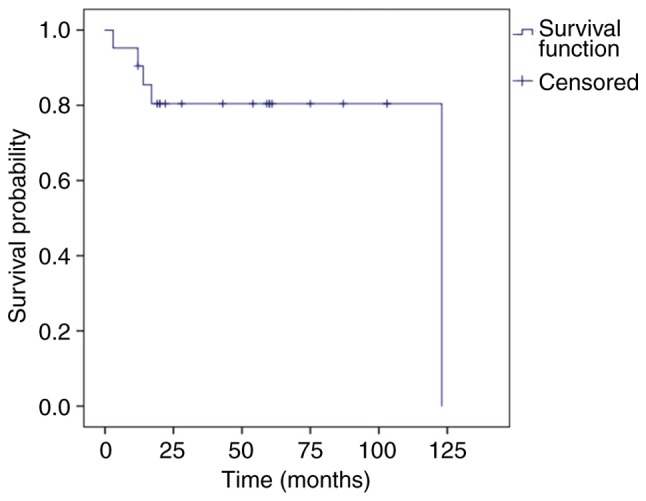
Kaplan-Meier curve displaying disease-specific survival. The 1-, 3-, and 5-year disease-specific survival was 90.5, 80.4 and 80.4%, respectively.
Table II.
Prognostic factors for recurrence and survival.
| Characteristics | Patients | Recurrences, n (%) | Recurrences, P-value | 1-year DSS, % | 3-year DSS, % | Survival, P-value |
|---|---|---|---|---|---|---|
| All patients | 21 | 9 (42.9) | / | 90.5 | 80.4 | / |
| Sex | >0.05 | 0.38 | ||||
| Male | 18 | 8 (44.4) | 88.9 | 77.0 | ||
| Female | 3 | 1 (33.3) | 100 | 100 | ||
| Age, years | 0.15 | 0.12 | ||||
| ≥65 | 7 | 1 (14.3) | 100 | 100 | ||
| <65 | 14 | 8 (57.1) | 85.7 | 70.1 | ||
| T stage | 0.03 | <0.01 | ||||
| T4 | 8 | 6 (75.0) | 75.0 | 45.0 | ||
| T1, T2, T3 | 13 | 3 (23.1) | 100 | 100 | ||
| Invasive orbital cavity | 0.03 | 0.22 | ||||
| Yes | 10 | 7 (70.0) | 90.0 | 67.5 | ||
| No | 11 | 2 (18.2) | 90.9 | 90.9 | ||
| Origin site | 0.57 | 0.92 | ||||
| Maxillary sinus | 11 | 4 (36.4) | 90.9 | 80.8 | ||
| Other sites | 10 | 5 (50.0) | 90.0 | 80.0 | ||
| Surgical margin | 0.33 | <0.01 | ||||
| Negative | 15 | 5 (37.5) | 93.3 | 93.3 | ||
| Positive | 6 | 4 (60.0) | 66.7 | 44.4 |
Prognostic factors
Prognostic factors for the treatment outcomes of SCC associated with IP are shown in Table II. T4 tumors influenced survival; 3-year DSS of these patients was 45.0%, in contrast to patients with a stage of T3 or less for whom 3-year DSS was 100% (P<0.01; Fig. 4). Positive surgical margins adversely affected patient outcome; these patients had a 44.4% 3-year cumulative survival probability, whereas negative surgical margins improved survival to 93.3% (P<0.01; Fig. 5). Although the 3-year DSS of patients aged ≥65 years (100%) was higher than those aged <65 years (70.1%), there was no significant difference between the two groups (P=0.12). Males were not significantly different in terms of DSS from females (P=0.38). The invasion of the orbital cavity showed lower survival rates compared with non-invasion, but the survival comparison did not reveal any significant differences (P=0.22). Furthermore, there was no significant difference in the survival rate between the maxillary sinus and other origin sites (P=0.92).
Figure 4.
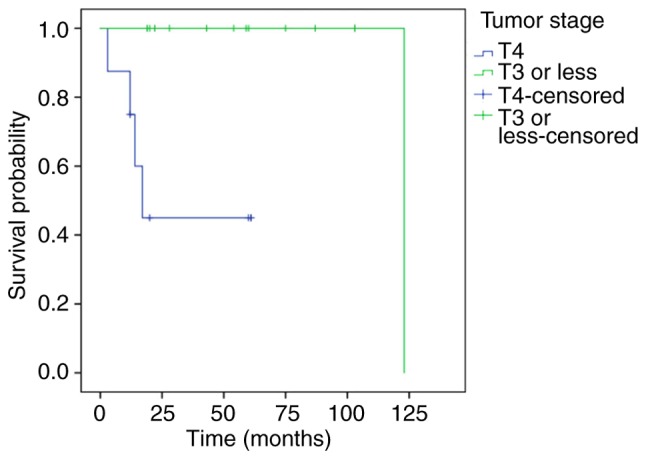
Kaplan-Meier displaying DSS of patients according to T stage. The DSS was significantly lower in patients with T4 vs. T3 tumors. P<0.01. DSS, disease-specific survival.
Figure 5.
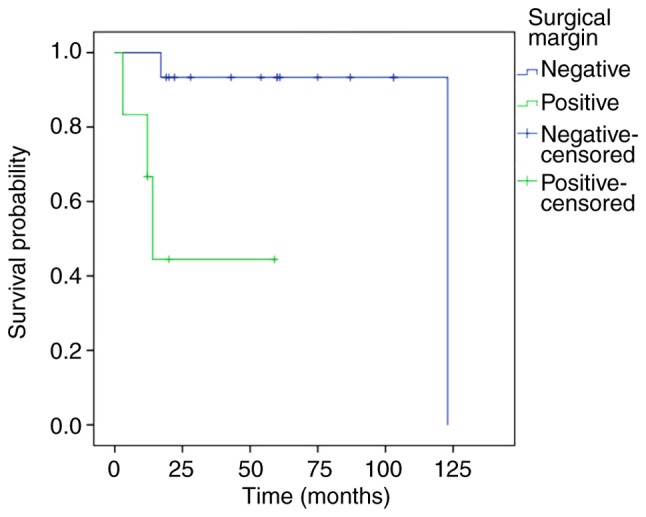
Kaplan-Meier curves displaying DSS of patients according to surgical margins. The DSS of patients was lower in those with surgical margins compared with those without surgical margins. P<0.01. DSS, disease-specific survival.
Discussion
The current literature suggests that the incidence of malignancy among patients with IP varies widely and ranges from 2–27% (6). Although adenocarcinomas, mucoepidermoid carcinomas, nasal undifferentiated carcinomas, small cell carcinomas, and NOS (not specifically specified) were also associated with IP, major histological findings most often confirmed SCC (12). The number of males with SCC associated with IP was larger than the number of women (13). The results of the present study coincided with these results, and the rate of association with malignancy was 5.1% (21/408), and all IP-associated carcinoma was SCC. Males were the predominant group of patients and accounted for 85.7% (18/21). There was a significant association between male sex and malignancy. In recent years, some studies have reported that sinonasal IP progression to SCC was significantly associated with the presence of human papillomavirus (HPV) infection (14,15). However, Mohajeri et al compared HPV positivity among sinonasal IP samples and those from patients with SCC by immunohistochemistry and found that HPV was not supported as an etiological driver of IP development or progression to SCC (16). Thus, further research is required to confirm the association between HPV infection and SCC with IP.
Lesperance et al described an average interval of 63 months (6 months to 13 years) between the onset of IP and the development of metachronous cancer (17). Another study showed that one of six cases (17%) developed metachronous cancer approximately 10 years after undergoing IP surgery (7). In this series, the average interval between benign IP and cancer onset was 44 months (range, 8 months-10 years) in 7 patients. Patient 15 underwent maxillary sinus fenestration due to the invasion of the tumor, and the pathological findings suggested benign IP. The patient remained disease-free for five years following the first surgery, when the tumor recurred again in the maxillary sinus and the patient underwent lateral rhinotomy resection. Ten years following the first surgery, maxillary sinus IP recurred for the second time and invaded the orbital floor, and endoscopic transnasal resection was performed. However, the pathological type changed from IP to SCC. Therefore, long-term follow-up is required to monitor metachronous carcinoma despite complete resection of benign IP, particularly IP presenting with severe atypical hyperplasia of mucosal epithelium.
Computed tomography (CT) scans are performed in patients with suspected IP, which can be helpful in predicting IP attachment sites (18). Bone erosion and orbital wall involvement on CT presentation are suggestive of an aggressive tumor, in accord with SCC associated with IP. However, orbital wall invasion can also be found in recurrent benign IP tumors. Therefore, the sensitivity and specificity in the diagnosis of SCC in IP are low (19). Magnetic resonance imaging (MRI) features are more valuable for distinguishing IP from SCC in IP. IP shows a distinctive gross mucosal morphology known as a convoluted cerebriform pattern (CCP). CCP is defined as a mixture of linear or curvilinear hypointensity and hyperintensity locally or diffusely presented in the solid components of tumors on enhanced T1-weighted or T2-weighted MRI. A partial loss of CCP was associated with SCC in IP patients and can be used in preoperative analysis (20,21).
Sinonasal IP is different from other benign tumors affecting paranasal sinuses notably, and its high recurrence rate is between 10 and 20%. Risk factors for local recurrence of IP remain controversial (22,23). Lisan et al reported that the history of previous resection was the only factor associated with recurrence when compared with those who underwent a first resection, which may correspond to incomplete initial resection (24). This was also supported by another study, which demonstrated that recurrence was associated with the thoroughness of the first surgical excision of the lesions (25). The risk factors associated with the recurrence of SCC in IP are seldom addressed in the literature. In the present study, the risk factors of T4 stage and invasive orbital cavity had a significant influence on local recurrence. This seemed to indicate that the invasion of areas outside the nasal cavity, such as the orbit, pterygopalatine fossa, and infratemporal fossa, led to the incomplete resection of tumors and recurrence. In addition, the recurrence rate of patients with positive margins was higher (4/6, 66.7%) compared with those with negative margins (5/15, 33.3%). However, there was no significant difference between the two groups. The reason may be that the number of patients in this study was not very large.
The prognosis of SCC associated with IP varies according to different reports. Lee et al (27) revealed that the median survival time of SCC in IP was >10 years, and ~60% of patients become long-term survivors (26). This result seemed to be comparable with the survival rate of patients with sinonasal SCC, which was reported to be between 50 and 60%. A study by Yu et al (28) compared the prognoses between the 21 patients with SCC in IP and 65 patients with SCC, and the 5-year DFS was not significantly different, which was 61.5% in the SCC in the IP group and 52.8% in the SCC group. However, some studies also suggested that SCC in IP had a more favorable prognosis compared with SCC only (7,29,30). Karligkiotis et al (31) observed a 5-year DFS of SCC in IP of 71.2% during an average follow-up period of 60 months. The same result was shown in a study of 32 patients, indicating a median survival time of 62.2 months with a 5-year OS of 72.5% (30). In the present study, the 5-year DFS and OS were 80.4 and 68.5%, respectively, which appeared to be a more favorable prognosis than most published cases of primary sinonasal SCC.
The AJCC staging system is valuable for maxillary sinus, nasal cavity and ethmoid sinus carcinomas. As SCC in IP patients rarely present with lymph node or distant metastasis, T staging regarding primary tumors is useful in predicting prognosis. Karligkiotis et al (31) reported that the 3-year DSS in the IP-associated malignancy was 100% for T1, 100% for T2, 77.8% for T3, and 40% for T4, indicating that the advanced T classification (T3 or greater) was significantly associated with poorer outcome. This was also supported by Kim et al (13) demonstrating that patients with a T stage of T2 or less had a significantly better DFS and longer disease-free interval than those with a T stage of T3 or more. In contrast, Choi et al (7) demonstrated that tumor stage was not associated with the clinical outcome of SCC in IP. In this series, the prognosis of stage T4 was not satisfactory with a 3-year DSS of 45%, and the clinical outcomes of T1, T2 and T3 were excellent, with a 3-year DSS of 100%. Furthermore, positive surgical margins also adversely affected outcomes. In addition, there were some limitations in this study: T4 and positive surgical margins were risk factors for an adverse prognosis. However, there may be a confounding factor between the two. Considering the small number of patients available at the AEENTH, more patients with SCC associated with IP should be enrolled, and confounding factors should be excluded for future studies.
Of the 5 patients who died as a result of disease progression, patient 6 with a stage of T3 died following 123 months, while 4 patients with a stage of T4 died during the first few years. These T4 tumors often invaded the sphenoid sinus, frontal sinus, infratemporal fossa and intraorbital region. The invasion range of these tumors was large and could not be entirely resected by surgery. Although radiotherapy and chemotherapy were administered following the operation, the lesions recurred, and patients died within a short time. This finding indicated that these invading regions from the primary site strongly associate with poor prognosis, but there were no statistically significant conclusions drawn, due to the small number of patients in the present study. In addition, among the other 4 patients with stage T4, patient 1 lived with disease and incurred brain metastasis 20 months following subtotal resection of the tumor in the initial operation, patient 7 and patient 20 lived without disease following complete resection and postoperative radiotherapy, and patient 19 was lost during follow-up. Thus, local lesions are difficult to control without entire resection in the treatment of T4 disease. Various surgical methods, such as endoscopic transnasal resection, the Caldwell-Luc approach and lateral rhinotomy, are used for treatment, but the complete removal of lesions is considered the key aspect not the removal method. In addition, some studies noted that postoperative radiotherapy was recommended for patients with advanced T3 or higher, and positive margins, critical areas (such as orbit or anterior skull base), and unresectable disease affected outcomes (32,33).
SCC associated with IP has a high recurrence rate, and the risk factors of T4 stage and invasive disease in the orbital cavity have a significant influence on local recurrence. In addition, SCC associated with IP has a favorable overall survival, and the prognosis of patients with stage T4 was not as satisfactory as those with a stage of T3 or less, and positive surgical margins adversely affected outcome. Thus, early diagnosis and completely resected lesions are required for a good prognosis for SCC associated with IP. Regarding tumors with stage T4, more aggressive surgical approaches combined with postoperative adjuvant therapy seem to be effective.
Acknowledgements
Not applicable.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant no. 81870703).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
DW, WL and HL conceived and designed the study. WL, HL, HZ, XS and LH acquired the data. WL, HL, HZ, XS and LH drafted the manuscript. WL and HL performed the statistical analysis. DW supervised the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Myers EN, Fernau JL, Johnson JT, Tabet JC, Barnes EL. Management of inverted papilloma. Laryngoscope. 1990;100:481–490. doi: 10.1288/00005537-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Outzen KE, Grøntveld A, Jørgensen K, Clausen PP, Ladefoged C. Inverted papilloma: Incidence and late results of surgical treatment. Rhinology. 1996;34:114–118. [PubMed] [Google Scholar]
- 3.Sham CL, Woo JK, van Hasselt CA, Tong MC. Treatment results of sinonasal inverted papilloma: An 18-year study. Am J Rhinol Allergy. 2009;23:203–211. doi: 10.2500/ajra.2009.23.3296. [DOI] [PubMed] [Google Scholar]
- 4.Govindaraj S, Wang H. Does human papilloma virus play a role in sinonasal inverted papilloma? Curr Opin Otolaryngol Head Neck Surg. 2014;22:47–51. doi: 10.1097/MOO.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 5.Justice JM, Davis KM, Saenz DA, Lanza DC. Evidence that human papillomavirus causes inverted papilloma is sparse. Int Forum Allergy Rhinol. 2014;4:995–1001. doi: 10.1002/alr.21358. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JS, Jr, Westra WH, Thompson LD, Barnes L, Cardesa A, Hunt JL, Williams MD, Slootweg PJ, Triantafyllou A, Woolgar JA, et al. The sinonasal tract: Another potential ‘hot spot’ for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2014;8:241–249. doi: 10.1007/s12105-013-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JW, Kim SG, Kim YM, Yoon YH, Kim AY, Rha KS. Clinical and histologic features of inverted papilloma-associated malignancy. Eur Arch Otorhinolaryngol. 2012;269:2349–2354. doi: 10.1007/s00405-012-1935-5. [DOI] [PubMed] [Google Scholar]
- 8.Mirza S, Bradley PJ, Acharya A, Stacey M, Jones NS. Sinonasal inverted papillomas: Recurrence, and synchronous and metachronous malignancy. J Laryngol Otol. 2007;121:857–864. doi: 10.1017/S002221510700624X. [DOI] [PubMed] [Google Scholar]
- 9.Lawson W, Patel ZM. The evolution of management for inverted papilloma: An analysis of 200 cases. Otolaryngol Head Neck Surg. 2009;140:330–335. doi: 10.1016/j.otohns.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Yasumatsu R, Nakashima T, Sato M, Nakano T, Kogo R, Hashimoto K, Sawatsubashi M, Nakagawa T. Clinical management of squamous cell carcinoma associated with sinonasal inverted papilloma. Auris Nasus Larynx. 2017;44:98–103. doi: 10.1016/j.anl.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Re M, Gioacchini FM, Bajraktari A, Tomasetti M, Kaleci S, Rubini C, Bertini A, Magliulo G, Pasquini E. Malignant transformation of sinonasal inverted papilloma and related genetic alterations: A systematic review. Eur Arch Otorhinolaryngol. 2017;274:2991–3000. doi: 10.1007/s00405-017-4571-2. [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Kim D, Koo Y, Kim CH, Choi EC, Lee JG, Yoon JH. Sinonasal carcinoma associated with inverted papilloma: A report of 16 cases. J Craniomaxillofac Surg. 2012;40:e125–e129. doi: 10.1016/j.jcms.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Udager AM, McHugh JB, Goudsmit CM, Weigelin HC, Lim MS, Elenitoba-Johnson KSJ, Betz BL, Carey TE, Brown NA. Human papillomavirus (HPV) and somatic EGFR mutations are essential, mutually exclusive oncogenic mechanisms for inverted sinonasal papillomas and associated sinonasal squamous cell carcinomas. Ann Oncol. 2018;29:466–471. doi: 10.1093/annonc/mdx736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahnane N, Ottini G, Turri-Zanoni M, Furlan D, Battaglia P, Karligkiotis A, Albeni C, Cerutti R, Mura E, Chiaravalli AM, et al. Comprehensive analysis of HPV infection, EGFR exon 20 mutations and LINE1 hypomethylation as risk factors for malignant transformation of sinonasal-inverted papilloma to squamous cell carcinoma. Int J Cancer. 2019;144:1313–1320. doi: 10.1002/ijc.31971. [DOI] [PubMed] [Google Scholar]
- 16.Mohajeri S, Lai C, Purgina B, Almutairi D, Baghai T, Dimitroulakos J, Kilty S. Human papillomavirus: An unlikely etiologic factor in sinonasal inverted papilloma. Laryngoscope. 2018;128:2443–2447. doi: 10.1002/lary.27207. [DOI] [PubMed] [Google Scholar]
- 17.Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995;105:178–183. doi: 10.1288/00005537-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Yousuf K, Wright ED. Site of attachment of inverted papilloma predicted by CT findings of osteitis. Am J Rhinol. 2007;21:32–36. doi: 10.2500/ajr.2007.21.2984. [DOI] [PubMed] [Google Scholar]
- 19.Karkos PD, Fyrmpas G, Carrie SC, Swift AC. Endoscopic versus open surgical interventions for inverted nasal papilloma: A systematic review. Clin Otolaryngol. 2006;31:499–503. doi: 10.1111/j.1365-2273.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan CH, Tong CCL, Penta M, Patel VS, Palmer JN, Adappa ND, Nayak JV, Hwang PH, Patel ZM. Imaging predictors for malignant transformation of inverted papilloma. Laryngoscope. 2019;129:777–782. doi: 10.1002/lary.27582. [DOI] [PubMed] [Google Scholar]
- 21.Jeon TY, Kim HJ, Chung SK, Dhong HJ, Kim HY, Yim YJ, Kim ST, Jeon P, Kim KH. Sinonasal inverted papilloma: Value of convoluted cerebriform pattern on MR imaging. AJNR Am J Neuroradiol. 2008;29:1556–1560. doi: 10.3174/ajnr.A1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DY, Hong SL, Lee CH, Jin HR, Kang JM, Lee BJ, Moon IJ, Chung SK, Rha KS, Cho SH, et al. Inverted papilloma of the nasal cavity and paranasal sinuses: A Korean multicenter study. Laryngoscope. 2012;122:487–494. doi: 10.1002/lary.22495. [DOI] [PubMed] [Google Scholar]
- 23.Busquets JM, Hwang PH. Endoscopic resection of sinonasal inverted papilloma: A meta-analysis. Otolaryngol Head Neck Surg. 2006;134:476–482. doi: 10.1016/j.otohns.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 24.Lisan Q, Laccourreye O, Bonfils P. Sinonasal inverted papilloma: Risk factors for local recurrence after surgical resection. Ann Otol Rhinol Laryngol. 2017;126:498–504. doi: 10.1177/0003489417705671. [DOI] [PubMed] [Google Scholar]
- 25.Xiao-Ting W, Peng L, Xiu-Qing W, Hai-Bo W, Wen-Hui P, Bing L, Er-Peng Z, Guang-Gang S. Factors affecting recurrence of sinonasal inverted papilloma. Eur Arch Otorhinolaryngol. 2013;270:1349–1353. doi: 10.1007/s00405-012-2216-z. [DOI] [PubMed] [Google Scholar]
- 26.Tanvetyanon T, Qin D, Padhya T, Kapoor R, McCaffrey J, Trotti A. Survival outcomes of squamous cell carcinoma arising from sinonasal inverted papilloma: Report of 6 cases with systematic review and pooled analysis. Am J Otolaryngol. 2009;30:38–43. doi: 10.1016/j.amjoto.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Hur DG, Roh HJ, Rha KS, Jin HR, Rhee CS, Min YG. Survival rates of sinonasal squamous cell carcinoma with the new AJCC staging system. Arch Otolaryngol Head Neck Surg. 2007;133:131–134. doi: 10.1001/archotol.133.2.131. [DOI] [PubMed] [Google Scholar]
- 28.Yu MS, Lim WS, Lee BJ, Chung YS. Squamous cell carcinoma associated with inverted papilloma of the maxillary sinus: Our experience with 21 patients. Clin Otolaryngol. 2017;42:1048–1052. doi: 10.1111/coa.12804. [DOI] [PubMed] [Google Scholar]
- 29.Shipchandler TZ, Batra PS, Citardi MJ, Bolger WE, Lanza DC. Outcomes for endoscopic resection of sinonasal squamous cell carcinoma. Laryngoscope. 2005;115:1983–1987. doi: 10.1097/01.mlg.0000178330.09881.6b. [DOI] [PubMed] [Google Scholar]
- 30.Yu HX, Liu G. Malignant transformation of sinonasal inverted papilloma: A retrospective analysis of 32 cases. Oncol Lett. 2014;8:2637–2641. doi: 10.3892/ol.2014.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karligkiotis A, Lepera D, Volpi L, Turri-Zanoni M, Battaglia P, Lombardi D, Accorona R, Bignami M, Nicolai P, Castelnuovo P. Survival outcomes after endoscopic resection for sinonasal squamous cell carcinoma arising on inverted papilloma. Head Neck. 2016;38:1604–1614. doi: 10.1002/hed.24481. [DOI] [PubMed] [Google Scholar]
- 32.von Buchwald C, Bradley PJ. Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr Opin Otolaryngol Head Neck Surg. 2007;15:95–98. doi: 10.1097/MOO.0b013e3280803d9b. [DOI] [PubMed] [Google Scholar]
- 33.Wormald PJ, Ooi E, van Hasselt CA, Nair S. Endoscopic removal of sinonasal inverted papilloma including endoscopic medial maxillectomy. Laryngoscope. 2003;113:867–873. doi: 10.1097/00005537-200305000-00017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.