Abstract
We aimed to study whether jugular venous distension (JVD) and peripheral edema were associated with worse outcomes in patients with acute heart failure in the ASCEND-HF trial.Of 7141 patients in ASCEND-HF, 7135 had complete data on baseline JVD and peripheral edema status. Patients were grouped according to baseline examination findings: 1) no JVD or peripheral edema; 2) JVD only; 3) peripheral edema only; 4) JVD and peripheral edema. We used unadjusted and adjusted logistic or Cox regression analyses to assess associations between groups and the outcomes of index length of stay (LOS), in-hospital mortality, 30- and 180-day all-cause mortality. Patients with peripheral edema (Groups 3 and 4) had higher body mass index, NT-proBNP and BNP values, and more comorbid disease, and reduced left ventricular ejection fraction (LVEF) compared with patients in Groups 1–2. The median (25th-75th) LOS for Groups 1–4 was 6 (4–9), 5 (4–8), 7 (4–11), and 6 days (4–10), respectively. For the 30-day and 180-day outcomes, adjusted analyses found no significant difference in risk for patients presenting with JVD only or peripheral edema only as compared with patients without evidence of JVD or peripheral edema (p>0.05 for all). The presence of both JVD and peripheral edema was associated with an adjusted 24% increase in risk for all-cause mortality at 30 days, but no risk difference at 180 days. In conclusion, in patients with heart failure presenting to the hospital with dyspnea, the presence of peripheral edema is associated with a longer hospital LOS, but no difference in short and long term clinical outcomes when compared with patients wihout peripheral edema. The combination of peripheral edema and JVD identifies the highest risk cohort for poor clinical outcomes.
Keywords: Congestion, heart failure, jugular venous distention, peripheral edema
Acute and chronic heart failure (HF) states are characterized by resting or exercise induced dyspnea with presence or absence of signs of clinical congestion. The bedside examination is still critical in the assessment and monitoring of patients presenting with acute HF. Jugular venous distension (JVD) and peripheral edema have been reported to predict poor prognosis in patients with chronic HF 1, 2. However, the relative prognostic value of these variables is unclear in patients with acute HF with dyspnea. Some evidence suggests that clinical examination findings could identify specific congestion phenotypes with a predominant central vascular or peripheral congestion component 3–5. Using data from the acute HF population enrolled in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial, we aimed to stratify dyspneic patients with HF by congestion phenotypes based on the presence or absence of JVD and peripheral edema, and to compare the in-hospital and postdischarge outcomes between the groups. We also explored whether nesiritide, an arterial vasodilator, is associated with improved outcomes in any of the HF groups.
METHODS
Briefly, ASCEND-HF was a global, randomized, double-blind, placebo-controlled trial designed to examine the short and long term efficacy and safety of nesiritide, a recombinant natriuretic peptide 6, 7. A total of 7141 patients hospitalized for HF were enrolled and randomized to receive either nesiritide or placebo, in addition to standard therapy, within 24 hours of the first intravenous HF-related treatment.
Physical examinations were conducted at randomization and patients were grouped post-hoc into the following 4 categories of volume overload: (1) no JVD or peripheral edema; (2) JVD only; (3) peripheral edema only; and (4) both JVD and peripheral edema. JVD was classified by the presence or absence of JVD only. Peripheral edema was classified by the extent of the edema as no edema, ankle, shins, knees, and sacrum. Patients without information on JVD or peripheral edema at randomization were excluded from the analysis. The primary outcomes of interest were 30- and 180-day all-cause death and the combination of all-cause death or HF rehospitalization at 30 days only. Additional secondary endpoints included in-hospital mortality and length of stay (LOS) (time from randomization to discharge). Finally, we compared 2 quality of life assessments across the 4 groups. We used the EuroQOL 5 dimensions (EQ-5D) questionnaire 8. The questionnaire has 2 components—a descriptive profile and a single-index visual analogue scale (VAS). The descriptive profile includes 5 dimensions (mobility, self-care, usual activity, pain/discomfort, and anxiety/depression), and the VAS provides a global assessment of health status ranging from 0 (“worst imaginable health state”) to 100 (“best imaginable health state”).
All continuous variables are presented as medians and 25th, 75th percentiles; all categorical variables are presented as frequencies and percentages. Comparisons of baseline and discharge characteristics between HF groups were conducted using ANOVA or Kruskal-Wallis tests for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. In-hospital mortality was compared using logistic regression analyses. Further, ANOVA models were used to compare the length of stay between the 4 groups. If a significant difference was found, then multiple comparisons using Tukey methods were conducted to determine differences in mean log length of stay between patients presenting with peripheral volume overload and patients without evidence of peripheral volume overload.
Cox regression models were used to determine the relationship between the 4 congestion groups and 180-day all-cause death. Logistic regression models were used to determine the relationship between the 4 congestion groups and 30-day all-cause death and 30-day combined death and readmission endpoints. Previously published adjustment models for 30-day and 180-day endpoints in the ASCEND-HF trial were available and used for these analyses 9. Imputed datasets were used for the Cox and logistic regression analyses conducted on 30- and 180-day endpoints, in which missing values for all baseline variables included in the selection models were imputed. In addition to these imputed variables, brain natriuretic peptide (BNP) was an a priori determined covariate. In ASCEND-HF, measurements of BNP varied by site (BNP vs. NT-proBNP); therefore, patients had 1 of 2 measurements for BNP that were on different scales and unable to be converted and combined. In order to combine these BNP measures into 1 variable to be used in analysis, we standardized each of the 2 BNP measurement types and then combined the standardized values into 1 BNP variable for use in adjusted analyses. There was also missing data for the outcomes measures that could not be imputed.
Interaction between nesiritide treatment and peripheral volume overload group in relation to the combination of 30-day all-cause mortality and HF rehospitalization was assessed in an unadjusted logistic regression. The model included main effects for treatment (nesiritide vs. placebo), peripheral volume overload group, and the interaction effect between the 2 variables. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC). A two-tailed P<0.05 was considered statistically significant.
Scios Inc., now Johnson & Johnson, provided financial and material support for ASCEND-HF. The authors take responsibility for the manuscript’s integrity and had complete control and authority over its preparation and the decision to publish.
RESULTS
The ASCEND-HF cohort included 7141 patients. Six patients were excluded due to missing physical assessment at baseline. The remaining 7135 patients were categorized into the following 4 congestion groups: (1) no JVD or peripheral edema (n=1096); (2) JVD only (n=714); (3) peripheral edema only (n=2036); and (4) both JVD and peripheral edema (n=3289).
At baseline, patients with peripheral edema (with or without JVD) were more commonly white and had more comorbidities compared with those without peripheral edema (Table 1). Patients with peripheral edema were more likely to be taking guideline-directed medical therapy, including beta blockers, angiotensin converting enzyme (ACE) inhibitors, and diuretics. Patients with JVD only had the lowest left ventricular ejection fraction and patients with JVD with or without peripheral edema had worse clinical signs of intravascular congestion such as orthopnea and pulmonary rales. Additionally, patents with JVD had higher BNP values and worse renal function on admission. Patients with JVD and peripheral edema at discharge continued to have worse renal function with higher rates of beta blocker and diuretic use (Supplemental Table 1). Measures of QOL at discharge indicated a lower rating on the EQ-5D (mobility, self-care, usual activity, pain/discomfort, and anxiety/depression) for patients with JVD and peripheral edema, despite similar global QOL as measured by VAS across all 4 groups.
Table 1.
Patient characteristics at acute HF presentation by congestion group
| Characteristic | No JVP or Peripheral Edema (N=1096) |
JVP Only (N=714) |
Peripheral Edema Only (N=2036) |
Both JVP and Peripheral Edema (N=3289) |
P- Value |
|---|---|---|---|---|---|
| Age (years) | 65 (55, 76) | 64 (53, 73) | 69 (59, 77) | 66 (56, 76) | <.001 |
| Female | 391 (35.7%) | 248 (34.7%) | 714 (35.1%) | 1088 (33.1%) | 0.300 |
| White | 482 (44.0%) | 318 (44.5%) | 1467 (72.1%) | 1717 (52.2%) | |
| Black | 74 (6.8%) | 90 (12.6%) | 235 (11.5%) | 678 (20.6%) | |
| Asian | 528 (48.2%) | 267 (37.4%) | 286 (14.0%) | 685 (20.8%) | |
| Other | 12 (1.1%) | 39 (5.5%) | 48 (2.4%) | 208 (6.3%) | |
| Baseline BMI (kg/m2) | 24.5 (21.9, 28.1) | 24.7 (21.5, 29.1) | 29.6 (25.5, 35.1) | 28.2 (24.2, 33.1) | <.001 |
| LVEF (%) | 28 (21, 35) | 25 (20, 34) | 30 (22, 40) | 28 (20, 35) | <.001 |
| Orthopnea | 667 (61.0%) | 537 (75.3%) | 1470 (72.3%) | 2806 (85.4%) | <.001 |
| Rales >1/3 lung fields | 503 (45.9%) | 387 (54.2%) | 963 (47.3%) | 1880 (57.2%) | <.001 |
| NYHA classification | <.001 | ||||
| I | 46 (4.2%) | 23 (3.2%) | 66 (3.2%) | 120 (3.6%) | |
| II | 200 (18.2%) | 101 (14.1%) | 358 (17.6%) | 439 (13.3%) | |
| III | 405 (37.0%) | 269 (37.7%) | 724 (35.6%) | 1454 (44.2%) | |
| IV | 306 (27.9%) | 210 (29.4%) | 419 (20.6%) | 748 (22.7%) | |
| Baseline SBP (mm Hg) | 122 (110, 139) | 120 (110, 135) | 127 (113, 140) | 122 (110, 139) | <.001 |
| Prior MI | 384 (35.0%) | 258 (36.1%) | 712 (35.0%) | 1133 (34.4%) | 0.853 |
| Prior AF/Flutter | 313 (28.6%) | 204 (28.6%) | 875 (43.0%) | 1280 (38.9%) | <.001 |
| Hypertension by history | 689 (62.9%) | 440 (61.6%) | 1539 (75.6%) | 2478 (75.3%) | <.001 |
| Prior diabetes mellitus | 380 (34.7%) | 259 (36.3%) | 866 (42.5%) | 1538 (46.8%) | <.001 |
| Prior cerebrovascular disease | 92 (8.4%) | 82 (11.5%) | 252 (12.4%) | 415 (12.6%) | 0.002 |
| Prior PAD | 83 (7.6%) | 60 (8.4%) | 226 (11.1%) | 371 (11.3%) | <.001 |
| ACE inhibitor or ARB | 619 (56.5%) | 419 (58.8%) | 1274 (62.6%) | 2024 (61.5%) | 0.005 |
| Beta blockers | 556 (50.8%) | 393 (55.1%) | 1243 (61.1%) | 1964 (59.7%) | <.001 |
| MRAs (aldosterone antagonists) | 272 (24.8%) | 194 (27.2%) | 604 (29.7%) | 922 (28.0%) | 0.037 |
| Calcium antagonists | 116 (10.6%) | 79 (11.1%) | 296 (14.5%) | 432 (13.1%) | 0.006 |
| Loop diuretics | 562 (51.4%) | 408 (57.1%) | 1332 (65.5%) | 2235 (68.0%) | <.001 |
| Baseline creatinine (mg/dL) | 1.2 (1.0, 1.4) | 1.2 (1.0, 1.5) | 1.2 (1.0, 1.5) | 1.3 (1.0, 1.7) | <.001 |
| Baseline BUN (mg/dL) | 22 (17, 33) | 25 (18, 38) | 24 (18, 37) | 27 (19, 42) | <.001 |
| Baseline sodium (mmol/L) | 139 (136, 141) | 138 (136, 141) | 139 (136, 142) | 139 (136, 141) | <.001 |
| Baseline BNP (pg/mL) | 1029 (541, 2130) | 921 (417, 1586) | 907 (558, 1592) | 1067 (572, 1953) | <.001 |
| Baseline NT-proBNP (pg/mL) | 3694 (1690, 7781) | 4842 (2456, 8973) | 3909 (1875, 8004) | 5150 (2490, 10745) | <.001 |
| ACE inhibitor or ARB | 619 (56.5%) | 419 (58.8%) | 1274 (62.6%) | 2024 (61.5%) | 0.005 |
| Beta blockers | 556 (50.8%) | 393 (55.1%) | 1243 (61.1%) | 1964 (59.7%) | <.001 |
| MRAs (aldosterone antagonists) | 272 (24.8%) | 194 (27.2%) | 604 (29.7%) | 922 (28.0%) | 0.037 |
| Calcium antagonists | 116 (10.6%) | 79 (11.1%) | 296 (14.5%) | 432 (13.1%) | 0.006 |
| Loop diuretics | 562 (51.4%) | 408 (57.1%) | 1332 (65.5%) | 2235 (68.0%) | <.001 |
Variables expressed as median (25th, 75th) percentile or no. (%) if continuous or categorical, respectively, unless otherwise specified. P value
ACE=angiotensin converting enzyme; AF=atrial fibrillation; ARB=angiotensin receptor blocker; BMI=body mass index; BNP=brain natriuretic peptide; BUN=blood urea nitrogen; JVP=jugular venous pressure; LVEF=left ventricular ejection fraction; MI=myocardial infarction; MRA= mineralocorticoid receptor antagonist; NYHA=New York Heart Association; PAD=peripheral artery disease; SBP=systolic blood pressure.
Of the 7135 patients included in the final analysis, 151 (2%) experienced in-hospital mortality. The highest in-hospital mortality was seen in patients with both JVD and peripheral edema (n=90 [2.7%]) (Supplemental Table 2). In unadjusted analyses, odds ratios (ORs) determined that patients with both JVD and peripheral edema were significantly more likely to experience in-hospital mortality (OR 1.79, 95% confidence interval [CI] 1.06–3.01; p=0.0298), but this trend did not hold after adjustment (OR 1.17, 95% CI 0.67–2.03; p=0.59). The overall median hospital length of stay was 6 days. Patients with peripheral edema only had the longest median length of stay (7 days), while patients with JVD only had the shortest median length of stay (5 days) (p<0.001).
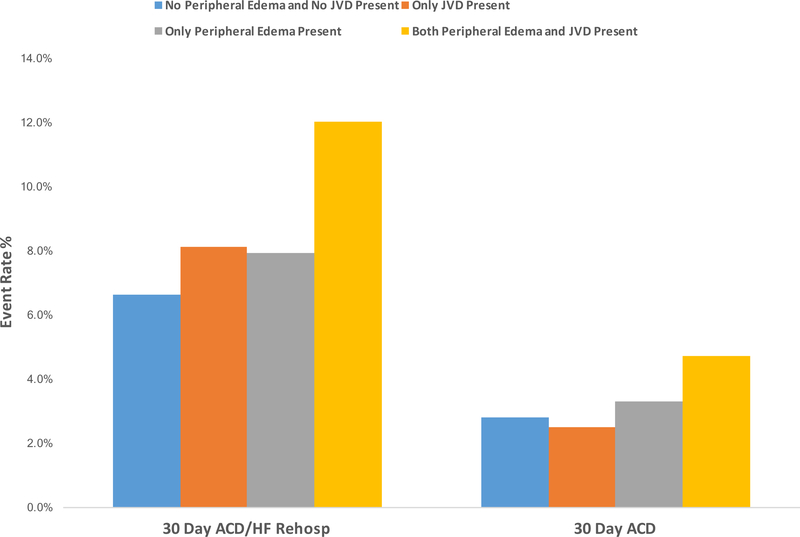
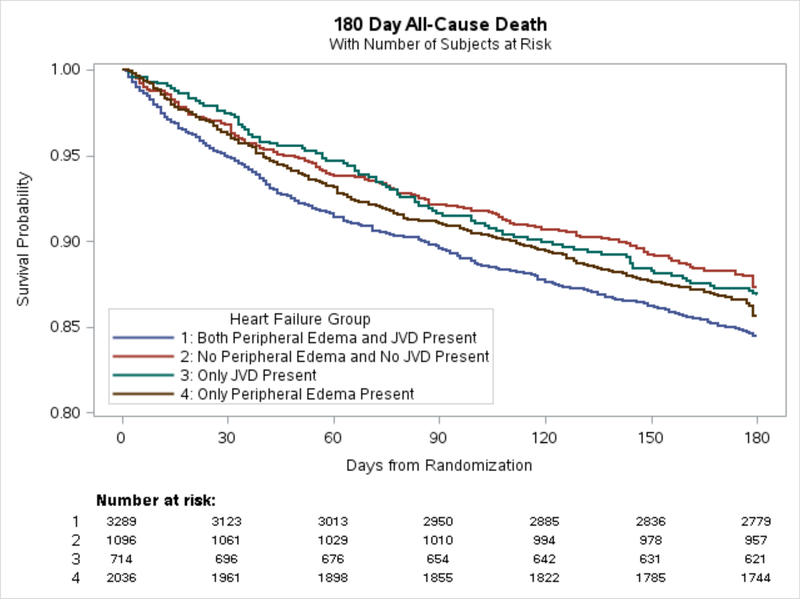
Of the 7135 patients in our analysis, 686 (10%) experienced primary the endpoint of the combination of 30-day all-cause death or HF rehospitalization; 273 (4%) experienced the endpoint of 30-day all-cause death; and 1036 (15%) experience the endpoint of 180-day all-cause death (Figure 1 and Table 2). For the primary endpoint of combined 30-day all-cause death or HF rehospitalization, adjusted analyses found no significant difference in risk for patients presenting with JVD only or peripheral edema only as compared with patients without evidence of JVD or peripheral edema (p=0.48 and p=0.14, respectively). However, adjusted logistic regression analyses found that patients presenting with both JVD and peripheral edema had a significantly increased risk of 30-day all-cause death or HF rehospitalization compared with patients without evidence of peripheral volume overload (OR 1.25, 95% CI 1.08–1.43; p=0.002) (Table 3). In adjusted analyses for the endpoint of 30-day all-cause death, only patients presenting with both JVD and peripheral edema showed significant (borderline) increased risk compared with patients without evidence of peripheral volume overload (OR 1.24, 95% CI 1.00–1.54; p=0.050). Finally, for the endpoint of 180-day all-cause death, patients with JVD and peripheral edema were at increased risk compared with patients without edema or JVD (unadjusted HR 1.26, 95% CI 1.04–1.51; p= 0.018) (Figure 2). This association with 180-day all-cause death was no longer present after adjustment (Table 3). Unadjusted analyses are presented in Supplemental Table 3.
Figure 1.
30-day event rate (%) for all-cause death and heart failure hospitalization by congestion group. ACD=all-cause death, HF=heart failure, JVD=jugular venous distension
Table 2.
Distribution of events by congestion groups
| Group | 30-Day All-cause Death/ HF Rehospitalization |
30-Day All-cause Death |
180-Day All-cause Death |
|---|---|---|---|
| No JVP or peripheral edema | 72 (6.6%) | 31 (2.8%) | 139 (12.7%) |
| JVP only | 58 (8.1%) | 18 (2.5%) | 94 (13.2%) |
| Peripheral edema only | 160 (7.9%) | 68 (3.3%) | 292 (14.3%) |
| Both JVP and peripheral edema | 396 (12.0%) | 156 (4.7%) | 511 (15.5%) |
HF = heart failure; JVP=jugular venous pressure.
Table 3.
Adjusted survival analysis for 30- and 180-day all-cause death endpoints
| 30 Day All-Cause Death* |
30-Day All-Cause Death/ HF Rehospitalization* |
180-Day All-Cause Death* | ||||
|---|---|---|---|---|---|---|
| Group Comparison |
OR (95% CI) | P-Value | OR (95% CI) | P-Value | HR (95% CI) |
P-Value |
| Group 2 vs. Group 1 | 0.87 (0.60–1.27) | 0.477 | 1.08 (0.87–1.36) | 0.482 | 0.92 (0.69–1.22) | 0.5578 |
| Group 3 vs. Group 1 | 0.94 (0.73–1.22) | 0.652 | 0.88 (0.74–1.07) | 0.139 | 1.03 (0.83–1.28) | 0.7734 |
| Group 4 vs. Group 1 | 1.24 (1.00–1.54) | 0.050 | 1.25 (1.08–1.43) | 0.002 | 1.05 (0.86–1.29) | 0.6225 |
Group 1 = No JVP or peripheral edema; Group 2 = JVP only; Group 3 = Peripheral edema only; Group 4 = Both JVP and peripheral edema.
Adjusted for age, baseline BUN (log), cerebrovascular disease, baseline creatinine (log), depression, dyspnea, prior HF hospitalization is past year, baseline sodium, baseline SBP, chronic renal disease, and BNP (standardized).
CI=confidence interval; HR=hazard ratio; OR=odds ratio
Figure 2.
180-day all-cause mortality. Only significant relationship in unadjusted analysis group 1 vs 2 [HR 1.3 (1.0–1.5), p=0.02]. JVD=jugular venous distension
There was no association between nesiritide treatment and congestion phenotype in relation to the combination of 30-day outcome (interaction p=0.42).
DISCUSSION
There were 4 major findings from our analysis of the largest clinical trial population with acute HF. First, the majority (75%) of patients hospitalzed with acute dyspnea had at least some degree of peripheral edema, while a minority (10%) presented with JVD without signs of extravascular fluid accumulation. In 15% of cases, patients were hospitalized for acute HF without signs of central or peripheral congestion. Second, while patients with peripheral edema alone have longer hospital length of stay, when compared with patients with JVD alone, the 2 groups have comparable in-hospital, 30-day and 180-day outcomes. Third, patients with signs of peripheral and central congestion have a significantly higher rate of 30-day combined all-cause death or HF rehospitalziation. Fourth, treatment with the vasodilator nesiritide was not associated with differential outcomes in any of the groups tested.
The bedside examination is critical in the assessment of and initial decision making for patients presenting with acute or chronic HF 10. In patients with acute and chronic HF, central (JVD) 11 and peripheral congestion (edema) 1, 2, 5, 12 have been reported to predict poor prognosis. While it is well accepted that the extent of fluid volume congestion is closely associated with clinical outcomes 13–15, the concept of fluid and salt overload as the primary driver of cardiac decompensation with resting or exercise-induced dyspnea has been challenged 3, 4. Notably, about 50% of patients gain only minor weight (<2 pounds or <2 kg based on the study) in the days prior to a hospitalization 16, 17. In a previous analysis of the ASCEND-HF trial, 26% of patients had no weight loss (+/−1 kg change) and 8% actually experienced weight gain (≥1 kg) during hospitalization 18. Finally, an increase in central filling pressures 19, 20 occurs in many cases in the absence of weight gain or total body volume increase 15, 21, suggesting a complimentary contribution of volume redistribution to the mechanism of cardiac decompensation 22, 23.
Patients with acute and chronic HF have been previously classified as “puffers” and “bloaters” 5, 24, 25. A typical “bloater” presents with signs of fluid retention, weight gain, peripheral edema, and renal impairment. These patients are characterized by more advanced right ventricular dysfunction and pulmonary hypertension 26. On the contrary, the classic “puffers” have exercise-induced dyspnea in the absence of objective findings of fluid retention. Their predominant mode of decompensation appears to be redistribution of fluid from the periphery and abdominal compartment to the central compartment as a result of impaired vascular compliance and increased systemic vascular resistance 3, 27. For that reason, this congestion phenotype is sometimes referred to as “vascular phenotype.” Arguably “puffers” are more likely to show signs of central vascular congestion (JVD) without peripheral edema. It has been suggested that patients with HF and preserved ejection fraction (HFpEF) might be overrepresented among “puffers” given that HFpEF is known to be especially fluid sensitive and those with HFpEF are prone to developing exercise induced symptoms with minimal fluid retention 28. Our analysis does not appear to support the notion that “puffers” are more commonly associated with preserved LVEF, given comparably low mean LVEF in patients with peripheral edema alone and JVD alone.
In our study, patients with JVD alone and peripheral edema had distinct phenotypes. Presence of peripheral edema was associated with longer hospital length of stay compared with patients with JVD alone, but the short and long term clinical outcomes did not differ between the groups. Whether the presence of JVD and peripheral edema represents differing pathophysiologies or the same congestive process on a continuum, the combined presence of central vascular and peripheral congestion identified an advanced phenotype, likely with more severe biventricular impairment, which was associated with significantly worse 30-day outcomes. Our findings are in concordance with previous analyses of chronic HF populations 1, 2. Similarly, a greater extent of congestion upon discharge has been associated with poor clinical outcomes, suggesting that congestion phenotypes are linked to outcomes irrespective of timing during a HF hospitalization 29.
Patients with a more preload- and afterload-sensitive physiology and a component of volume redistribution (“puffers”) could be more responsive to vasodilator therapy. These patients may infer less benefit from volume removal given only minor-to-no fluid overload on presentation to the hospital for dyspnea 3, 27, 28. This concept did not seem to hold up in a retrospective analysis of the RELAX-AHF trial testing the direct vasodilator serelaxin. Serelaxin had no benefit in patients with acute HF with no or only minor peripheral edema (“puffers”), but had more absolute benefit in patients with overt peripheral edema on presentation to the hospital 5. The drug nesiritide tested in ASCEND-HF, a natriuretic peptide with primary vasodilator properties, did not appear to have any association with better outcomes compared with placebo in any of the groups tested. Previous work suggested that the physiology underlying the vascular phenotype is primarily based on an increased venous vascular tone, causing decreased vascular compliance of the peripheral and abdominal vascular beds. Thus, neuromodulatory agents (renin-angiotensin blockers) and venodilators (nitrates) have been proposed as preferential drug choices to affect physiology in patients with elevated filling pressures without significant volume gain 3–5. The predominant arterio-dilatory properties of agents such as serelaxin (48 h) and nesiritide (24–168 h) or the short exposure to them could explain a lack of therapeutic effectiveness in the vascular phenotype/puffers. Whether a more nuanced classification of congestion (congestion scores) can provide more insight into different congestion phenotypes needs to be further explored. At the same time, we need to test the best approach to the safe management of fluid overload in acute and chronic HF.
We acknowledge that there are limitations to the assessment of volume overload based on clinical examination parameters such as presence of JVD, peripheral edema, orthopnea, and an S3 and positive hepatojugular 30, 31. Most notably, these limitations apply to many other commonly accepted markers of congestion measures such as increases in body weight 17, 32, natriuretic peptides 17, 33, and even invasive hemodynamic measurements 34, 35, all of which have modest sensitivity and specificity to assess a patient’s intra- and extravascular volume status. Nevertheless, physical examination findings retain a strong prognostic value for short and long term clinical outcomes.
In patients with HF presenting to the hospital with dyspnea, the presence of peripheral edema is associated with a longer hospital length to stay, but no difference in short and long term clinical outcomes when compared with patients wihout peripheral edema. The combination of peripheral edema and JVD identifies the highest risk cohort for poor clinical outcomes. Use of an arterial vasodilator, nesiritide, does not appear to be associated with improved outcomes in any of the studied groups.
Supplementary Material
Acknowledgments
Sources of funding: Scios Inc., now Johnson & Johnson, provided financial and material support for the ASCEND-HF trial.
MF: AxonTherapies, Coridea, Cibiem. GE Healthcare, Supported by AHA Grant 17MCPRP33460225 and the NHLBI T32 postdoctoral training grant 5T32HL007101–42. RJM: Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Novartis, Otsuka, ResMed, Thoratec. JAE: Abbott Labs, Amgen, Johnson&Johnson, Pfizer, Servier. AAV: Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Servier. CMO: Amgen, Astellas, GE Healthcare, Gilead, Novella, Otsuka, Roche Diagnostics, Resmed. AFH: Sanofi, Johnson & Johnson, AstraZeneca, Corthera.
Footnotes
Conflict of Interest: All other authors report no disclosures.
References
- 1.Drazner MH, Rame JE, Stevenson LW and Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. The New England journal of medicine. 2001;345:574–581. [DOI] [PubMed] [Google Scholar]
- 2.Damman K, Voors AA, Hillege HL, Navis G, Lechat P, van Veldhuisen DJ, Dargie HJ, Investigators C- and Committees. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. European journal of heart failure. 2010;12:974–982. [DOI] [PubMed] [Google Scholar]
- 3.Fudim M, Hernandez AF and Felker GM. Role of Volume Redistribution in the Congestion of Heart Failure. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fallick C, Sobotka PA and Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circulation Heart failure. 2011;4:669–675. [DOI] [PubMed] [Google Scholar]
- 5.Gimpelewicz C, Metra M, Cleland JGF, Szecsody P, Chang Wun CC, Boer-Martins L, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Pang P, Ponikowski P, Severin T, Voors AA and Teerlink JR. Effects of serelaxin on the outcome of patients with or without substantial peripheral edema: A subgroup analysis from the RELAX-AHF trial. American heart journal. 2017;190:113–122. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez AF, O’Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K and Califf RM. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF). American heart journal. 2009;157:271–217. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F and Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine. 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosy AP, Hernandez AF, Armstrong PW, Butler J, Dunning A, Ezekowitz JA, Felker GM, Greene SJ, Kaul P, McMurray JJ, Metra M, O’Connor CM, Reed SD, Schulte PJ, Starling RC, Tang WH, Voors AA and Mentz RJ. The clinical course of health status and association with outcomes in patients hospitalized for heart failure: insights from ASCEND-HF. European journal of heart failure. 2016;18:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fudim M, O’Connor CM, Dunning A, Ambrosy AP, Armstrong PW, Coles A, Ezekowitz JA, Greene SJ, Metra M, Starling RC, Voors AA, Hernandez AF, Michael Felker G and Mentz RJ. Aetiology, timing and clinical predictors of early vs. late readmission following index hospitalization for acute heart failure: insights from ASCEND-HF. European journal of heart failure. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CS, FitzGerald JM, Schulzer M, Mak E and Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA : the journal of the American Medical Association. 2005;294:1944–1956. [DOI] [PubMed] [Google Scholar]
- 11.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB and Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. Journal of the American College of Cardiology. 2009;53:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleland JG, Chiswell K, Teerlink JR, Stevens S, Fiuzat M, Givertz MM, Davison BA, Mansoor GA, Ponikowski P, Voors AA, Cotter G, Metra M, Massie BM and O’Connor CM. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: a report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ Heart Fail. 2014;7:76–87. [DOI] [PubMed] [Google Scholar]
- 13.Yoshihisa A, Abe S, Sato Y, Watanabe S, Yokokawa T, Miura S, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Saitoh SI and Takeishi Y. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. 2017:2048872617690889. [DOI] [PubMed] [Google Scholar]
- 14.Hudson SR, Chan D and Ng LL. Change in plasma volume and prognosis in acute decompensated heart failure: an observational cohort study. J R Soc Med. 2016;109:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J and Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. The American journal of cardiology. 2004;93:1254–1259. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhry SI, Wang Y, Concato J, Gill TM and Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116:1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewin J, Ledwidge M, O’Loughlin C, McNally C and McDonald K. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? European journal of heart failure. 2005;7:953–957. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, Ezekowitz JA, Felker GM, Fudim M, Greene SJ, Hernandez AF, O’Connor CM, Schulte P, Starling RC, Teerlink JR, Voors AA and Mentz RJ. Body Weight Change During and After Hospitalization for Acute Heart Failure: Patient Characteristics, Markers of Congestion, and Outcomes: Findings From the ASCEND-HF Trial. JACC Heart Fail. 2017;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr., ,Abraham WT, Smart FW, Stevenson LW, Kueffer FJ and Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–1441. [DOI] [PubMed] [Google Scholar]
- 20.Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Current heart failure reports. 2009;6:287–292. [DOI] [PubMed] [Google Scholar]
- 21.Miller WL. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circulation Heart failure. 2016;9:e002922. [DOI] [PubMed] [Google Scholar]
- 22.Fudim M, Jones WS, Boortz-Marx RL, Ganesh A, Green CL, Hernandez AF and Patel MR. Splanchnic Nerve Block for Acute Heart Failure. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fudim M, Yalamuri S, Herbert JT, Liu PR, Patel MR and Sandler A. Raising the pressure: Hemodynamic effects of splanchnic nerve stimulation. J Appl Physiol (1985). 2017;123:126–127. [DOI] [PubMed] [Google Scholar]
- 24.Clark AL and Cleland JG. Causes and treatment of oedema in patients with heart failure. Nat Rev Cardiol. 2013;10:156–170. [DOI] [PubMed] [Google Scholar]
- 25.Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M and Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. The American journal of cardiology. 2005;96:11G–17G. [DOI] [PubMed] [Google Scholar]
- 26.Damy T, Kallvikbacka-Bennett A, Goode K, Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Rande JL, Hittinger L, Clark AL and Cleland JG. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. Journal of cardiac failure. 2012;18:216–225. [DOI] [PubMed] [Google Scholar]
- 27.Cotter G, Felker GM, Adams KF, Milo-Cotter O and O’Connor CM. The pathophysiology of acute heart failure--is it all about fluid accumulation? American heart journal. 2008;155:9–18. [DOI] [PubMed] [Google Scholar]
- 28.Cotter G, Metra M, Milo-Cotter O, Dittrich HC and Gheorghiade M. Fluid overload in acute heart failure--re-distribution and other mechanisms beyond fluid accumulation. European journal of heart failure. 2008;10:165–169. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr., Grinfeld L, Udelson JE, Zannad F, Gheorghiade M and Investigators ET. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. European heart journal. 2013;34:835–843. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson LW and Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA : the journal of the American Medical Association. 1989;261:884–888. [PubMed] [Google Scholar]
- 31.Chakko S, Woska D, Martinez H, de Marchena E, Futterman L, Kessler KM and Myerberg RJ. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. The American journal of medicine. 1991;90:353–359. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RH, Rogers JG, Hasselblad V, Tasissa G, Binanay C, Califf RM, O’Connor CM, Evaluation Study of Congestive Heart F and Pulmonary Artery Catheterization Effectiveness Trial I. Association of weight change with subsequent outcomes in patients hospitalized with acute decompensated heart failure. The American journal of cardiology. 2009;103:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James KB, Troughton RW, Feldschuh J, Soltis D, Thomas D and Fouad-Tarazi F. Blood volume and brain natriuretic peptide in congestive heart failure: a pilot study. American heart journal. 2005;150:984. [DOI] [PubMed] [Google Scholar]
- 34.Shippy CR, Appel PL and Shoemaker WC. Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit Care Med. 1984;12:107–112. [DOI] [PubMed] [Google Scholar]
- 35.Oohashi S and Endoh H. Does central venous pressure or pulmonary capillary wedge pressure reflect the status of circulating blood volume in patients after extended transthoracic esophagectomy? J Anesth. 2005;19:21–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.