Abstract
Determining an effective biomarker for predicting the prognosis of patients with hepatocellular carcinoma (HCC) may improve patient survival rates. The present study aimed to investigate the expression of glucose transporter 3 (GLUT-3) in HCC and to determine its predictive value for the survival of patients with HCC. Immunohistochemistry was used to detect GLUT-3 expression in HCC tissues of 275 and 140 patients with HCC from training and validation cohorts, respectively. The association between GLUT-3 expression and the clinicopathological characteristics of patients with HCC, and between GLUT-3 expression and patient survival rates were analyzed. The predictive value of GLUT-3 expression was confirmed using the validation cohort. The results demonstrated that the high GLUT-3 expression in HCC tissues was significantly associated with elevated α-fetoprotein level, large tumor size, poor histological differentiation and Tumor-Node-Metastasis stages III and IV (P<0.05). In addition, GLUT-3 high expression was also significantly associated with reduced overall survival of patients with HCC in the training and validation cohorts. In conclusion, the results from the present study suggested that GLUT-3 may be considered as a potential independent prognostic factor for predicting the survival of patients with HCC.
Keywords: GLUT3, hepatocellular carcinoma, prognosis, biomarker, mechanism
Introduction
Hepatocellular carcinoma (HCC) is the seventh most common malignant tumor and the second most frequent cause of cancer-associated mortality worldwide in 2016 (1). Although progress has been made in the diagnosis of HCC, the treatment and prevention of the disease and prognosis prediction remain poor (2). At present, the classification and prognosis evaluation of patients with HCC depend on clinical staging systems, including Tumor-Node-Metastasis (TNM) stage, Barcelona Clinic Liver Cancer (BCLC) stage and the Cancer of the Liver Italian Program stage (3). Although the clinical stage can predict the risk of tumor recurrence to a certain extent, it rarely directly reflects the prognosis of patients with HCC after hepatectomy. It is therefore crucial to identify an effective prognostic molecular marker to predict the clinical prognosis of patients with HCC.
A total of 14 subtypes of facilitative glucose transporters (GLUTs) have been described in humans, of which role is to transport glucose to different tissues in the body (4). Previous studies have reported that GLUT-3 is overexpressed in numerous solid tumors, including oral squamous cell carcinoma, laryngeal carcinoma, nonsmall cell lung carcinoma and bladder cancer, which may be due to the rapid proliferation of tumor cells in hypoxic condition (5–9). Since the rate of ATP produced by glycolysis under anaerobic conditions is significantly lower than during aerobic metabolism, high GLUTs expression is required by tumor cells to satisfy the increased need for glucose (4). GLUT-3 may therefore be a potential tumor cell marker. To the best of our knowledge, the expression of GLUT-3 in HCC and its association with the clinicopathological characteristics of patients have not yet been identified. In the present study, the association between GLUT-3 expression in HCC tissues and the clinicopathological characteristics and clinical prognosis of patients with HCC was evaluated.
Materials and methods
Patients and tissue specimens
Formalin-fixed paraffin-embedded tissues of 275 patients with HCC who underwent surgical resection between April 2003 and December 2008 at the Shandong Provincial Hospital Affiliated to Shandong University (Shandong, China) were included in the training cohort. In parallel, in order to verify the prognostic efficacy of GLUT-3 as a predictive marker in HCC, 140 formalin-fixed paraffin-embedded tissues of patients with HCC who underwent surgery during the same period at the Sun Yat-Sen University Cancer Center (Guangdong, China) were randomly selected and included in the validation cohort. The inclusion criteria were as follows: i) Child-Pugh classification (10) was A or B; ii) patients did not receive antitumor therapy prior to surgery; iii) radical resection was performed; iv) HCC pathology was confirmed after surgery; v) no evidence of extrahepatic metastasis or primary cancer of other organs; and vi) complete follow-up information was available. The exclusion criteria were as follows: i) Patients received preoperative antitumor therapy, including radiotherapy or chemotherapy; ii) preoperative extrahepatic metastasis was observed; iii) malignant tumors associated with other organs were identified; and iv) follow-up information was missing.
In the training cohort, the median age of the patients was 55 years (age range, 24–74 years), 38 patients were women and 237 patients were men. In the validation cohort, the median age of the patients was 52 years (age range, 28–72 years), 15 patients were women and 125 patients were men. Clinical baseline and complete follow-up information were reviewed from the hospital databases. This study was approved by the Institutional Review Boards of Sun Yat-Sen University Cancer Center and Shandong Provincial Hospital Affiliated to Shandong University. Written informed consent was obtained from all patients included in this study.
Isolation of RNA and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the tissue samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The quality and quantity of RNA were assessed using the Agilent 2100 Bioanalyzer and NanoDrop ND-1000 Spectrophotometer (Agilent Technologies, Inc.). cDNA was synthesized from immunoprecipitated RNA using reverse transcriptase followed by second strand synthesis to generate double-stranded cDNA using SuperScript IV Reverse Transcriptase kit (Thermo Fisher Scientific, Inc.) under the following conditions: 25°C for 6 min, 55°C for 20 min, and 80°C for 10 min. The qPCR was performed using SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories, Inc.) according to the manufacturer's protocols. The GAPDH was used as an endogenous control, and fold changes were calculated via relative quantification (2−ΔΔCq). Transcripts were assessed using the following primers: GLUT-3 (forward, CAGCGAGACCCAGAGATGC; reverse, GACCCCAGTGTTGTAGCCAA) and GAPDH (forward, TGCACCACCAACTGCTTAGC; reverse, GGCATGGACTGTGGTCATGAG).
IHC staining
The formalin-fixed paraffin-embedded specimens were cut into 5-µm sections and placed on polylysine-coated slides (Sigma-Aldrich; Merck KGaA). Sections were deparaffinized in xylene and rehydrated using a gradient series of alcohol (100% for 5 min; 90% for 5 min; 80% for 5 min; and 70% for 5 min). Antigen retrieval was performed by heating sections in citrate buffer (pH 6.0; Dako; Agilent Technologies, Inc.) at 95°C for 10 min. Samples were blocked with 10% goat serum (Beijing Solarbio Science & Technology Co., Ltd) at 37°C for 2 h and with Peroxidase-Blocking Solution (Dako; Agilent Technologies, Inc.) at 37°C for 30 min. Sections were incubated with the primary antibody against GLUT-3 (1:50; cat. no. ab95256; Abcam) and with an isotype-matched immunoglobulin G (1:100; cat. no. Ab83567; Abcam) used as a negative control at room temperature for 2 h. Immunohistochemical staining was performed using the Dako Envision Plus system [Dako; Agilent Technologies, Inc. Dako, EnVisio+System/HRP, Mo(DAB+), K400611-2] according to the manufacturer's instructions (magnification, ×400). The number of tumor cells with a strong membrane signal for GLUT-3 was counted in ten low magnification fields with light microscope, and expressed as a percentage of the total number of cells. The mean percentage of immunoreactive tumor cells was calculated and scored according to the following 5-point scale: 0, 1, 2, 3 or 4 points for 0, 1–25, 26–50, 51–75 or 76–100% of positively stained cells, respectively. GLUT-3 was considered to be not expressed if the final score was 0. GLUT-3 expression was considered to be low if the final score was 1 or 2, and high if the final score was 3 or 4.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 statistical software for Windows (SPSS, Inc.). GLUT-3 mRNA level in different types of tissue was analyzed using ANOVA followed by Scheffe post hoc test. χ2 or Fisher's exact tests were used to determine the association between GLUT-3 expression levels and the clinicopathological characteristics of patients. Disease-free survival (DFS) time was calculated as the time between surgical resection and the appearance of recurrence evidence at any site or the last follow-up contact. Overall survival (OS) time was calculated as the time between surgical resection and the time of death or the last follow-up.
Receiver operating characteristic (ROC) curves were used in the training cohort and the validation cohort to validate the prognostic ability of GLUT-3 expression levels. Survival rate was calculated using Kaplan-Meier method, and log-rank test was used to compare differences in survival between groups. The Kaplan-Meier method was used for univariate analysis, whereas Cox proportional hazards regression model was used for multivariate analysis. Variables with P<0.05 in the univariate analysis were selected as variables for multivariate analysis. A two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics and expression of GLUT-3 in patients with HCC
The clinicopathological characteristics of all patients in the two cohorts included age, sex, etiology, liver cirrhosis, Child-Pugh classification, serum α-fetoprotein (AFP) level, tumor size, tumor number, vascular invasion, histological differentiation, BCLC stage and TNM stage, and are summarized in Tables I and II.
Table I.
Expression of GLUT-3 and its relationship with clinicopathological characteristics of the training cohort.
| GLUT-3 expression (n=275) | |||
|---|---|---|---|
| Characteristic | No and low (n=193) | High (n=82) | P-value |
| Age (≥55/<55 years) | 60/133 | 29/53 | 0.489 |
| Sex (F/M) | 28/165 | 10/72 | 0.611 |
| Etiology | 0.473 | ||
| Hepatitis B virus | 176 | 74 | |
| Hepatitis C virus | 2 | 1 | |
| Other | 15 | 7 | |
| Cirrhosis | 0.487 | ||
| Yes | 143 | 64 | |
| No | 50 | 18 | |
| Child-Pugh classification | 0.381 | ||
| A | 191 | 80 | |
| B | 2 | 2 | |
| AFP level | 0.007 | ||
| ≤400 ng/ml | 115 | 34 | |
| >400 ng/ml | 78 | 48 | |
| Tumor size | 0.166 | ||
| ≤5 cm | 100 | 35 | |
| >5 cm | 93 | 47 | |
| Tumor number | 0.039 | ||
| Single | 160 | 59 | |
| Multiple | 33 | 23 | |
| Vascular invasion | 0.097 | ||
| Yes | 40 | 24 | |
| No | 153 | 58 | |
| Histological differentiation | 0.028 | ||
| Well | 33 | 6 | |
| Moderate | 125 | 54 | |
| Poor | 35 | 22 | |
| TNM stage | 0.018 | ||
| I and II | 153 | 54 | |
| III and IV | 40 | 28 | |
| BCLC stage | 0.227 | ||
| 0 and A | 154 | 60 | |
| B and C | 39 | 22 | |
AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; F, female; GLUT-3, glucose transporter 3; M, male.
Table II.
Expression of GLUT-3 and its relationship with clinicopathological characteristics of the validation cohort.
| GLUT-3 expression (n=140) | |||
|---|---|---|---|
| Characteristic | No and low (n=99) | High (n=41) | P-value |
| Age (≥55/<55 years) | 31/68 | 17/24 | 0.250 |
| Sex (F/M) | 9/90 | 6/35 | 0.335 |
| Etiology | 0.473 | ||
| Hepatitis B virus | 93 | 37 | |
| Hepatitis C virus | 1 | 1 | |
| Others | 5 | 3 | |
| Cirrhosis | 0.087 | ||
| Yes | 74 | 36 | |
| No | 25 | 5 | |
| Child-Pugh classification | 0.580 | ||
| A | 92 | 38 | |
| B | 7 | 3 | |
| AFP level, | 0.001 | ||
| ≤400 ng/ml | 63 | 14 | |
| >400 ng/ml | 36 | 27 | |
| Tumor size | 0.031 | ||
| ≤5 cm | 56 | 15 | |
| >5 cm | 43 | 26 | |
| Tumor number | 0.660 | ||
| Single | 80 | 31 | |
| Multiple | 19 | 10 | |
| Vascular invasion | 0.022 | ||
| Yes | 8 | 9 | |
| No | 91 | 32 | |
| Histological differentiation | 0.004 | ||
| Well | 21 | 0 | |
| Moderate | 60 | 26 | |
| Poor | 18 | 15 | |
| TNM stage | 0.144 | ||
| I and II | 79 | 28 | |
| III and IV | 20 | 13 | |
| BCLC stage | 0.403 | ||
| 0 and A | 81 | 31 | |
| B and C | 18 | 10 | |
AFP, α-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; F, female; GLUT-3, glucose transporter 3; M, male.
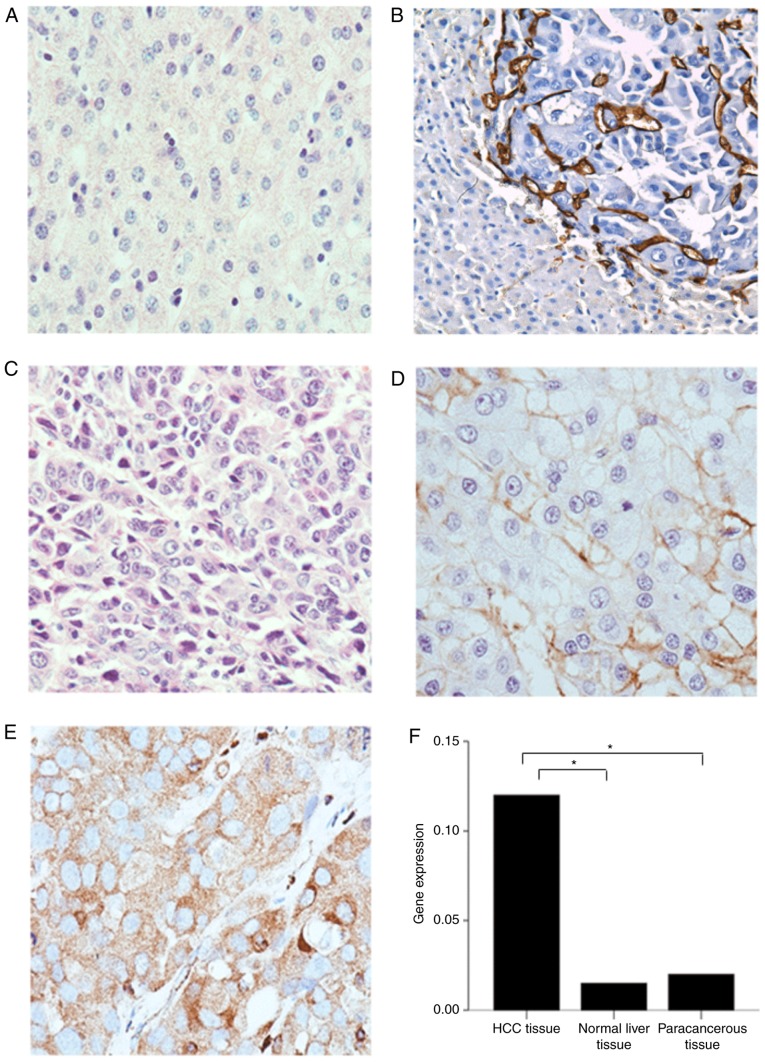
IHC was performed to investigate GLUT-3 expression. The results demonstrated that GLUT-3 was not expressed in normal liver (Fig. 1A) and paracancerous tissues of patients with HCC (Fig. 1B). However, GLUT-3 was expressed in variable ways in HCC tissues (Fig. 1C-E). Representative IHC images are presented in Fig. 1.
Figure 1.
Representative immunohistochemistry images of GLUT-3 expression in normal liver and HCC tissues and GLUT-3 gene expression in different tissues. (A) Normal liver tissue with no GLUT-3 expression. (B) Paracancerous tissues with no GLUT-3 expression. (C) Liver cancer tissues with no GLUT-3 expression. (D) Liver cancer tissues with low GLUT-3 expression. (E) Liver cancer tissues with high GLUT-3 expression. Magnification, ×40. (F) GLUT-3 mRNA level in different tissues. *P<0.05. GLUT-3, glucose transporter 3; HCC, hepatocellular carcinoma.
GLUT-3 expression level in tumor tissues was significantly higher compared with normal liver tissues (P<0.05) and paracancerous tissues (P<0.05). However, there was no statistical difference in GLUT-3 expression level between normal liver and paracancerous tissues (P>0.05; Fig. 1F).
High GLUT-3 expression tissue score in HCC was significantly and positively associated with elevated AFP level, large tumor size, poor histological differentiation and TNM stages III and IV (P<0.05).
Prognostic values of serum AFP level, GLUT-3 expression and tumor size for the OS and DFS of patients with HCC
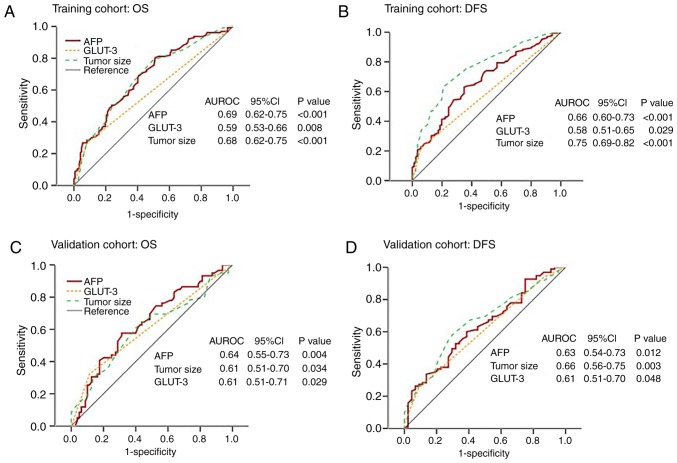
The area under curves (AUCs) among serum AFP level, GLUT-3 expression and tumor size in predicting OS and DFS in patients with HCC were analyzed by ROC curves analysis in the training and validation cohorts. In the training cohort, the AUCs for GLUT-3 expression predicting the OS and DFS of patients with HCC were 0.59 [95% confidence interval (CI), 0.53–0.66] and 0.58 (95% CI, 0.51–0.65), respectively. In the validation cohort, the AUCs for GLUT-3 expression predicting the OS and DFS of patients with HCC were 0.61 (95% CI, 0.51–0.71) and 0.61 (95% CI, 0.51–0.70), respectively (Fig. 2).
Figure 2.
ROC curves of serum AFP level, GLUT-3 expression level and tumor size for predicting OS and DFS in the training and validation cohorts. (A) AUCs of AFP, GLUT-3 and tumor size were 0.69, 0.59 and 0.68, respectively. (B) AUCs of AFP, GLUT-3 and tumor size were 0.66, 0.58 and 0.75, respectively. (C) AUCs of AFP, GLUT-3 and tumor size were 0.64, 0.61 and 0.61, respectively. (D) AUCs of AFP, GLUT-3 and tumor size were 0.63, 0.61 and 0.66, respectively. AUC, area under curve; AFP, α-fetoprotein; CI, confidence interval; DFS, disease-free survival; GLUT-3, glucose transporter 3; HCC, hepatocellular carcinoma; OS, overall survival; ROC, receiver operating characteristic; AUROC, area under the receiver operating characteristic curve.
Survival and expression of GLUT-3
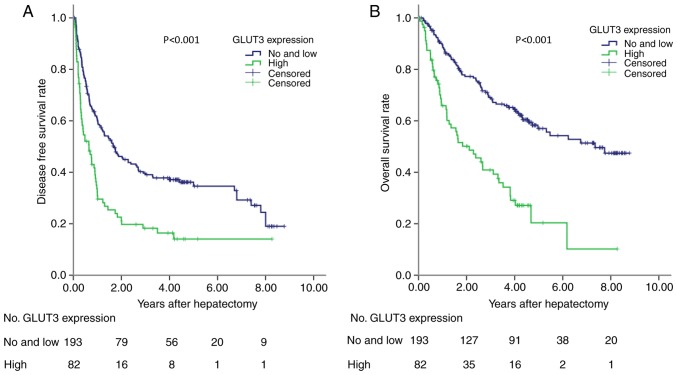
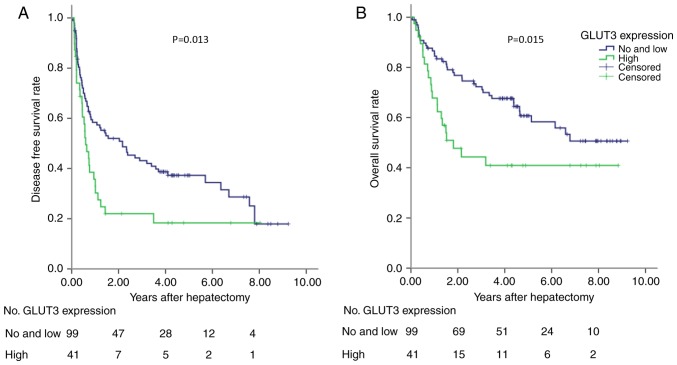
In the training cohort, the prognostic ability of GLUT-3 expression was analyzed in 275 patients with HCC. High GLUT-3 tissue score was significantly associated with reduced DFS and OS (P<0.001; Fig. 3). To validate these findings, a validation cohort containing patients with HCC was tested. The results demonstrated that high GLUT-3 expression level in the validation cohort was also associated with poor DFS and OS (P<0.05; Fig. 4). The predictive value of GLUT-3 expression in the validation cohort was therefore validated for OS and DFS. The results from multivariate Cox regression analysis demonstrated that GLUT-3 expression level, BCLC, vascular invasion and tumor size were independent prognostic factors for the OS of patients with HCC (Table III).
Figure 3.
DFS and OS in the training cohort. (A) DFS of 275 patients with HCC according to GLUT-3 expression. (B) OS of 275 patients with HCC according to GLUT-3 expression. GLUT-3 expression was predictive for DFS and OS in patients with HCC. P<0.001. DFS, disease-free survival; GLUT-3, glucose transporter 3; HCC, hepatocellular carcinoma; OS, overall survival.
Figure 4.
DFS and OS in the validation cohort. (A) DFS of 140 patients with HCC according to GLUT-3 expression. (B) OS of 140 patients with HCC according to GLUT-3 expression. GLUT-3 expression was predictive for DFS (P=0.013) and OS (P=0.015) in patients with HCC. DFS, disease-free survival; GLUT-3, glucose transporter 3; HCC, hepatocellular carcinoma; OS, overall survival.
Table III.
Cox regression model analysis in training cohort.
| 95.0% CI for Exp (B) | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | B | SE | Wald | P-value | Exp (B) | Down | Upper |
| BCLC stage | 0.671 | 0.232 | 8.368 | 0.004 | 1.957 | 1.242 | 3.084 |
| GLUT-3 expression | 0.891 | 0.208 | 18.388 | <0.001 | 2.436 | 1.622 | 3.660 |
| Vascular invasion | 0.636 | 0.253 | 6.341 | 0.012 | 1.889 | 1.151 | 3.099 |
| Tumor size | 0.687 | 0.208 | 10.957 | 0.001 | 1.988 | 1.323 | 2.985 |
BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; GLUT-3, glucose transporter 3.
Discussion
HCC is a common malignant tumor associated with high mortality rate (11). Surgical resection is the most effective treatment for patients with liver cancer; however, the postoperative long-term survival rate of patients is limited due to tumor recurrence (70% at 5 years) (12,13). Traditional stratification schemes that are based on clinical characteristics, including the American Joint Committee on Cancer (14), TNM and BCLC stages, provide limited prognostic guidance in the management of patients with HCC due to disease heterogeneity (3,15). Specific biomarkers would therefore allow better stratification of the disease.
High serum AFP levels were associated with poor prognosis of patients with HCC (16); however, the optimal cut-off value of serum AFP level that could be used to predict a poor prognosis in patients with HCC has not yet been determined. To our knowledge, no molecular profiles have been established to date as the widely satisfactory prognostic biomarker in HCC, although some biomarkers have potentially predictive value (EpCAM signature, G3-proliferation subclass, and SUOX) (17–20). Prognostic molecular biomarkers should significantly predict the survival prognosis and be indicated for most patients in clinical practice. Therefore, more acceptable markers should be explored according to standard criteria. In the present study, GLUT-3 expression and its prognostic value in patients with HCC were analyzed. The results demonstrated that increased GLUT-3 expression level was associated with decreased OS in patients with HCC following tumor resection. In addition, GLUT-3 expression level was also associated with elevated serum AFP level, large tumor size, poor histological differentiation and TNM stages III and IV. Taken together, these results demonstrated that GLUT-3 overexpression may be considered as a biomarker for predicting the survival of patients with HCC.
Increased energy metabolism has been accepted as a hallmark of cancer, and is widely observed in cancer cells (21). Increased glucose use by glycolysis is an exclusive property of invasive cancer cells (22). In tumor cells, glucose uptake across the plasma membrane, which is mediated by facilitative GLUTs, is thought to be the rate-limiting step of glucose metabolism (23). Enhanced glucose uptake in tumors can be therefore mediated by overexpression of GLUTs overexpression (4). Of the 14 subtypes of human GLUTs, the most closely associated with glucose metabolism are GLUT-1-5, which have different body distributions under physiological conditions. For instance, GLUT-l and 3 are the two most widely studied GLUTs, and the ones that are most strongly associated with malignant tumors. It has been reported that the upregulation of specific glucose transporters may represent a key mechanism by which malignant cells may achieve increased glucose uptake to support the high rate of glycolysis (24,25). In addition, GLUT-3 is overexpressed in human brain tumors, oral tongue carcinoma, endometrial and breast cancers, non-small lung carcinoma, oral squamous cell carcinoma and laryngeal carcinoma (5,6,8,26,27). To the best of our knowledge, the present study was the first to analyze GLUT-3 expression and its association with the prognosis of patients with HCC.
Compared with other GLUTs, GLUT-1 and 3 have a higher affinity for glucose under physiological conditions (4). GLUT-3 is mainly expressed in the brain and testicles (28). In addition, GLUT-3 is present in the intracellular vesicles of various types of leukocyte and can be transferred to plasma membrane under the activation of proliferative stimuli. For instance, in T-lymphocytes, activation is characterized by the emergence of insulin receptors on the plasma membrane; however, their physiological significance is unclear (29). As aforementioned, numerous studies demonstrated that GLUT-3 is also expressed in various types of tumor tissue. Malignant cells grow faster and require more oxygen and glucose than normal cells. Although mitochondrial oxidative phosphorylation is considered to be a more efficient metabolic process for ATP synthesis compared with glycolysis (30), tumor cells use glycolysis as the main metabolic mode, even when sufficient oxygen is present. This phenomenon is known as the Warburg effect (31). Although glycolysis produces less ATP, a large number of intermediate metabolites can be used to construct macromolecular structures, including RNA, proteins, lipids and NADP (30). As tumors grow, cells may encounter hypoxic conditions that lead to the induction of the hypoxia inducible factor 1 (HIF-1) transcription factor, which increases the transcription of glucose transporters (32). The decrease of ATP production efficiency and the high energy requirement of tumor cells can stimulate the increase of glucose uptake by malignant tumor cells as aforementioned (30,32). Furthermore, GLUT-3 overexpression can participate in the transport of more glucose into tumor cells in order to satisfy their high metabolism and rapid growth. However, the mechanism of GLUT-3 overexpression in tumor cells is unknown, particularly in HCC, which was investigated, to the best of our knowledge, in only one study to date (33). At present, there are several hypotheses about the role of GLUT-3 overexpression in tumor cells, including IL-6/signal transducer and activator of transcription 3 (STAT3), PI3K-Akt and hypoxia-induciblefactor-1 (HIF-1) signaling pathways. A previous study demonstrated that activation of IL-6/STAT3 pathway can stimulate expression of GLUT isoforms, and therefore increase glucose uptake capacity in HCCs (33). STAT3 is a membrane receptor-mediated nuclear transcription factor (34). Cytokines, including IL-6, and growth factors (such as epidermal growth factor and platelet-derived growth factor) activate STAT3 through phosphorylation. Phosphorylated STAT3 enters then the nucleus, binds to the DNA regulatory regions of target genes and induces their expression (35). High expression of GLUT-3 may therefore be facilitated by the activation of the IL-6/STAT3 pathway. The involvement of PI3K-Akt in GLUTs regulation suggests that uncontrolled Akt activation, caused by disturbances in PI3K α subunit or phosphate and tension homolog, may mediate the increased glucose uptake and overexpression of GLUTs observed in tumors. A previous study reported that in hypoxic BeWo choriocarcinoma cells, HIF-1 mediates transcriptional regulation of glycolytic genes with hypoxia-response elements in their promoter regions, including GLUT-1 and GLUT-3 (36). GLUT-3 is overexpressed following HIF-1α complex stabilization in response to hypoxia in BeWo choriocarcinoma cells (36). However, the underlying mechanisms of GLUT-3 overexpression in HCC remain unclear and require further investigation.
The current study presented some limitations. Firstly, there were inherent biases due to the retrospective nature of the study. Secondly, the number of patients involved in this study was relatively small, and results should be confirmed in a larger patient cohort. Thirdly, the molecular mechanism of GLUT-3 overexpression in liver cancer tissues remains unclear and requires further investigations.
In conclusion, the present study demonstrated the association between GLUT-3 expression level and the clinical prognosis of patients with HCC. Furthermore, the results demonstrated that increased GLUT-3 expression level was associated with poor prognosis of patients with HCC, suggesting that GLUT-3 may be considered as a potential prognostic in HCC. This finding provided a basis for investigating GLUT-3 as a potential target in the treatment of HCC, which may lead to the development of novel treatment strategies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AFP
α-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- DFS
disease-free survival
- GLUT
glucose transporter
- HCC
hepatocellular carcinoma
- HIF-1
hypoxia-inducible factor-1
- OS
overall survival
- STAT3
signal transducer and activator of transcription3
- TNM
Tumor-Node-Metastasis
Funding
The research was supported by The Natural Science Foundation of China Youth Project (grant no. 81802379) and the Jinan Science and Technology Development Project (grant no. 201805029).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
HG, JL and YH participated in the conception and design of the study. All authors collected and interpreted the data. HG, XZ, MC, HL, FL, YH, XS, HZ, ZN and QN performed the statistical analysis. YH drafted the manuscript, and HG and JL edited it critically. All authors gave final approval of the version to be published.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Sun Yat-Sen University Cancer Center and Shandong Provincial Hospital Affiliated to Shandong University. Written informed consent was obtained from all the patients who participated in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, Anderson BO, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 3.Sirivatanauksorn Y, Tovikkai C. Comparison of staging systems of hepatocellular carcinoma. HPB Surg. 2011;2011:818217. doi: 10.1155/2011/818217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism. 2016;65:124–139. doi: 10.1016/j.metabol.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Ayala FR, Rocha RM, Carvalho KC, Carvalho AL, da Cunha IW, Lourenço SV, Soares FA. GLUT1 and GLUT3 as potential prognostic markers for Oral squamous cell carcinoma. Molecules. 2010;15:2374–2387. doi: 10.3390/molecules15042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estilo CL, O-charoenrat P, Talbot S, Socci ND, Carlson DL, Ghossein R, Williams T, Yonekawa Y, Ramanathan Y, Boyle JO, et al. Oral tongue cancer gene expression profiling: Identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer. 2009;9:11. doi: 10.1186/1471-2407-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baer S, Casaubon L, Schwartz MR, Marcogliese A, Younes M. Glut3 expression in biopsy specimens of laryngeal carcinoma is associated with poor survival. Laryngoscope. 2002;112:393–396. doi: 10.1097/00005537-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 8.Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80:1046–1051. doi: 10.1002/(SICI)1097-0142(19970915)80:6<1046::AID-CNCR6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Conde VR, Oliveira PF, Nunes AR, Rocha CS, Ramalhosa E, Pereira JA, Alves MG, Silva BM. The progression from a lower to a higher invasive stage of bladder cancer is associated with severe alterations in glucose and pyruvate metabolism. Exp Cell Res. 2015;335:91–98. doi: 10.1016/j.yexcr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 11.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong SS, Kim TK, Sung KB, Kim PN, Ha HK, Kim AY, Lee MG. Extrahepatic spread of hepatocellular carcinoma: A pictorial review. Eur Radiol. 2003;13:874–882. doi: 10.1007/s00330-002-1519-7. [DOI] [PubMed] [Google Scholar]
- 13.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: Patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 14.Chun YH, Kim SU, Park JY, Kim DY, Han KH, Chon CY, Kim BK, Choi GH, Kim KS, Choi JS, Ahn SH. Prognostic value of the 7th edition of the AJCC staging system as a clinical staging system in patients with hepatocellular carcinoma. Eur J Cancer. 2011;47:2568–2575. doi: 10.1016/j.ejca.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D'Amico F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. doi: 10.1186/1477-7819-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 18.Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M, Kumada H, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140:1501–1512.e2. doi: 10.1053/j.gastro.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin GZ, Yu WL, Dong H, Zhou WP, Gu YJ, Yu H, Yu H, Lu XY, Xian ZH, Liu YK, et al. SUOX is a promising diagnostic and prognostic biomarker for hepatocellular carcinoma. J Hepatol. 2013;59:510–517. doi: 10.1016/j.jhep.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Nault JC, De Reynies A, Villanueva A, Calderaro J, Rebouissou S, Couchy G, Decaens T, Franco D, Imbeaud S, Rousseau F, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology. 2013;145:176–187. doi: 10.1053/j.gastro.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 23.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 24.Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res. 2012;18:721–728. doi: 10.1007/s12253-012-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha TK, Her NG, Lee MG, Ryu BK, Lee JH, Han J, Jeong SI, Kang MJ, Kim NH, Kim HJ, Chi SG. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res. 2012;72:4097–4109. doi: 10.1158/0008-5472.CAN-12-0448. [DOI] [PubMed] [Google Scholar]
- 26.Boado RJ, Black KL, Pardridge WM. Gene expression of GLUT3 and GLUT1 glucose transporters in human brain tumors. Brain Res Mol Brain Res. 1994;27:51–57. doi: 10.1016/0169-328X(94)90183-X. [DOI] [PubMed] [Google Scholar]
- 27.Starska K, Forma E, Jozwiak P, Brys M, Lewy-Trenda I, Brzezinska-Blaszczyk E, Krzeslak A. Gene and protein expression of glucose transporter 1 and glucose transporter 3 in human laryngeal cancer-the relationship with regulatory hypoxia-inducible factor-1α expression, tumor invasiveness, and patient prognosis. Tumour Biol. 2015;36:2309–2321. doi: 10.1007/s13277-014-2838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haber RS, Weinstein SP, O'Boyle E, Morgello S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology. 1993;132:2538–2543. doi: 10.1210/endo.132.6.8504756. [DOI] [PubMed] [Google Scholar]
- 29.Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, Raptis SA. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. 2007;37:282–290. doi: 10.1111/j.1365-2362.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 30.Lunt SY, Vander Heiden MG. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 31.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ. Identification of hypoxically inducible mRNAs in HeLa cells using differential-display PCR. Role of hypoxia-inducible factor-1. Eur J Biochem. 1996;241:403–410. doi: 10.1111/j.1432-1033.1996.00403.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang HL, Wang MD, Zhou X, Qin CJ, Fu GB, Tang L, Wu H, Huang S, Zhao LH, Zeng M, et al. Blocking preferential glucose uptake sensitizes liver tumor-initiating cells to glucose restriction and sorafenib treatment. Cancer Lett. 2017;388:1–11. doi: 10.1016/j.canlet.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 35.Zhong Z, Wen Z, Darnell JE., Jr Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 36.Baumann MU, Zamudio S, Illsley NP. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2007;293:C477–C485. doi: 10.1152/ajpcell.00075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.