Abstract
Background
Reduced nutrient intake is common in patients after hospitalization, contributing to increased risk for readmission and mortality. Oral nutrition supplements can improve nutrition status and clinical outcomes, but intake of food is prioritized by clinicians. This study examines the impact of a high‐protein oral nutrition supplement (S‐ONS) on nutrient intake post discharge.
Methods
In a subset of patients (14 S‐ONS and 16 placebo) from the NOURISH (Nutrition effect On Unplanned ReadmIssions and Survival in Hospitalized patients) trial, 24‐hour dietary recalls were conducted on 3 randomly selected days during the weeks of 30, 60, and 90 days post discharge. Nutrient intake was estimated using Nutrition Data System for Research software. Adequate energy and protein intake were defined as 30 kcal/kg/d and 1.2 g/kg/d, respectively. Dietary Reference Intakes (DRIs) were used for other nutrients.
Results
Less than half of patients met the requirements for energy, protein, and 12 micronutrients from food intake alone during the study. Energy and protein intakes from food were not diminished relative to placebo. Considering nutrient intake from both food and S‐ONS, 50% and 71% of patients receiving S‐ONSs met energy and protein goals respectively at 90 days (compared with 29% and 36%, in the placebo group), and 100% met the DRI for total carbohydrate, iron, phosphorus, copper, selenium, thiamin, and riboflavin at all time points, all of which were consumed at higher amounts vs placebo.
Conclusion
Three months of S‐ONS consumption increases intake of numerous nutrients without decreasing nutrient intake from food in older malnourished adults post discharge.
Keywords: malnutrition, minerals/trace element intake, nutrient intake, oral nutrition supplement, vitamin intake
Clinical Relevancy Statement
This article provides evidence supporting consumption of oral nutrition supplements (ONSs) in hospitalized older adults in postdischarge settings to improve overall nutrient intake. Routine clinical practice often prioritizes nutrient intake in the form of food over ONSs; however, this belief may need to be reassessed. In fact, ONS is an effective intervention to increase intake of numerous macronutrients and micronutrients without disrupting normal food consumption.
Background
Malnutrition is a prevalent but underdiagnosed risk factor associated with poor health outcomes including increased risk for infection, decreased cardiopulmonary and immune function, delayed wound healing, and lower activities of daily living.1, 2, 3 Furthermore, malnourished individuals experience increased hospital length of stay, readmission, and long‐term medical costs, which are associated with decreased quality of life and increased mortality,2, 4, 5, 6, 7 particularly those with cardiopulmonary conditions.7
One‐third to one‐half of patients are assessed as malnourished at hospital admission.3, 5, 8, 9, 10 Unfortunately, patients may experience further nutrition decline during their hospitalization.11, 12 This exacerbation is ill addressed. The Canadian Malnutrition Task Force reported that at least one‐third of patients discharged to home are malnourished.13 Maeda et al3 reported that nearly two‐thirds of Japanese patients transferred to post–acute care are malnourished. To further compound these issues, multiple factors influence reduced food intake and increased nutrient needs after discharge. These include underlying illness, anorexia, difficulty preparing and consuming food independently, and oral problems.14, 15
Oral nutrition supplements (ONSs) are developed to help patients overcome these factors to meet nutrition needs, thus improving overall nutrition status, quality of life, and clinical outcomes. Consumption of ONSs, particularly those with high protein content, improves clinical and economic outcomes in older patients who are frail, at nutrition risk, and/or have multiple morbidities.16, 17, 18 In a review of 36 randomized controlled trials including 2790 older patients,18 the use of high‐protein ONSs (>20% energy from protein) reduces complications and readmissions with no impact on normal food intake. Despite all of this, dietary planning for the postdischarge period is often deficient,19, 20 and <10% of patients receive a prescription for ONS at discharge.10
Although the importance of meeting energy and protein goals is a primary clinical focus, intake of micronutrients also supports patient recovery.18, 21, 22 There is a dearth of data on dietary micronutrient consumption in older malnourished patients. The small amount of available data suggests that older adult patients have low vitamin D intake,23 and that those with chronic obstructive pulmonary disease (COPD) in particular have deficient intake of calcium, potassium, and vitamins A, D, B1, and B9 as compared with the recommended daily allowance (RDA).24
To comprehensively assess the effect of ONS on overall nutrient intake, this analysis examines supplementation of a high‐protein specialized oral nutrition supplement (S‐ONS) on macronutrient and micronutrient intake for 90 days after hospital discharge. Furthermore, it provides suggested sample sizes to facilitate design of future studies aiming to assess nutrient intake and provide targeted nutrition intervention in older adults.
Methods
Study Design
The data for this analysis were derived from 30 patients (14 S‐ONS and 16 placebo) from the NOURISH (Nutrition effect On Unplanned ReadmIssions and Survival in Hospitalized patients) study cohort.25 The NOURISH study is a prospective, randomized, double‐blind study that was designed to examine the effect of the S‐ONS in 652 malnourished (subjective global assessment B or C) older adults (≥65 years of age) hospitalized with chronic heart failure, acute myocardial infarction, pneumonia, or COPD (clinicaltrials.gov NCT01626742). Patients with diabetes, liver or kidney failure, dementia, or receiving active cancer treatment were excluded. Detailed inclusion and exclusion criteria and primary study findings were reported previously.25
Interventions
Patients who were randomized to the S‐ONS group received standard of care and a specialized, nutrient‐dense, high‐protein, ready‐to‐drink supplement (Abbott Nutrition, Columbus, OH, USA), 2 servings per day during hospitalization and up to 90 days after discharge. Each serving (237 mL) of the S‐ONS provided 350 kcal, 20 g of protein, 11 g of fat, 44 g of carbohydrate, 1.5 g of calcium β‐hydroxy β‐methylbutyrate, and 26 essential vitamins and minerals. Patients in the placebo group received standard of care and a ready‐to‐drink liquid (Abbott Nutrition) that contained 48 kcal, 12 g of carbohydrate, and 10 mg of vitamin C, but no other macronutrients or micronutrients. Notably, patients were instructed to consume the supplements between meals, before bed, or each time with oral medications to minimize impact on regular food intake. As previously reported, approximately one‐third of patients in both groups reported product consumption of >75% over the 90‐day follow‐up period.25
Dietary Data Analysis
After hospital discharge, 24‐hour dietary recalls were conducted by phone on 3 randomly selected days (2 weekdays and 1 weekend day) during the weeks of days 30, 60, and 90 post discharge by the Pennsylvania State University Diet Assessment Center. The recalls were conducted on unannounced, random, nonconsecutive days, using standardized multiple‐pass methodology and portion size estimation tools. Nutrient intake was estimated using Nutrition Data System for Research Version 2012 (University of Minnesota, Minneapolis, MN, USA). Because many patients found that this procedure was challenging, which impacted patient retention and study compliance, dietary recall was terminated after completion of the 30 patients, whose data were included in this analysis.
Nutrient adequacy was determined as follows: the adequacy of caloric intake was defined as 30 kcal/kg body weight/d,26 and protein adequacy was defined as 1.2 g/kg body weight/d27 given the fact that the population was malnourished, with chronic diseases and acute illness. The Dietary Reference Intakes (DRIs) were used to assess adequacies of other nutrients.28
Statistical Analyses
Nutrient intakes from the diet (food alone and food + ONS), as well as percentage of DRIs met from the diet, were analyzed using Wilcoxon rank sum test to compare the placebo and S‐ONS groups. The proportion of subjects who met DRIs by nutrient was compared between groups using Fisher exact test. Within each group, the relationship between study product consumed vs the number of DRIs met was modeled using repeated‐measures analysis of variance. P‐values ≤ .05 were considered statistically significant. No adjustment in the significance level for multiplicity of testing was made for these exploratory outcomes. SAS version 9.4 was used in the analyses.
To facilitate the design of future studies aiming to assess individual nutrient intake in this diseased population, we conducted a series of power analyses focusing on individual nutrients using nQuery Advisor 7.0 with power set at 80% and statistical significance at .05 using 2‐sided t‐tests; the effect sizes appear in Table 3.
Table 3.
Number of Patients Required to Detect Differences in Standardized Effect Sizes of Percentage of Dietary Reference Intake Met at Day 90 From Food Intake Alone in This Population
| Nutrient | Common SD | Effect Size | n Per Group |
|---|---|---|---|
| Dietary fiber (g) | 19.7 | 0.49 | 68 |
| Added sugars (% kcal) | 3.98 | 0.47 | 73 |
| Total fat (% kcal) | 8.14 | 0.69 | 35 |
| Linoleic acid (g) | 50.9 | 0.34 | 137 |
| Linolenic acid (g) | 43.6 | 0.56 | 52 |
| Calcium (mg) | 30.5 | 0.78 | 27 |
| Potassium (mg) | 14.1 | 0.52 | 60 |
| Vitamin E (mg) | 31.5 | 0.82 | 25 |
| Vitamin D (mcg) | 20.9 | 0.71 | 32 |
| Vitamin C (mg) | 53.9 | 0.46 | 74 |
| Thiamin (mg) | 40.7 | 0.66 | 37 |
| Riboflavin (mg) | 64.4 | 0.88 | 22 |
| Niacin (mg) | 35.4 | 0.73 | 31 |
| Vitamin B6 (mg) | 36.1 | 0.60 | 45 |
| Vitamin K (mcg) | 162 | 0.14 | 802 |
| Folate (mcg) | 53.5 | 0.83 | 24 |
Results
Baseline Characteristics
Baseline demographic and clinical characteristics of the NOURISH population and this subset of patients are presented in Table 1. For the 30 patients included in the dietary analysis, age, weight, body mass index, primary diagnosis, gender, race, marital status, number of household members, and average household income were all comparable between S‐ONS and placebo groups, except for nutrition risk assessment, as measured by subjective global assessment, which tended to be worse (C status) in the S‐ONS group (P = .051).
Table 1.
Baseline Patient Characteristics
| Characteristics | Placebo (n = 16) | S‐ONS (n = 14) | Total | P‐Valuea | NOURISH Group (n = 622) |
|---|---|---|---|---|---|
| Age, y, mean (SEM) | 77.69 (2.49) | 76.64 (1.91) | 77.20 (1.58) | NSD | 77.93 (0.34) |
| Weight, kg, mean (SEM) | 61.70 (3.07) | 60.84 (3.84) | 61.30 (2.39) | NSD | 66.88 (0.67) |
| Body mass index, mean (SEM) | 22.34 (1.05) | 21.85 (1.14) | 22.11 (0.76) | NSD | 24.07 (0.20) |
| Sex, n (%) | NSD | ||||
| Male | 7 (43.8) | 7 (50.0) | 14 (46.7) | 298 (47.9) | |
| Female | 9 (56.3) | 7 (50.0) | 16 (53.3) | 324 (52.1) | |
| Race, n (%) | NSD | ||||
| Black/African American | 2 (12.5) | 1 (7.1) | 3 (10.0) | 67 (10.8) | |
| White/Caucasian | 14 (87.5) | 13 (92.9) | 27 (90.0) | 541 (87.0) | |
| Subjective global assessment, n (%) | .051 | ||||
| B | 16 (100) | 11 (78.6) | 27 (90) | 543 (87.3) | |
| C | 0 (0) | 3 (21.4) | 3 (10) | 79 (12.7) | |
| Primary diagnosis, n (%) | NSD | ||||
| Heart failure | 2 (12.5) | 1 (7.1) | 3 (10.0) | 157 (25.3) | |
| Acute myocardial infarction | 3 (18.8) | 1 (7.1) | 4 (13.3) | 55 (8.9) | |
| Pneumonia | 7 (43.8) | 7 (50.0) | 14 (46.7) | 195 (31.4) | |
| Chronic obstructive pulmonary disease | 4 (25.0) | 5 (35.7) | 9 (30.0) | 214 (34.5) | |
| Marital status | NSD | ||||
| Single | 1 (6.3) | 0 (0.0) | 1 (3.3) | 71 (11.4) | |
| Married/Common law | 6 (37.5) | 6 (42.9) | 12 (40.0) | 234 (37.6) | |
| Divorced/Separated | 2 (12.5) | 1 (7.1) | 3 (10.0) | 80 (12.8) | |
| Widowed | 7 (43.8) | 7 (50.0) | 14 (46.7) | 237 (38.1) | |
| No. of household members, mean (SD) | 2.27 (0.46) | 1.86 (0.42) | 2.07 (0.31) | NSD | 1.95 (0.04) |
| Average household income, n (%) | NSD | ||||
| <$10,000 | 3 (18.8) | 1 (7.1) | 4 (13.3) | 90 (14.5) | |
| $10,000–$24,999 | 3 (18.8) | 3 (21.4) | 6 (20.0) | 194 (31.2) | |
| $25,000–$49,999 | 2 (12.5) | 3 (21.4) | 5 (16.7) | 120 (19.3) | |
| $50,000–$75,000 | 1 (6.3) | 1 (7.1) | 2 (6.7) | 75 (12.1) | |
| $75,000–$99,999 | 0 | 0 | 28 (4.5) | ||
| >$100,000 | 0 (0.0) | 1 (7.1) | 1 (3.3) | 19 (3.1) | |
| No response | 7 (43.8) | 5 (35.7) | 12 (40.0) | 95 (15.3) |
NOURISH, nutrition effect on unplanned readmission and survival in hospitalized patients; NSD, no significant difference; SEM, standard error of the mean; S‐ONS, specialized oral nutrition supplement.
P‐values reflect difference between placebo and S‐ONS groups; significance was set at P < .05.
Patient Compliance
In the subset of patients, the median product consumption was 1.46 servings/d (first quartile = 0.62, third quartile = 1.90). The number of supplements consumed was not significantly different between placebo and S‐ONS groups, or across time points within group (all P > .05).
Nutrient Intake From Food Alone
As expected, most patients did not meet energy or protein requirements from food alone at any time point (Figure 1A and 1B). Macronutrient intake from food alone was similar between the 2 groups except that, at 30 days only, the proportion of total calories from fat tended to be higher in the placebo group (P = .052; Figure 1C). As a percentage of DRI, micronutrient intake from food alone was not different between placebo and S‐ONS groups for most nutrients (Table 2, Figure 1). However, at 90 days, dietary intake of the micronutrients iron, magnesium, phosphorus, zinc, copper, manganese, selenium, vitamin A, vitamin B12, and choline were higher in the S‐ONS treatment group compared with placebo controls (all P < .05).
Figure 1.
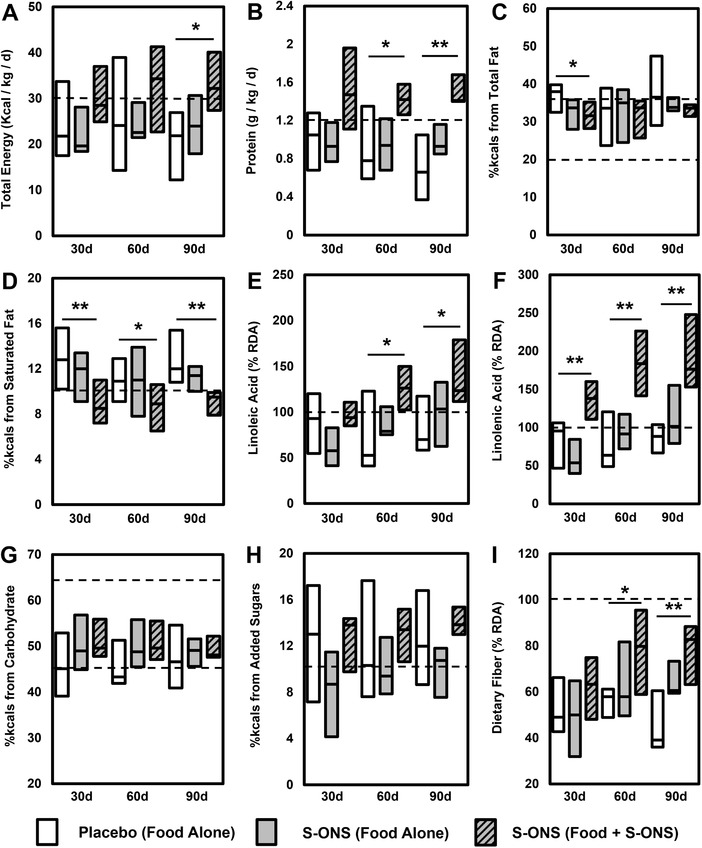
Specialized oral nutrition supplement (S‐ONS) improves overall macronutrient consumption profile. Boxes represent interquartile range with median as the middle (horizontal line). Recommended intake is represented by dashed lines. Macronutrients included (A) total kilocalories, (B) protein, (C) total fat, (D) saturated fat, (E) linoleic acid, (F) linolenic acid, (G) total carbohydrate, (H) added sugars, and (I) dietary fiber. Significant difference between placebo and S‐ONS (food + S‐ONS): *P < .05; **P < .01. RDA, recommended dietary allowance.
Table 2.
Percentage of DRI Met From Food Alone or With S‐ONS by Time and Treatment
| Percentage of DRI Met, Median (1st quartile, 3rd quartile) | Effect, P‐Valuea | |||||
|---|---|---|---|---|---|---|
| Nutrients | Day | PL (Food) | S‐ONS (Food) | S‐ONS (Food + S‐ONS) | PL vs S‐ONS (Food) | PL vs S‐ONS (Food + ONS) |
| Calcium | 30 | 34.3 (24.6, 63.7) | 44.3 (29.8, 63.4) | 109.0 (90.2, 139.0) | .6157 | S‐ONS > PL: P < .0001 |
| 60 | 39.3 (35.4, 52.4) | 50.0 (37.0, 85.0) | 125.9 (119.7, 144.3) | .2778 | S‐ONS > PL: P = .0003 | |
| 90 | 47.8 (22.3, 57.2) | 49.9 (47.9, 98.0) | 130.4 (110.5, 144.0) | .1286 | S‐ONS > PL: P = .0004 | |
| Iron | 30 | 130.6 (96.5, 189.0) | 129.5 (89.1, 172.2) | 216.82 (165.0, 266.1) | .6157 | S‐ONS > PL: P = .0107 |
| 60 | 128.1 (110.3, 224.2) | 145.8 (125.6, 201.2) | 242.0 (202.7, 298.1) | .2778 | S‐ONS > PL: P = .0101 | |
| 90 | 128.6 (73.4, 210.8) | 194.8 (182.3, 223.4) | 290.4 (269.1, 335.9) | S‐ONS > PL: P = .0334 | S‐ONS > PL: P = .0006 | |
| Magnesium | 30 | 48.0 (41.9, 53.7) | 47.5 (31.4, 63.7) | 88.3 (80.0, 111.4) | .9131 | S‐ONS > PL: P = .0001 |
| 60 | 48.8 (41.1, 61.3) | 73.2 (43.7, 75.2) | 121.1 (96.5, 126.0) | .0586 | S‐ONS > PL: P = .0003 | |
| 90 | 40.4 (34.1, 65.5) | 64.0 (58.3, 75.8) | 111.4 (90.6, 124.3) | S‐ONS > PL: P = .0402 | S‐ONS > PL: P = .0002 | |
| Phosphorus | 30 | 121.5 (77.7, 164.4) | 109.8 (100.8, 157.3) | 192.9 (163.7, 242.3) | .9131 | S‐ONS > PL: P = .0024 |
| 60 | 129.4 (88.3, 164.1) | 142.2 (110.6, 190.3) | 241.4 (195.6, 285.0) | .2036 | S‐ONS > PL: P = .0014 | |
| 90 | 80.6 (51.3, 152.0) | 146.6 (129.2, 185.9) | 231.6 (204.4, 284.4) | S‐ONS > PL: P = .0276 | S‐ONS > PL: P = .0006 | |
| Potassium | 30 | 42.5 (28.6, 51.6) | 36.7 (29.3, 49.1) | 56.2 (45.8, 74.4) | .7107 | S‐ONS > PL: P = .0154 |
| 60 | 35.4 (31.4, 58.0) | 41.8 (33.5, 47.3) | 63.2 (47.1, 72.2) | .9259 | S‐ONS > PL: P = .0277 | |
| 90 | 33.1 (25.0, 54.3) | 40.13 (39.0, 55.8) | 60.5 (55.0, 65.2) | .3233 | S‐ONS > PL: P = .0018 | |
| Sodium | 30 | 89.0 (64.3, 135.2) | 92.6 (77.2, 127.5) | 105.3 (89.7, 144.8) | 1 | .2475 |
| 60 | 91.4 (81.4, 164.6) | 103.6 (64.4, 128.5) | 111.3 (85.3, 146.8) | .5558 | .7802 | |
| 90 | 90.3 (45.1, 140.8) | 97.4 (47.9, 140.5) | 118.2 (67.2, 147.0) | .8792 | .4474 | |
| Zinc | 30 | 99.8 (67.1, 119.5) | 71.1 (56.1, 111.4) | 339.3 (240.2, 421.4) | .2301 | S‐ONS > PL: P < .0001 |
| 60 | 93.1 (73.2, 111.3) | 91.0 (55.4, 116.6) | 387.7 (359.6, 461.1) | .9259 | S‐ONS > PL: P = .0006 | |
| 90 | 61.9 (49.7, 79.0) | 111.7 (96.9, 133.7) | 431.2 (369.7, 473.3) | S‐ONS > PL: P = .0062 | S‐ONS > PL: P = .0002 | |
| Copper | 30 | 96.1 (65.9, 138.0) | 89.7 (60.2, 107.9) | 194.5 (151.0, 246.7) | .5854 | S‐ONS > PL: P = .0006 |
| 60 | 101.4 (73.7, 144.0) | 110.6 (84.6, 124.7) | 228.1 (170.4, 263.6) | .6869 | S‐ONS > PL: P = .0006 | |
| 90 | 87.8 (51.0, 112.9) | 113.8 (102.9, 161.8) | 250.4 (199.4, 300.7) | S‐ONS > PL: P = .0482 | S‐ONS > PL: P = .0003 | |
| Manganese | 30 | 107.9 (71.8, 127.5) | 126.5 (59.3, 163.0) | 164.1 (111.5, 215.1) | .4715 | S‐ONS > PL: P = .0154 |
| 60 | 130.2 (73.0, 162.4) | 150.3 (71.5, 181.5) | 207.7 (134.8, 239.8) | .5981 | .0586 | |
| 90 | 74.2 (61.0, 132.3) | 153.1 (129.6, 182.0) | 195.7 (163.1, 234.1) | S‐ONS > PL: P = .0276 | S‐ONS > PL: P = .0039 | |
| Selenium | 30 | 149.0 (96.6, 230.9) | 160.2 (112.3, 183.1) | 243.3 (172.4, 262.0) | .9131 | S‐ONS > PL: P = .0094 |
| 60 | 159.0 (97.7, 187.2) | 150.1 (131.8, 185.0) | 237.5 (206.1, 276.3) | .7802 | S‐ONS > PL: P = .0143 | |
| 90 | 111.0 (76.3, 170.9) | 194.8 (146.0, 222.8) | 279.8 (243.9, 321.1) | S‐ONS > PL: P = .0098 | S‐ONS > PL: P = .0008 | |
| Vitamin A | 30 | 59.6 (47.7, 136.1) | 74.6 (33.7, 95.1) | 124.4 (95.3, 173.1) | .3261 | S‐ONS > PL: P = .0307 |
| 60 | 67.2 (33.3, 78.5) | 80.0 (43.4, 106.6) | 154.8 (115.0, 163.9) | .3062 | S‐ONS > PL: P = .0026 | |
| 90 | 65.2 (46.8, 84.2) | 119.2 (94.9, 138.1) | 167.8 (154.8, 223.1) | S‐ONS > PL: P = .0098 | S‐ONS > PL: P = .0004 | |
| Vitamin E | 30 | 36.2 (19.1, 54.3) | 28.9 (22.3, 48.4) | 578.1 (382.3, 635.9) | .7434 | S‐ONS > PL: P < .0001 |
| 60 | 37.0 (20.1, 73.6) | 48.0 (39.5, 60.5) | 635.8 (446.0, 646.7) | .2512 | S‐ONS > PL: P < .0001 | |
| 90 | 39.3 (24.9, 53.1) | 54.8 (36.5, 79.4) | 625.7 (564.8, 660.0) | .1106 | S‐ONS > PL: P = .0002 | |
| Vitamin D | 30 | 20.2 (13.4, 34.0) | 18.9 (11.5, 42.4) | 69.1 (46.7, 93.7) | .7434 | S‐ONS > PL: P = .0002 |
| 60 | 19.9 (12.1, 26.3) | 28.9 (12.9, 45.3) | 76.5 (60.4, 94.7) | .3364 | S‐ONS > PL: P = .0017 | |
| 90 | 14.3 (4.7, 27.6) | 27.6 (18.4, 54.0) | 72.1 (69.7, 83.9) | .0946 | S‐ONS > PL: P = .0004 | |
| Vitamin Cb | 30 | 55.2 (34.1, 87.9) | 75.2 (27.8, 142.1) | 211.0 (150.3, 246.3) | .6157 | S‐ONS > PL: P = .0010 |
| 60 | 51.8 (26.9, 81.6) | 67.7 (24.9, 163.0) | 186.5 (174.1, 242.3) | .5558 | S‐ONS > PL: P = .0017 | |
| 90 | 39.3 (23.7, 71.0) | 85.4 (42.2, 111.6) | 208.3 (165.5, 257.9) | .1965 | S‐ONS > PL: P = .0033 | |
| Thiamin | 30 | 92.3 (65.9, 146.2) | 94.7 (78.9, 125.1) | 156.9 (132.5, 178.7) | .7107 | S‐ONS > PL: P = .0032 |
| 60 | 112.1 (79.4, 129.6) | 117.4 (92.6, 139.4) | 175.8 (124.9, 196.6) | .5558 | S‐ONS > PL: P = .0026 | |
| 90 | 111.5 (55.3, 134.9) | 134.1 (103.6, 145.4) | 171.5 (160.2, 208.7) | .1489 | S‐ONS > PL: P = .0011 | |
| Riboflavin | 30 | 130.3 (91.8, 222.9) | 121.3 (84.5, 172.3) | 170.8 (155.0, 234.6) | .5557 | .0847 |
| 60 | 149.6 (116.6, 167.4) | 135.7 (105.2, 196.2) | 206.1 (169.3, 264.6) | .9259 | S‐ONS > PL: P = .0279 | |
| 90 | 139.7 (96.3, 157.6) | 183.8 (158.2, 245.4) | 249.9 (202.4, 290.6) | .0575 | S‐ONS > PL: P = .0018 | |
| Niacin | 30 | 103.6 (70.2, 144.0) | 102.1 (76.5, 142.9) | 154.3 (132.0, 205.2) | .8443 | S‐ONS > PL: P = .0275 |
| 60 | 95.7 (76.5, 142.5) | 131.2 (113.4, 140.6) | 179.1 (171.2, 188.9) | .2036 | S‐ONS > PL: P = .0017 | |
| 90 | 114.5 (47.1, 123.2) | 116.1 (103.5, 131.0) | 174.4 (137.2, 201.2) | .2545 | S‐ONS > PL: P = .0008 | |
| Vitamin B6 | 30 | 96.8 (63.1, 127.1) | 72.8 (47.4, 98.5) | 119.9 (99.2, 146.3) | .2849 | S‐ONS > PL: P = .0343 |
| 60 | 97.3 (47.9, 107.7) | 82.8 (69.9, 116.1) | 143.3 (114.8, 161.6) | .5981 | S‐ONS > PL: P = .0039 | |
| 90 | 87 (34.9, 108.4) | 107.4 (81.0, 122.2) | 146.5 (127.0, 183.8) | .2875 | S‐ONS > PL: P = .0024 | |
| Vitamin B12 | 30 | 117.6 (86.1, 255.0) | 102.9 (86.9, 169.6) | 328.2 (269.7, 416.3) | .4987 | S‐ONS > PL: P = .0011 |
| 60 | 147.0 (105.3, 218.5) | 143.1 (110.2, 199.3) | 369.4 (307.8, 400.7) | .6869 | S‐ONS > PL: P = .0032 | |
| 90 | 101.3 (55.5, 221.4) | 224.2 (135.6, 273.5) | 450.6 (348.1, 523.5) | S‐ONS > PL: P = .0402 | S‐ONS > PL: P = .0004 | |
| Choline | 30 | 53.2 (40.5, 92.6) | 44.4 (36.0, 60.0) | 71.6 (60.8, 90.8) | .2301 | .1831 |
| 60 | 41.0 (34.1, 77.2) | 44.6 (36.0, 71.1) | 77.0 (66.2, 110.2) | .5981 | S‐ONS > PL: P = .0438 | |
| 90 | 42.1 (29.9, 51.4) | 69.5 (53.4, 78.6) | 101.8 (83.6, 108.7) | S‐ONS > PL: P = .0334 | S‐ONS > PL: P = .0008 | |
| Vitamin K | 30 | 49.9 (34.5, 102.1) | 41.2 (19.4, 61.7) | 69.4 (48.6, 106.2) | .1692 | .2301 |
| 60 | 62.3 (30.5, 77.5) | 61.8 (51.3, 86.5) | 97.9 (87.6, 122.2) | .4025 | S‐ONS > PL: P = .0236 | |
| 90 | 63.5 (47.3, 182.9) | 71.4 (45.9, 103.4) | 104.7 (84.8, 122.2) | .9394 | .1965 | |
| Folate | 30 | 86.2 (62.1, 122.3) | 104.0 (74.7, 113.7) | 228.2 (198.3, 280.3) | .5557 | S‐ONS > PL: P < .0001 |
| 60 | 105.8 (74.5, 141.0) | 113.2 (77.5, 118.6) | 242.4 (208.6, 287.0) | .8768 | S‐ONS > PL: P = .0002 | |
| 90 | 81.9 (64.7, 124.8) | 126.7 (109.0, 187.9) | 277.5 (225.9, 355.1) | .0682 | S‐ONS > PL: P = .0004 | |
DRI, Dietary Reference Intake; ONS, oral nutrition supplement; PL, placebo; S‐ONS, specialized oral nutrition supplement.
Significance was set at P < .05.
Placebo supplement contained 10 mg of vitamin C each, included in food + ONS.
It is also important to consider how many patients actually met or exceeded nutrient intake goals. Reflective of the percentage of DRI met data, the percentage of patients meeting most nutrient intake goals from food alone did not differ between placebo and S‐ONS groups (Table S1). The majority of patients (70%) did not meet energy and protein goals regardless of treatment group or time point. However, at 90 days, more patients in the S‐ONS treatment group met the RDA for iron, zinc, manganese, vitamin A, and vitamin B12 (all P < .05). Notably, no patients in either group met the RDA for choline (90 days), dietary fiber (60 and 90 days), magnesium (60 and 90 days), potassium (all days), or vitamin D (all days) from food alone, indicating that these patients may be at highest risk for deficiency of these nutrients.
Nutrient Intake From Food and ONSs
As anticipated, nutrient intake from food + S‐ONS was higher for almost all micronutrients as a percentage of DRI met (Table 2). Exceptions were sodium (all days), choline (30 days), and vitamin K (30 and 90 days). Macronutrient intakes of total energy (90 days), protein (60 and 90 days), dietary fiber (60 and 90 days), linoleic acid (60 and 90 days), and linolenic acid (all days) were also increased by S‐ONS (Figure 1). Conversely, percent calories from saturated fat intake was greater in the placebo group at all time points, whereas total fat was higher at 30 days (Figure 1C) vs the S‐ONS group.
Again considering the proportion of patients who met the DRI, intake of about one‐third of nutrients was improved by S‐ONS as indicated by more patients meeting or exceeding nutrient intake goals (Table S1). Including nutrient intake from both food and S‐ONS within the S‐ONS group, numerically more patients met energy (50%) and protein (71%) goals compared with food alone (29% and 36%, respectively) regardless of time. Furthermore, more patients in the S‐ONS group met the DRI for total fat (30 days) and saturated fat (30 and 90 days). More patients receiving S‐ONS (vs placebo) met the DRI at all time points for linolenic acid, calcium, magnesium, phosphorus, zinc, copper, vitamin E, vitamin C, thiamin, and folate. In fact, all patients in the S‐ONS group met the RDA for iron, phosphorus, copper, selenium, thiamin, and riboflavin with supplement consumption. At 90 days, the percentage of patients meeting the DRI was not different between placebo and S‐ONS groups for dietary fiber, added sugars, potassium, sodium, vitamin D, riboflavin, or vitamin K.
Relationship Between Patient Supplement Consumption and Number of DRIs Met
Regression analysis, accounting for repeated measurements per subject, of supplement intake vs number of DRIs met is displayed in Figure 2. Regardless of day, higher patient compliance (ie, consumption of S‐ONSs per day) was associated with a higher number of DRIs met (regression coefficient, β = 4.46, P = .0002), whereas there was no significant relationship for the placebo group (β = 1.21, P = .41).
Figure 2.
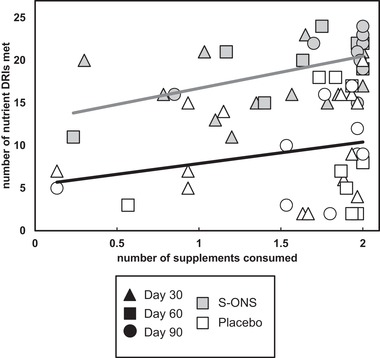
Increased compliance to specialized oral nutrition supplement (S‐ONS) consumption, but not placebo, is associated with meeting more Dietary Reference Intakes (DRIs), regardless of time point. Time points were pooled within treatment groups after no significant differences in regression slopes were found. Relationships were tested via repeated‐measures analysis. S‐ONS group: P = .0002, β = 4.46; placebo group: P = .41, β = 1.21. Black triangles indicate day 30; squares indicate day 60; circles indicate day 90; gray‐filled shapes represent S‐ONS; white‐filled shapes represent placebo.
Power Analysis for Individual Nutrients
Results of the power analysis for the 16 nutrients found not to be significantly different between treatment groups at day 90 from food alone are displayed in Table 3. Number of patients needed to adequately power future studies in this population ranged from 22 (riboflavin) to 802 (vitamin K). Standardized effect sizes ranged from 0.140 (vitamin K) to 0.88 (riboflavin).
Discussion
This study highlights the effectiveness of using S‐ONSs to improve daily macronutrient and micronutrient intake in malnourished older adult patients with cardiopulmonary diseases after hospital discharge. The results suggest that an S‐ONS, when consumed between meals, does not reduce intake of nutrients from food; in fact, it increases intake of some food‐derived micronutrients, while concurrently improving overall diet quality. It may take a longer time (eg, 3 months) of S‐ONS therapy for some changes in dietary patterns to become evident.
This study emphasizes the nutrition deficits in patients with cardiopulmonary diseases post discharge, and how a majority of these needs can be met with daily ONS intake in conjunction with normal food intake. The notion that nutrient intake should be consumed primarily from food, with nutrition supplements serving only as a secondary source or stopgap, should be reconsidered; encouraging intake of both food and ONSs can fill the gaps in macronutrient and micronutrient intake. As shown in this study, some patients still had gaps of certain nutrients even with the ONS consumption. Hence it is not one or the other; both food and ONSs need to be considered when addressing the nutrition needs of the individual. Overall diet quality was improved via S‐ONSs in several respects, while maintaining (days 30 and 60) or increasing (day 90) total energy consumed. First, nutrients of concern for overconsumption in this high‐risk population, such as added sugars and sodium, were not increased, and percent calories from saturated fat intake was lower in the group consuming S‐ONS. Second, consumption of protein and practically all micronutrients was higher in the S‐ONS group 90 days after discharge. Finally, higher compliance to the recommendation of consuming 2 S‐ONSs per day was associated with patients meeting more nutrient intake goals. However, 0 or very few patients met recommendations for dietary fiber, magnesium, potassium, or vitamin D at any time point included in this study, indicating that there is a role for dietary counseling to improve intake of these key nutrients from food, and that future nutrition supplements can be improved to address these gaps as well. Dietary interventions should consider both food and ONSs to achieve optimal nutrient intake in patients who are at increased risk for malnutrition.
Small sample size is a weakness of this study, yet it did not hamper our ability to detect differences in consumption for the majority of nutrients. This subgroup of patients had better compliance to ONS intake compared with other studies,29 which allowed us to demonstrate the positive impact of ONS consumption, because our data indicate that higher compliance is related to meeting more nutrient intake goals. In addition, results from this study lay the groundwork for future studies by providing the estimated number of patients needed to investigate specific nutrients in this population. Our power analyses suggest that some nutrients may be more difficult to study in this context, given that >100 patients would need to be followed in this great amount of detail to make an adequately powered comparison between groups. However, the biological and clinical relevance of small effect sizes observed for some nutrients in this study should be considered when designing future studies. This study used a validated method, 3‐day dietary recall, to estimate nutrient intake. As with all self‐reported estimates of dietary intake, there is a tendency for misreporting, typically underreporting of intake. Much of the error in dietary recall is due to errors in memory, portion size estimation, and day‐to‐day variation in intake, all of which may impact on dietary outcomes. Using standardized multiple‐pass methodology, intake data from 3 randomly selected days, and portion size estimation tools (as were used in this trial) may significantly reduce the extent to which these errors occur.30, 31, 32
A strength of this study lies in its longitudinal collection of consumption data for all nutrients with an RDA. These data demonstrate the need for long‐term follow‐up with this population to monitor changes in nutrition adequacy. No differences in nutrient intake from food alone were detected between groups before 90 days of intervention (as both percent DRI met and proportion of patients meeting DRIs), and 37% more nutrients had a higher percentage of patients meeting the DRI after 90 days of treatment (compared with 30 days). Although this study used DRI as the nutrition goalpost for these patients, it is important to remember that these dietary guidelines are designed for healthy individuals, and nutrient requirements may differ for patients with cardiopulmonary diseases. Future studies should also strive to collect biomarkers confirming nutrition status in a larger patient population, which will contribute to setting nutrient recommendations for this population.
In conclusion, S‐ONS intake increases oral nutrient intake to help meet most nutrient requirements without decreasing nutrient intake from food in malnourished clinical populations.
Statement of Authorship
D. C. Mitchell, T. R. Ziegler, N. E. Deutz, and L. E. Matarese contributed to the conception/design of the research; B. R. Loman, M. Luo, G. E. Baggs, N. E. Deutz, and L. E. Matarese contributed to acquisition, analysis, or interpretation of the data; B. R. Loman, M. Luo, and G. E. Baggs drafted the manuscript; B. R. Loman, M. Luo, G. E. Baggs, D. C. Mitchell, J. L. Nelson, T. R. Ziegler, N. E. Deutz, and L. E. Matarese critically revised the manuscript; and B. R. Loman, M. Luo, G. E. Baggs, D. C. Mitchell, J. L. Nelson, T. R. Ziegler, N. E. Deutz, and L. E. Matarese agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
Supporting information
Table S1 Number of patients meeting the DRI from food alone or with S‐ONS by time and treatment.
Financial disclosure: This study was funded by Abbott Nutrition.
Conflicts of interest: None declared.
[This article was modified on December 12, 2019, after initial online publication to correct the copyright line.]
References
- 1. Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease‐related malnutrition. Clin Nutr. 2008;27(1):5‐15. [DOI] [PubMed] [Google Scholar]
- 2. Allaudeen N, Vidyarthi A, Maselli J, Auerbach A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54‐60. [DOI] [PubMed] [Google Scholar]
- 3. Maeda K, Koga T, Akagi J. Nutritional variables predict chances of returning home and activities of daily living in post‐acute geriatric care. Clin Interv Aging. 2018;13:151‐157. 10.2147/CIA.S154129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr. 2004;79(4):613‐618. [DOI] [PubMed] [Google Scholar]
- 5. Hudson L, Chittams J, Griffith C, Compher C. Malnutrition identified by Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition is associated with more 30‐day readmissions, greater hospital mortality, and longer hospital stays: a retrospective analysis of nutrition assessment data in a major medical center. JPEN J Parenter Enteral Nutr. 2018;42(5):892‐897. [DOI] [PubMed] [Google Scholar]
- 6. Curtis LJ, Bernier P, Jeejeebhoy K, et al. Costs of hospital malnutrition. Clin Nutr. 2017;36(5):1391‐1396. [DOI] [PubMed] [Google Scholar]
- 7. Ruiz AJ, Buitrago G, Rodriguez N, et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients [published online ahead of print June 1, 2018]. Clin Nutr. 10.1016/j.clnu.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 8. Somanchi M, Tao X, Mullin GE. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. JPEN J Parenter Enteral Nutr. 2011;35(2):209‐216. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez‐Rodriguez D, Marco E, Annweiler C, et al. Malnutrition in postacute geriatric care: basic ESPEN diagnosis and etiology based diagnoses analyzed by length of stay, in‐hospital mortality, and functional rehabilitation indexes. Arch Gerontol Geriatr. 2017;73:169‐176. 10.1016/j.archger.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 10. Sherry CL, Sauer AC, Thrush KE. Assessment of the nutrition care process in US hospitals using a web‐based tool demonstrates the need for quality improvement in malnutrition diagnosis and discharge care. Curr Dev Nutr. 2017;1(11):e001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braunschweig C, Gomez S, Sheean PM. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. J Am Diet Assoc. 2000;100(11):1316‐1322; quiz 1323‐1314. [DOI] [PubMed] [Google Scholar]
- 12. Allard JP, Keller H, Jeejeebhoy KN, et al. Decline in nutritional status is associated with prolonged length of stay in hospitalized patients admitted for 7 days or more: a prospective cohort study. Clin Nutr. 2016;35(1):144‐152. [DOI] [PubMed] [Google Scholar]
- 13. Keller H, Laporte M, Payette H, et al. Prevalence and predictors of weight change post discharge from hospital: a study of the Canadian Malnutrition Task Force. Eur J Clin Nutr. 2017;71(6):766‐772. [DOI] [PubMed] [Google Scholar]
- 14. Hiesmayr M, Schindler K, Pernicka E, et al. Decreased food intake is a risk factor for mortality in hospitalised patients: the NutritionDay survey 2006. Clin Nutr. 2009;28(5):484‐491. [DOI] [PubMed] [Google Scholar]
- 15. Pilgrim AL, Robinson SM, Sayer AA, Roberts HC. An overview of appetite decline in older people. Nurs Older People. 2015;27(5):29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elia M, Normand C, Laviano A, Norman K. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in community and care home settings. Clin Nutr. 2016;35(1):125‐137. [DOI] [PubMed] [Google Scholar]
- 17. Milne AC, Avenell A, Potter J. Meta‐analysis: protein and energy supplementation in older people. Ann Intern Med. 2006;144(1):37‐48. [DOI] [PubMed] [Google Scholar]
- 18. Cawood AL, Elia M, Stratton RJ. Systematic review and meta‐analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11(2):278‐296. [DOI] [PubMed] [Google Scholar]
- 19. Keller HH, Vesnaver E, Davidson B, et al. Providing quality nutrition care in acute care hospitals: perspectives of nutrition care personnel. J Hum Nutr Diet. 2014;27(2):192‐202. [DOI] [PubMed] [Google Scholar]
- 20. Tappenden KA, et al. Critical role of nutrition in improving quality of care: an interdisciplinary call to action to address adult hospital malnutrition. JPEN J Parenter Enteral Nutr. 2013;37(4):482‐497. [DOI] [PubMed] [Google Scholar]
- 21. Stratton RJ, Elia M. A review of reviews: a new look at the evidence for oral nutritional supplements in clinical practice. Clin Nutr Suppl. 2007;2(1):5‐23. [Google Scholar]
- 22. Beck AM, Holst M, Rasmussen HH. Oral nutritional support of older (65 years+) medical and surgical patients after discharge from hospital: systematic review and meta‐analysis of randomized controlled trials. Clin Rehabil. 2013;27(1):19‐27. [DOI] [PubMed] [Google Scholar]
- 23. Gariballa SE, Forster SJ. Dietary intake of older patients in hospital and at home: the validity of patient kept food diaries. J Nutr Health Aging. 2008;12(2):102‐106. [DOI] [PubMed] [Google Scholar]
- 24. Laudisio A, Costanzo L, Di Gioia C, et al. Dietary intake of elderly outpatients with chronic obstructive pulmonary disease. Arch Gerontol Geriatr. 2016;64:75‐81. 10.1016/j.archger.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 25. Deutz NE, Matheson EM, Matarese LE, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35(1):18‐26. [DOI] [PubMed] [Google Scholar]
- 26. Adademy of Nutrition and Dietetics . Recommendations summary. UWL: energy needs 2009. https://www.andeal.org/template.cfm?key=2066. Accessed October 3, 2017.
- 27. Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans . 8th ed. December 2015. http://health.gov/dietaryguidelines/2015/guidelines/. Accessed December 2015.
- 29. Hubbard GP, Elia M, Holdoway A, Stratton RJ. A systematic review of compliance to oral nutritional supplements. Clin Nutr. 2012;31(3):293‐312. [DOI] [PubMed] [Google Scholar]
- 30. Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five‐step multiple‐pass method in men: an observational validation study. J Am Diet Assoc. 2004;104(4):595‐603. [DOI] [PubMed] [Google Scholar]
- 31. Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple‐Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324‐332. [DOI] [PubMed] [Google Scholar]
- 32. Jonnalagadda SS, Mitchell DC, Smiciklas‐Wright H, et al. Accuracy of energy intake data estimated by a multiple‐pass, 24‐hour dietary recall technique. J Am Diet Assoc. 2000;100(3):303‐308; quiz 309‐311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Number of patients meeting the DRI from food alone or with S‐ONS by time and treatment.


