Abstract
The aim of the present study was to compare the safety and efficacy of cryoablation (CA) and microwave ablation (MWA) as treatments for non-small cell lung cancer (NSCLC). Patients with stage IIIB or IV NSCLC treated with CA (n=45) or MWA (n=56) were enrolled in the present study. The primary endpoint was progression-free survival (PFS); the secondary endpoints included overall survival (OS) time and adverse events (AEs). The median PFS times between the two groups were not significantly different (P=0.36): CA, 10 months [95% confidence interval (CI), 7.5–12.4] vs. MWA, 11 months (95% CI, 9.5–12.4). The OS times between the two groups were also not significantly different (P=0.07): CA, 27.5 months (95% CI, 22.8–31.2 months) vs. MWA, 18 months (95% CI, 12.5–23.5). For larger tumors (>3 cm), patients treated with MWA had significantly longer median PFS (P=0.04; MWA, 10.5 months vs. CA, 7.0 months) and OS times (P=0.04; MWA, 24.5 months vs. CA, 14.5 months) compared patients treated with CA. However, for smaller tumors (≤3 cm), median PFS (P=0.79; MWA, 11.0 months vs. CA, 13.0 months) and OS times (P=0.39; MWA, 30.0 months vs. CA, 26.5 months) between the two groups did not differ significantly. The incidence rates of AEs were similar in the two groups (P>0.05). The number of applicators, tumor size and length of the lung traversed by applicators were associated with a higher risk of pneumothorax and intra-pulmonary hemorrhage in the two groups. Treatment with CA resulted in significantly less intraprocedural pain compared with treatment with MWA (P=0.001). Overall, the present study demonstrated that CA and MWA were comparably safe and effective procedures for the treatment of small tumors. However, treatment with MWA was superior compared with CA for the treatment of large tumors.
Keywords: cryoablation, microwave ablation, lung cancer, progression-free survival, overall survival
Introduction
According to the 2018 global cancer statistic estimates of cancer incidence and mortality, lung cancer is the leading cause of cancer-associated mortality worldwide, with 2.1 million new cases (11.6% of the total cases) and 1.8 million deaths (18.4% of the total cancer-associated mortalities) each year (1). Of these deaths, 85% are the result of non-small cell lung cancer (NSCLC), as two-thirds of patients with NSCLC are diagnosed with an advanced disease stage, where curative surgery is not an option (2). Despite advances in diagnosis and treatment, the 5-year relative survival rate remains at 19% for overall lung cancer and 23% for NSCLC (3). Systemic chemotherapy remains the primary means of treatment for patients with advanced NSCLC. Progression-free survival (PFS) of patients with NSCLC undergoing platinum-based doublet chemotherapy ranges between 3.6 and 4.8 months, and OS ranges between 7.9 and 10.3 months (4,5). Thus, the unsatisfactory PFS and OS times have necessitated the development and use of alternative treatment options, particularly for patients with unresectable NSCLC. In recent years, there have been significant developments and refinements in several novel, minimally invasive techniques, such as percutaneous image-guided ablation therapy for patients who cannot undergo surgery (6).
The potential of various thermal ablation technologies, including radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation (CA) and irreversible electroporation for the treatment of NSCLC has been demonstrated (7). RFA and MWA are thermal-based ablative techniques, where thermal ablation of the tumor is achieved by radiofrequency waves in RFA and microwaves in MWA (8). In recent years, MWA has been increasingly used for the treatment of pulmonary tumors. MWA offers many theoretical advantages over RFA, including enhanced thermocoagulation of tumor cells as a result of improved energy deposition in an aerated lung, and increased heating near blood vessels, which allows for increased intratumoral temperatures with larger ablation zones (≤2 cm from the probe tip) in a shorter period of time compared with RFA (9). In comparison to RFA, MWA has been reported to be effective for lesions near vascular structures with a decreased heat sink effect (10–12). Furthermore, MWA also offers benefits of decreased treatment times and pain between treatments over RFA (9,10).
Contrary to RFA and MWA, CA is a relatively novel ablation technique, which uses pressurized argon gas to create a temperature as low as −140°C to destroy tumor cells (13). The advantages of CA over other ablative techniques include good visualization under computed tomography (CT) or magnetic resonance imaging guidance, preservation of the collagenous architecture and lower intraprocedural pain (14). CA also has the advantage of having the probe placed inside the tumor, thus preventing probe displacement during treatment, which can occur with expandable RFA electrodes frequently used in lung ablation (15). Several studies have demonstrated the efficacy of pulmonary CA in cases of primary (16,17) and recurrent (18) lung cancer, as well as pulmonary metastasis (19,20). Furthermore, CA is more cost-effective compared with the other ablative techniques (21). However, although a number of studies have compared MWA with RFA for the treatment of primary and secondary neoplasms of the lung, comparisons between MWA and CA have not been performed. Therefore, the aim of the present study was to compare the effectiveness of CA and MWA in the treatment of patients with advanced stage NSCLC.
Patients and methods
Ethics approval and consent to participate
The study protocol was developed in accordance with the Declaration of Helsinki (22) and was approved by the Institutional Review Board of the Affiliated Hospital of North Sichuan Medical College (Nanchong, China) and Xuzhou City Center Hospital (Xuzhou, China). Informed consent to undergo the procedure and to provide clinical follow-up data was obtained from all patients.
Patients
The present study was a retrospective analysis of patients with stage IIIB or IV NSCLC who had undergone MWA or CA at the Interventional Radiology department of the Affiliated Hospital of North Sichuan medical College (Nanchong, China) or Xuzhou Central Hospital (Xuzhou, China) between March 2011 and September 2016. All patients were histologically or cytologically diagnosed with stage IIIB or IV NSCLC according to the 8th edition of the Tumor-Node-Metastasis (TNM) classification (23) and had an Eastern Co-operative Oncology Group performance status of 0 or 1 (24). Patients whose tumors were considered to be surgically inoperable and unresponsive to standard chemotherapy or radiotherapy were included in the present study. In addition, according to the criteria used to perform ablation therapy (23–25), only patients with ≤3 lesions per hemithorax and with the largest lesion diameter ≤5.0 cm were treated with MWA or CA. The exclusion criteria were as follows: i) Age <18 years; ii) uncontrolled malignant pleural effusion; iii) symptomatic brain metastases; iv) life expectancy ≤3.0 months; v) history of current extra pulmonary malignancies or previous malignancies within the last 5 years; and vi) inadequate hematologic, hepatic or renal function.
Pre-ablation assessment
The decision to perform lung ablation (MWA or CA) was made by an interventional radiologist following consultation with the patient and subsequent referral to a physician. Patients attended the tumor ablation clinic for a pre-procedural visit ~1 month prior to percutaneous ablation procedures. Following the completion of medical history and physical examinations, suggestions for performing relevant imaging studies were reviewed with the patient. The indications, risks and benefits of the procedures were discussed. Pre-ablation complete blood cell counts, platelet counts and prothrombin time and international normalized ratio were routinely obtained. Patients receiving anticoagulant and anti-platelet medications were instructed to stop taking them 2–7 days prior to ablation. Patients fasted for 12 h prior to arriving at the computed tomography (CT) suite on the day of the procedure.
CA procedure
An argon-based CA delivery system (AccuTarget MediPharma Co. Ltd.) was used with 14–18-gauge cryoprobes. The number, type and configuration of the needles were based on the necessity to maintain a distance of ≤15 mm between adjacent CA needles and ≤10 mm from the tumor margin, while avoiding or displacing adjacent normal anatomical structures. CA was performed using a three-cycle freeze-thaw phase protocol. The times for each phase were recorded and varied depending on the size of the tumor (target times: Freeze, 3 min; thaw, 3 min; freeze, 8 min; thaw, 5 min; freeze, 8 min; followed by active thawing). For lesions ≤3.0 cm in diameter, one cryoprobe was inserted, whereas two cryoprobes were used for lesions >3.0 cm. Each procedure was monitored using non-contrast CT imaging at 3–5 min intervals to visualize the growing ablation zone, with the goal of achieving a circumferential margin of 0.5 cm beyond the tumor. Ablation time and power were recorded during all procedures. All procedures were performed under local anesthesia. Cardiac status and vital signs were continuously monitored throughout the ablation procedure.
All patients underwent an immediate post-ablation contrast-enhanced CT scan and were admitted for overnight observation. To prevent renal failure, which is induced by myoglobinuria, a prophylactic regimen consisting of three ampules of sodium bicarbonate (50.0 mEq per ampule) in 5.0% dextrose in water was administered at 150.0 ml/h for 24 h if the post-procedural serum myoglobin levels increased >1,000 mg/l.
MWA procedure
The MWA procedure was performed under CT guidance (Philips MX16; Koninklijke Philips N.V.). The MWA instrument used was a KY-2000 microwave multi-function therapeutic instrument (Kangyou Medical Co., Ltd.). A microwave antenna, 14–20 gauge depending on tumor size and location, was inserted into the lesion. For lesions <3.0 cm in diameter, one antenna was inserted, whereas two antennas were inserted for lesions >3.0 cm. The lesion was ablated by maintaining an output power of 50–80 W, with the aim of obtaining and ablative margin of 0.5 cm. If the tumor was not ablated in one session, based on tumor size, location and geometry, multiple sequential ablations were performed to achieve complete necrosis. All procedures were performed with conscious sedation and local anesthesia. Throughout the session, cardiac status and vital signs were continuously monitored. At the end of every procedure, a CT scan was performed to prevent complications, and the patients were transferred to the in-patient ward for 24-h observation.
Intraprocedural pain assessment
Pain experienced by the patient during the MWA and CA procedures was compared. Patients reported on the experienced pain using the visual analogue score (VAS) criteria, where the minimum score of 0 indicates no pain experienced and the maximum score of 10 indicates severe extreme pain (26).
Follow-up
Patients were followed up post-ablation as outpatients, with CT scans performed at 1, 3 and 6 months and subsequently every 6 months.
Outcome measures
The primary outcome measures of the present study were technical success, clinical effectiveness, safety and OS. Technical success was defined as the correct placement of the ablation device into the target lesion and completion of the planned ablation protocol, with no detectable enhancement observed in the CT scans performed in the first 30 days following ablation. Clinical effectiveness was defined as local disease control. The areas of hypoattenuation that were not enhanced in the CT scan were considered to represent the ablation zone. Irregular focal enhancement of the lesion >15 Hounsfield units (HU) compared with the initial post-ablation non-enhanced lesion was considered as a sign of local tumor progression. A circumferential rim of enhancement ≤0.5 cm around the ablation zone at 6 months post-ablation was considered to indicate benign peritumoral enhancement. Survival was assessed as PFS and OS. PFS was calculated from the start of the ablation treatment to disease progression, including progression in ablative sites, distant metastasis or death. OS was calculated from the start of treatment to death or the last follow-up. The safety was defined according to the frequency of procedural and procedure-related complications. These were evaluated using the common terminology criteria for adverse events (AEs) (version 4.0) model (27).
Survival analysis of sub-groups
Tumor size has been reported as a prognostic marker of disease progression in a number of previous studies (2,16,25,28–32). Therefore, the survival function of patients treated with MWA and CA were analyzed according to tumor size. In the present study, tumors size ranged from 0.8–5.0 cm (mean ± standard deviation; 2.9±1.17 cm). Therefore, 3.0 cm was used as the threshold.
Statistical analysis
All statistical analyses were performed using SPSS version 23.0 (IBM Corp.) and GraphPad Prism version 5.0 (GraphPad Software, Inc.). The PFS and OS times were assessed using Kaplan-Meier analysis. The comparisons of survival functions were performed using a log-rank test. The median survival estimates were reported with 95% confidence intervals (CIs). The associations between AEs and clinicopathological characteristics were evaluated using χ2 test. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
The present retrospective study included data from 101 patients with stage IIIB or IV primary NSCLC. The patients who had undergone MWA were denoted as the MWA group, and those treated with CA as the CA group. The MWA group comprised 56 patients (34 male and 22 female; mean age, 59.1 years; age range, 29–77 years), whereas the CA group comprised 45 patients (26 male and 19 female; mean age, 57.7 years; age range, 32–78 years). Of the 56 patients in the MWA group, 32 (57.1%) had stage IIIB NSCLC and 24 (42.9%) had stage IV NSCLC; 43 patients (76.8%) had adenocarcinoma, 10 (17.8%) had squamous cell carcinoma and 3 (5.4%) had large cell carcinoma. In the CA group, 27 patients (57.1%) had stage IIIB NSCLC and 18 (42.9%) had stage IV NSCLC; 33 patients (73.3%) had adenocarcinoma and 12 (26.6%) had squamous cell carcinoma. The baseline patient characteristics did not differ significantly (Table I).
Table I.
Baseline patient clinicopathological characteristics.
| Variable | CA (n=45), n (%) | MWA (n=56), n (%) | P-value |
|---|---|---|---|
| Sex | 0.76 | ||
| Male | 26 (57.8) | 34 (60.7) | |
| Female | 19 (42.2) | 22 (39.3) | |
| Age, years | 0.91 | ||
| <60 | 27 (60.0) | 33 (58.9) | |
| ≥60 | 18 (40.0) | 23 (41.1) | |
| Pathology | 0.52 | ||
| ADC | 33 (73.3) | 43 (76.8) | |
| Non-ADCa | 12 (26.7) | 13 (23.2) | |
| Stageb | 0.77 | ||
| IIIB | 27 (60.0) | 32 (57.1) | |
| IV | 18 (40.0) | 24 (42.9) | |
| Tumor site (side) | 0.84 | ||
| Right lung | 29 (64.4) | 35 (62.5) | |
| Left lung | 16 (35.6) | 21 (37.5) | |
| Tumor site (lobe) | 0.69 | ||
| Upper and middle | 28 (62.2) | 37 (66.1) | |
| Lower | 17 (37.8) | 19 (33.9) | |
| Tumor size, cm | 0.95 | ||
| ≤3.0 | 26 (57.8) | 32 (57.1) | |
| >3.0 | 19 (42.2) | 24 (42.9) | |
| Tumor location | 0.43 | ||
| Central | 12 (26.7) | 19 (33.9) | |
| Peripheral | 33 (73.3) | 37 (66.1) | |
| Tumor distance from pleura, cm | 0.83 | ||
| ≤1 | 17 (37.8) | 20 (35.7) | |
| >1 | 28 (62.2) | 36 (64.3) | |
| Tumor distance from vessel, mm | 0.78 | ||
| ≤3 | 14 (31.1) | 16 (28.6) | |
| >3 | 31 (68.9) | 40 (71.4) | |
| Metastatic site | |||
| Lymph node | 27 (60.0) | 30 (58.9) | 0.55 |
| Intra-pulmonary | 13 (28.9) | 17 (30.3) | 1.00 |
| Distant | 30 (66.7) | 32 (57.1) | 0.41 |
| Metastases, n | 0.38 | ||
| 1 | 24 (53.3) | 25 (44.6) | |
| ≥2 | 21 (46.7) | 31 (55.4) |
Squamous cell, large cell and other undifferentiated carcinomas are grouped together as non-ADC.
Staging according to The Eighth Edition Lung Cancer Stage Classification (23). CA, cryoablation; MWA, micro-wave ablation; ADC, adenocarcinoma.
In the MWA group, 35 primary tumors were located in the right lung and 21 in the left lung; 37 tumors were located in the upper and middle lobes, whereas 19 were in the lower lobes. The mean diameter of the primary tumors in the MWA group was 2.9 cm (range, 0.8–5.0 cm), and 24 tumors (42.9%) were >3.0 cm. In the CA group, 29 primary tumors were located in the right lung and 28 in the upper and middle lobes. The mean diameter of the primary tumors was 2.6 cm (range, 0.9–5.0 cm), and 19 tumors (42.2%) were >3.0 cm.
Effectiveness
In the MWA group, a total of 56 MWA sessions were performed, with 80 antennas used for 56 primary tumor sites. Among these, 32 patients were treated using one antenna, whereas 24 patients were treated using two antennas. The median ablation time was 7 min (range, 5–10 min). Initial technical success (no detectable enhancement in the initial post-ablation CT scan) was achieved in 52 (92.86%) ablations. Re-ablation within a 6-month period was performed in 4 patients (7.14%), which resulted in 100% secondary technical success.
In the CA group, a total of 45 CA sessions were performed, with 64 cryoprobes used for 45 primary tumor sites. Among these, 26 patients were treated with one cryoprobe, whereas 19 were treated with two cryoprobes. Initial technical success was achieved in 42 ablations (93.33%). Re-ablation within a 6-month period was performed in 3 patients (6.67%) with 100% secondary technical success.
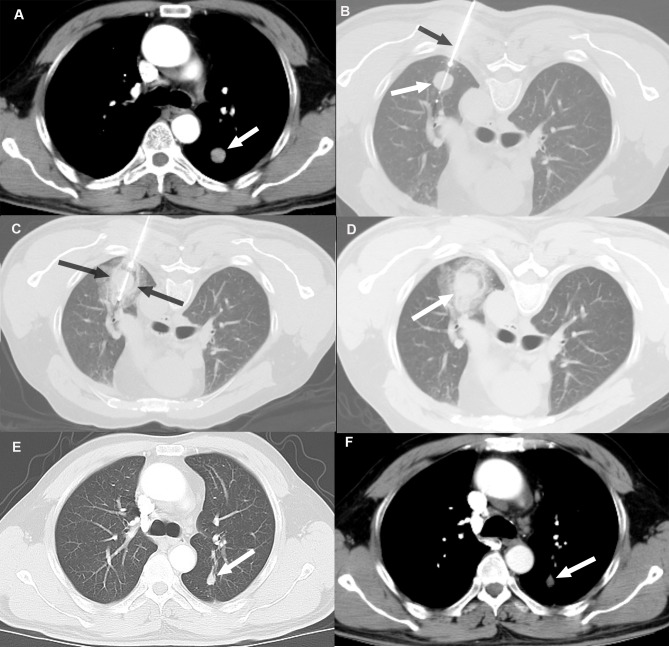
At 6 months post-ablation, local disease control was evaluated in terms of recurrence at the ablation site (residual disease). In the MWA group, 15 patients (26.78%) exhibited disease progression at the ablative sites, whereas in the CA group, 11 patients (24.44%) exhibited disease progression at the ablative sites. The difference was not statistically significant (P>0.05). An example case of local disease control following CA based on tumor size reduction without evidence of enhancement compared with the pre-ablation image is presented in Fig. 1.
Figure 1.
Local disease control at six months post-CA in a 48-year old male patient with a 14.8-mm left lower lobe primary tumor. (A) Pre-ablation contrast-enhanced axial CT images in soft-tissue windows showing a primary tumor (white arrow). (B and C) Axial CT images during the CA procedure showing (B) cryoprobe (black arrow) positioned within the tumor (white arrow) and (C) excellent coverage of tumor with the ice ball (between black arrows) following freezing. (D) Images captured immediately post-CA. (E and F) Contrast-enhanced follow-up CT images in (E) lung and (F) soft-tissue windows 6 months after CA demonstrating tumor size reduction without evidence of enhancement (white arrow). CA, cryoablation.
Regarding disease progression at distant sites from the ablation site, 41 patients (73.21%) in the MWA group developed metastases in lobes other than the ablative site or distant sites, and 7 patients (12.50%) presented with metastases at both the ablative and a distant site during the 3-year follow-up. In the CA group, 34 patients (75.55%) developed metastases in lobes other than the ablative site or distant sites, and 6 patients (13.33%) exhibited metastases at both the ablative and a distant site after 3 years of follow-up. The difference in disease progression rate was not statistically significant between the two groups (P>0.05).
OS analysis
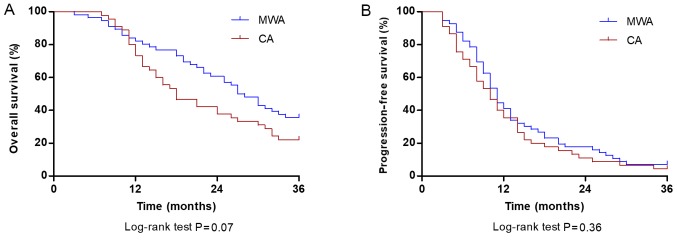
The mean duration of follow-up in patients was 24.10±17.3 months, with a median duration of 19.5 months (range, 4.3–46.4 months). During the follow-up period, the cumulative OS rate at 1, 2 and 3 years was 78.57, 51.78 and 35.71%, respectively, for patients treated with MWA and 73.33, 40.00 and 22.22%, respectively, for patients treated with CA. The median OS was 27.5 months (95% CI, 22.8–31.2 months) in the MWA group and 18.0 months (95% CI, 12.5–23.5 months) in the CA group; however, the difference was not significant (P=0.07; Fig. 2A). The cumulative PFS rates at 1, 2 and 3 years were 41.07, 17.85 and 7.14%, respectively, in patients treated with MWA, and 35.55, 11.11 and 4.44%, respectively, in patients treated with CA. The median PFS time of the MWA group was 11.0 months (95% CI, 9.5–12.4 months) and did not significantly differ from the CA group (10.0 months; 95% CI, 7.5–12.4 months; P=0.36; Fig. 2B).
Figure 2.
Comparison of OS and PFS rate at 36 months between patients with stage IIIB/IV non-small cell lung carcinoma in the CA and MWA groups. (A) OS rates at 1, 2 and 3 years were 78.57, 51.78 and 35.71% in patients treated with MWA (blue line) and 73.33, 40.00 and 22.22% in patients treated with CA (red line), respectively (P=0.07). (B) PFS rates at 1, 2 and 3 years were 41.07, 17.85 and 7.14% in patients treated with MWA (blue line), and 35.55, 11.11 and 4.44% in patients treated with CA (red line), respectively (P=0.36). OS, overall survival; PFS, progression-free survival; CA, cryoablation; MWA, microwave ablation.
Survival analysis of the subgroups
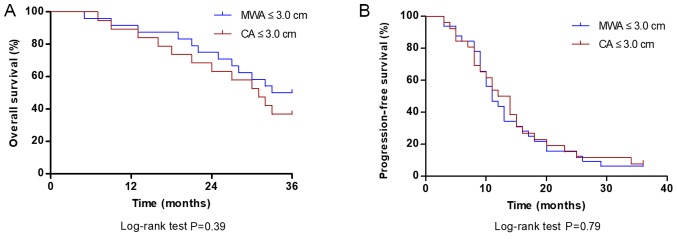
For patients with tumor diameter ≤3.0 cm, the median OS in the MWA group was 30.0 months, which was not significantly different compared with the CA group (26.5 months; P=0.39; Fig. 3A). The median PFS of patients with tumors ≤3.0 cm in the MWA group was 11.0 months, which was not significantly different compared with the CA group (13.0 months; P=0.79; Fig. 3B).
Figure 3.
Comparison of OS and PFS rate at 36 months between MWA and CA groups for tumors ≤3.0 cm. (A) OS rate of patients treated with MWA was not significantly different compared with those treated with CA (P=0.39). (B) The PFS rate in patients treated with MWA was not significantly different compared with those treated with CA. P=0.79. OS, overall survival; PFS, progression-free survival; CA, cryoablation; MWA, microwave ablation.
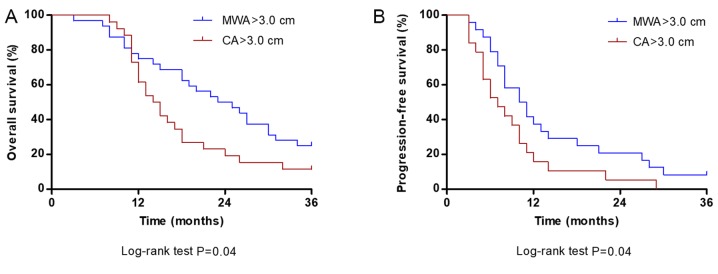
For tumors >3.0 cm, the median OS (24.5 months) and PFS (10.5 months) times in the MWA group were significantly longer compared with the median OS (14.5 months) and PFS (7.0 months) in the CA group (both P=0.04; Fig. 4A and B, respectively).
Figure 4.
Comparison of OS and PFS at 36 months between MWA and CA groups for tumors >3.0 cm. (A) OS rate of patients treated with MWA was significantly higher compared with patients treated with CA (P=0.04). (B) PFS rate of patients treated with MWA was significantly higher compared with the PFS of patients treated with CA (P=0.04). OS, overall survival; PFS, progression-free survival; CA, cryoablation; MWA, microwave ablation.
Intraprocedural pain
The intra-procedural VAS scores in the MWA group (6.01±2.06) were significantly higher compared with the CA group (2.43±1.39; P=0.001; data not shown).
AEs
Complications associated with the MWA and CA procedures are presented in Table II. No intraprocedural deaths occurred and no mortality-associated AEs were observed. The differences in the incidence rates of complications observed between the two groups were not statistically significant (P>0.05). The most common procedural complication observed in the two groups was pneumothorax, which occurred in 23 patients (41.1%) treated with MWA and 17 patients (37.8%) treated with CA. The majority of cases of pneumothorax were clinically insignificant. However, 7 cases (12.5% of procedures) in the MWA group and 5 cases (11.1% of procedures) in the CA group required the use of a chest tube drainage for pneumothorax. The second most commonly observed complication was intrapulmonary hemorrhage, which occurred in 19 patients (33.9%) in the MWA group and 11 patients (24.4%) in the CA group. Therefore, the association between the clinicopathological characteristics of the patients with the two most common complications was determined (Tables III and IV).
Table II.
Adverse events associated with the procedures.
| Adverse eventsa | CA (n=45), n (%) | MWA (n=56), n (%) | P-value |
|---|---|---|---|
| Pneumothorax | 17 (37.8) | 23 (41.1) | 0.74 |
| Grade 1, asymptomatic | 12 (26.7) | 16 (28.6) | |
| Grade 2, symptomatic requiring chest tube | 5 (11.1) | 7 (12.5) | |
| Intra-pulmonary hemorrhage | 11 (24.4) | 19 (33.9) | 0.30 |
| Grade 1, mild symptoms; intervention not indicated | 11 (24.4) | 16 (28.6) | |
| Grade 2, moderate symptoms; medical intervention indicated | 0 (0.0) | 3 (5.4) | |
| Pleural effusion | 8 (17.8) | 14 (25.0) | 0.38 |
| Grade 1, asymptomatic; clinical or diagnostic observations only | 8 (17.8) | 14 (25.0) | |
| Hemoptysis | 7 (15.6) | 10 (17.9) | |
| Grade 1, mild, <100 ml, intervention not required | 7 (15.6) | 10 (17.9) | 0.80 |
| Infection | 5 (11.1) | 7 (12.5) | 0.83 |
| Post-ablation syndrome | 1 (2.2) | 2 (3.6) | 0.69 |
| Burn | 0 (0.0) | 2 (3.6) | 0.20 |
| Complication requiring admission (mean length of stay, 1–2 days) | 7 (15.6) | 12 (21.4) | 0.54 |
The grade of adverse events was evaluated according to the common terminology criteria for adverse events version 4.0 (22). CA, cryoablation; MWA, micro-wave ablation.
Table III.
Association between the occurrence of pneumothorax and intra-pulmonary hemorrhage with CA (n=45) procedure and clinical characteristics.
| Pneumothorax | Intra-pulmonary hemorrhage | |||||
|---|---|---|---|---|---|---|
| Variable | Yes (n=17, 37.8%) | No (n=28, 62.2%) | P-value | Yes (n=11, 24.4%) | No (n=34, 75.6%) | P-value |
| Sex | 0.76 | 0.65 | ||||
| Male | 9 (20.0) | 17 (37.8) | 7 (15.5) | 19 (42.2) | ||
| Female | 8 (17.8) | 11 (24.4) | 4 (8.9) | 15 (33.4) | ||
| Age, years | 0.45 | 0.78 | ||||
| <60 | 9 (20.0) | 18 (40.0) | 6 (13.3) | 21 (46.7) | ||
| 60 | 8 (17.8) | 10 (22.2) | 5 (11.1) | 13 (28.9) | ||
| Stagea | 0.98 | 0.32 | ||||
| IIIB | 10 (22.2) | 17 (37.8) | 7 (15.6) | 20 (44.4) | ||
| IV | 7 (15.6) | 11 (24.4) | 4 (8.9) | 14 (31.1) | ||
| Nodule size, cm | 0.03 | 0.80 | ||||
| >3 | 6 (13.3) | 20 (44.4) | 6 (13.3) | 20 (44.4) | ||
| ≤3 | 11 (24.5) | 8 (17.8) | 5 (11.1) | 14 (31.1) | ||
| Nodule location (side) | 0.79 | 0.31 | ||||
| Right lung | 11 (24.5) | 18 (40.0) | 8 (17.8) | 21 (46.7) | ||
| Left lung | 6 (13.3) | 10 (22.2) | 3 (6.7) | 13 (28.9) | ||
| Nodule location (lobe) | 0.13 | 0.91 | ||||
| Upper and middle | 13 (28.9) | 15 (33.3) | 7 (15.6) | 21 (46.7) | ||
| Lower | 4 (8.9) | 13 (28.9) | 4 (8.9) | 13 (28.9) | ||
| Tumor distance to pleura, cm | 0.71 | 0.41 | ||||
| >1 | 10 (22.2) | 18 (40.0) | 8 (17.8) | 20 (44.4) | ||
| ≤1 | 7 (15.6) | 10 (22.2) | 3 (6.7) | 14 (31.1) | ||
| Tumor distance from vessel, mm | 0.85 | 0.05 | ||||
| >3 | 12 (26.7) | 19 (42.2) | 5 (11.1) | 26 (57.8) | ||
| ≤3 | 5 (11.1) | 9 (20.0) | 6 (13.3) | 8 (17.8) | ||
| Nodule location (region) | 0.03 | 0.02 | ||||
| Central (close to hilum) | 8 (17.8) | 4 (8.9) | 6 (13.3) | 6 (13.3) | ||
| Peripheral | 9 (20.0) | 24 (53.3) | 5 (11.1) | 28 (62.2) | ||
| Number of cryoprobes | 0.03 | 0.80 | ||||
| 1 | 6 (13.3) | 20 (44.4) | 6 (13.3) | 20 (44.4) | ||
| >1 | 11 (24.5) | 8 (17.8) | 5 (11.1) | 14 (31.1) | ||
Staging according to The Eighth Edition Lung Cancer Stage Classification (23). CA, cryoablation.
Table IV.
Association between occurrence of pneumothorax and intra-pulmonary hemorrhage with MWA (n=56) procedure and clinical characteristics.
| Pneumothorax | Intra-pulmonary hemorrhage | |||||
|---|---|---|---|---|---|---|
| Variable | Yes (n=23, 41.1%) | No (n=33, 58.9%) | P-value | Yes (n=19, 33.9%) | No (n=37, 66.1%) | P-value |
| Sex | 0.27 | 0.79 | ||||
| Male | 12 (21.4) | 22 (39.4) | 12 (21.4) | 22 (39.4) | ||
| Female | 11 (19.6) | 11 (19.6) | 7 (12.5) | 15 (26.7) | ||
| Age, years | 0.16 | 0.645 | ||||
| <60 | 11 (19.6) | 22 (39.4) | 12 (21.4) | 21 (37.5) | ||
| 60 | 12 (21.4) | 11 (19.6) | 7 (12.5) | 16 (28.6) | ||
| Stagea | 0.24 | 0.62 | ||||
| IIIB | 11 (19.6) | 21 (37.5) | 10 (17.8) | 22 (39.4) | ||
| IV | 12 (21.4) | 12 (21.4) | 9 (16.1) | 15 (26.7) | ||
| Nodule size, cm | 0.03 | 0.04 | ||||
| >3 | 9 (16.1) | 23 (41.1) | 7 (12.5) | 25 (44.6) | ||
| ≤3 | 14 (25.0) | 10 (17.8) | 12 (21.4) | 12 (21.4) | ||
| Nodule location (side) | 0.18 | 0.09 | ||||
| Right lung | 12 (21.4) | 23 (41.1) | 9 (16.1) | 26 (46.4) | ||
| Left lung | 11 (19.6) | 10 (17.9) | 10 (17.8) | 11 (19.6) | ||
| Nodule location (lobe) | 0.07 | 0.13 | ||||
| Upper and middle | 12 (21.4) | 25 (44.7) | 10 (17.8) | 27 (48.2) | ||
| Lower | 11 (19.6) | 8 (14.3) | 9 (16.1) | 10 (17.8) | ||
| Tumor distance from pleura, cm | 0.11 | 0.47 | ||||
| >1 | 12 (21.4) | 24 (42.9) | 11 (19.6) | 25 (44.7) | ||
| ≤1 | 11 (19.6) | 9 (16.1) | 8 (14.3) | 12 (21.4) | ||
| Tumor distance from vessel, mm | 0.14 | 0.13 | ||||
| >3 | 14 (25.0) | 26 (46.4) | 11 (19.6) | 29 (51.8) | ||
| ≤3 | 9 (16.1) | 7 (12.5) | 8 (14.3) | 8 (14.3) | ||
| Nodule location (region) | 0.02 | 0.03 | ||||
| Central (close to hilum) | 12 (21.4) | 7 (12.5) | 10 (17.8) | 9 (16.1) | ||
| Peripheral | 11 (19.6) | 26 (46.4) | 9 (16.1) | 28 (50.0) | ||
| Number of antennas | 0.03 | 0.04 | ||||
| 1 | 9 (16.1) | 23 (41.1) | 7 (12.5) | 25 (44.7) | ||
| >1 | 14 (25.0) | 10 (17.8) | 12 (21.4) | 12 (21.4) | ||
Staging according to The Eighth Edition Lung Cancer Stage Classification (23).
For the MWA and CA procedures, the following were all associated with the occurrence of pneumothorax and intrapulmonary hemorrhage: i) Central tumors for which needles had to traverse a large distance through the lung field; ii) the use of more than one applicator (antennas in MWA and cryoprobes in CA) to ablate a nodule; and iii) tumor size, which was directly associated with the number of applicators required for ablation. However, nodule distance ≤1 cm from the pleura was not associated with the occurrence of pneumothorax in the MWA and CA groups. In the MWA group, 14 of the 23 patients with post-procedural pneumothorax were treated with two antennas. Additionally, 5 of 7 patients requiring chest tube drainage were treated with two antennas for tumor ablation. Similarly, in the CA group, of the 17 patients who developed post-procedural pneumothorax, 11 patients were treated with two cryoprobes. Furthermore, 3 of the 5 patients who developed pneumothorax and required chest tube drainage in the CA group were treated with two cryoprobes.
Intrapulmonary hemorrhage in the MWA group was not associated with the distance between the tumor and a major vessel. Of the 16 patients with tumors ≤3 mm from a major vessel (≥3 mm in diameter), 8 patients developed intrapulmonary hemorrhage post-ablation, and of these, 3 developed symptomatic hemorrhage (hypotension); the patients stabilized following fluid resuscitation. In the CA group, intrapulmonary hemorrhage was also not associated with the distance between the tumor and a major vessel. Of the 14 patients with tumors ≤3 mm from a major vessel, 6 patients developed intrapulmonary hemorrhage post-ablation, although none exhibited symptomatic hemorrhage.
Discussion
MWA and CA have received increasing attention in recent years for treating malignancies of the lung (6,28,32). However, whether MWA or CA should be used in specific patients remains unclear. In clinical practice, the decision should depend on the safety and effectiveness of the particular ablation technique on a case-by-case basis (33). However, to the best of our knowledge, the relative rate of complications and the oncologic effectiveness of these two ablative modalities have not previously been compared. In the present study, the efficacy (progression and survival rates) and safety (major complication rates) of CA and MWA in patients with stage IIIB or IV NSCLC were compared.
Regarding technical success, MWA and CA displayed complete tumor ablation in the majority of cases. For patients with residual tumors, a total complete ablation was achieved following second ablation in the two groups, and local recurrence rates were similar between the groups.
The survival estimates obtained following MWA and CA in the present study were similar to previous studies (2,34–36). A limited number of studies on the long-term survival effects of CA in patients with advanced lung cancer are available. Niu et al (34) analyzed the efficacy of CA in patients with stage IV lung cancer and reported that median OS was 14 months. In another study by the same authors, the 1- and 2-year OS of patients with stage IIIB and IV lung cancer treated with CA was 58 and 48%, respectively (35). Li et al (36) investigated the long-term effects of CA in 253 patients with advanced lung cancer and reported that the median survival time was 11.98 months. The survival estimates of the CA group in the present study were similar to the aforementioned studies, with median survival times of 18 months and 1-, 2- and 3-year survival rates of 78.57, 51.78 and 35.71%, respectively. Similarly, a low number of studies have examined the effectiveness of MWA solely in advanced stage primary lung malignancy, with the majority of studies either including primary tumors of various stages or combining primary tumors with metastatic tumors; in addition, studies reporting the survival effects of MWA in advanced stage lung cancer are primarily based on patients treated with a combination of chemotherapy and MWA (2,30,37,38). However, the survival estimates of the MWA group in the present study were similar to the results of previous studies. Wei et al (2) reported median PFS and OS times of 10.9 and 23.9 months, respectively, in patients with advanced NSCLC treated with MWA in combination with chemotherapy. In another study, treatment with MWA in combination with chemotherapy resulted in median PFS and OS times of 8.7 and 21.3 months, respectively (37). Despite treatment with a combination of chemotherapy and MWA, aforementioned studies yielded lower survival rates compared with the present study. The differences may be due to larger tumor sizes in the previous studies [tumor size range, 1–9 cm in Wei et al (2); and 1–11 cm in Wei et al (37)]. To the best of our knowledge, no previous studies have compared MWA with CA for the treatment of lung carcinoma. In the present study, median PFS and OS times were similar between the MWA and CA groups and did not differ significantly.
There remains a lower probability of an ablation technique successfully obtaining complete tumor necrosis in patients with larger tumors. Larger tumors exhibit irregular tumor shapes, increasing the difficulty of the use of ablation applicators to optimize the entry route and completely kill the tumor cells resulting in tumor residues (25). Studies have reported that both MWA and CA have lower survival rates in patients with larger tumors compared with smaller tumor sizes (16,25,28–30,39,40). However, the threshold tumor size used to differentiate small and large tumors varied across previous studies and depended on the maximum size of the tumor observed in each study. In a study by Pusceddu et al (28), 4 cm was used as the threshold, where the tumor size ranged between 3 and 14 cm in size, with a mean (± standard deviation) size of 5±1.8 cm. Wei et al (2) used 3.5 cm as the threshold, where the tumor sizes ranged between 1 and 7 cm. Other studies enrolled patients with tumors ≤5 cm and thus used 3 cm as the threshold (28–30). Furthermore, previous studies did not compare the survival functions of both the ablation technique (MWA and CA) based on tumor size. In the present study, tumor size of 3.0 cm was used as the threshold, and survival function of both techniques, MWA and CA, was compared for larger as well as smaller tumors. PFS and OS were not significantly different between MWA and CA groups in patients with tumors ≤3.0 cm; however, treatment with MWA resulted in significantly improved PFS and OS compared with CA in patients with tumors >3.0 cm, which may be due to the ability of MWA to form a larger ablation zone.
The most frequently observed complications for the CA and MWA procedures in the present study were pneumothorax and intrapulmonary hemorrhage. The incidence of pneumothorax between the two groups was not significantly different. The incidence of pneumothorax in the CA group (37.7%) was similar to the 38% incidence rate observed by Mcdevitt et al (39) and 37% in Zemlyak et al (41), and within the previously reported range of 12–62% (39–44). The pneumothorax rate in the MWA group (41.1%) was similar to that of 39.1% observed by Wei et al (2) and within the reported range of 13–63% (2,25,28,29,37).
No significant differences were observed in the rates of intrapulmonary hemorrhage between the CA and MWA groups in the present study. The rate of intrapulmonary hemorrhage in the CA group was 24.4%, similar to the 24% reported by Chou et al (45). The rate of intra-pulmonary hemorrhage in the MWA group was 33.9% in the present study, which was higher compared with the 25% incidence rate reported by Yang et al (46). The lower rate observed in the previously published study may be due to the low number of tumors treated (n=11), which was lower than the number of tumors located centrally (n=19) and tumors treated with two antennas (n=24) in the present study. Data regarding intrapulmonary hemorrhage post-ablation therapy has not been commonly reported. This may partly be due to the spontaneous resolution of pulmonary hemorrhage or an inherited bias towards underreporting the incidence of minor clinical complications (47). However, it is important to consider intrapulmonary hemorrhage in the clinical setting, as large pulmonary hemorrhages can be fatal, particularly in patients with co-morbidities, and their management during ablation therapy may be difficult.
Previous studies have reported that the use of multiple applicators and the length of the lung traversed by the applicator(s) (central tumor/close to hilum) are associated with an increased risk of pneumothorax and intra-parenchymal hemorrhage (48–51). In agreement with these studies, patients with centrally located tumors and patients treated with two applicators exhibited a higher incidence of pneumothorax and intrapulmonary hemorrhage in the CA and MWA groups in the present study. As the number of applicators used was directly associated with the size of the tumor, a larger tumor size was one of the risk factors of developing pneumothorax or intrapulmonary hemorrhage. Tumor distance ≤3 mm from a major vessel (≥3 mm in diameter) was not determined to be a risk factor of intrapulmonary hemorrhage for either of the treatment groups in the present study, in agreement with Lyons et al (52). Several studies have reported that heat-based ablation is associated with the occurrence of pleural effusion as there is an increase in the pleural temperature during this procedure, which may induce pleural effusion, secondary to pleuritis, induced by thermal injury (49,53,54). Furthermore, for ablation techniques like RFA and MWA, nodule distance ≤1 cm from the pleura has been reported to be a significant risk factor for the development of pleural effusion (49,51,54). In the present study, although the difference between the rates of pleural effusion between CA and MWA was not significantly different, the rate of pleural effusion was higher in the MWA group, particularly in patients with tumors closer to the pleura. Furthermore, in the present study, a small number of patients with tumors in contact with the pleura experienced skin burn in the MWA group. Of note, patients in the CA group experienced significantly less intraprocedural pain compared with those in the MWA group.
Several previous studies have reported CA to be less painful compared with other ablation techniques (43,55,56). Extreme cold acts as an anesthetic and may be the reason for less intraprocedural pain during CA. Electrophysiologic experiments have confirmed that the cold temperature blocks nerve conduction (57,58). In addition, vasoconstriction of blood vessels from cooling may minimize the resulting edema and reduce the release of pain-inducing substances from damaged tissue (56).
The present study had certain limitations that should be considered. The study was designed retrospectively, contained data from a single center and had a relatively small cohort in both groups. Biopsies were not routinely performed during follow-up. Therefore, the present study lacks histopathological proof of treatment success. In addition, as the evaluation of local tumor progression was based only on CT images, evaluation of the viability of parts of the tumor was difficult and CT resolution was insufficient to allow the detection of microscopic relapses or lymphatic involvement. However, all imaging modalities have difficulties in detecting microscopic relapse, demonstrating a limitation of non-invasive approaches in general (36).
In conclusion, the present study demonstrated that the CA and MWA procedures were comparably safe for treating patients with advanced stage NSCLC. MWA exhibited improved treatment outcomes with significantly higher survival rates compared with CA in patients with large tumors. However, both CA and MWA were comparably effective treatment modalities with similar survival benefits in patients with advanced NSCLC with small tumors. In addition, treatment with CA had the advantage of decreased intra-procedural pain compared with MWA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SKD, YYH and HFY conceived and designed the study. SKD, YYH, BL, XXY and HFY analyzed and interpreted the data. SKD, YYH, BL and RHX collected the data. XXY and RHX performed statistical analysis. SKD wrote the manuscript. SKD, YYH and HFY critically revised the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The current study was approved by the Institutional Review Board of Affiliated Hospital of North Sichuan Medical College (Nanchong, China) and Xuzhou City Center Hospital (Xuzhou, China), and written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wei Z, Ye X, Yang X, Huang G, Li W, Wang J, Han X. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015;32:464. doi: 10.1007/s12032-014-0464-z. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society's (ACS) publication; Jan, 2019. Cancer Facts & Figures 2019. [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 6.Palussière J, Catena V, Buy X. Percutaneous thermal ablation of lung tumors-Radiofrequency, microwave and cryotherapy: Where are we going? Diagn Interv Imaging. 2017;98:619–625. doi: 10.1016/j.diii.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 7.de Baere T, Tselikas L, Catena V, Buy X, Deschamps F, Palussière J. Percutaneous thermal ablation of primary lung cancer. Diagn Interv Imaging. 2016;97:1019–1024. doi: 10.1016/j.diii.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Baisi A, De Simone M, Raveglia F, Cioffi U. Thermal ablation in the treatment of lung cancer: Present and future. Eur J Cardiothorac Surg. 2013;43:683–686. doi: 10.1093/ejcts/ezs558. [DOI] [PubMed] [Google Scholar]
- 9.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics. 2005;25(Suppl 1):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 10.Chi J, Ding M, Shi Y, Wang T, Cui D, Tang X, Li P, Zhai B. Comparison study of computed tomography-guided radiofrequency and microwave ablation for pulmonary tumors: A retrospective, case-controlled observational study. Thorac Cancer. 2018;9:1241–1248. doi: 10.1111/1759-7714.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ierardi AM, Floridi C, Fontana F, Chini C, Giorlando F, Piacentino F, Brunese L, Pinotti G, Bacuzzi A, Carrafiello G. Microwave ablation of liver metastases to overcome the limitations of radiofrequency ablation. Radiol Med. 2013;118:949–961. doi: 10.1007/s11547-013-0968-1. [DOI] [PubMed] [Google Scholar]
- 12.van Tilborg AA, Scheffer HJ, de Jong MC, Vroomen LG, Nielsen K, van Kuijk C, van den Tol PM, Meijerink MR. MWA versus RFA for perivascular and peribiliary CRLM: A retrospective patient- and lesion-based analysis of two historical cohorts. Cardiovasc Intervent Radiol. 2016;39:1438–1446. doi: 10.1007/s00270-016-1413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu L, Xu K, Mu F. Cryosurgery for lung cancer. J Thorac Dis. 2012;4:408–419. doi: 10.3978/j.issn.2072-1439.2012.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonntag PD, Hinshaw JL, Lubner MG, Brace CL, Lee FT., Jr Thermal ablation of lung tumors. Surg Oncol Clin N Am. 2011;20(ix):369–387. doi: 10.1016/j.soc.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Baere T, Tselikas L, Gravel G, Deschamps F. Lung ablation: Best practice/results/response assessment/role alongside other ablative therapies. Clin Radiol. 2017;72:657–664. doi: 10.1016/j.crad.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi Y, Izumi Y, Hashimoto K, Yashiro H, Inoue M, Nakatsuka S, Goto T, Anraku M, Ohtsuka T, Kohno M, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One. 2012;7:e33223. doi: 10.1371/journal.pone.0033223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore W, Talati R, Bhattacharji P, Bilfinger T. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol. 2015;26:312–319. doi: 10.1016/j.jvir.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Goto T, Izumi Y, Nakatsuka S, Nomori H. Percutaneous cryoablation as a salvage therapy for local recurrence of lung cancer. Ann Thorac Surg. 2012;94:e31–e33. doi: 10.1016/j.athoracsur.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura M, Izumi Y, Tsukada N, Asakura K, Sugiura H, Yashiro H, Nakano K, Nakatsuka S, Kuribayashi S, Kobayashi K. Percutaneous cryoablation of small pulmonary malignant tumors under computed tomographic guidance with local anesthesia for nonsurgical candidates. J Thorac Cardiovasc Surg. 2006;131:1007–1013. doi: 10.1016/j.jtcvs.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 20.de Baere T, Tselikas L, Woodrum D, Abtin F, Littrup P, Deschamps F, Suh R, Aoun HD, Callstrom M. Evaluating cryoablation of metastatic lung tumors in patients-safety and efficacy: The ECLIPSE trial-interim analysis at 1 year. J Thorac Oncol. 2015;10:1468–1474. doi: 10.1097/JTO.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 21.Welch BT, Brinjikji W, Schmit GD, Callstrom MR, Kurup AN, Cloft HJ, Woodrum DA, Nichols FC, Atwell TD. A national analysis of the complications, cost, and mortality of percutaneous lung ablation. J Vasc Interv Radiol. 2015;26:787–791. doi: 10.1016/j.jvir.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association: World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Ye X, Zheng A, Huang G, Ni X, Wang j, Han X, Li W, Wei Z. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: Clinical evaluation of 47 cases. J Surg Oncol. 2014;110:758–763. doi: 10.1002/jso.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Common terminology criteria for adverse events (version 4.0) https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdf. [Aug 19;2018 ]; doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed]
- 27.Wewes ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 28.Pusceddu C, Melis L, Sotgia B, Guerzoni D, Porcu A, Fancellu A. Usefulness of percutaneous microwave ablation for large non-small cell lung cancer: A preliminary report. Oncol Lett. 2019;18:659–666. doi: 10.3892/ol.2019.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: Effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Wang J, Shao JB, Zhu LM, Sun ZG, Zhang N. Microwave ablation combined with chemotherapy improved progression free survival of IV stage lung adenocarcinoma patients compared with chemotherapy alone. Thorac Cancer. 2019;10:1628–1635. doi: 10.1111/1759-7714.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Tian J, Zhao L, Wu B, Kacher DS, Ma X, Liu S, Ren C, Xiao YY. CT-guided conformal cryoablation for peripheral NSCLC: Initial experience. Eur J Radiol. 2012;8:3354–3362. doi: 10.1016/j.ejrad.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 32.Mahnken AH, König AM, Figiel JH. Current technique and application of percutaneous cryotherapy. Rofo. 2018;190:836–846. doi: 10.1055/a-0598-5134. (In English, German) [DOI] [PubMed] [Google Scholar]
- 33.Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT, Jr, Brace CL. Percutaneous tumor ablation tools: Microwave, radiofrequency, or cryoablation-what should you use and why? Radiographics. 2014;34:1344–1362. doi: 10.1148/rg.345140054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu L, Chen J, Yao F, Zhou L, Zhang C, Wen W, Bi X, Hu Y, Piao X, Jiang F, et al. Percutaneous cryoablation for stage IV lung cancer: A retrospective analysis. Cryobiology. 2013;67:151–155. doi: 10.1016/j.cryobiol.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Niu L, Xu K, He W, GuoZ Q, Zhang SP, He TS, Zuo JS. Percutaneous Cryoablation for patients with advanced non-small cell lung cancer. Technol Cancer Res T. 2007;6:451–452. [Google Scholar]
- 36.Li Y, Feng H, Nie Z. The long-term effects and risk factors analysis in 253 cases advanced non-small cell lung cancer treated with percutaneous cryosurgery. Chin Clin Oncol. 2010;15:346–349. [Google Scholar]
- 37.Wei Z, Ye X, Yang X, Zheng A, Huang G, Li W, Ni X, Wang J, Han X. Microwave ablation in combination with chemotherapy for the treatment of advanced non-small cell lung cancer. Cardiovasc Intervent Radiol. 2015;38:135–142. doi: 10.1007/s00270-014-0895-0. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Zhao M, Wang J, Fan W, Li W, Pan T, Wu P. Percutaneous CT-guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non-small cell lung cancer. AJR Am J Roentgenol. 2013;201:1362–1367. doi: 10.2214/AJR.12.10511. [DOI] [PubMed] [Google Scholar]
- 39.McDevitt JL, Mouli SK, Nemcek AA, Lewandowski RJ, Salem R, Sato KT. Percutaneous cryoablation for the treatment of primary and metastatic lung tumors: Identification of risk factors for recurrence and major complications. J Vasc Interv Radiol. 2016;27:1371–1379. doi: 10.1016/j.jvir.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Yashiro H, Nakatsuka S, Inoue M, Kawamura M, Tsukada N, Asakura K, Yamauchi Y, Hashimoto K, Kuribayashi S. Factors affecting local progression after percutaneous cryoablation of lung tumors. J Vasc Interv Radiol. 2013;24:813–821. doi: 10.1016/j.jvir.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010;211:68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Pusceddu C, Sotgia B, Fele RM, Melis L. CT-guided thin needles percutaneous cryoablation (PCA) in patients with primary and secondary lung tumors: A preliminary experience. Eur J Radiol. 2013;82:e246–e253. doi: 10.1016/j.ejrad.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Inoue M, Nakatsuka S, Yashiro H, Ito N, Izumi Y, Yamauchi Y, Hashimoto K, Asakura K, Tsukada N, Kawamura M, et al. Percutaneous cryoablation of lung tumors: Feasibility and safety. J Vasc Interv Radiol. 2012;23:295–302. doi: 10.1016/j.jvir.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: Initial experience with more than 200 procedures. Radiology. 2005;235:289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 45.Chou HP, Chen CK, Shen SH, Sheu MH, Wu MH, Wu YC, Chang CY. Percutaneous cryoablation for inoperable malignant lung tumors: Midterm results. Cryobiology. 2015;70:60–65. doi: 10.1016/j.cryobiol.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Ye X, Zhang L, Geng D, Du Z, Yu G, Ren H, Wang J, Huang G, Wei Z, et al. Microwave ablation for lung cancer patients with a single lung: Clinical evaluation of 11 cases. Thorac Cancer. 2018;9:548–554. doi: 10.1111/1759-7714.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinke K, King J, Glenn D, Morris DL. Pulmonary hemorrhage during percutaneous radiofrequency ablation: A more frequent complication than assumed? Interact Cardiovasc Thorac Surg. 2003;2:462–465. doi: 10.1016/S1569-9293(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 48.Nour-Eldin NE, Naguib NN, Mack M, Abskharon JE, Vogl TJ. Pulmonary hemorrhage complicating radiofrequency ablation, from mild hemoptysis to life-threatening pattern. Eur Radiol. 2011;21:197–204. doi: 10.1007/s00330-010-1889-1. [DOI] [PubMed] [Google Scholar]
- 49.Hiraki T, Tajiri N, Mimura H, Yasui K, Gobara H, Mukai T, Hase S, Fujiwara H, Iguchi T, Sano Y, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: Incidence and risk factors. Radiology. 2006;241:275–283. doi: 10.1148/radiol.2411051087. [DOI] [PubMed] [Google Scholar]
- 50.Welch BT, Brinjikji W, Schmit GD, Callstrom MR, Kurup AN, Cloft HJ, Woodrum DA, Nichols FC, Atwell TD. A national analysis of the complications, cost, and mortality of percutaneous lung ablation. J Vasc Interv Radiol. 2015;26:787–791. doi: 10.1016/j.jvir.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Zheng A, Wang X, Yang X, Wang W, Huang G, Gai Y, Ye X. Major complications after lung microwave ablation: A single-center experience on 204 sessions. Ann Thorac Surg. 2014;98:243–248. doi: 10.1016/j.athoracsur.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Lyons GR, Askin G, Pua BB. Clinical outcomes after pulmonary cryoablation with the use of a triple freeze protocol. J Vasc Interv Radiol. 2018;29:714–721. doi: 10.1016/j.jvir.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 53.Ye X, Fan W, Wang H, Wang J, Wang Z, Gu S, Feng W, Zhuang Y, Liu B, Li X, et al. Expert consensus workshop report: Guidelines for thermal ablation of primary and metastatic lung tumors (2018 edition) J Cancer Res Ther. 2018;14:730–744. doi: 10.4103/jcrt.JCRT_221_18. [DOI] [PubMed] [Google Scholar]
- 54.Hiraki T, Gobara H, Fujiwara H, Ishii H, Tomita K, Uka M, Makimoto S, Kanazawa S. Lung cancer ablation: Complications. Semin Intervent Radiol. 2013;30:169–175. doi: 10.1055/s-0033-1342958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colak E, Tatlı S, ShynP B, Tuncalı K, Silverman SG. CT-guided percutaneous cryoablation of central lung tumors. Diagn Interv Radiol. 2014;20:316–322. doi: 10.5152/dir.2014.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allaf ME, Varkarakis IM, Bhayani SB, Inagaki T, Kavoussi LR, Solomon SB. Pain control requirements for percutaneous ablation of renal tumors: Cryoablation versus radiofrequency ablation-initial observations. Radiology. 2005;237:366–370. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- 57.Li CL. Effect of cooling on neuromuscular transmission in the rat. Am J Physiol. 1958;194:200–206. doi: 10.1152/ajplegacy.1958.194.1.200. [DOI] [PubMed] [Google Scholar]
- 58.Douglas WW, Malcolm LL. The effect of localized cooling on conduction in cat nerves. J Physiol. 1955;130:53–71. doi: 10.1113/jphysiol.1955.sp005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.