Abstract
Background:
Once-weekly exenatide (EQW) had a neutral effect on hospitalization for heart failure (hHF) in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL), with no differential treatment effect on major adverse cardiac events (MACE) by baseline heart failure (HF) status. EQW’s effects on secondary endpoints based on hHF status have not been reported. The objective was to explore the effects of EQW on secondary endpoints in patients with and without baseline HF and test the effects of EQW on recurrent hHF events.
Methods:
The prespecified analysis of the randomized controlled EXSCEL trial, which enrolled patients with type 2 diabetes with and without additional cardiovascular disease, analyzed EQW effects on all-cause death, each MACE component, first hHF and repeat hHF by baseline HF status (regardless of ejection fraction). A subgroup analysis of the population stratified by preserved or reduced baseline ejection fraction was performed.
Results:
Of 14,752 EXSCEL participants, 2389 (16.2%) had HF at baseline. Compared with those without HF at baseline, patients with preexisting HF were older, more likely to be male and White, and with a higher burden of other cardiovascular diseases. Overall, those assigned to EQW had a lower incidence of all-cause death (HR 0.86, 95%CI 0.77–0.97) and the composite outcome of all-cause death or hHF (HR 0.89, 95%CI 0.80–0.99). When stratified by presence or absence of baseline HF, there was no observed reduction in all-cause death with EQW with baseline HF (HR 1.05, 95%CI 0.85–1.29), while the risk of mortality was reduced with EQW in the no-HF group (HR 0.79, 95%CI 0.68–0.92) with an interaction p-value of 0.031. Reduction in all-cause death or hHF seen with EQW in patients without baseline HF (HR 0.81, 95%CI 0.71–0.93) was not seen in patients with baseline HF (HR 1.07, 95%CI 0.89–1.29) (interaction p=0.015). First plus recurrent hHF was reduced in the exenatide group versus placebo (HR 0.82, 95%CI 0.68–0.99; p=0.038).
Conclusions:
In EXSCEL, the use of EQW in patients with or without HF was well tolerated, but benefits of EQW on reduction in all-cause death and first hospitalization for HF were attenuated in patients with baseline HF.
Trial Registration:
Keywords: GLP-1R agonist, exenatide, diabetes, heart failure, outcomes
Diabetes and heart failure (HF) are frequent comorbid conditions that can complicate disease management and worsen quality of life and clinical outcomes.1–2 As mandated by the US Food and Drug Administration, new medications for the treatment of type 2 diabetes (T2D) are required to demonstrate cardiovascular (CV) safety. Yet, the focus to date for the primary study endpoints in most pivotal CV outcomes trials has been on CV death, myocardial infarction, and stroke, with relegation of HF effects to secondary endpoints.3,4 Novel glucose-lowering agents such as the glucagon-like peptide-1 receptor agonists (GLP-1 RAs) albiglutide, dulaglutide, liraglutide, and semaglutide,5–8 and the sodium-glucose cotransporter-2 inhibitors canagliflozin, dapagliflozin, empagliflozin and canagliflozin,9–12 have demonstrated improved outcomes across a broad range of major adverse cardiac events (MACE) in trials that aimed for glycemic equipoise. However, no significant effect on MACE was seen with dipeptidyl peptidase-4 (DPP-4) inhibitors13–16 or with the GLP-1 RA exenatide and lixisenatide.17,18
Concerns around the possibility of HF-specific effects of incretin drugs arose following the observation of a 27% significantly increased risk of hospitalization for HF (hHF) with saxagliptin in the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI) 53 trial13 and a non-significant 19% increase with aloglipitin in the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) study.14,19 The observed risk does not appear to have a class effect based on more recent data from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) and the Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: (CARMELINA) showing a neutral impact with sitagliptin/linagliptin.15,16
Despite positive effects seen with GLP-1 RAs on blood glucose and decreases in body weight, blood pressure, and lipid levels18,20,21 benefits specifically for patients with HF have not been seen to date. On the contrary, concerns have been raised regarding the effect of GLP-1 RAs on the heart given the observed heart rate elevation, and small HF-specific studies found an either a lack of CV22 benefit or increased risk of cardiac adverse events23.
In the recent Exenatide Study of Cardiovascular Event Lowering (EXSCEL), a large pragmatic clinical trial that included patients with T2D, with and without additional CV disease,18,24,25 exenatide once-weekly (EQW) had a neutral effect on time to first hHF, without evidence of a differential treatment effect on the primary endpoint of MACE by baseline HF status. In a prespecified analysis, the aim was to further explore the effects of EQW on secondary endpoints in patients with and without baseline HF and test the effects of EQW on recurrent hHF events.
METHODS
Requests to access the data for this study from qualified researchers trained in human subject confidentiality protocols may be submitted at dcri.org/data-sharing.
Population
Between June 2010 and September 2015, EXSCEL enrolled 14,752 patients at 687 sites in 35 countries. The design, baseline characteristics, and primary results of EXSCEL (including the CONSORT flow diagram for the trial) have been published.18,24,25 In brief, the trial studied the effects of EQW at a dose of 2 mg compared to placebo. The study included adults with T2D (defined as a glycated hemoglobin [A1c] of 6.5 to 10.0%) with the goal to enroll approximately 70% of participants with prior CV events including previous coronary, cerebrovascular, or peripheral vascular events or stenosis. Key exclusion criteria were a history of type 1 diabetes, two or more episodes of severe hypoglycemia during the preceding 12 months, and an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. Race was self-reported. Presence or absence of clinical HF was captured upon enrolment into the trial. HF status at baseline was prospectively recorded by the clinician-investigator based on all available clinical data including patients’ signs/symptoms and objective measures such as echocardiography and biomarker data (e.g., natriuretic peptide levels). A blinded, independent clinical events classification committee adjudicated all the components of the primary composite outcome and secondary outcomes including hHF. The definitions for these events are outlined in the Supplementary Appendix of the primary results publication.18 All patients provided informed consent. Ethics committees at each participating site approved the protocol. The trial was conducted by the Duke Clinical Research Institute and the University of Oxford Diabetes Trials Unit in collaboration with industry sponsorship.
Statistical Analysis
The study population was stratified by baseline HF status. Baseline characteristics were summarized by counts and percentages for categorical variables and by medians with interquartile ranges for continuous variables. The treatment effects of EQW on the secondary endpoints of all-cause death, the individual components of the primary composite endpoint (CV death, myocardial infarction, and stroke), and hHF were analyzed. In a pre-specified analysis, a potentially different effect of EQW in patients with and without baseline HF was evaluated. To do so, a Cox regression analysis stratified by prior CV event with treatment, history of HF, and their interaction in the model was performed.
The relationship of EQW on hHF was analyzed as time-to-first event and recurrent hHF. Time to first hHF was analyzed using a Cox model with additional adjustment for age, sex, ethnicity, race, region, diabetes duration, diabetes therapy at baseline (any oral agent, insulin, DPP-4 inhibitor, or biguanide), prior coronary artery disease, prior cerebrovascular disease, prior peripheral arterial disease, prior myocardial infarction, prior HF, qualifying A1c, baseline eGFR (mL/min/1.73 m2), body mass index, cigarette smoking status, hypertension, hyperlipidemia/dyslipidemia, baseline systolic blood pressure, baseline diastolic blood pressure, baseline heart rate, baseline use of angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, beta-blockers, and aldosterone antagonists. For the recurrent hHF analysis, the pre-specified Andersen-Gill method within the framework of Cox proportional hazard regression, stratified by prior CV event with treatment group and baseline of HF as explanatory variables was used. Briefly, Andersen-Gill method uses the traditional Cox regression model, except after having the first event, the patient is not removed from the analysis and remains “at risk” for the subsequent events. The method estimates the common treatment effect for preventing any occurrence of the hHF.
All analyses were performed on the intention-to-treat population. Analyses were conducted with the use of SAS software, version 9.4 (SAS Institute Inc.).
RESULTS
Preexisting Heart Failure – Baseline Characteristics and Outcomes
In EXSCEL, the 2389 (16.2%) patients with known HF were randomized to EQW (N=1161, 7.9%) or placebo (N=1228, 8.3%). Compared with those without HF at baseline, patients with preexisting HF were older, more likely to be male and White, and with a higher burden of other CV diseases such as coronary artery disease and cerebrovascular disease (Table 1, Supplemental Table 1). Patients with HF had a greater body mass index and lower eGFR, and >85% of these patients had New York Heart Association (NYHA) class I or II symptoms. Use of oral glucose-lowering drugs was less common for patients with HF versus those without HF, whereas use of insulin therapy was more common. The breakdown of CV-specific drugs by presence or absence of HF is presented in Supplemental Table 2. In patients with HF, left ventricular ejection fraction (LVEF) was preserved (>55%) in 22% (516/2389), borderline (40–55%) in 24% (574/2389) or reduced in 13% (303/2389). In 42% (996/2389) of patients with HF, LVEF was not documented.
Table 1.
Baseline characteristics of patients with and without heart failure
| History of Heart Failure | |||
|---|---|---|---|
| Yes N=2389 | No N=12,362 | P-value | |
| Age at randomization (years)* | <0.001 | ||
| Median (Q1, Q3) | 64.0 (58.0, 69.0) | 62.0 (55.0, 68.0) | |
| Sex | 0.007 | ||
| Male | 1540 / 2389 (64.5%) | 7609 / 12362 (61.6%) | |
| Race | <0.001 | ||
| White | 2120 / 2389 (88.7%) | 9055 / 12357 (73.3%) | |
| Black | 83 / 2389 (3.5%) | 795 / 12357 (6.4%) | |
| Asian | 79 / 2389 (3.3%) | 1373 / 12357 (11.1%) | |
| Indian (American) or Alaska Native | 5 / 2389 (0.2%) | 68 / 12357 (0.6%) | |
| Native Hawaiian or Other Pacific Islander | 4 / 2389 (0.2%) | 31 / 12357 (0.3%) | |
| Hispanic | 98 / 2389 (4.1%) | 1035 / 12357 (8.4%) | |
| Region | <0.001 | ||
| Europe | 1666 / 2389 (69.7%) | 5122 / 12362 (41.4%) | |
| North America | 403 / 2389 (16.9%) | 3305 / 12362 (26.7%) | |
| Latin America | 243 / 2389 (10.2%) | 2483 / 12362 (20.1%) | |
| Asia Pacific | 77 / 2389 (3.2%) | 1452 / 12362 (11.7%) | |
| Prior CV event at randomization | <0.001 | ||
| Yes | 2067 / 2389 (86.5%) | 8714 / 12362 (70.5%) | |
| No | 322 / 2389 (13.5%) | 3648 / 12362 (29.5%) | |
| Coronary artery disease | 1733 / 2389 (72.5%) | 6060 / 12362 (49.0%) | <0.001 |
| Cerebrovascular disease | 500 / 2389 (20.9%) | 2009 / 12360 (16.3%) | <0.001 |
| Peripheral arterial disease | 425 / 2389 (17.8%) | 2375 / 12361 (19.2%) | 0.104 |
| Prior MI | 1258 / 2389 (52.7%) | 3420 / 12362 (27.7%) | <0.001 |
| NYHA class | |||
| I | 738 / 2387 (30.9%) | - | |
| II | 1333 / 2387 (55.8%) | - | |
| III | 303 / 2387 (12.7%) | - | |
| IV | 13 / 2387 (0.5%) | - | |
| Most recent assessment of left ventricular function (ejection fraction) | |||
| Normal (>55%) | 516 / 2389 (21.6%) | 2409 / 12362 (19.5%) | |
| Mild Dysfunction (40–55%) | 574 / 2389 (24.0%) | 924 / 12362 (7.5%) | |
| Moderate Dysfunction (25–39%) | 247 / 2389 (10.3%) | 141 / 12362 (1.1%) | |
| Severe Dysfunction (<25%) | 56 / 2389 (2.3%) | 25 / 12362 (0.2%) | |
| Unknown | 996 / 2389 (41.7%) | 8863 / 12362 (71.7%) | |
| Duration of type 2 diabetes (years) | 0.051 | ||
| Median (Q1, Q3) | 12.0 (7.0, 18.0) | 12.0 (7.0, 18.0) | |
| Antihyperglycemic agents therapy | |||
| None | 25 / 2389 (1.0%) | 203 / 12362 (1.6%) | 0.031 |
| Oral agents use | 1904 / 2389 (79.7%) | 10587 / 12362 (85.6%) | <0.001 |
| Insulin use | 1222 / 2389 (51.2%) | 5613 / 12362 (45.4%) | <0.001 |
| DPP-4 inhibitor therapy | 280 / 2389 (11.7%) | 1923 / 12362 (15.6%) | <0.001 |
| Biguanides | 1681 / 2389 (70.4%) | 9614 / 12362 (77.8%) | <0.001 |
| SGLT-2 inhibitors‡ | 4/1334 (0.3%) | 73/7205 (1.0%) | 0.011 |
| A1c (%) | <0.001 | ||
| Median (Q1, Q3) | 8.1 (7.4, 9.0) | 8.0 (7.3, 8.8) | |
| Body mass index (kg/m2) | <0.001 | ||
| Median (Q1, Q3) | 32.9 (29.3, 37.4) | 31.6 (28.1, 35.9) | |
| eGFR (mL/min/1.73 m2)** | <0.001 | ||
| Median (Q1, Q3) | 70.4 (56.6, 88.2) | 77.2 (62.4, 92.9) | |
Total cohort of 14,752. One patient’s history of heart failure status was missing.
Age = ((date of randomization - date of birth) + 1) / 365.25 rounded down to the nearest whole year.
Information regarding SGLT-2 inhibitor use and ‘other antihyperglycemic agent’ use was added to the electronic case report form on May 9, 2013.
MDRD formula was used to calculate the eGFR. Site-reported values are presented in the table.
A1c, glycated hemoglobin; CV, cardiovascular; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; NYHA, New York Heart Association; Q1, Q3, interquartile range; SGLT-2, sodium-glucose co-transporter 2.
Over a median follow-up period of 3.2 years (interquartile range 2.2–4.4), 353 (14.8%) patients died in the HF group and 738 (6.0%) in the non-HF group. HF-related hospitalizations were more commonly encountered amongst patients with a baseline of HF (N=177, 7.4%) than patients without HF (N=273, 2.2%).
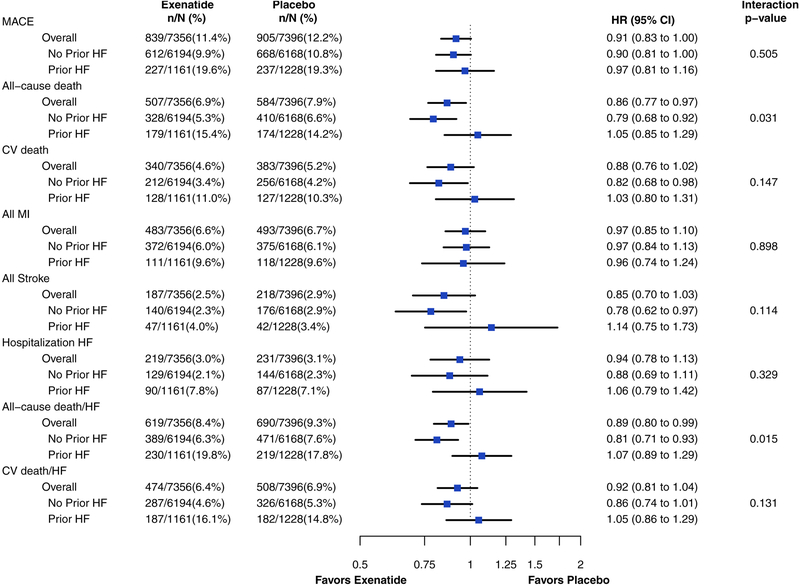
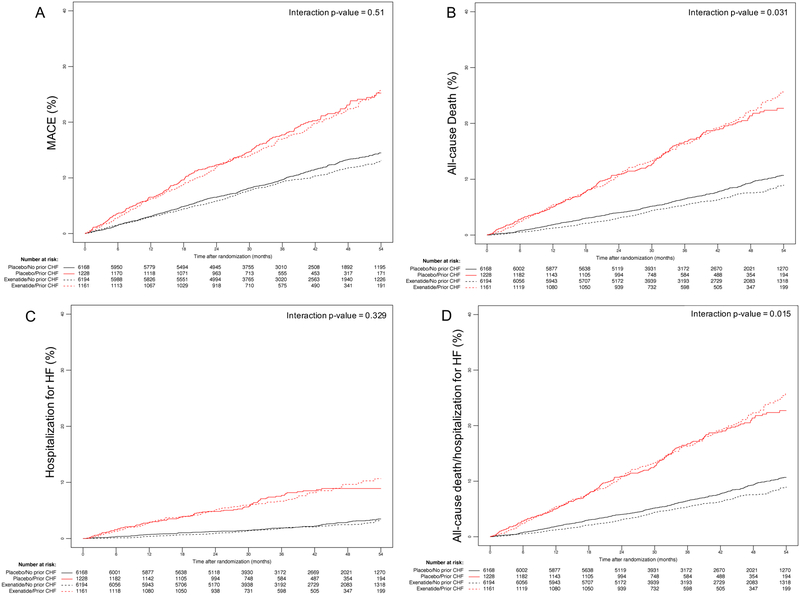
The effects of EQW on clinical outcomes, stratified by presence or absence of HF, are presented in Figure 1. Overall, those assigned to EQW had a lower incidence of all-cause death (HR 0.86, 95% CI 0.77–0.97) and the composite outcome of all-cause death or hHF (HR 0.89, 95% CI 0.80–0.99) (Figure 2, Supplemental Table 3 for breakdown by mode of death). No statistically significant change was observed in CV death (HR 0.88, 95% CI 0.76–1.02) and the composite outcome of CV death or hHF (HR 0.92, 95% CI 0.81–1.04). When stratified by presence or absence of baseline HF, there was no observed change in all-cause death with exenatide in the group with baseline HF (HR 1.05, 95% CI 0.85–1.29), while the risk of mortality was reduced with EQW in the no HF group (HR 0.79, 95% CI 0.68–0.92) with an interaction p value of 0.031. Similarly, the reduction in the composite outcome of all-cause death or hHF seen with EQW in patients without baseline HF (HR 0.81, 95% CI 0.71–0.93) was not seen in patients with baseline HF (HR 1.07, 95% CI 0.89–1.29) (interaction p=0.015). The treatment effect of EQW in CV death (HR 1.03, 95% CI 0.80–1.31) versus non-CV death (HR 1.11, 95% CI 0.74–1.64) in patients with prior HF showed no interaction (p=0.756). For the remaining clinical endpoints, there was no evidence of a differential effect of EQW on clinical endpoints in those with vs. without baseline HF (all interaction p>0.1).
Figure 1.
Forest plot for clinical outcomes stratified by treatment group and baseline of heart failure.
Abbreviations: MACE, major adverse cardiac event; CV, cardiovascular; HF, heart failure; MI, myocardial infarction.
Figure 2.
Kaplan-Meier event curves for (A) MACE, (B) all-cause death, (C) time to first HF hospitalization and (D) all-cause death and time to first HF hospitalization.
Abbreviations: MACE, major adverse cardiac event; HF, heart failure.
An LVEF-based subgroup analysis (<40% vs. ≥40%) was performed in patients with baseline HF and documented LVEF, which represents 9.4% of the total trial population (Supplemental Table 4). The incidence of primary and secondary outcomes during study follow-up appeared not different between LVEF subgroups.
Hospitalization for Heart Failure During Follow-up
In EXSCEL, patients who experienced a first hHF event during study follow-up tended to be older than patients without HF episodes, regardless of the study intervention that they received (Table 2). Further, patients with hHF were more commonly male, with a higher burden of comorbid CV disease, such as known HF (more than twice the prevalence at baseline), coronary artery disease, and cerebrovascular disease. Further, hHF was more common among patients with a longer duration of diabetes, higher body mass index, and worse renal function, again irrespective of treatment assignment (Table 2).
Table 2.
Baseline characteristics by incidence of first heart failure event requiring hospitalization and treatment
| Heart Failure Event Requiring Hospitalization | No Heart Failure Events | |||
|---|---|---|---|---|
| Exenatide N=219 | Placebo N=231 | Exenatide N=7137 | Placebo N=7165 | |
| Age at randomization (years)* | ||||
| Median (Q1, Q3) | 66.0 (9.3) | 65.4 (8.3) | 61.7 (9.4) | 61.8 (9.4) |
| Sex | ||||
| Male | 155 / 219 (70.8%) | 164 / 231 (71.0%) | 4407 / 7137 (61.7%) | 4423 / 7165 (61.7%) |
| Ethnicity | ||||
| White | 190 / 219 (86.8%) | 193 / 231 (83.5%) | 5364 / 7135 (75.2%) | 5428 / 7162 (75.8%) |
| Black | 15 / 219 (6.8%) | 20 / 231 (8.7%) | 427 / 7135 (6.0%) | 416 / 7162 (5.8%) |
| Asian | 4 / 219 (1.8%) | 6 / 231 (2.6%) | 721 / 7135 (10.1%) | 721 / 7162 (10.1%) |
| Indian (American) or Alaska Native | 0 / 219 (0.0%) | 2 / 231 (0.9%) | 38 / 7135 (0.5%) | 33 / 7162 (0.5%) |
| Native Hawaiian or Other Pacific Islander | 0 / 219 (0.0%) | 0 / 231 (0.0%) | 18 / 7135 (0.3%) | 17 / 7162 (0.2%) |
| Hispanic | 10 / 219 (4.6%) | 10 / 231 (4.3%) | 567 / 7135 (7.9%) | 547 / 7162 (7.6%) |
| Region | ||||
| Europe | 84 / 219 (38.4%) | 99 / 231 (42.9%) | 3305 / 7137 (46.3%) | 3300 / 7165 (46.1%) |
| North America | 112 / 219 (51.1%) | 111 / 231 (48.1%) | 1722 / 7137 (24.1%) | 1763 / 7165 (24.6%) |
| Latin America | 12 / 219 (5.5%) | 12 / 231 (5.2%) | 1352 / 7137 (18.9%) | 1351 / 7165 (18.9%) |
| Asia Pacific | 11 / 219 (5.0%) | 9 / 231 (3.9%) | 758 / 7137 (10.6%) | 751 / 7165 (10.5%) |
| Coronary artery disease | 172 / 219 (78.5%) | 169 / 231 (73.2%) | 3726 / 7137 (52.2%) | 3727 / 7165 (52.0%) |
| Cerebrovascular disease | 52 / 219 (23.7%) | 52 / 231 (22.5%) | 1181 / 7135 (16.6%) | 1224 / 7165 (17.1%) |
| Peripheral arterial disease | 41 / 219 (18.7%) | 61 / 231 (26.4%) | 1359 / 7136 (19.0%) | 1339 / 7165 (18.7%) |
| Prior MI | 112 / 219 (51.1%) | 110 / 231 (47.6%) | 2236 / 7137 (31.3%) | 2221 / 7165 (31.0%) |
| NYHA class | ||||
| I | 21 / 89 (23.6%) | 21 / 87 (24.1%) | 344 / 1071 (32.1%) | 352 / 1140 (30.9%) |
| II | 42 / 89 (47.2%) | 53 / 87 (60.9%) | 586 / 1071 (54.7%) | 652 / 1140 (57.2%) |
| III | 24 / 89 (27.0%) | 10 / 87 (11.5%) | 138 / 1071 (12.9%) | 131 / 1140 (11.5%) |
| IV | 2 / 89 (2.2%) | 3 / 87 (3.4%) | 3 / 1071 (0.3%) | 5 / 1140 (0.4%) |
| Most recent assessment of left ventricular function (ejection fraction) | ||||
| Normal (>55%) | 37 / 219 (16.9%) | 51 / 231 (22.1%) | 1410 / 7137 (19.8%) | 1427 / 7165 (19.9%) |
| Mild Dysfunction (40–55%) | 37 / 219 (16.9%) | 48 / 231 (20.8%) | 706 / 7137 (9.9%) | 707 / 7165 (9.9%) |
| Moderate Dysfunction (25–39%) | 34 / 219 (15.5%) | 18 / 231 (7.8%) | 176 / 7137 (2.5%) | 160 / 7165 (2.2%) |
| Severe Dysfunction (<25%) | 15 / 219 (6.8%) | 9 / 231 (3.9%) | 27 / 7137 (0.4%) | 30 / 7165 (0.4%) |
| Unknown | 96 / 219 (43.8%) | 105 / 231 (45.5%) | 4818 / 7137 (67.5%) | 4841 / 7165 (67.6%) |
| Duration of type 2 diabetes (years) | ||||
| Median (Q1, Q3) | 14.0 (8.0, 21.0) | 15.0 (9.0, 22.0) | 12.0 (7.0, 17.0) | 12.0 (7.0, 18.0) |
| Antihyperglycemic therapy | ||||
| None | 3 / 219 (1.4%) | 4 / 231 (1.7%) | 104 / 7137 (1.5%) | 117 / 7165 (1.6%) |
| Oral agents use | 148 / 219 (67.6%) | 165 / 231 (71.4%) | 6065 / 7137 (85.0%) | 6113 / 7165 (85.3%) |
| Insulin use | 140 / 219 (63.9%) | 152 / 231 (65.8%) | 3257 / 7137 (45.6%) | 3287 / 7165 (45.9%) |
| DPP-4 inhibitor therapy | 27 / 219 (12.3%) | 24 / 231 (10.4%) | 1091 / 7137 (15.3%) | 1061 / 7165 (14.8%) |
| Biguanides | 125 / 219 (57.1%) | 138 / 231 (59.7%) | 5493 / 7137 (77.0%) | 5539 / 7165 (77.3%) |
| Insulin use, alone or in combination | 140 / 219 (63.9%) | 152 / 231 (65.8%) | 3257 / 7137 (45.6%) | 3287 / 7165 (45.9%) |
| A1c (%) | ||||
| Median (Q1, Q3) | 62.8 (57.4, 73.8) | 65.0 (56.6, 73.8) | 63.9 (56.3, 73.8) | 63.9 (56.3, 73.8) |
| Body mass index (kg/m2) | ||||
| Median (Q1, Q3) | 34.6 (30.7, 39.4) | 35.2 (31.0, 40.9) | 31.8 (28.1, 36.1) | 31.6 (28.2, 36.0) |
| eGFR group (mL/min/1.73 m2)** | ||||
| eGFR ≥60 | 122 / 216 (56.5%) | 142 / 231 (61.5%) | 5647 / 7118 (79.3%) | 5603 / 7140 (78.5%) |
| eGFR <60 | 94 / 216 (43.5%) | 89 / 231 (38.5%) | 1471 / 7118 (20.7%) | 1537 / 7140 (21.5%) |
Age = ((date of randomization - date of birth) + 1) / 365.25 rounded down to the nearest whole year.
MDRD formula was used to calculate the eGFR. Site reported values are presented in the table.
A1c, glycated hemoglobin; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; NYHA, New York Heart Association.
In total, 219 patients experienced at least one hHF in the exenatide arm and 231 in the placebo arm. Time to first adjudicated hHF event was not different in both treatment arms (HR 0.95, 95% CI 0.79–1.14; p=0.55). Similar results were observed in a multivariable-adjusted Cox proportional hazards regression analysis (HR 0.92, 95% CI 0.76–1.12; p=0.42). Due to recurrent hHF among patients, the total number of first plus recurrent hHF was more than 50% higher than first hHF (exenatide, N=351 vs. placebo, N=362). However, the risk for first plus recurrent hHF was lower in the EQW group compared with placebo (HR 0.82, 95% CI 0.68–0.99; p=0.038) (Supplemental Table 5). There was no interaction when comparing the treatment effect of EQW vs placebo on first plus recurrent hHF by baseline HF status (p=0.53). Among patients who experienced a hHF, the subsequent risk for MACE was not different between patients treated with exenatide or placebo (Supplemental Table 6).
DISCUSSION
New glucose-lowering drugs such as GLP-1 RAs hold promise to change the landscape of CV disease management in T2D.7–12 Patients with HF and T2D are at the highest risk for poor CV outcomes,4,26 with only few medical therapies targeting the cardiometabolic pathology. Initial concerns of an increased hHF risk with some glucose-lowering agents used to treat T2D (including DPP-4 inhibitors) were extended to GLP-1 RAs use in patients with HF. In previously published analyses, there was no evident benefit for GLP-1 RAs with respect to hHF. In the analysis of EXSCEL, the largest GLP-1 RA trial reported to date, the use of EQW among patients with and without baseline HF was associated with no difference in clinical outcomes across most endpoints. The 14% risk reduction seen with EQW on all-cause death in the full study cohort and the 21% risk reduction seen in the subset of patients without baseline HF was not observed in patients with baseline HF. Further, when compared with placebo, EQW was associated with no difference in risk for time to first hHF event but an 18% lower risk for recurrent hHF during study follow-up.
Mounting evidence supports the CV safety and efficacy of GLP-1 RAs. In regard to HF, safety concerns were raised following the observation of an increased hHF risk with DPP-4 inhibitors. The risk of hHF was significantly increased with saxagliptin in SAVOR-TIMI 5313 and with a non-significant trend in the same direction with aloglipitin in EXAMINE.14,19 With respect to GLP-1 RAs, two small studies raised awareness of potential HF-specific safety concerns. The Effect of LIraglutide on Left VEntricular Function in Chronic Heart Failure Patients With and Without Type 2 Diabetes (LIVE) trial tested the efficacy of liraglutide in patients with chronic stable systolic HF and found no improvement in LVEF, with a statistical increase in serious cardiac events (combination of arrhythmic, coronary, death and HF events).23 Further, the Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) study examined the effects of liraglutide in patients with systolic HF and recent hHF, thus deemed to have advanced HF.22 The authors found no benefit in clinical outcomes, with a non-significant trend toward higher rate of recurrent hHF. Compared with EXSCEL, LIVE (N=241) and FIGHT (N=300) were small, with limited follow-up, restricted to patients with systolic HF and not powered for clinical outcomes. However, the observed signals from these trials paired with the repeated observation of an increased heart rate with all GLP-1 RAs have been linked to worse outcomes in patients with systolic or diastolic HF,27 and demands close evaluation of the risk for HF-related events in patients with and without HF.
Among the patients enrolled in EXSCEL, 16.2% (N=2,387) had a baseline of HF at enrollment, which is greater than the number of HF patients in any of the three other major GLP-1 RA trials (Harmony Outcomes8 [N=1922, 20%]; Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results [LEADER]6 [N=1305, 14%]; and Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes [SUSTAIN-6]7 [N=777, 23.6%]). EXSCEL patients with HF at baseline were older, had more comorbid CV conditions, and were at greater risk for adverse clinical outcomes during follow-up when compared to patients without HF. The beneficial effects of EQW on all-cause death seen in the overall cohort were not observed in the HF subgroup. Similarly, LEADER6 found a reduced effect of liraglutide on the primary outcome (first occurrence of death from CV causes, nonfatal myocardial infarction, or nonfatal stroke) in patients with baseline HF, which reached statistical significance for heterogeneity for between-group differences (liraglutide vs. placebo). The interaction analysis in SUSTAIN-6 indicated a trend toward attenuation of the semaglutide effect in patients with baseline HF.7 However, the recently presented Harmony Outcomes8 indicated no such attenuation of GLP-1 RA effect in patients with baseline HF, if at all, the risk of patients with baseline HF was trending lower with albiglutide versus placebo (HR 0.70, 95% CI 0.54–0.90 vs. HR 0.82, 95% CI 0.69–0.98; p=0.278). Despite some evidence for an attenuated effect, the lack of therapeutic effect of EQW or the class of GLP-1 RAs in patients with pre-existing HF remains speculative at this time and requires dedicated HF-specific clinical trials. The overall modest length of exposure to the study drug, particularly in EXSCEL, and modest medication adherence could have contributed to a weakened effect on patients with HF, who were at the highest risk of adverse clinical outcomes. Specifically, patients with preexisting HF had more advanced CV and non-CV comorbidities which were unlikely to be fully accounted for by statistical adjustment. Given the minimal evidence of heterogeneity, it also needs to be considered that the finding of attenuated exenatide effect on all-cause mortality in patients with HF could have been the result of chance. Finally, the exploratory analysis of LVEF subgroups restricted to a small subpopulation of the EXSCEL-HF trial did not appear to support differential outcomes in patients with HF and reduced or preserved LVEF.
Preclinical and clinical work supports the CV protective properties of GLP-1 RAs. In animal models, GLP-1 RAs were found to slow the development of HF.28 In humans, there is evidence of cardiometabolic protection given sustained reduction in HbA1c, blood pressure, weight loss,29 and improved LVEF.30 Additional cardioprotective properties of GLP-1 RAs might derive from the anti-inflammatory effects seen in small human studies.31, 32 Ischemic HF events could be more favorably affected by GLP-1 RAs, given evidence of limited infarct size in preclinical and clinical myocardial infarction studies.33–35 This hypothesis needs to be further explored in future studies, since HF etiology and serial LVEF measurements were not performed in EXSCEL.
Since HF is characterized by repeated hospitalizations, the analysis of all events is more likely to give a complete picture of treatment effect than the evaluation of the time to first event alone.36 In the whole patient cohort, the non-significant trend toward fewer HF events in the EQW group strengthened further with the increased power in the prespecified first plus recurrent hHF analyses. Here EQW was associated with a significant reduction in the burden of HF rehospitalizations.
Limitations
Despite the size of EXSCEL and the formal adjudication of clinical outcome events, the study has several limitations. First, although this analysis was prespecified, it can be subject to unmeasured bias and no adjustments were made for multiple testing. However, given that the study populations were randomly assigned to study treatment, the HF and no-HF study groups were balanced between the treatment arms upon entry into the study. Second, baseline HF status was clinically defined by the treatment team and was not formally adjudicated. Functional assessment was limited to NYHA status and assessment of LVEF was missing in about 42% of the cases, so serial evaluation was not performed. Although the present findings are hypothesis-generating, the analysis offers the largest HF-specific analysis of patients treated with a GLP-1 RA.
Conclusion
In EXSCEL, the use of EQW in patients with or without HF was well tolerated, but benefits of EQW on reduction in all-cause death and first hospitalization for HF were attenuated in patients with baseline HF. EQW did not increase the risk for hHF, but on the contrary, EQW use was associated with a lower risk of recurrent hHF.
Supplementary Material
Clinical Perspective:
What is new?
In EXSCEL, the largest GLP-1 RA trial reported to date, the use of EQW among patients with and without baseline HF was associated with no difference in clinical outcomes across most endpoints.
The 14% risk reduction seen with EQW on all-cause death in the full study cohort and the 11% risk reduction seen in the subset of patients without baseline HF was not observed in patients with baseline HF.
Compared with placebo, EQW was associated with no difference in risk for time to first HF hospitalization event but 18% lower risk for first plus recurrent HF hospitalization during study follow-up.
What are the clinical implications?
In EXSCEL, in the overall analysis the use of EQW was well tolerated with no difference in first HF hospitalization, but benefits of EQW on reduction in all-cause death and first hospitalization for HF were attenuated in patients with baseline HF.
EQW use was associated with a lower risk of first plus recurrent HF hospitalizations.
Acknowledgment:
Peter Hoffmann of the Duke Clinical Research Institute provided editorial assistance.
Source of Funding: This work was supported by Amylin Pharmaceuticals Inc. (San Diego, CA), a wholly owned subsidiary of AstraZeneca (Gaithersburg, MD).
Disclosures: MF is supported by an American Heart Association Grant, 17MCPRP33460225 and NIH T32 grant 5T32HL007101; he consults for Coridea, and Galvani. J White has no disclosures. NJP has received grants from Amarin Pharmaceutical, AstraZeneca, Novo Nordisk, Regeneron Pharmaceuticals, Sanofi, and Verily Life Sciences. YL has received grants from Merck, AstraZeneca, and GlaxoSmithKline. J Wainstein has received speaker’s bureau compensation from Novo-Nordisk, Eli Lilly, Sanofi, MSD, Novartis, BI, Astra-Zeneca, and has served as an advisory board member for Boehringer Ingelheim, Novo Nordisk, Sanofi, and MSD. JM has received speaker’s bureau compensation from Boehringer Ingelheim and Sanofi, and his institution received compensation from AstraZeneca for EXSCEL trial patient follow-up. NI is an employee of AstraZeneca. PÖ is an employee of AstraZeneca. RDL has received research grants from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, and Sanofi-Aventis; and personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, and Portola. BR is an employee of AstraZeneca. RRH reports receiving grants from AstraZeneca, Bayer AG, and Merck Sharp & Dohme, and personal fees from Amgen, Bayer AG, Boehringer Ingelheim, Novo Nordisk, and Servier. AFH reports receiving research funding from AstraZeneca, GlaxoSmithKline, Merck, and Novartis; and consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Merck, Novartis, and Pfizer. RJM reports receiving grants or consulting fees from Abbott, Akros Pharmaceuticals, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Gilead, GlaxoSmithKline, Janssen Pharmaceuticals, Luitpold Pharmaceuticals, Medtronic Vascular, Merck & Co., Novartis Pharmaceutical Co., Otsuka America Pharmaceutical, and ResMed.
Abbreviations:
- CARMELINA
Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk
- CV
cardiovascular
- DPP-4
dipeptidyl peptidase-4
- eGFR
estimated glomerular filtration rate
- EQW
Once-weekly exenatide
- EXAMINE
Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care
- EXSCEL
Exenatide Study of Cardiovascular Event Lowering
- FIGHT
Functional Impact of GLP-1 for Heart Failure Treatment
- GLP-1 RA
glucagon-like peptide-1 receptor agonists
- HF
heart failure
- hHF
hospitalization for heart failure
- LIVE
The Effect of LIraglutide on Left VEntricular Function in Chronic Heart Failure Patients With and Without Type 2 Dia
- LVEF
left ventricular ejection fraction
- MACE
major adverse cardiac events
- NYHA
New York Heart Association
- T2D
type 2 diabetes
- TECOS
Trial Evaluating Cardiovascular Outcomes with Sitagliptin
- SAVOR TIMI
Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus Thrombolysis in Myocardial Infarction 53 trial
REFERENCES
- 1.Mentz RJ, Felker GM. Noncardiac comorbidities and acute heart failure patients. Heart Fail Clin. 2013;9:359–367, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–851. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Bhatt DL, Calvo G, Brown NJ, Zannad F, Mentz RJ. Heart failure event definitions in drug trials in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. [DOI] [PubMed] [Google Scholar]
- 6.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. [DOI] [PubMed] [Google Scholar]
- 9.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 12.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 13.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 14.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 15.McGuire DK, Van de Werf F, Armstrong PW, Standl E, Koglin J, Green JB, Bethel MA, Cornel JH, Lopes RD, Halvorsen S, et al. Association Between Sitagliptin Use and Heart Failure Hospitalization and Related Outcomes in Type 2 Diabetes Mellitus: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2016;1:126–135. [DOI] [PubMed] [Google Scholar]
- 16.McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, Wanner C, Kahn SE, Toto RD, Zinman B, et al. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation. 2019;139:351–361. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, et al. ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 18.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. [DOI] [PubMed] [Google Scholar]
- 20.Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. [DOI] [PubMed] [Google Scholar]
- 21.Blonde L, Pencek R, MacConell L. Association among weight change, glycemic control, and markers of cardiovascular risk with exenatide once weekly: a pooled analysis of patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, Nilsson B, Møller JE, Hjort JL, Rasmussen J, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. [DOI] [PubMed] [Google Scholar]
- 24.Holman RR, Bethel MA, George J, Sourij H, Doran Z, Keenan J, Khurmi NS, Mentz RJ, Oulhaj A, Buse JB, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103–110. [DOI] [PubMed] [Google Scholar]
- 25.Mentz RJ, Bethel MA, Gustavson S, Thompson VP, Pagidipati NJ, Buse JB, Chan JC, Iqbal N, Maggioni AP, Marso SP, et al. Baseline characteristics of patients enrolled in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL). Am Heart J. 2017;187:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dauriz M, Mantovani A, Bonapace S, Verlato G, Zoppini G, Bonora E, Targher G. Prognostic impact of diabetes on long-term survival outcomes in patients with heart failure: a meta-analysis. Diabetes Care. 2017;40:1597–1605. [DOI] [PubMed] [Google Scholar]
- 27.Vazir A, Claggett B, Jhund P, Castagno D, Skali H, Yusuf S, Swedberg K, Granger CB, McMurray JJ, Pfeffer MA, et al. Prognostic importance of temporal changes in resting heart rate in heart failure patients: an analysis of the CHARM program. Eur Heart J. 2015;36:669–675. [DOI] [PubMed] [Google Scholar]
- 28.Vyas AK, Yang KC, Woo D, Tzekov A, Kovacs A, Jay PY, Hruz PW. Exenatide improves glucose homeostasis and prolongs survival in a murine model of dilated cardiomyopathy. PLoS One. 2011;6:e17178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–649. [DOI] [PubMed] [Google Scholar]
- 30.Munaf M, Pellicori P, Allgar V, Wong K. A meta-analysis of the therapeutic effects of glucagon-like peptide-1 agonist in heart failure. Int J Pept. 2012;2012:249827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Scholten BJ, Persson F, Rosenlund S, Eugen-Olsen J, Pielak T, Faber J, Hansen TW and Rossing P. Effects of liraglutide on cardiovascular risk biomarkers in patients with type 2 diabetes and albuminuria: A sub-analysis of a randomized, placebo-controlled, double-blind, crossover trial. Diabetes Obes Metab. 2017;19:901–905. [DOI] [PubMed] [Google Scholar]
- 32.Hogan AE, Gaoatswe G, Lynch L, Corrigan MA, Woods C, O’Connell J and O’Shea D. Glucagon-like peptide 1 analogue therapy directly modulates innate immune-mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57:781–784. [DOI] [PubMed] [Google Scholar]
- 33.Lønborg J, Kelbæk H, Vejlstrup N, Bøtker HE, Kim WY, Holmvang L, Jørgensen E, Helqvist S, Saunamäki K, Terkelsen CJ, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv. 2012;5:288–295. [DOI] [PubMed] [Google Scholar]
- 34.Bernink FJ, Timmers L, Diamant M, Scholte M, Beek AM, Kamp O, Marques KM, Denham RN, Chen WJ, Doevendans PA, et al. Effect of additional treatment with EXenatide in patients with an Acute Myocardial Infarction: the EXAMI study. Int J Cardiol. 2013;167:289–290. [DOI] [PubMed] [Google Scholar]
- 35.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. [DOI] [PubMed] [Google Scholar]
- 36.Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Östergren J, Pfeffer MA, Solomon SD, Swedberg K, Yusuf S. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. EurJ Heart Fail. 2014;16:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.