Abstract
Alcohol use and chronic pain are highly comorbid. Acute alcohol use typically produces an analgesic effect. However, chronic use can worsen the progression of chronic pain. In rodent models, acute models of pain have primarily been used to investigate the relationship between alcohol and pain analgesia. Here, we use two models of chronic pain, chronic inflammatory and peripheral neuropathic pain, to investigate acute alcohol's anti-nociceptive and analgesic properties. We hypothesize that acute ethanol is acting through opioid receptors to create an analgesic-like effect in both reflexive and affective dimensions of pain.
Using male and female C57BL/6J mice, oral ethanol administration (0–1.25 g/kg) showed a dose-dependent reversal of mechanical hypersensitivity in both Complete Freund's Adjuvant (CFA) and chronic constriction injury (CCI) models of chronic inflammatory and neuropathic pain. No sex differences were observed. Using the conditioned place preference (CPP) task to assess the subjective responses to ethanol's anti-nociceptive properties, CCI-injured animals showed a preference for the ethanol-paired side, suggesting a reduction in an aversive and pain-like state produced by nerve injury. These effects are likely mediated through the kappa and possibly the mu opioid systems, since ethanol-induced anti-nociception following CCI was fully reversed by pretreatment with the kappa selective antagonist, nor-BNI, or high doses of naltrexone. These data show that ethanol possesses analgesic-like properties in chronic inflammatory and neuropathic pain models in mice and provide new insight into ethanol as it relates to chronic pain.
Keywords: Alcohol pain mice chronic
1. Introduction
Alcohol use and chronic pain share strong comorbidity. Chronic pain, defined as continuous pain that persists for at least three months, affects just over 20% of the adult population in the U.S. (Dahlhamer et al., 2018). Relative to the general population, chronic pain patients have higher rates of excessive alcohol consumption and are up to two times more likely to meet criteria for an alcohol use disorder (AUD (Ditre et al., 2019);). Similarly, individuals who drink alcohol tend to report greater prevalence and intensity of pain (Boissoneault et al., 2019). While alcohol confers an analgesic effect (Thompson et al., 2017), chronic use can exacerbate the progression of chronic pain, and pain may be heightened during abstinence (Zale et al., 2015; Ditre et al., 2019). Additionally, pain can be a potent motivator for drinking as shown in human (Moskal et al., 2018) and animal studies (Yu et al., 2019), and it may contribute to escalating use and relapse (Powers et al., 2019). Furthermore, withdrawal from chronic alcohol use can produce hyperalgesia individuals with alcohol use disorder (Jochum et al., 2010) and in alcohol-dependent rats (Roltsch Hellard et al., 2017). Thus, the relationship between pain and alcohol use has been proposed to act in a positive feedback loop, worsening both conditions over time (Ditre et al., 2019).
Alcohol has analgesic properties in humans (Perrino et al., 2008; Ralevski et al., 2010) and animal models (Mogil et al., 1993; Bell et al. 1998a, 1998b; Gatch and Lal, 1999; Campbell et al., 2006), most notably, in alcohol's ability to modulate acute pain. Intravenous alcohol was antinociceptive against acute pain using noxious electrical stimulation (Perrino et al., 2008; Ralevski et al., 2010) and capsaicin-induced hyperalgesia in human subjects (Arout et al., 2016). In rodent models, alcohol's antinociceptive effects were observed in response to acute thermal stimulation after intraperitoneal (i.p.) administration in hot plate and tail-flick tests (Gatch and Lal, 1999; Campbell et al., 2006). However, support for the effects of alcohol on chronic pain conditions is derived from either observational correlational studies (Edlund et al., 2013), or prospective work (Caldeiro et al., 2008), which do not always allow causal inferences. Therefore, experimental studies examining the direct effects of alcohol on chronic pain are warranted.
Extant rodent studies have been limited in their translation value due to their experimental focus on reflexive or evoked measures of pain, or for using the i.p. route of administration for ethanol. This route shows a different blood ethanol concentration (BEC) profile than intragastric administration in rodents (Livy et al., 2003), and alcohol is most commonly consumed by drinking in humans. The pain experience also involves multi-dimensional aspects such as affective (i.e. anxiety, depression), cognitive, behavioral, and genetic factors (Gatchel et al., 2007; Elman and Borsook, 2016), some of which have been recently implicated in pain-alcohol relations (e.g., pain-related anxiety) (Zale et al., 2019). While reflexive measures have been adequate to model the sensory dimension of pain, they have fallen short in capturing these other dimensions (Yezierski and Hansson, 2018). To address this deficit, the present study characterized ethanol's analgesic-like properties and tolerance in three mouse models of acute and chronic pain, including the hot-plate test, the chronic inflammatory Complete Freund's adjuvant (CFA) model, and the chronic constrictive injury (CCI) of the sciatic nerve model of neuropathic pain. We examined the effects of ethanol in two assays that have been developed to better assess the affective dimensions of pain including voluntary wheel running (Cobos et al., 2012) and conditioned place preference (Navratilova et al., 2013). We also performed these tests in standard C57BL/6J male and female mice, to assess sex differences in response to both pain and ethanol.
2. Materials and methods
2.1. Animals
Adult male and female C57BL/6J mice (25–30 g; 8–10 weeks) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were housed in a 21 °C humidity-controlled animal care facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Mice were housed in groups of four by sex and had free access to standard rodent chow (#7012, Envigo Teklad, Madison, WI, United States) and water throughout the study. Rooms were on a 12-h light/dark cycle (lights on at 6:00 a.m.). Mice were housed with Teklad corn cob bedding (#7097, Envigo Teklad, Madison, WI, United States) and cages were changed weekly. All experiments were performed during the light cycle (between 6:00 a.m. and 6:00 p.m.) and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Observers of the behavioral tests were blind to the treatment group of all subjects. Only one trained observer was used for each behavioral assay. Mice were randomly selected to be in treatment or control groups.
2.2. Chemicals
For antinociception tests, ethanol was diluted in water and prepared as a 20% (v/v) solution and delivered by oral gavage (i.g.) for all experiments. Ethanol doses (0.5–2.0 g/kg) were chosen based on effective doses obtained in dose response curves conducted before each study, which were consistent with those found in the literature (Browman et al., 2000). Naltrindole (NTI) (with > 98% purity) was provided by Dr. Susruta Majumdar (Memorial Sloan Kettering Cancer Centre, New York, USA). Norbinaltorphimine dihydrochloride (norBNI) was a generous gift from the NIDA Drug Supply Program (Research Triangle Park, NC) and naloxone HCl was purchased from Sigma-Aldrich (St. Louis, MO). NTL, norBNI and naloxone were dissolved in physiologic saline (0.9% sodium chloride) and injected subcutaneous (s.c.) at a total volume of 1 ml/100 g body weight, unless noted otherwise. All doses of opiates antagonists are expressed as the free base of the drug.
2.3. Acute thermal pain test
The antinociceptive effect of alcohol was assessed by the hot-plate method. Male and female mice were placed into a 10-cm wide glass cylinder on a hot-plate (Thermojust Apparatus, Columbus, OH) maintained at 55 °C. The device was connected to a manually operated timer that recorded the amount of time the mouse spent on the heated surface before showing signs of nociception (e.g. jumping, paw licks). Two control latencies at least 10 min apart were determined for each mouse. Mice with baseline latencies of less than 8 s or more than 12 s were excluded from the study. To avoid tissue damage, the hot-plate would automatically disengage after 40 s. Antinociceptive response was calculated as a percentage of maximum possible effect (% MPE), where % MPE = [test value - control]/[cut-off time (40 s) - control] * 100. The reaction time was recorded when the animal jumped or licked its paws. Experiments were carried out by injecting the mice with either vehicle or alcohol (2 mg/kg, i.g.) and different group of animals were tested 15, 30, 60 and 90 min after administration. Alcohol dose-response curve (0.5, 1 and 2 g/kg, i.g.) was determined 30 min after administration in a separate cohort of mice.
2.4. Peripheral neuropathy by chronic constriction injury of the sciatic nerve
Mice were anesthetized with 4% isoflurane and maintained with 1.5–2% isoflurane in oxygen using a face mask and a vaporizer (VetEquip Inc, Pleasanton, CA). An incision was made just below the hip bone, parallel to the sciatic nerve. The left common sciatic nerve was exposed at the level proximal to the sciatic trifurcation and a nerve segment 3–5 mm long was separated from surrounding connective tissue. Two loose ligatures with 5-0 silk suture were made around the nerve with a 1.0–1.5 mm interval between each of them. Muscles were closed, and the wound was sutured. This procedure results in CCI of the ligated nerve and peripheral neuropathy/mechanical hypersensitivity continues at least 2 months (Bagdas et al., 2015). For sham surgery, the same protocol was used without ligating of sciatic nerve. All animals were randomly assigned to CCI or sham surgeries. Animals were used between 2 and 3 weeks post-surgery and tested for their mechanical thresholds. While all sham mice showed similar mechanical thresholds compared to their baseline values, CCI mice showed a robust reduction on their left paw mechanical thresholds.
2.5. Complete Freund's adjuvant (CFA)-induced inflammatory pain model
To induce chronic inflammation, mice were placed into a restraint tube and the left hind paw was injected intraplantar (i.pl.) with complete Freund's adjuvant (CFA; Sigma-Aldrich, MO, USA), using a 1710 TLL Hamilton microsyringe (Hamilton Company, NV, USA) and a 30½-gauge needle. Mice were injected in the left hindpaw with 20 μl of undiluted CFA (100% pure). Control animals received i.pl. injections of sterile mineral oil (Sigma-Aldrich, MO, USA). Animals were tested for their mechanical hypersensitivity using the von Frey test at least three days after administration of CFA or vehicle.
2.6. Thermal hypersensitivity with the Hargreaves test
Thermal withdrawal latencies were measured using the Hargreaves test as previously described (Bagdas et al., 2015; Wodarski et al., 2018). Mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus, Columbus, OH) as a measure of antinociception. The hot plate was a rectangular heated surface surrounded by Plexiglas and maintained at 55 °C. The device was connected to a manually operated timer that recorded the amount of time the mouse spent on the heated surface before showing signs of nociception (e.g., jumping, paw licks). Two control latencies at least 10 min apart were determined for each mouse. The basal latency (reaction time) of 8–12 s was assessed with a saline injection.
2.7. Mechanical hypersensitivity with von Frey testing
Mechanical withdrawal thresholds were determined using von Frey filaments as previously described (Bagdas et al., 2015). Mice were acclimated to a Plexiglas cage on mesh metal flooring for 30 min prior to testing. Withdrawal thresholds were measured by applying a series of calibrated von-Frey filaments (Stoelting, Wood Dale, IL; logarithmically incremental force from 2.83 to 5.88 expressed in dsLog 10 of [10 pound force in milligram]) to the hind paw. Using a modified up-down method (Bagdas et al., 2015), in the absence of a paw withdrawal response (paw withdrawn, licking, or shaking) to the initially selected filament, a thicker filament corresponding to a stronger stimulus was presented. Once a paw withdrawal occurred, the next weaker stimulus was chosen. Each filament was presented vertically against the paw, with sufficient force to cause slight bending, and held for 2–3 s. A stimulation of the same intensity was applied 3 times at intervals of a few seconds. Mechanical hypersensitivity values are reported as %MPE = (Test Force – Post Injury Force)/(Baseline Force – Post Injury Force).
2.8. Blood ethanol concentration (BEC) analysis
Following oral gavage of 20% v/v ethanol at 1.25 g/kg, blood was collected from the mouse facial vein into heparinized (Elkins-Sinn, Inc., Cherry Hill, NJ) tubes. The blood was then prepared as described below for ethanol extraction and quantification via gas chromatography/mass spectrometry (GC/MS). Calibrations were prepared from ethanol-naïve whole blood. Fifty μl of deuterated ethanol (1 ng/μl, Radian Corporation, Austin TX) was added to 250 μl of calibrator blood and samples were incubated overnight. The following day, 2.5 ml of cold acetonitrile (Fisher Scientific, Raleigh, NC) was added drop-wise, the mixture was vortexed, centrifuged at 4000 rpm for 10 min, and incubated at −20 °C overnight, allowing three layers to form. The acetonitrile layer was removed, 2 ml of 0.2 N NaOH was added, and the mixture was vortexed. Next, 4 ml of 9:1 hexane:ethyl acetate (Fisher Scientific, Raleigh, NC) was added and the vials were then vortexed and centrifuged at 30 rpm for 30 min. After mixing, the vials were centrifuged (4000 rpm) for 10 min. Once again, the organic layer was removed and evaporated to dryness while heated to 40 °C. Upon drying, 50 μl of derivatizing agent (Regisil plus 10% TMCS, Regis Technologies, Morton Grove, Il) was added and vortexed. The vials were heated at 40 °C for 1 h. Each sample was injected into a GC/MS (Hewlett Packard 6890, Palo Alto, CA) with a split/splitless injection port and a Hewlett Packard 7683 autosampler for quantitative analysis. The mass selective detector (MSD) was a Hewlett Packard model 5973. The initial oven temperature was 190 °C and the final temperature was 230 °C. The injection-port temperature was 230 °C and the transfer temperature was 280 °C. An HP-1 column, 12 m × 0.2 mm, 0.33 μm film thickness was used.
2.9. Ethanol tolerance following CCI
Prior to any treatments, mice were randomly divided into two groups: ethanol-treated and control. Mice in the ethanol group were given an initial dose of 1.25 g/kg ethanol before assessing their mechanical hypersensitivity. CCI surgeries were performed for all mice as described above and mice were allowed to recover and induce peripheral neuropathy for 14 days. Chronic tolerance was established by a regimen of daily gavage with 1.25 g/kg of ethanol or vehicle for 4 or 10 days in separate cohorts of mice. After the 4 or 10 days of ethanol administration, all mice were given a challenge dose of 1.25 g/kg and their mechanical hypersensitivity was reassessed. Animals in the vehicle group went through a similar paradigm as the ethanol treated mice except 4 or 10 doses of vehicle were given between initial testing and challenge testing with 1.25 g/kg of ethanol.
2.10. Opioid receptor antagonism and mechanical hypersensitivity
Antagonism studies were performed in male and female mice 14 days following CCI surgery using von Frey filaments. Naloxone and Naltrindole (NAL) were given as a s.c. injection 30 min (unless otherwise noted) before a 1.25 g/kg ethanol (20% w/w) oral gavage. Naloxone, a mu opioid receptor antagonist, was given at doses of 2 and 4 mg/kg. Naltrindole (NAL), a delta opioid-selective antagonist, was given at 10 mg/kg. Nor-BNI, a kappa-selective antagonist, was given as 8-h pretreatment at a dose of 10 mg/kg. These doses were reported to fully block the behavioral effects of delta and kappa receptor agonists, respectively (Takemori et al., 1988; Shah et al., 1994). Mechanical hypersensitivity was assessed using the von Frey filaments over a 2-h period following ethanol gavage. Results from the 30 min time point are reported and correspond to the antinociceptive time course of ethanol as determined in dose-response curves of ethanol in the CCI model (see Fig. 2).
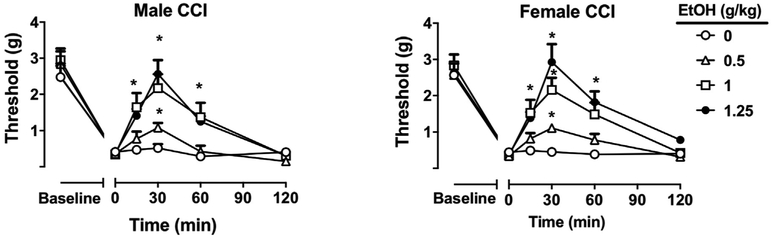
Fig. 2. Antinociceptive effects of ethanol in the CCI model.
Mechanical thresholds in male and female C57BL/6J mice before and after CCI surgery. Mechanical hypersensitivity before and after CCI surgery with time and dose after ethanol (0.5–1.25 g/kg, i.g.) and their antinociceptive effect in male and female CCI animals. *P < 0.05 vs. water at a given timepoint. Data are expressed as mean ± S.E.M. of n = 8 per group.
2.11. Ethanol-induced locomotor activity following intragastric administration
Mice were acclimated to the testing room at least 1 h prior to behavioral testing. Locomotor activity induced by acute ethanol was assessed in locomotor activity boxes (28 × 16.5 cm, Omnitech, Columbus, OH) with two banks of eight photocells. Two weeks following CCI surgery, animals were injected with either saline or ethanol 0.5, 1.25 g/kg (i.g.). Locomotor activity scores were defined as the number of interruptions of the photobeam cells measured for 120-min test period. Data were expressed as mean ± SEM of the number of photocell interruptions.
2.12. Ethanol conditioned place preference (CPP)
An unbiased eight-day CPP paradigm was performed as described (Kota et al., 2007; Harenza et al., 2014; Dawson et al., 2018). Our CPP apparatus (ENV3013; Med Associates, St Albans, VT) consists of two conditioning chambers (20 × 20 × 20 cm each) that differ in color (black and white) and floor texture (white mesh and black rod) to provide two distinct conditioning environments, separated by a small grey chamber with smooth flooring. White and black chambers were used to condition animals to 1.25 g/kg ethanol or water. On day 1 (pretest), CCI and sham animals (14 days after CCI surgery) were allowed to freely move in all chambers (two conditioning chambers with a central acclimation chamber) for a 15-min duration and the time spent for each chamber was recorded as baseline. Based on the time spent in each conditioning chamber, animals were divided into equal groups of mice: ethanol and water treated. On days 2–7, mice were confined to differing chambers after water or ethanol administration (1.25 g/kg, i.g.) for 20 min for a six-day conditioning period. Two sessions were conducted each day during conditioning; animals were confined in one chamber (e.g. white) in the morning and in other chamber (e.g. black) in the afternoon. While control groups received water in both morning and afternoon sessions, the drug group received ethanol in one session and water in other session. Pretreatment time for ethanol was 5 min. Treatments were counterbalanced equally in order to ensure that some mice received the unconditioned stimulus in the morning while others received it in the afternoon. The ethanol-paired chamber was randomized among all groups. Morning and afternoon sessions were 4 h apart from each other. All sessions were conducted by the same experimenter, who was blind to the treatment groups. On day 8, mice were given access to move freely in all chambers for a 15-min duration without any drug administration. The preference score was found by determining the difference between time spent in the drug paired side on day 8 minus the time in drug paired side on day 1. A significant positive response in time spent in the drug-paired chamber was interpreted as ethanol CPP.
2.13. Voluntary wheel running following CFA-induced inflammatory pain
Voluntary wheel running was assessed in polycarbonate wheels (diameter 21.5 cm; width 5 cm) with a steel rod axle. Mice were placed in this wheel directly from their home cage and testing was initiated immediately for baseline testing. Complete rotations were assessed by electronic counter over a 2-h period and converted to a distance traveled by the following formula: distance traveled = (rotations completed) × (wheel circumference). All mice were naïve to the running wheels prior to initial baseline testing and no habituation or training was performed in the wheels. Wheels were free rotating, unidirectional, and allowed mice to stop and start running at will. After the baseline testing, the mice were i.pl. injected in the left hindpaw with 20 μL CFA to induce peripheral inflammation, or with mineral oil as a control. The effects of i.g. ethanol on CFA-induced decreases in voluntary wheel running was assessed 3 days after CFA injection. Before being placed in the wheel, mice were given an oral gavage of 1.25 g/kg and returned to their home cages for 5 min. A noxious stimulus (e.g. CFA) is expected to reduce the distance traveled in 2-h, while an anti-nociceptive effect of ethanol is expected to reverses that decrease.
2.14. Statistical analysis
Percent maximal potential effect (%MPE) calculations for the Hargreaves test were determined with %MPE = [(Test value-Post Injury value)/(Baseline value-Post Injury value)] *100. One-way repeated measures analysis of variance (RM-ANOVA) was used to determine significance at p < 0.05 for the Hargreaves test, the von Frey mechanical sensitivity and voluntary wheel running. For tolerance or antagonism to the antinociceptive effects of ethanol and ethanol conditioned place preference, two-way ANOVA was used with treatment and time as factors. For all studies with both sexes, 2-way repeated measures analysis of variance (ANOVA) was used to assess the primary variables of sex and treatment over time. For BEC, one-way ANOVA was used to determine ethanol concentrations over time. Each analysis was followed by Holm-Sidak post-hoc tests to further analyze significant data with the alpha level set at 0.05.
3. Results
3.1. Ethanol-induced acute antinociception in the Hargreaves hot-plate test
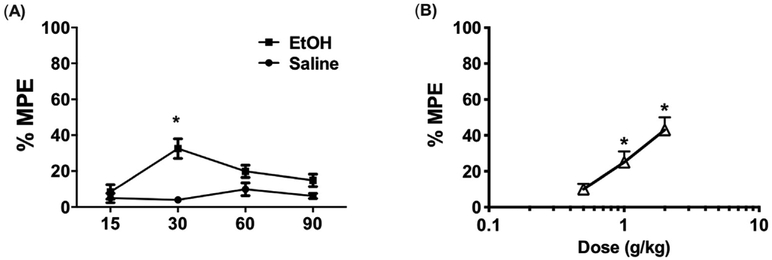
In C57BL/6J mice, treatment with 2 g/kg ethanol revealed a time-dependent antinociceptive effect in the hot-plate test (Fig. 1A; F (3,56) = 4.323 p = 0.0082). The peak antinociceptive response occurred 30 min post injection. Subsequent treatment with (1–2) g/kg ethanol in a separate cohort of mice produced a dose-dependent and significant increase in the time to respond on the hot plate assay (Fig. 1B; F(3,56) = 5.143 p = 0.0033).
Fig. 1. Antinociceptive effects of ethanol in the Hargreaves test.
(A) Time course of antinociceptive effects of 2 g/kg EtOH in the hot plate assay *P < 0.01 vs saline at a given time point. Data are expressed as mean ± S.E.M. of n = 8–10 per group. (B) Dose response curve of EtOH (0.5–2 g/kg, i.g.) in mice. *P < 0.05 vs 0.5 g/kg EtOH. EtOH = ethanol.
3.2. Ethanol-induced antinociception in the CCI model of peripheral neuropathy
To assess the effects of ethanol antinociception in peripheral neuropathy, male and female mice underwent CCI surgery and were tested for mechanical hypersensitivity using von Frey filaments. Treatment with ethanol (0.5–1.25 g/kg, i.g.) induced a dose-dependent antinociceptive effect in neuropathic CCI male (Ftreatment × dose (5,140) = 53.63 p < 0.0001) and female mice (Ftreatment × dose (5,140) = 42.67 p < 0.0001; Fig. 2). Ethanol fully restored mechanical withdrawal thresholds at the highest dose of 1.25 g/kg while lower doses had a proportionately lower antinociceptive response. Peak effects were observed at 30 min post-gavage, while a significant but reduced effect was observed at 60 min post-gavage. There were no significant differences in sex with regard to dose [F (3,42) = 0.794 p = 0.6724; Fig. S1A] or time [F (3,42) = 1.721 p = 0.0833; Suppl. Figure 1A] as assessed by comparison of %MPE reversal of mechanical hypersensitivity at 30 min after administration of alcohol (Suppl. Figure 1A).
3.3. Ethanol induced antinociception in CFA-induced model of inflammation
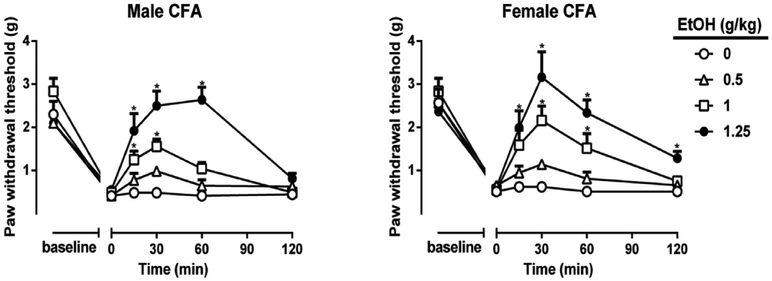
Using the CFA model for inflammatory pain, treatment with ethanol (0.5–1.25 g/kg, i.g.) induced a dose-dependent antinociceptive effect in CFA-treated male (Ftreatment × dose(5,140) = 45.88 p < 0.0001) and female mice (Ftreatment × dose (5,140) = 57.90 p < 0.0001; Fig. 3). Ethanol fully reversed mechanical hypersensitivity in the CFA-treated mice at a dose of 1.25 g/kg, while lower doses had proportionately lower antinociceptive responses. Peak effects were observed at 30 min post gavage while a significant but reduced effect was observed at both 15 and 60 min post gavage. There were no significant sex differences with regard to dose or time course as determined by % reversal of mechanical hypersensitivity [F (3,42) = 0.961 p = 0.5273; Fig. S1B (dose)] [F (3,42) = 1.134 p = 0.1722; Fig. S1B (time)]. Thus, male and female data were collapsed in further analyses.
Fig. 3. Antinociceptive effects of ethanol in the CFA model.
Mechanical thresholds in male and female C57BL/6J mice before and after CFA injection. Mechanical hypersensitivity with time and dose after ethanol (0.5–1.25 g/kg, i.g.) and their antinociceptive effect in male and female CFA animals. *P < 0.05 vs. water at a given timepoint. Data are expressed as mean ± S.E.M. of n = 8 per group.
3.4. Comparison of time course of antinociceptive response with BEC
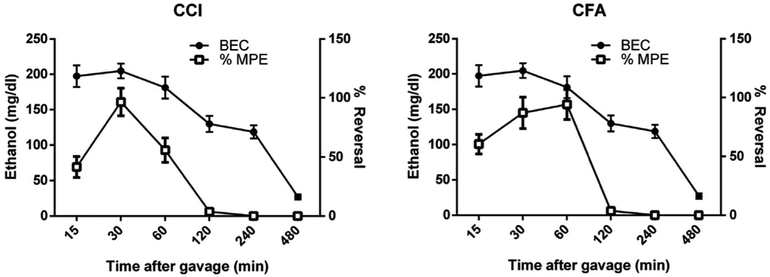
In both the CCI model for neuropathic pain (Fig. 4A) and the CFA model of inflammatory pain (Fig. 4B), an acute gavage of 1.25 g/kg ethanol, the maximally effective antinociceptive observed dose, rapidly increased blood ethanol concentration (BEC) followed by a slow reduction across 4 h. The maximum antinociceptive effect, based on previously observed mechanical hypersensitivity, occurred at 30 min and dissipated by 120 min after oral ethanol gavage.
Fig. 4. Pharmacological and BEC time course relationship of the antinociceptive effects of ethanol in mice.
Subjects were male and female C57BL/6J mice. Left axis shows the BEC over time. Right axis shows the percent reversal of mechanical hypersensitivity. Combined to show a time course of %MPE and blood ethanol content of C57BL/6J mice dosed with EtOH (1.25 g/kg, i.g) % MPE = Test Value-Baseline)/(Pre CCI/CFA Values).(A) Time-course relationship in CCI mice. (B) Time-course relationship in CFA mice. Data are expressed as mean ± S.E.M. of n = 8 for time points 60, 120, 240 and 480 min and n = 12 for time points 15 and 30.
3.5. Antinociceptive tolerance induced by repeated ethanol exposure
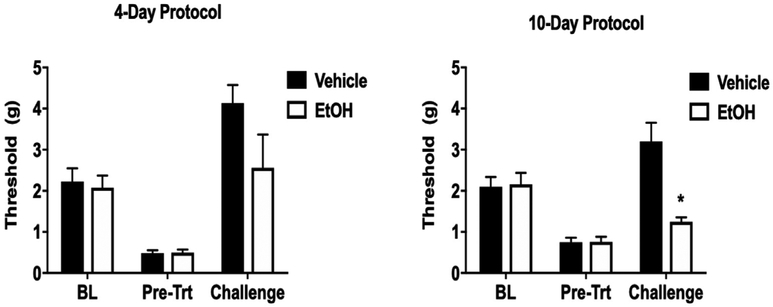
To assess potential tolerance to the antinociceptive effects of ethanol, mice were first dosed with 1.25 g/kg ethanol (i.g.) and tested for baseline (BL) mechanical hypersensitivity prior to CCI surgery. Two weeks following CCI surgeries, mice were tested again for mechanical hypersensitivity using the von Frey filaments (pre-treatment, Pre-Trt). Mice were then treated with 1.25 g/kg ethanol or water daily by gavage for four or ten days and tested a third time for mechanical hypersensitivity with an acute ethanol challenge following induction of potential ethanol tolerance. Repeated treatment with 1.25 g/kg ethanol for four days did not produce a significant level of tolerance in the von Frey test as determined by a change in the final assessed mechanical threshold compared with the baseline threshold (F (2,26) = 2.846 p = 0.1155; Fig. 5A). However, repeated daily treatment with 1.25 g/kg ethanol for ten days produced a significant level of tolerance as assessed by mechanical allodynia following a final challenge dose of 1.25 g/kg (F (2,26) = 13.65 p < 0.0001; Fig. 5B). Tolerance was indicated at the 30-min time point after administration of alcohol on the challenge day. A significantly reduced mechanical threshold in the 10-day ethanol mice was observed, while mice repeatedly gavaged with water instead of ethanol still show a high mechanical threshold after an ethanol challenge, indicative of antinociception.
Fig. 5. Tolerance to the antinociceptive effects of ethanol following repeated exposure.
(A) Mechanical hypersensitivity in C57BL/6J mice following 4 days of gavage using vehicle or 1.25 g/kg ethanol. BL indicates pre-surgical mechanical thresholds while Pre-Trt indicates the threshold following CCI surgery to induce neuropathy. Challenge indicates the mechanical threshold 30 min after a treatment with 1.25 g/kg ethanol by oral gavage on challenge day following repeated gavage. n = 8–10 per group. (B) Mechanical hypersensitivity in C57BL/6J mice following 10 days of gavage using vehicle or 1.25 g/kg ethanol. Data are expressed as mean ± S.E.M. of n = 8–10 per group. *p < 0.05 vs. vehicle.
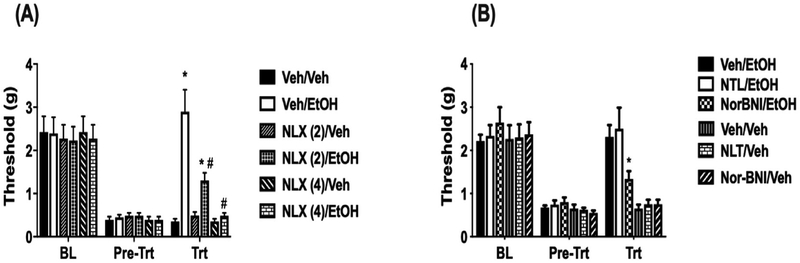
3.6. Antagonism of ethanol induced antinociception in neuropathic mice
Due to previous evidence showing the role of the mu opioid system in the antinociceptive effect of ethanol in models of acute pain (Bell et al. 1998a, 1998b; Campbell et al., 2007), we used a pharmacological approach to investigate the role of opioid receptors. Following CCI, the opiate antagonist, naloxone, at a dose of 2 mg/kg was capable of partially blocking the antinociceptive effect of ethanol. A higher dose of 4 mg/kg of naloxone was capable of fully blocking the antinociceptive effect of ethanol (F (2,26) = 12.44 p = 0.0087; Fig. 6A). Since naloxone is also partially active at the kappa and delta opioid receptors (Gouarderes et al., 1985), the complete blockade at the 4 mg/kg dose of naloxone may be due to additional effects at the delta and kappa receptors. Thus, we used the selective antagonists delta and kappa receptors respectively, nor-BNI and naltrindole (NAL), to assess their involvement in ethanol-induced antinociception. Pretreatment with the delta-selective antagonist, NAL (10 mg kg, i.p.), did not alter ethanol-induced antinociception (F (2, 26 = 0.8442 p = 0.5312; Fig. 6B). Pretreatment with the kappa selective antagonist, nor-BNI (10 mg/kg, s.c.), however fully blocked the antinociceptive effects of ethanol in neuropathic mice following CCI surgery (F(2,26) = 8.32 p = 0.032; Fig. 6B). Together, these data suggest that both the kappa and mu opioid receptors are necessary for the antinociceptive effects of ethanol in chronic pain.
Fig. 6. Antagonism of ethanol-induced antinociception in CCI mice assessed using mechanical sensitivity.
(A) BL indicates the threshold for animals following CCI surgery to induce neuropathy. Pre-Trt indicates the threshold for animals after CCI that have received naloxone at 2 or 4 mg/kg. Trt indicates withdrawal threshold 30 min after EtOH-induced mechanical sensitivity in CCI animals pretreated with naloxone. *P < 0.05 vs. veh + veh; #P < 0.05 vs. Veh + EtOH. n = 8/group. (B) Withdrawal threshold for male C57BL/6J mice after CCI surgery. BL indicates the baseline threshold for animals after CCI. Pre-Trt indicates threshold for animals after naltrindole (NTL) or nor-BNI. Trt indicates mechanical withdrawal threshold 30 min after EtOH administration in CCI animals pretreated with the antagonists. *P < 0.05 vs. Veh/EtOH. Data are expressed as mean ± S.E.M. of n = 8/group. Veh = Vehicle; EtOH = ethanol.
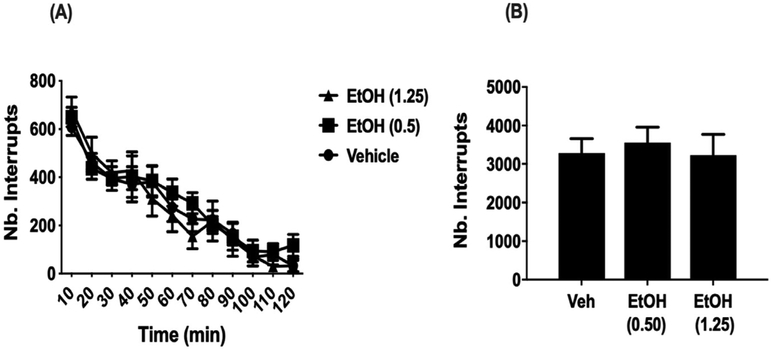
3.7. Impact of ethanol on locomotion
To determine whether analgesic-like effects of ethanol in our assays of pain could be affected by potential motor impairment, we assessed general locomotor activity following low (0.5 g/kg, i.g.) and high dose (1.25 g/kg, i.g.) of ethanol in male C57BL/6J mice. No significant differences in locomotion were found between animals treated with saline and those treated with either 0.5 or 1.25 g/kg ethanol (F (2,21) = 0.1533 p = 0.8588; Fig. 7). This lack of effect on locomotion suggests the doses of ethanol used to attain behaviorally effective results in our assays of pain were not confounded by motor impairment of animals.
Fig. 7. Impact of ethanol on locomotion following CCI.
(A) General activity assessed by beam breaks over a 120-min period in mice after a 5 min pre-treatment with water, 0.5 or 1.25 g/kg ethanol. (B) Total number of beams breaks over the entire 120 min test period. Male and female C57BL/6J mice were collapsed. No significant differences are observed between any treatment given. Data are expressed as mean ± S.E.M. of n = 6 per group.
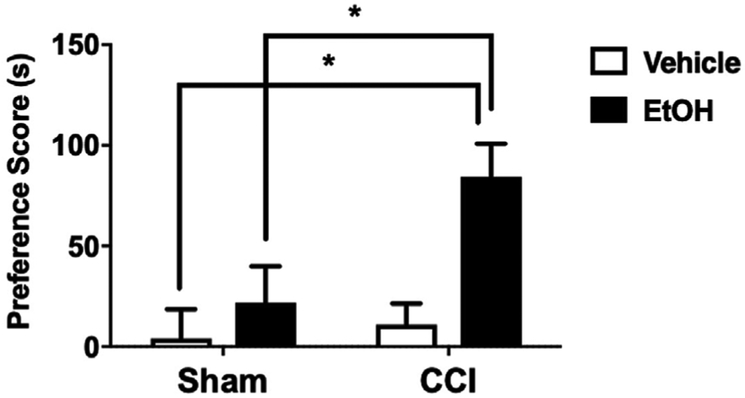
3.8. Ethanol place preference and antinociception in CCI models of neuropathy
We used the CPP test in our model of peripheral neuropathy to evaluate the ability of ethanol to induce preference in CCI injured mice, which could be interpreted as pain-induced motivation to seek alcohol (e.g., for pain relief) in mice experiencing ongoing, spontaneous pain (Navratilova et al., 2016). In CCI-injured mice, administration of ethanol (1.25 g/kg, i.g.) induced a significant preference for the ethanol-paired side of the CPP chamber (FCCI × EtOH (1, 36) = 8.956; P = 0.005; Fig. 8). Treatment with the vehicle did not show a significant preference in mice. This testing was performed while CCI injured mice still showed mechanical hypersensitivity suggesting that ethanol is antinociceptive in the CCI model of neuropathic pain. In sham mice, ethanol at the same dose (1.25 g/kg, i.g.) did not induce a significant preference compared to vehicle-treated mice.
Fig. 8. Antinociceptive effects of ethanol in CCI animals assessed by CPP.
EtOH induced preference for the ethanol-paired side in CCI but not sham mice. C57BL/6J male mice conditioned with 1.25 g/kg or vehicle daily for 6 days as either sham or CCI animals. No preference was established in sham animals at doses that are antinociceptive. CCI animals conditioned with EtOH display preference for the EtOH paired chamber. Preference score is calculated as the (time spent drug side) - (time spent on veh side). *P < 0.05 vs. sham. Data are expressed as mean ± S.E.M. of n = 12 per treatment group.
3.9. Effects of ethanol on CFA-induced decreases in voluntary wheel running
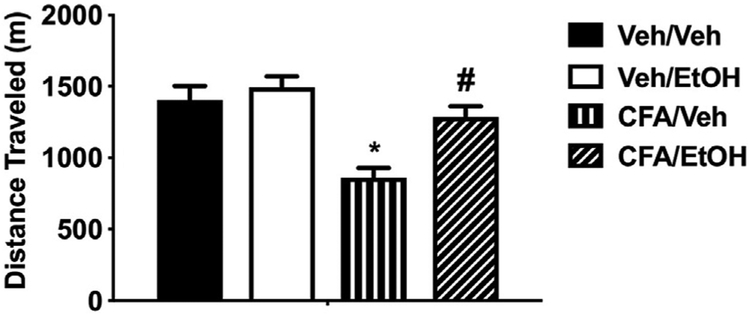
Intraplantar injection with CFA significantly reduced the distance traveled in a voluntary wheel running task as compared to their ethanol-naïve vehicle counterparts (F(1,56) = 13.65 p < 0.00001, Fig. 9). The CFA-induced decrease was significantly reversed when animals were treated with ethanol at 1.25 g/kg following CFA injection (F (1,56) = 13.65 p < 0.00001). In vehicle-treated mice, ethanol at the same dose did not significantly alter wheel running.
Fig. 9. Ethanol-induced antinociception assessed by voluntary wheel running.
Three days after CFA or vehicle injection, distance traveled was reduced in CFA male mice treated with vehicle compared to non-inflamed animals, *p < 0.05. No significant difference in distance traveled was seen between non-inflamed animals treated with ethanol and non-inflamed animals treated with vehicle. CFA-inflamed animals treated with ethanol ran significantly further than inflamed animals treated with vehicle, #p < 0.05. Distance traveled by CFA-inflamed animals treated with ethanol was not significantly different from non-inflamed animals treated with ethanol. Data are expressed as mean ± S.E.M. of n = 12 per group.
4. Discussion
The overall goal of our study was to determine the antinociceptive effects of ethanol on peripheral neuropathic pain and inflammatory pain models after intragastric administration in mice. In uninjured mice, oral ethanol administration induced a dose-dependent antinociceptive effect in the Hargreaves hot-plate test. We then employed two models of chronic pain based on CCI-induced neuropathy and CFA-induced inflammation in C57BL/6J mice. Oral ethanol administration was antinociceptive in a time- and dose-dependent manner. At a dose of 1.25 g/kg ethanol fully reversed mechanical hypersensitivity in both models of chronic pain after oral ethanol administration. Tolerance to the antinociceptive effects of ethanol developed after 10 days of chronic oral administration. Antagonists of mu and kappa opioids receptors blocked ethanol-induced antinociception in the CCI model. Finally, we found that ethanol was also effective in two non-reflexive assays of pain: conditioned place preference test and voluntary wheel running in C57BL/6J mice.
Prior to our studies, there have been a few reports describing the antinociceptive effect of ethanol in rodent models of acute thermal pain (Mogil et al., 1993; Bell et al. 1998a, 1998b). These studies found that doses of 1–2.5 g/kg ethanol i.p. were capable of producing significant antinociception in the tail-flick and hot-plate tests using adult male rats and mice (Mogil et al., 1993; Gatch and Lal, 1999; Campbell et al., 2006). However, higher doses of 2–3 g/kg ethanol i.p. have been shown to impair rotarod performance in male C57BL/6J mice (Stromberg, 1988). Given the high comorbidity of excessive alcohol use in chronic pain patients (Ditre et al., 2019), it is important to complement these prior results in acute pain assays by studying the effect of ethanol in chronic pain models. We found that ethanol was antinociceptive in assays of inflammatory and neuropathic pain as assessed by reflexive von Frey filament testing. Ethanol was maximally effective in reversing mechanical hypersensitivity at a dose of 1.25 g/kg without producing any sedative effects. This suggests that ethanol is more efficacious in models of chronic pain than acute pain when comparing their %MPE in hot plate (40% MPE) and mechanical threshold testing. Though there have been previous reports of sex differences in the effects of ethanol on mice, such as increased anxiety behaviors following chronic ethanol intake in males only (Jury et al., 2017), and more rapid increases in intake in female mice compared with males (Sneddon et al., 2019), no major sex differences were found across our studies in C57BL/6J mice.
Additionally, peak BEC was observed at 30 min post administration, which corresponds to peak antinociception also at 30 min post-gavage in CCI-injured mice and CFA-injected mice. BEC levels in mice given i.g. at 1.25 g/kg of ethanol reached a maximum of 215 mg/dl. According to CDC reports of safe drinking, this is equivalent to 10 drinks over a 2-h timespan, or 3-times the safe legal drinking limit in most states ((CDC) 2016). However, the antinociceptive effect drops off rapidly over 2 h while the blood ethanol levels remain relatively stable up to 4 h. This suggests that animals may be rapidly acclimating to the ethanol and are showing reduced behavioral responses despite a relatively constant BEC. This phenomenon has been previously described as acute functional tolerance and has been observed in various other behavioral measures of ethanol's sedative and hypnotic effects (Ponomarev and Crabbe, 2004; Radcliffe et al., 2013).
Tolerance to ethanol's antinociceptive effect in our CCI model can also develop after chronic exposure to the drug. In animals treated with a 10-day regimen of intragastric ethanol, the mechanical sensitivity thresholds were dramatically reduced as compared to their initial assessment before their 10-days of tolerance induction. This effect appeared to be time dependent since a shorter 4-day treatment regimen demonstrated a slight but non-significant reduction in the effect of ethanol, similar to other studies showing 4 days of ethanol exposure is insufficient to produce tolerance to the antinociceptive effects in rats (Gatch, 2006). This data shows that, similar to other behavioral effects, the antinociceptive effects of ethanol can be reduced after repeated or chronic exposure to ethanol (Bell et al. 1998a, 1998b; Werner et al., 2009).
Consistent with previous reports (Campbell et al., 2007), we also demonstrated that both the mu and kappa opioid receptors play a central role in ethanol's antinociceptive effects in chronic pain. Indeed, norBNI blockade of alcohol's effects suggest the involvement of kappa opioid receptors. Despite its high κ-selectivity in vitro, nor-BNI produces transient μ antagonism in vivo. To the best of our knowledge, the μ-antagonistic action of norBNI (5 and 20 mg/kg, s.c.) declined to the control level at 2 or 4 h after administration in mice (Endoh et al., 1992). Nor-BNI was given as 8-h pretreatment at a dose of 10 mg/kg in our studies. We therefore believe that μ-antagonistic action of norBNI are probably not involved in ethanol's effects in our studies. In addition, our results show that delta opioid receptors did not play a significant role, since the delta-selective antagonist naltrindole was unable to alter the antinociceptive effect of ethanol. Other non-opiate receptors such as the NMDA receptor and GABA receptors are likely contributing to this effect potentially through anxiolytic mechanisms (Mogil et al., 1993; Bell et al. 1998a, 1998b) and will be investigated in future studies.
Our studies also showed that ethanol has analgesic-like effects in two affective assays of chronic pain. Voluntary wheel running can be used to model motivation and CFA-treated animals have been shown to decrease their voluntary wheel running (Cobos et al., 2012). In the current study, ethanol reversed CFA-induced reduction in wheel running, while not significantly altering voluntary wheel running in non-inflamed animals. Additionally, ethanol administration in CCI-injured mice induced a significant place preference for the ethanol-paired treatment chamber, but not their sham counterparts in the CPP test. This suggests that the CCI injury is inducing a neuropathic state and this negative state can be reversed with ethanol to create a preference in the CPP assay. Demonstrated preference for the ethanol-paired chamber among CCI-injured mice is also consistent with human experimental evidence that pain can be a potent motivator of addictive substance seeking in general (Ditre et al., 2019), and the urge to drink alcohol in particular (Moskal et al., 2018). Indeed, CPP outcomes are often viewed as indicative of classically-conditioned reward seeking and incentive motivational effects (Huston et al., 2013; Lees et al., 2015). Collectively, these results provide a new insight into ethanol as it relates to chronic pain while confirming and expanding on knowledge gained from previous reports of the antinociceptive effect of ethanol in acute pain models.
Given our results that ethanol possesses analgesic-like properties in chronic inflammatory and neuropathic pain models in mice, it is important to consider how this can translate to human studies of chronic pain and alcohol. In a survey of population level behavior, 25–27% of chronic pain patients “at least sometimes” used alcohol as a pain-relieving strategy (Riley and King, 2009). Greater pain-related physical impairment has been associated with a 23–45% increased likelihood of meeting the criteria for alcohol use disorder (AUD) or nicotine use disorder (NUD (McDermott et al., 2018);). Similarly, when rates of pain are examined as a function of substance misuse, the same patterns are observed. Whereas 18% of the general population reports current moderate-severe pain, that rate increases to 43% among problem drinkers and up to 75% among those with AUD (Brennan et al., 2005; Larson et al., 2007; Brennan and Soohoo, 2013). Relative to the general population, individuals with chronic pain endorse higher rates of excessive alcohol consumption and are up to two times more likely to meet criteria for AUD (Zale et al., 2015; Ditre et al., 2019). Our results add to the growing direct evidence that ethanol possesses analgesic-like properties in chronic pain states, with one important implication being that the analgesic properties of alcohol could contribute to increased drinking and the development/maintenance of AUD among individuals with persistent pain (Zale et al., 2015; Ditre et al., 2019). Furthermore, the accessibility and relative low cost of alcohol is likely to encourage its use as an analgesic in comparison to more difficult-to-obtain substances (e.g., illegal drugs) or interventions. Alcohol-induced analgesia and the development of tolerance to analgesic-like properties following repeated use could help to explain observed rates of alcohol misuse among those with persistent pain despite its substantial threat to long-term health, including risk for developing or exacerbating chronically painful conditions (Kim et al., 2013).
Supplementary Material
HIGHLIGHTS.
Alcohol induces antinociceptive effects in acute thermal pain models.
Alcohol induces analgesic-like properties in mouse chronic pain models.
Tolerance develops to the antinociceptive effects of alcohol.
Opiate receptors mediate the antinociceptive effects of alcohol.
Acknowledgments
NIH P30 DA033934 and R01AA027175 to MID and MFM and NIH P50 AA022537 to MFM. The authors have no conflicts of interest to declare.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://doi.org/10.1016/j.neuropharm.2019.107793.
References
- Arout CA, Perrino AC Jr., Ralevski E, Acampora G, Koretski J, Limoncelli D, Newcomb J, Petrakis IL, 2016. Effect of intravenous ethanol on capsaicin-induced hyperalgesia in human subjects. Alcohol Clin. Exp. Res 40 (7), 1425–1429. http://10.1111/acer.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI, 2015. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem. Pharmacol 97 (4), 590–600. https://10.1016/j.bcp.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Olson RD, Vaccarino AL, 1998a. Tolerance to ethanol analgesia is not accompanied by cross-tolerance to morphine analgesia in rats. Pharmacol. Biochem. Behav 59 (1), 123–127. https://10.1016/s0091-3057(97)00380-8. [DOI] [PubMed] [Google Scholar]
- Bell RL, Soignier RD, Olson RD, Vaccarino AL, 1998b. Reduction of stress-induced analgesia following ethanol exposure in mice. Life Sci. 63 (9), 731–736. https://10.1016/s0024-3205(98)00328-2. [DOI] [PubMed] [Google Scholar]
- Boissoneault J, Lewis B, Nixon SJ, 2019. Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol 75, 47–54. https://10.1016/j.alcohol.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Brennan PL, Soohoo S, 2013. Pain and use of alcohol in later life: prospective evidence from the health and retirement study. J. Aging Health 25 (4), 656–677. https://10.1177/0898264313484058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, Moos RH, 2005. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction 100 (6), 777–786. https://10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Browman KE, Rustay NR, Nikolaidis N, Crawshaw L, Crabbe JC, 2000. Sensitivity and tolerance to ethanol in mouse lines selected for ethanol-induced hypothermia. Pharmacol. Biochem. Behav 67 (4), 821–829. https://10.1016/s0091-3057(00)00427-5. [DOI] [PubMed] [Google Scholar]
- Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, Saxon AJ, 2008. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction 103 (12), 1996–2005. https://10.1111/j.1360-0443.2008.02358.x. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y, 2006. Antinociceptive effects of alcohol and nicotine: involvement of the opioid system. Brain Res. 1097 (1), 71–77. https://10.1016/j.brainres.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y, 2007. Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception. Alcohol Clin. Exp. Res 31 (8), 1435–1440. https://10.1111/j.1530-0277.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- CDC, C. f. D. C. a. P, 2016. Fact Sheets- Moderate Drinking – Alcohol.
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ, 2012. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153 (4), 876–884. http://10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C, 2018. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb. Mortal. Wkly. Rep 67 (36), 1001–1006. https://10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Wolstenholme JT, Roni MA, Campbell VC, Jackson A, Slater C, Bagdas D, Perez EE, Bettinger JC, De Biasi M, Miles MF, Damaj MI, 2018. Knockout of alpha 5 nicotinic acetylcholine receptors subunit alters ethanol-mediated behavioral effects and reward in mice. Neuropharmacology 138, 341–348. http://10.1016/j.neuropharm.2018.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, LaRowe LR, 2019. A reciprocal model of pain and substance use: transdiagnostic considerations, clinical implications, and future directions. Annu. Rev. Clin. Psychol 15, 503–528. https://10.1146/annurev-clinpsy-050718-095440. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Sullivan MD, Han X, Booth BM, 2013. Days with pain and substance use disorders: is there an association? Clin. J. Pain 29 (8), 689–695. https://10.1097/AJP.0b013e318270fa77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2016. Common brain mechanisms of chronic pain and addiction. Neuron 89 (1), 11–36. https://10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H, 1992. Mar-Apr Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther 316, 30–42. [PubMed] [Google Scholar]
- Gatch MB, 2006. Tolerance to the antinociceptive effects of ethanol during ethanol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 30 (5), 946–952. http://10.1016/j.pnpbp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Lal H, 1999. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin. Exp. Res 23 (2), 328–333. [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC, 2007. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol. Bull 133 (4), 581–624. https://10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Gouarderes C, Cros J, Quirion R, 1985. Autoradiographic localization of mu, delta and kappa opioid receptor binding sites in rat and Guinea pig spinal cord. Neuropeptides 6 (4), 331–342. [DOI] [PubMed] [Google Scholar]
- Harenza JL, Muldoon PP, De Biasi M, Damaj MI, Miles MF, 2014. Genetic variation within the Chrna7 gene modulates nicotine reward-like phenotypes in mice. Genes Brain Behav. 13 (2), 213–225. https://10.1111/gbb.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JP, Silva MA, Topic B, Muller CP, 2013. What's conditioned in conditioned place preference? Trends Pharmacol. Sci 34 (3), 162–166. https://10.1016/j.tips.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Burkhardt C, Juckel G, Bar KJ, 2010. Increased pain sensitivity in alcohol withdrawal syndrome. Eur. J. Pain 14 (7), 713–718. https://10.1016/j.ejpain.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A, 2017. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol 58, 53–60. https://10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Vincent A, Clauw DJ, Luedtke CA, Thompson JM, Schneekloth TD, Oh TH, 2013. Association between alcohol consumption and symptom severity and quality of life in patients with fibromyalgia. Arthritis Res. Ther 15 (2), R42 http://10.1186/ar4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI, 2007. Nicotine dependence and reward differ between adolescent and adult male mice. J. Pharmacol. Exp. Ther 322 (1), 399–407. https://10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH, 2007. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction 102 (5), 752–760. https://10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- Lees A, Sikk K, Taba P, 2015. The story of "speed" from "cloud nine" to brain gain. Int. Rev. Neurobiol 120, 1–7. https://10.1016/bs.irn.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Parnell SE, West JR, 2003. Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol 29 (3), 165–171. [DOI] [PubMed] [Google Scholar]
- McDermott KA, Joyner KJ, Hakes JK, Okey SA, Cougle JR, 2018. Pain interference and alcohol, nicotine, and cannabis use disorder in a national sample of substance users. Drug Alcohol Depend. 186, 53–59. https://10.1016/j.drugalcdep.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Marek P, Yirmiya R, Balian H, Sadowski B, Taylor AN, Liebeskind JC, 1993. Antagonism of the non-opioid component of ethanol-induced analgesia by the NMDA receptor antagonist MK-801. Brain Res. 602 (1), 126–130. [DOI] [PubMed] [Google Scholar]
- Moskal D, Maisto SA, De Vita M, Ditre JW, 2018. Effects of experimental pain induction on alcohol urge, intention to consume alcohol, and alcohol demand. Exp. Clin. Psychopharmacol 26 (1), 65–76. https://10.1037/pha0000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, King T, Porreca F, 2013. Evaluation of reward from pain relief. Ann. N. Y. Acad. Sci 1282, 1–11. https://10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Morimura K, Xie JY, Atcherley CW, Ossipov MH, Porreca F, 2016. Positive emotions and brain reward circuits in chronic pain. J. Comp. Neurol 524 (8), 1646–1652. https://10.1002/cne.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrino AC Jr., Ralevski E, Acampora G, Edgecombe J, Limoncelli D, Petrakis IL, 2008. Ethanol and pain sensitivity: effects in healthy subjects using an acute pain paradigm. Alcohol Clin. Exp. Res 32 (6), 952–958. https://10.1111/j.1530-0277.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC, 2004. Characterization of acute functional tolerance to the hypnotic effects of ethanol in mice. Alcohol Clin. Exp. Res 28 (7), 991–997. [DOI] [PubMed] [Google Scholar]
- Powers JM, Zvolensky MJ, Ditre JW, 2019. An integrative review of personalized feedback interventions for pain and alcohol. Curr Opin Psychol 30, 48–53. http://10.1016/j.copsyc.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe RA, Larson C, Bennett B, 2013. Genetic studies of acute tolerance, rapid tolerance, and drinking in the dark in the LXS recombinant inbred strains. Alcohol Clin. Exp. Res 37 (12), 2019–2028. https://10.1111/acer.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Perrino A, Acampora G, Koretski J, Limoncelli D, Petrakis I, 2010. Analgesic effects of ethanol are influenced by family history of alcoholism and neuroticism. Alcohol Clin. Exp. Res 34 (8), 1433–1441. https://10.1111/j.1530-0277.2010.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL 3rd, King C, 2009. Self-report of alcohol use for pain in a multi-ethnic community sample. J. Pain 10 (9), 944–952. https://10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roltsch Hellard EA, Impastato RA, Gilpin NW, 2017. Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict. Biol 22 (3), 692–701. https://10.1111/adb.12360. [DOI] [PubMed] [Google Scholar]
- Shah S, Davis T, Yoburn BC, 1994. The effect of naltrindole on spinal and supraspinal delta opioid receptors and analgesia. Life Sci. 55 (19), 1451–1458. https://10.1016/0024-3205(94)00685-7. [DOI] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK, 2019. Sex differences in binge-like and aversion-resistant alcohol drinking in C57BL/6J mice. Alcohol Clin. Exp. Res 43 (2), 243–249. https://10.1111/acer.13923. [DOI] [PubMed] [Google Scholar]
- Stromberg C, 1988. Interactions of antidepressants and ethanol on spontaneous locomotor activity and rotarod performance in NMRI and C57BL/6 mice. J. Psychopharmacol 2 (2), 61–66. https://10.1177/026988118800200201. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Ho BY, Naeseth JS, Portoghese PS, 1988. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J. Pharmacol. Exp. Ther 246 (1), 255–258. [PubMed] [Google Scholar]
- Thompson T, Oram C, Correll CU, Tsermentseli S, Stubbs B, 2017. Analgesic effects of alcohol: a systematic review and meta-analysis of controlled experimental studies in healthy participants. J. Pain 18 (5), 499–510. https://10.1016/j.jpain.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Werner DF, Swihart AR, Ferguson C, Lariviere WR, Harrison NL, Homanics GE, 2009. Alcohol-induced tolerance and physical dependence in mice with ethanol insensitive alphal GABA A receptors. Alcohol Clin. Exp. Res 33 (2), 289–299. http://10.1111/j.1530-0277.2008.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarski R, Bagdas D, Paris JJ, Pheby T, Toma W, Xu R, Damaj MI, Knapp PE, Rice ASC, Hauser KF, 2018. Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type-1 Tat-expressing female mice: involvement of Tat during early stages of HIV-associated painful sensory neuropathy. Pain Rep 3 (3), e654 https://10.1097/PR9.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezierski RP, Hansson P, 2018. Inflammatory and neuropathic pain from bench to bedside: what went wrong? J. Pain 19 (6), 571–588. https://10.1016/j.jpain.2017.12.261. [DOI] [PubMed] [Google Scholar]
- Yu W, Hwa LS, Makhijani VH, Besheer J, Kash TL, 2019. Chronic inflammatory pain drives alcohol drinking in a sex-dependent manner for C57BL/6J mice. Alcohol 77, 135–145. https://10.1016/j.alcohol.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, Ditre JW, 2015. Interrelations between pain and alcohol: an integrative review. Clin. Psychol. Rev 37, 57–71. https://10.1016/j.cpr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, LaRowe LR, Boissoneault J, Maisto SA, Ditre JW, 2019. Gender differences in associations between pain-related anxiety and alcohol use among adults with chronic pain. Am. J. Drug Alcohol Abuse 45 (5), 479–487. https://10.1080/00952990.2019.1578968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.