Abstract
Aim:
The aim of this study was to determine the prevalence of restless legs syndrome (RLS) and Pittsburgh Sleep Quality Index (PSQI) in patients with type 2 diabetes mellitus (T2DM) attending primary healthcare.
Subjects and Methods:
This is a cross-sectional study and participants were between 25 and 70 years old who visited the diabetes and endocrinology department of Mega Medipol University Teaching Hospital, Istanbul. The diagnosis of RLS was performed according to the International Restless Legs Syndrome Study Group consensus criteria. The RLS and PSQI instruments were conducted on 871 patients with T2DM. Good sleep quality was defined as PSQI score <5. RLS severity was assessed by the Restless Legs Syndrome-6 Scales (RLS-6). The scale development and validation was carried out using Rasch measurement model.
Results:
The prevalence of RLS was 22.8% including 60.3% of females and 39.7% of males. This study showed significant differences between RLS and no RLS patients with respect to their age (years), body mass index (BMI) (kg/m2), physical activity, smoking habit, sheesha smoking, income, and sleeping quality with PSQI. Also, the analysis presented that statistically significant differences between both RLS and no RLS reported sleep complaints including difficulty falling asleep, inadequate sleep, anytime fatigue, and leg discomfort. There were statistically significant differences between RLS and no RLS patients regarding hypoglycemia, numbness in legs, retinopathy, neuropathy, nephropathy high blood pressure, depression, stroke, anemia, diabetic foot, ulcer, arthritis, respiratory disease, metabolic syndrome, and coronary heart disease. Furthermore, there were statistically significant differences between RLS and no RLS concerning the number of sleeping hours, wake-up time (AM), sleeping time (PM), BMI (kg/m2), HbA1c, vitamin D, calcium, creatinine, fasting blood glucose, low-density lipoprotein, triglyceride, uric acid, and systolic and diastolic blood pressure (mmHg).
Conclusion:
This study confirms positive relation and high prevalence of RLS among patients with T2DM visiting primary healthcare. The results suggest that physical activity is associated with a better perception of functional capacity and pain in diabetic patients with RLS, and thus a more active lifestyle should be encouraged.
Keywords: Pittsburgh Sleep Quality Index, restless leg syndrome, risk factors, sleeping disturbances, type 2 diabetes mellitus
Introduction
Restless legs syndrome (RLS) is a chronic neurosensorimotor disease characterized by an urge to move the legs which is usually accompanied by unpleasant or uncomfortable often painful sensations in the legs.[1,2,3] RLS is a burdensome sleep disorder affecting 10%–15% of the general population and over 20% of primary care patients.[4,5,6] RLS can have profound negative effects on the quality of life and daily activities and is associated with significant economic, social, and healthcare burdens.[7,8,9,10] RLS has been significantly associated with diabetes, hypertension, obesity, and metabolic syndrome;[5,6] meanwhile, the situation and role of these relationships remain unclear.
At present, many experimental and epidemiological studies have reported that poor sleep quantity and quality are related to greater prevalence of high fasting plasma glucose and high HbA1c level.[11,12,13,14] Most recently, studies have been reported that long durations of sleep are associated with severity of diabetes.[15,16,17,18] Many studies provided evidence that sleep quality influences the glycemic control among patients with type 2 diabetes mellitus (T2DM) and approximately 37%–50% of patients with T2DM have sleep problems, which is higher than the general population.[19]
The aim of this study was to determine the prevalence of RLS and Pittsburgh Sleep Quality Index (PSQI) in patients with T2DM attending primary healthcare. The ethical approval obtained from The Committee as Institutional Review Board (IRB) of Istanbul Medipol University, (Research Protocol and IRB# 10840098-604.01.01-E.20326 Dated: 26/02/2016 and IRB# 10840098-604.01.01-E.40791 Date: 26/10/2017).
Subjects and Methods
This is a cross-sectional study and participants were patients age 25–70 years who visited the diabetes and endocrinology unit of Mega Medipol University Teaching Hospital, Istanbul, and Primary Health Care Clinics. Data used in this report were used to investigate the relationship between RLS, sleep, and glycemic control in people with T2DM.[20] A systematic random sample of 1000 patients administered in the endocrinology unit in four general hospitals were recruited between January 2016 and April 2018, and 871 agreed and gave their consent to take part in this study, thus giving a response rate of 87.1%. The inclusion criteria were as follows: (1) diagnosed with T2DM for over 3 years, additionally verified by the medical record; (2) age 25 years or over; and (3) able to communicate in Turkish. Participants were excluded if they had gestational diabetes, severe heart, lung, and cerebral disease and mental illness or disorders.
Laboratory measurements
People living with T2DM were considered as “case” patients if they had a history of DM and were taking any oral diabetes medications for at least a period of 3 years.[20] These “case' subjects were investigated for their lipid profile [total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein, triglyceride (TG)], glycosylated hemoglobin (HbA1c), postprandial glucose, blood pressure, serum creatinine, thyroid and presence related medical comorbidities. On the other hand, “control” subjects were not taking any DM medications and their HbA1c was less than 6.5% and their fasting blood glucose (FBG) was less than 7.0 mmol/L (126 mg/dL), which were confirmed by their medical records.[20]
This study was based on questionnaire, which assessed participant sociodemographics, physiological parameters, clinical and biochemistry parameters, blood pressure, and HbA1C. The level of HbA1C ≤7% was defined as good glycemic control based on the American Diabetes Association 2010 Guidelines, whereas a level of HbA1C >7% was considered poor glycemic control.[20]
Pittsburgh Sleep Quality Index
The PSQI was developed by Buysse et al.[21] to evaluate subjective sleep disturbance over the past month. The questionnaire measures seven groups for sleep difficulty including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each component is scored on a 4-point Likert scale from 0 to 3; the sum of the seven components results in a global PSQI score between 0 and 21. Based on the total PSQI score, the patients were divided into three groups: the “good sleep quality group” with PSQI score of ≤5, “average sleep quality group” with PSQI 6–8, and “poor sleep quality group” with PSQI ≥9.[16,17] The internal consistency Cronbach's alpha was 0.84, the split-half reliability was 0.87, and the 2-week test–retest reliability was 0.81; meanwhile, the sensitivity was 98.3% and specificity was 90.2% with a cut-off point set at 8.[21,22]
Restless Legs Syndrome-6 Scales
The RLS-6 includes six items, each one scoring on a 0–10 scale from 0 (no symptoms) to 10 (very severe). RLS severity was classified into mild (0–10), moderate (11–20), severe (21–30), and very severe (31–40) according to total International RLS Study Group Rating Scale scores.[23,24]
Statistical analysis and Rasch measurement
To determine the internal validity of the scale, we conducted a Rasch analysis.[25,26,27] The Rasch method is used to examine a participant's response to an item that is a function of the difference between an individual's ability and the characteristics of the item. Rasch measurement determines the relationship between the difficulty of an item and the ability of an individual. It is expected there is a higher probability in answering easier items correctly and a lower probability in answering more difficult items incorrectly.[25,26,27] According to the model, the probability of an individual n responding in category x to item i is given by

where τ0 = 0 so that
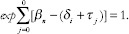
βn is the individual's position on the variable, δi is the scale value (difficulty to endorse) estimated for each item i, and τ1, τ2,...,τ3 are the m response thresholds estimated for the m + 1 rating categories.[25,26,27]
Measuring goodness of fit for the Rasch model
Testing the normality of residuals is the most frequently used goodness-of-fit measure for the Rasch model.[25,26,27] The Rasch analysis tests how well the observed data fit the model. Misfits statistics consist of the infit and outfit test statistics which are based on the standardized residuals. The most commonly used misfit statistics for Rasch analysis are the mean-square (MNSQ) misfit statistics and z-standardized misfit statistics. The z-standardized misfit statistics are usually used when the MNSQ statistics fail.[27]
Data were analyzed using the Statistical Package for the Social Sciences (SPSS Statistics for Windows, Version 22.0; IBM Corp, Armonk, NY, USA). Student's t-test was conducted to reveal whether any significant difference exists between mean values of two continuous variables. Fisher's exact test (two-tailed) and Chi-square were used to display differences in proportions of categorical variables between two or more groups. The scale development and validation was carried out using Rasch measurement model. The level of statistical significance was considered as P < 0.05 for all tests.
Results
The prevalence of RLS was 22.8% including 60.3% of females and 39.7% of males. Age in years was 49.30 ± 13.67 years with RLS and without RLS was 50.63 ± 14.47. Table 1 shows significant differences between RLS and no RLS patients with respect to their age in years, physical activity, smoking habit, sheesha smoking, income, and sleeping quality with PSQI. Also, the analysis presented that statistically significant differences between both RLS and no RLS reported sleep complaints including difficulty falling asleep, inadequate sleep, anytime fatigue, leg discomfort, number of sleeping hours, wake-up time (AM), and sleeping time (PM). Table 2 presents the clinical characteristics and comorbid condition by RLS among patients with T2DM. There were statistically significant differences between RLS and no RLS patients regarding hypoglycemia, numbness in legs, retinopathy, neuropathy, nephropathy high blood pressure, depression, stroke, anemia, diabetic foot, ulcer, arthritis, respiratory disease, metabolic syndrome, and coronary heart disease (CHD).
Table 1.
Sociodemographic characteristics of sleeping disorder studied by RLS among patients with T2DM (n=871)
| Variables | RLS=199 n (%) | No RLS=672 n (%) | P |
|---|---|---|---|
| Age groups (years) | |||
| <40 | 51 (25.6) | 183 (24.6) | 0.019 |
| 40-49 | 63 (31.7) | 147 (21.9) | |
| 50-59 | 37 (18.6) | 162 (24.1) | |
| >60 and above | 48 (24.6) | 200 (29.8) | |
| Gender | |||
| Male | 79 (39.7) | 251 (37.4) | 0.549 |
| Female | 120 (60.3) | 421 (62.6) | |
| Married | |||
| Single | 20 (10.1) | 101 (15.0) | 0.128 |
| Married | 29 (78.9) | 515 (76.7) | |
| Divorce/widow | 22 (11.0) | 56 (8.3) | |
| BMI (kg/m2) | |||
| Normal (<25 kg/m2) | 50 (25.1) | 195 (29.0) | 0.347 |
| Overweight (29-30 kg/m2) | 80 (45.3) | 309 (46.0) | |
| Obese (>30 kg/m2) | 59 (29.6) | 168 (29.0) | |
| Physical activity 30 min/day | |||
| Yes | 74 (37.2) | 161 (24.0) | 0.001 |
| No | 125 (62.8 | 511 (76.0) | 0.007 |
| Household income | 88 (44.2) | 376 (56.0) | 0.008 |
| Low | 72 (36.2) | 174 (25.9) | |
| Medium | 39 (19.6) | 122 (18.1) | |
| High | 35 (17.6) | 71 (10.6) | |
| Sheesha smoking | 164 (82.4) | 601 (88.4) | |
| Yes | |||
| No | |||
| Cigarette smoking | |||
| Never | 154 (77.4) | 577 (85.9) | 0.007 |
| Current smoker | 33 (16.6) | 60 (8.9) | |
| Past smoker | 12 (6.0) | 35 (5.2) | |
| Reported sleep complaints | |||
| Difficulty falling asleep | 49 (24.6) | 75 (11.2) | 0.001 |
| Inadequate sleep | 31 (15.6) | 56 (8.3) | 0.003 |
| Daytime fatigue | 43 (21.6) | 71 (10.6) | 0.001 |
| Leg discomfort | 66 (33.2) | 82 (12.2) | 0.001 |
| PSQI sleep quality | |||
| Good (PSQI <5) | 40 (20.1) | 199 (29.6) | 0.024 |
| Average (6 <PSQI ≤8) | 73 (36.7) | 203 (30.2) | |
| Poor (PSQI >8) | 86 (43.2) | 270 (40.2) | |
| Number of sleeping hours | 5.36±1.12 | 6.12±1.31 | 0.008 |
| Wake-up time (AM) | 6.60±0.81 | 6,73±0.86 | 0.016 |
| Sleeping time (PM) | 11.50±0.72 | 11.39±0.72 | 0.001 |
RLS=restless legs syndrome; T2DM=type 2 diabetes mellitus; BMI=body mass index; PSQI=Pittsburgh Sleep Quality Index
Table 2.
Clinical characteristics and comorbid condition by RLS among patients with T2DM (n=871)
| Variables | RLS n=199 n (%) | No RLS n=672 n (%) | OR and 95% confidence interval | P |
|---|---|---|---|---|
| Hypoglycemia | 72 (28) | 94 (14.0) | 1.95 (1.32-2.89) | 0.001 |
| Numbness in legs | 36 (18.1) | 77 (11.9) | 1.70 (1.08-2.62) | 0.014 |
| Retinopathy | 48 (24.1) | 106 (15.8) | 1.69 (1.15-2.49) | 0.007 |
| Neuropathy | 29 (14.6) | 45 (6.7) | 1.37 (1.44-3.90) | 0.001 |
| Nephropathy | 26 (13.1) | 43 (6.4) | 2.19 (1.31-3.68) | 0.002 |
| High blood pressure | 49 (24.6) | 105 (15.6) | 1.76 (1.20-2.59) | 0.003 |
| Depression | 41 (20.6) | 67 (10.0) | 2.34 (1.53-3.61) | 0.001 |
| Stroke | 25 (12.6) | 42 (6.3) | 2.15 (1.27-2.63) | 0.003 |
| Iron deficiency | 45 (22.6) | 57 (8.5) | 3.27 (2.12-5.05) | 0.001 |
| Diabetic foot | 34 (17.1) | 53 (7.9) | 2.40 (1.51-3.82) | 0.001 |
| Ulcer | 18 (9.0) | 56 (8.3) | 1.09 (0.62-1.90) | 0.752 |
| Arthritis | 34 (17.1) | 71 (10.6) | 1.74 (1.11-2.71) | 0.013 |
| Respiratory disease | 17 (8.9) | 26 (3.9) | 2.32 (1.32-4.37) | 0.008 |
| Metabolic syndrome | 53 (26.6) | 126 (18.8) | 1.57 (1.10-2.27) | 0.016 |
| Coronary heart disease | 72 (28) | 72 (28) | 1.95 (1.32-2.89) | 0.002 |
RLS=restless legs syndrome; T2DM=type 2 diabetes mellitus; OR=odds ratio
Table 3 reports the baseline values of biochemical indices by RLS and no RLS among patients with T2DM. Significant differences were reported between RLS and no RLS concerning body mass index (kg/m2), HbA1c, vitamin D (mmol/L), calcium (mmol/L), creatinine (mmol/L), FBG (mmol/L), LDL (mmol/L), TG (mmol/L), uric acid (mmol/L), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), and vitamin D deficiency.
Table 3.
Clinical biochemistry baseline value by RLS among patients with T2DM (n=871)
| Variables | RLS n=199 Mean±SD | No RLS n=672 Mean±SD | P |
|---|---|---|---|
| BMI (kg/m2) | 28.30±4.89 | 27.35±4.31 | 0.006 |
| Hemoglobin (g/dL) | 12.21±1.49 | 12.95±1.62 | 0.001 |
| HbA1c | 7.89±0.80 | 7.48±0.88 | 0.001 |
| Vitamin D (mmol/L) | 19.11±8.94 | 21.63±9.46 | 0.002 |
| Calcium (mmol/L) | 1.59±0.69 | 1.71±0.52 | 0.010 |
| Creatinine (mmol/L) | 65.18±27.49 | 63.18±22.87 | 0.387 |
| Fasting blood glucose (mmol/L) | 7.45±0.95 | 7.15±0.89 | 0.024 |
| Cholesterol (mmol/L) | 4.83±1.18 | 4.67±1.20 | 0.959 |
| HDL (mmol/L) | 1.14±0.35 | 1.36±0.31 | 0.590 |
| LDL (mmol/L) | 1.45±0.72 | 1.89±0.99 | 0.001 |
| Triglyceride (mmol/L) | 1.82±0.72 | 1.75±0.66 | 0.258 |
| Uric acid (mmol/L) | 294.22±62.17 | 273.26±60.10 | 0.001 |
| TSH | 2.73±1.00 | 2.67±0.99 | 0.363 |
| Systolic blood pressure (mmHg) | 131.86±14.84 | 128.07±13.78 | 0.001 |
| Diastolic blood pressure (mmHg) | 80.09±9.70 | 79.10±9.97 | 0.037 |
| Vitamin D | n (%) | n (%) | |
| Deficiency<20 ng/mL | 122 (61.3) | 289 (43) | |
| Insufficiency 20-29 ng/mL | 43 (21.6) | 214/31.8) | 0.001 |
| Sufficiency>30 ng/mL | 34 (17.1) | 169 (25.1) | |
RLS=restless legs syndrome; T2DM=type 2 diabetes mellitus; SD=standard deviation; BMI=body mass index; HDL=high-density lipoprotein; LDL=low-density lipoprotein; TSH= Thyroid stimulating hormone
In Figure 1, Rasch analysis was performed in Winsteps 4.0.1. Each “#” symbol indicates eight people and each “.” indicates one to seven people in the left-hand column. Each entry symbolizes a scale item in the right column. The items at the top are the most difficult items, while the people at the top are the highest scorers and versa vice. Items 4, 6, and 8 are difficult for people to endorse. The vertical line between the two columns represents the scale for parameter estimates measured in logits. Along the vertical line, M shows the mean, S shows one standard deviation above or below the mean, and T indicates two standard deviations above or below the mean.
Figure 1.

Person-item map for Restless Leg Scale (N = 871)
In Table 4, the person and item reliability indexes were 0.43 and 0.98, respectively, by Rasch analysis. Reliability ranged from 0 to 1.0. If a reliability coefficient is 0, there is no reliability, and if the coefficient is 1.0, it indicates perfect reliability. Furthermore, the person and item separation indexes were 0.87 and 6.78, respectively. Table 4 demonstrates that the restless leg scale has acceptable characteristics because the model fit MNSQ values range from 0.99 to 1.02, outfit MNSQ is 1.02, and infit MNSQ is 1.02. The values of infit and outfit MNSQs are in the acceptable range of 0.5–1.5 for these statistics.[25,26]
Table 4.
Fit statistics for Restless Leg scale (n=871, # item of scale=10)
| Person | 871 Input | 871 Measured | Infit | Outfit | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Count | Measure | RealSe | IMNSQ | ZSTD | OMNSQ | ZSTD | |
| Mean | 10.8 | 10.0 | −0.59 | 0.40 | 0.99 | −0.1 | 1.02 | 0.0 |
| P.Sd | 3.9 | 0.0 | 0.54 | 0.07 | 0.43 | 1.2 | 0.47 | 1.2 |
| Real RMSE 0.41 true Sd | 0.35 | Separation | 0.87 | Person reliability | 0.43 | |||
| Item | 10 Input | 10 Measured | Infit | Outfit | ||||
| Total | Count | Measure | RealSe | IMNSQ | ZSTD | OMNSQ | ZSTD | |
| Mean | 942.8 | 871 | 0.00 | 0.04 | 1.01 | −0.03 | 1.02 | −0.3 |
| P.Sd | 191.8 | 0.0 | 0.29 | 0.00 | 0.28 | 6.8 | 0.31 | 6.8 |
| Real RMSE 0.04 true Sd | 0.28 | Separation | 6.78 | Item reliability | 0.98 | |||
In Figure 2, each “#” symbol indicates 27 people and each “.” indicates 1 to 7 people in the left-hand column. Items 5-I, 11, 8, 9, 5-B, and 5-H are difficult for people to endorse.
Figure 2.
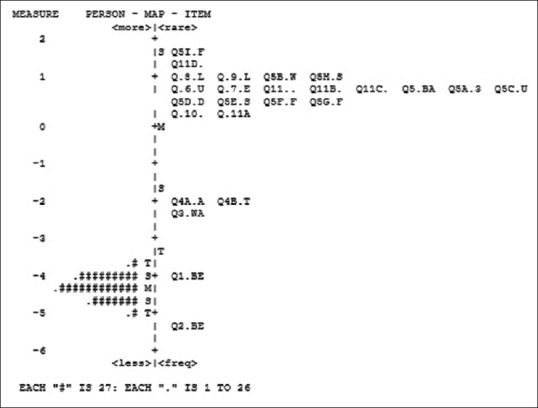
Person-item map for Pittsburgh Sleep Scale (N = 871)
In Table 5, the person and item reliability indexes were 0.63 and 1.00, respectively, by Rasch analysis. The person and item separation indexes were 1.31 and 46.69, respectively. Table 5 demonstrates that the Pittsburgh Sleep Scale has acceptable characteristics because the model fit MNSQ values range from 0.83 to 0.93, outfit MNSQ is 0.83, and infit MNSQ is 0.84.
Table 5.
Fit statistics for Pittsburgh Sleep Scale (n=871, # item of scale=25)
| Person | 871 Input | 871 Measured | Infit | Outfit | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Count | Measure | RealSe | IMNSQ | ZSTD | OMNSQ | ZSTD | |
| Mean | 78.3 | 25.0 | −4.32 | 0.17 | 0.93 | −0.1 | 0.83 | −0.6 |
| P.Sd | 11.1 | 0.0 | 0.29 | 0.04 | 0.64 | 0.9 | 0.31 | 1.1 |
| Real RMSE 0.18 true Sd | 0.23 | Separation | 1.31 | Person reliability | 0.63 | |||
| Item | 25 Input | 25 Measured | Infit | Outfit | ||||
| Total | Count | Measure | RealSe | IMNSQ | ZSTD | OMNSQ | ZSTD | |
| Mean | 2726.6 | 871 | 0.00 | 0.03 | 0.84 | −3.5 | 0.83 | −3.7 |
| P.Sd | 4856.7 | 0.0 | 1.67 | 0.01 | 0.25 | 4.8 | 0.25 | 4.7 |
| Real RMSE 0.04 true Sd | 1.67 | Separation | 46.69 | Item reliability | 1.00 | |||
Discussion
RLS is a common comorbidity in patients with diabetes which can be considered a significant burden on the quality of sleep and quality of life of patients, although this condition is frequently underdiagnosed. The prevalence of RLS in diabetics in Italy was 17.7%, significantly higher than that reported in the general population, ranging from 5% to 10%.[7] The prevalence of RLS was 22.8%, observed to be high in this population compared with the study done in Italy. In the Italian study,[7] there was a significantly higher prevalence of women with diabetes with RLS than in the diabetic no RLS group (64% vs 30%), and this is consistent with the current Turkish population (females 60.3% vs males 39. 7%).
The RLS was diagnosed with diabetes; the participants had a variety of comorbid health conditions including hypoglycemia, numbness in legs, retinopathy, neuropathy, nephropathy, high blood pressure, depression, stroke, anemia, diabetic foot, ulcer, arthritis, respiratory disease, metabolic syndrome, and CHD.
The RLS was diagnosed in 22.6% of the women with iron deficiency anemia. Iron deficiency is a well-known condition correlating with several forms of symptomatic RLS in the diabetic population; our study was in consistent with a previously reported study that showed an association between RLS and iron status in diabetic patients.[19] The association between RLS and hypertension and heart diseases could be due to the effects of a prolonged sleep loss in increasing the probability of developing hypertension and diabetes,[13,14,16,17,18] which may lead to vascular diseases.
More recently, study and data suggest that poor sleep quality as measured by the PSQI contributes to suboptimal diabetes control.[12] Sleep disturbances have effect and increase HbA1c level. In fact, the mechanism for the relationship between sleep disturbances and HbA1c was presented very clearly in the current study and it is consistent with previously reported studies.[11,17,18,22]
In this study, diabetic patients reported high prevalence of RLS (22.8%), and this is confirmative with other studies conducted in Iran (19.5%),[8] Bosnia and Herzegovina (21%),[13] the United States (24.5%),[5] Brazil (27%),[11] and Turkey (28.3%).[6] This result supports the study findings of other studies[13,14,16,17,18] that sleep disorders correlate highly with hypertension and diabetic population in the present study.
In patients affected by RLS, the unpleasant sensations may lead to a severe difficulty in initiating and maintaining sleep, disrupting sleep situation, and cause an important sleep disorder.[7] A chronic sleep debt, as observed in RLS patients, has been shown to be a predictor of morbidity or mortality. Patients with restless legs appear to have a significantly higher risk of ischemic stroke and hypertension than do subjects without this sleep disorder. The association between RLS and cerebrovascular diseases could be due to the effects of a prolonged sleep loss in increasing the probability of developing hypertension and diabetes[3,7,9,28] which are known to be the predictors of vascular diseases. Therefore, in the diabetic population, RLS and diabetes can interact in a vicious circle. Hence, RLS symptoms should be adequately treated to help with the management of the endocrine disease and, consequently, to reduce the risks of mortality caused by vascular diseases.[28]
This study is not without limitations. First, this is a cross-sectional design of the study which did not identify the causal relationship between the presence of sleep disturbance/insomnia symptoms and T2DM. Second, the sample of T2DM individuals was recruited from different hospitals, and there may be sampling bias. Third, some patients might be affected with T2DM or associated with other diseases. Fourth, participants who had subjective sleep disturbance have not been clinically diagnosed but have been assessed by higher score of the PSQI.
Conclusion
This study confirms positive relationship and high prevalence of RLS among patients with T2DM visiting primary healthcare. The results suggest that physical activity is associated with a better perception of functional capacity and pain in diabetic patients with RLS, and thus a more active lifestyle should be encouraged.
Financial support and sponsorship
This work was generously supported and funded by the Qatar Diabetes Association, Qatar Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank the Istanbul Medipol University for their support and ethical approval (Research Protocol and IRB# 10840098-604.01.01-E.20326 and Research Protool and IRB# 10840098-604.01.01-E.40791).
References
- 1.Ferri R, Fulda S, Allen RP, Zucconi M, Bruni O, Chokroverty S, et al. International and European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). World association of sleep medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG) Sleep Med. 2016;26:86–95. doi: 10.1016/j.sleep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – History, rationale, description, and significance. Sleep Med. 2014;15:860–73. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Akın S, Bölük C, Türk Börü Ü, Taşdemir M, Gezer T, Şahbaz FG, et al. Restless legs syndrome in type 2 diabetes mellitus. Prim Care Diabetes. 2019;13:87–91. doi: 10.1016/j.pcd.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: A systematic review. Sleep Med. 2011;12:623–34. doi: 10.1016/j.sleep.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trenkwalder C, Allen R, Högl B, Clemens S, Patton S, Schormair B, et al. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. 2018;17:994–1005. doi: 10.1016/S1474-4422(18)30311-9. [DOI] [PubMed] [Google Scholar]
- 6.Kalra S, Gupta A. Diabetic painful neuropathy and restless legs syndrome in diabetes. Diabetes Ther. 2018;9:441–7. doi: 10.1007/s13300-018-0376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merlino G, Fratticci L, Valente M, Del Giudice A, Noacco C, Dolso P, et al. Association of restless legs syndrome in type 2 diabetes: A case-control study. Sleep. 2007;30:866–71. doi: 10.1093/sleep/30.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modarresnia L, Golgiri F, Madani NH, Emami Z, Tanha K. Restless legs syndrome in Iranian people with type 2 diabetes mellitus: The role in quality of life and quality of sleep. J Clin Sleep Med. 2018;14:223–8. doi: 10.5664/jcsm.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuellar NG, Dorn JM. Peripheral diabetic neuropathy or restless legs syndrome in persons with type 2 diabetes mellitus: Differentiating diagnosis in practice. J Am Assoc Nurse Pract. 2015;27:671–5. doi: 10.1002/2327-6924.12311. [DOI] [PubMed] [Google Scholar]
- 10.Greco D. Restless legs syndrome and diabetes mellitus: An accidental association. Recenti Prog Med. 2011;102:212–6. doi: 10.1701/659.7671. [DOI] [PubMed] [Google Scholar]
- 11.Lopes LA, Lins Cde M, Adeodato VG, Quental DP, de Bruin PF, Montenegro RM, Jr, et al. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28:2633–6. doi: 10.2337/diacare.28.11.2633. [DOI] [PubMed] [Google Scholar]
- 12.Telford O, Diamantidis CJ, Bosworth HB, Patel UD, Davenport CA, Oakes MM, et al. The relationship between Pittsburgh sleep quality index subscales and diabetes control. Chronic Illn. 2018:1742395318759587. doi: 10.1177/1742395318759587. doi: 10.1177/1742395318759587. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabic A, Sinanovic O, Sabic D, Galic G. Restless legs syndrome in patients with hypertension and diabetes mellitus. Med Arch. 2016;70:116–8. doi: 10.5455/medarh.2016.70.116-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi-Wen T, Nai-Hsuan K, Tao HT, Chao YJ, Lin CJ, Chang KC, et al. Impact of subjective sleep quality on glycaemic control in type 2 diabetes. Fam Pract. 2012;29:30–5. doi: 10.1093/fampra/cmr041. [DOI] [PubMed] [Google Scholar]
- 15.Bener A, Al-Hamaq AOAA, Ozturk M, Çatan F, Haris P, Rajput KU, et al. Do Ramadan fasting and physical activity have effect on HbA1, sleeping quality, blood pressure and BMI in diabetes patients? Ann African Med. 2018;17:196–202. doi: 10.4103/aam.aam_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, et al. Association of usual sleep duration with hypertension: The Sleep Heart Health Study. Sleep. 2006;29:1009–1144. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YW, Kann NH, Tung TH, Chao YJ, Lin CJ, Chang KC, et al. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Fam Pract. 2012;29:30–5. doi: 10.1093/fampra/cmr041. [DOI] [PubMed] [Google Scholar]
- 18.Yagi A, Nishio Y, Ugi S, Kawai H, Uzu T, Imai M, et al. The role of sleep disturbance and depression in patients with type 2 diabetes. Diabetology International. 2011;2:79–85.20. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2017:S48e56. [Google Scholar]
- 19.patients with type 2 diabetes. Diabetology [Google Scholar]
- 20.International 2011;2:79–85.20. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2017:S48e56. [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh sleep quality index in primary insomnia. J Psychosom Res. 2002;53:737–40. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 23.Kohnen R, Martinez-Martin P, Benes H, Trenkwalder C, Högl B, Dunkl E, et al. Validation of the kohnen restless legs syndrome – Quality of life instrument. Sleep Med. 2016;24:10–7. doi: 10.1016/j.sleep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Kohnen R, Martinez-Martin P, Benes H, Trenkwalder C, Högl B, Dunkl E, et al. Rating of daytime and night-time symptoms in RLS: Validation of the RLS-6 scale of restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2016;20:116–22. doi: 10.1016/j.sleep.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Linacre JM. Rasch measurement and unidimensionality. Rasch Meas Trans. 2011;24:1310. [Google Scholar]
- 26.Linacre JM. Rasch model estimation: Further topics. J Applied Meas. 2004;5:95–110. [PubMed] [Google Scholar]
- 27.McNeely RN, Moutari S, Arba-Mosquera S, Verma S, Moore JE. An alternative application of Rasch analysis to assess data from ophthalmic patient-reported outcome instruments. PLoS One. 2018;13:e0197503. doi: 10.1371/journal.pone.0197503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: A novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol. 2005;99:2008–15. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]


