Abstract
Objective
To determine whether the size of THE birthmark in patients with Sturge-Weber syndrome (SWS) who have brain involvement can help predict neurological disability.
Study design
51 patients with SWS with facial birthmarks and brain involvement documented on MRI were included in this retrospective chart review. A neuroradiologist, blinded to all clinical information, assigned a previously validated SWS neuroimaging score. A pediatric neurologist prospectively assigned previously validated neurological severity scores, based on seizures, hemiparesis, visual field cut, and cognitive impairments. Three raters, blinded to clinical scores, independently graded the size of facial birthmark in each patient based on photographs. Their scores were averaged. Birthmark scores were compared with the imaging and neurological severity results using nonparametric correlation analysis.
Results
Size of facial PWB correlates with MRI scores on the left and right sides (ρ = 0.57 and 0.66 (p<0.001), respectively). Size is also positively associated with the neurological severity rating for patients age 6 and above (one-sided Fisher’s exact, p=0.032).
Conclusion
The size of facial PWB in SWS brain involvement can be developed as a tool to predict neurological severity of the disease.
Keywords: capillary malformation
Sturge-Weber syndrome (SWS) is generally defined by the constellation of a facial capillary malformation referred to as a port-wine birthmark (PWB), malformation of the vasculature of the eye, and/or vascular malformation in the brain (leptomeningeal angioma). The incidence of SWS is estimated at 1 in 20,000 to 50,000 live births, affecting males and females equally.1 Individuals with SWS may have impairment of neurological function including seizures or stroke-like episodes, cognitive impairment, visual field defects, and hemiparesis, all with a variable degree of severity. One challenge to developing optimal treatment strategies and providing prognoses to families of infants with SWS is the tremendous variability in neurologic outcomes from individuals with normal intelligence to those with severe epilepsy, neurologic deficits, and severe intellectual disability.
Not all facial PWB are associated with SWS. Traditionally, PWB occurring in the ophthalmic, or V1, division of the trigeminal nerve, especially with upper eyelid involvement, carried the highest risk of association with SWS. A more recent study has noted that the risk for SWS appears not to be determined by the dermatome but rather by embryonic vascular placodes, specifically one delineated by a line from the lateral canthus drawn to the top of the helix.2 This territory reflects the etiology of SWS, which is caused by an activating somatic mutation in GNAQ affecting the pluripotent progenitor cells that give rise to the brain, eye, and skin in this location.3 Various studies suggest that individuals born with a facial PWB on the forehead or upper eyelids have a 20–50% chance of SWS brain involvement depending on the extent of PWB.4,5,6 However, current knowledge of the relationship between the size of PWB and the severity of neurological dysfunction in those with SWS brain involvement is limited. We hypothesized that larger PWB would be associated with worse neurologic outcome. Our study aims to characterize that relationship in order to provide insight into future neurological outcomes at the time of diagnosis with SWS brain involvement early in life.
Methods
Subjects for this study are patients from the Hunter Nelson Sturge-Weber Center at Kennedy Krieger Institute participating in a multidisciplinary study protocol approved by the institutional review board. The inclusion criteria were: diagnosis of SWS, with presence of a facial PWB, and SWS brain involvement on brain MRI. For this retrospective study, neurologic scores were assigned prospectively at the time of each subject’s visit. However, the skin scores and MRI scores were assigned retrospectively to images previously obtained.
Conventional MR sequences including T1, T2, FLAIR, and post gadolinium contrast T1 and FLAIR images were evaluated. The MRI of each patient was rated by a single rater, a neuroradiologist (DDL) blinded to the size of PWB and the degree of clinical severity of each patient. In the case of patients who had multiple MRI images available, the MRI rated was the one obtained at the time closest to the time the neurological score was obtained. The scoring system was modified from a previously published system.7 Scores from 1 to 4 were assigned to each of the four brain regions (frontal, temporal, parietal, and occipital) for each hemisphere individually, where 1 signified no asymmetry, 2 mild asymmetry (angiomatosis only), 3 moderate asymmetry (angiomatosis and mild atrophy), and 4 severe asymmetry (angiomatosis and severe atrophy). The total score was then determined for each hemisphere, ranging from 4 to 16.
The neurological severity of the syndrome in each patient was evaluated by a single rater, a pediatric neurologist (AMC), who assigned ratings prospectively after carefully evaluating neurological function of the patients during their regular care or study visits utilizing a previously published system.8 The degree of visual field cut, hemiparesis, seizure frequency, and cognitive function (differently for each age group: infant/preschooler, child, and adult) was rated as shown in the Table (available at www.jpeds.com).
The individual scores were then summed, with the total score ranging from 0 to 15. In the case of patients for whom multiple scores were available from neurology visits at different time points, the score from the visit closest to the time of the MRI was used.
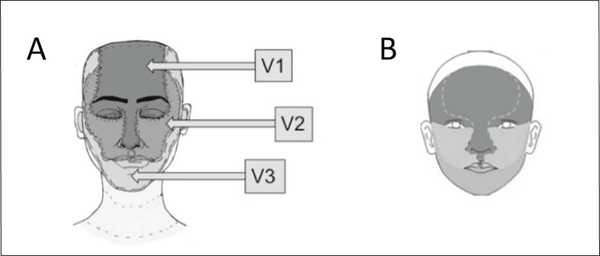
Photographs showing patients’ faces were obtained from their families. We asked families to provide photographs showing both sides of the face, as well as a full-frontal photograph. For the majority of our subjects, we were able to obtain all of these; however, we were able to obtain only full frontal photographs for some of the patients (n=10). The photographs reflected the original size of their facial PWB in the case of patients who underwent laser treatments, with the majority of patients’ photographs dating from infancy. Three independent raters scored each patient’s photo using both the traditional facial PWB classification system utilizing the trigeminal nerve distribution (raters: MD, AYK, BAC), shown in Figure 1, A, as well as the recently published embryonic facial vasculature distribution (raters: MD, EAO, BAC),2 shown in Figure 1, B. The photographs were scored using the two different systems on two separate occasions, with the raters blinded to the other scores, and to the results of the MRI and neurologic scores. For each territory (trigeminal dermatome or embryonic placode), the fraction of the area involving the PWB was visually approximated and scored from 0 to 4, assigning 0 to areas with no involvement, 1 to 1–25% of a particular surface area involved, 2 to 26–50% involvement, 3 to 51–75% involvement, and 4 to 76–100% involvement. The individual areas’ scores were either considered individually (V1 and placode 1) or summed to yield total facial (ranging from 0 to 24) and hemifacial (ranging from 0 to 12) scores. Scalp involvement was not evaluated, as the majority of photographs did not provide the opportunity to do so.
Figure 1:
Port-wine birthmark distribution: (A) utilizing trigeminal nerve distribution; (B) utilizing the embryonic facial vasculature distribution; top to bottom: placode 1, placode 2, and placode 3.2
Statistical analyses
Stata/IC 14.0 and IBM SPSS Statistics 23.0 were used for statistical analysis. The skin scores from the three raters were averaged, and inter-rater reliability was evaluated using Cohen kappa. The correlation between MRI scores and skin scores was calculated via Spearman correlation. Linear regression was applied to determine the correlation between skin scores (independent variable) and neurological scores. The association between better or worse neurological scores and skin scores was tested using Fisher exact test; because neurologic status tends to stabilize after 4–5 years of age, only patients age 6 and older at the time of their neurological evaluation were included in that analysis (n=21). Skin scores were classified into two categories: (1) <12 and (2) ≥12. Clinical severity scores were classified into (1) <4 and (2) ≥4, a cut-score previously shown to discriminate impaired and unimpaired individuals with regard to both intellectual and adaptive functioning.9
We used hierarchical multiple regression in order to determine the effect of using size of PWB as a predictor of neurological outcome in addition to the degree of brain involvement obtained from evaluation of the MRI. The first model used MRI score as the independent variable and neurological severity score as the dependent variable. The second model used both MRI score and total skin score as independent variables and neurological severity score as the dependent variable. These were computed only for patients whose neurological function was evaluated at age 6 and beyond. The difference in the R-squared value in the two models was then calculated.
Results
Photographs of patients were collected in July 2014, at which time the Center’s research database of study patients included 243 patients with signed consents. Of the 243 patients, 123 families or patients signed consents to allow the use of their child’s photographs for research purposes. Among these patients, 71 fit the criteria for our study, and we were able to obtain suitable photographs for 51 of them. These patients were followed by the Center for an average of 4.5 years (ranging from a single visit to 10 years and 10 months). The resulting study sample of 51 patients was 53% male, 47% female. The age at the time of neurological evaluation ranged from 1 week to 52 years, with a mean of 9.7 years. Bilateral brain involvement was found on MRI of 7 patients (14%). For the sub-group of subjects age 6 and older at the time of neurological score (n=21), the age at the time of MRI that was scored for this study ranged from 4 years to 52 years, with a mean of 19.9 years.
The total average skin scores obtained using the traditional trigeminal nerve distribution system were almost identical to the scores obtained using the embryonic facial vasculature distribution system, with a Spearman correlation coefficient between the two scores of ρ = 0.97 (p<0.001). Therefore, we focused on the analysis of results obtained using method 2.
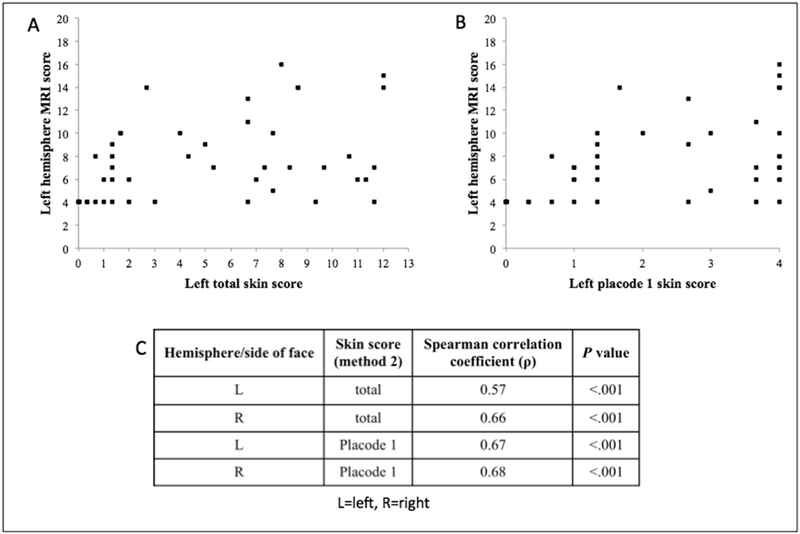
There was moderate agreement between the three raters, with the following values: left facial total score κ=0.43 (moderate), right facial total κ=0.46 (moderate), placode 1 on the left κ=0.59 (moderate), placode 1 on the right κ=0.54 (moderate). The distribution of left hemisphere MRI scores and their corresponding left face skin scores is shown in panel A and B of Figure 2. The results for the right side are similar and thus not shown. The size of PWB was positively correlated with MRI findings. Panel C of Figure 2 shows the Spearman coefficients for the correlation between the MRI score and the skin score for the totality of the left and right side, as well as for placode 1 on each side.
Figure 2:
Method 2 skin score and MRI score correlation results: (A) correlation between left face skin score and left hemisphere MRI score, (B) correlation between left placode 1 skin score and left hemisphere MRI score, (C) Spearman correlation coefficients for the relationship between MRI score and skin score.
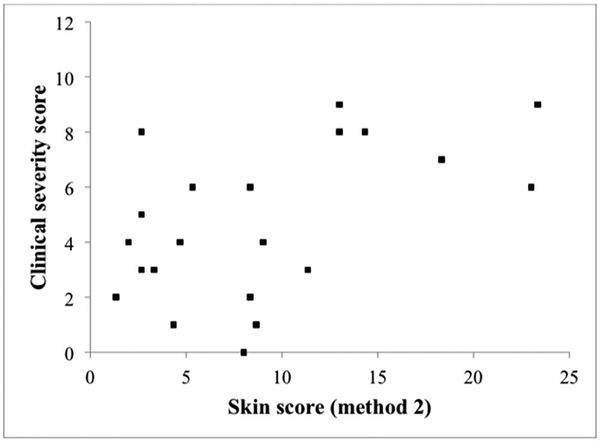
The distribution of clinical severity scores and corresponding total skin scores is shown in Figure 3 (n=21). The correlation under linear regression resulted in R-squared of 0.27 with p=0.015. Using the groupings described above, and utilizing one-sided Fisher exact test, the size of facial PWB is positively associated with clinical severity rating, with p=0.032. More specifically, skin scores less than 12 occurred in patients with clinical severity scores ranging from 0 to 8. However, skin scores of 12 and above were associated with clinical severity scores of 6 and above, indicating that patients with large PWB tend to have greater neurological dysfunction. The same result was obtained for analysis using method 1 for scoring the extent of PWB.
Figure 3:
Size of facial port-wine birthmark (method 2) is positively associated with the clinical severity rating.
The hierarchical multiple regression yielded R-squared of 0.507 for model 1 (independent variable: MRI score, dependent: neurological score) and 0.606 for model 2 (independent variables: MRI score, skin score, dependent: neurological score). The difference in R- squared, 0.099, means that the predictive power of the model increased by 9.9% with the addition of the skin score as a predictor in addition to the MRI score.
Additionally, we identified patients with a special pattern of distribution in the frontonasal prominence, occupying a particular portion of placode 1, as they did not appear to conform to the general trend described above. An example of the pattern is shown in Figure 4. This pattern was found in 5 of our patients. Patients had relatively small PWB involving the glabella or extending onto the nose (total skin score of 1 −3), and yet they had high MRI abnormality scores (10–14), and high neurological dysfunction scores (5–8). More specifically, all patients had high hemiparesis scores (3–4), indicating significant and severe fine and gross motor impairments, but variable seizure (0–3) and cognitive function scores (0–3). The MRI findings indicated involvement of the left hemisphere in 3 of these patients (MRI scores ranging from 10 to 14), right hemisphere in 1 patient (MRI score of 14), and bilateral involvement in 1 patient (MRI score of 14). All 5 of these patients were under age 6 at the time of their neurological evaluation; hence they were not taken into consideration when establishing the relationship between clinical severity scores and total skin scores as reported above.
Figure 4:
An example of the special frontonasal prominence pattern that was identified.
Discussion
Our study demonstrates that the size of facial PWB involved on each side of the face, i n subjects known to have SWS brain involvement, correlates positively with ipsilateral size and severity of brain involvement seen on brain MRI. Furthermore, in these subjects, age 6 and older, the greater extent of facial PWB was associated with more severe neurological impairment. Using the size of facial PWB, in addition to the degree of brain involvement on MRI, in assessing a patient’s eventual neurological outcome increases predictive value by a substantial amount (9.9%), and can be done without the need for invasive or expensive testing.
Up to this point, the emphasis in the literature has been on demonstrating how the pattern and extent of facial port-wine birthmark in undiagnosed infants relates to the likelihood of developing SWS brain or eye involvement. That is a different question from the one investigated here. The focus of our study (that is, determining how the extent of the birthmark is associated with neurologic outcome) is becoming more relevant now as more treatment options are becoming available and more treatment trials are being designed. Based on these data, the extent and pattern of the birthmark in pre-symptomatic infants with known SWS brain involvement will be one of the criteria used to select infants at high risk for developing seizures and a poor outcome.
It is perhaps not entirely surprising to those clinicians who see many of these patients that greater extent of PWB would be associated with greater brain involvement and worse neurologic outcome. However, it is important to demonstrate this association and provide this data for clinicians who may see only a handful of these patients over the course of their career. Furthermore, these results do not support the use of the PWB score instead of an appropriate MRI evaluation, but rather in addition to it.
It has been previously noted that those with no facial PWB at all, but with SWS brain involvement (about 6–7%),10,11,12 have later onset of seizures and better neurologic outcomes, suggesting that the brain involvement is different in those patients than in those with facial PWB. We have two hypotheses to explain our findings and the cases of patients with no PWB. One potential explanation is that a somatic mutation that occurs earlier in the process of development could lead to greater skin involvement and additional cell types involved in both the skin and the brain, increasing the eventual severity of the syndrome. Another possibility is that the presence of the PWB has a psychological effect on the development and learning of affected patients, intensifying the brain changes associated with the syndrome. Regardless of the reason, patients with extensive bilateral PWB involvement and brain involvement are at particular risk of severe neurologic outcomes, and close monitoring and aggressive treatment should be considered in their management.
The results of this study for scoring the extent of PWB, one based on the distribution of the trigeminal nerve, and another based on the embryonic facial vasculature distribution, were almost identical. The difference in those two methods is subtle, and, with our non-standardized view of the patient’s face on photographs supplied by families, differences may not be able to be fully appreciated. However, our result supports the theory that the developing facial vasculature may dictate the extent of a PWB.2 Furthermore, the special frontonasal distribution described above resembles an area commonly occupied by infantile hemangiomas,13 further corroborating the vasculature-based distribution theory.
An exception to the general pattern of correlation between the size of PWB and the severity of neurological disease, as described above, is the frontonasal prominence PWB. Those patients had relatively small PWB in the middle of their foreheads, and yet they had high MRI abnormality scores, and high neurological dysfunction scores, indicating significant neurological impairment. This specific PWB pattern has been described previously as a distribution associated with high risk for SWS.2,14 It is hypothesized that the embryologic origin of the frontonasal prominence is the prosencephalon and mesencephalon. Malformations of these critical embryonic structures during development could result in severe abnormalities in the cerebral cortex and eye. Our patients with this pattern of PWB had high hemiparesis scores, indicating significant and severe fine and gross motor impairments, which suggests possible motor cortex involvement.
One significant limitation of the study is the variability of facial position and quality in the photographs of our patients, which were supplied by patients’ families and therefore are not standardized. The borders of the area of the PWB were visually approximated by the rater from the image. Although there is a degree of subjectivity to these approximations, the subjectivity was partially resolved with the use of three independent raters whose scores were averaged, with good inter-rater reliability. A future study should be set up using standardized photographs of patients from multiple angles taken during clinic visits.
In conclusion, we have shown that it may be helpful to use the size of facial PWB to guide expectation of eventual neurological disease severity (after the age of 6) in patients with SWS with brain involvement, especially when combined with the results of brain MRI. Patients with extensive facial PWB, and those whose birthmarks have the frontonasal distribution, are at risk of severe neurological dysfunction, and should be followed closely over time. Our results are almost identical when analyzing the size of PWB using the trigeminal nerve dermatomes and the distribution of embryonic facial vasculature, which supports the theory that it may be the developing facial vasculature that determines the extent of a PWB.
Table 1:
Scoring system for the evaluation of clinical severity of the syndrome in each patient.8 (Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005; 20: 867–70.)
| Rating range | Meaning of assigned rating | |
|---|---|---|
| Visual field cut | 0–2 | 0 = no field cut 1 = partial homonymous hemianopsia 2 = full homonymous hemianopsia |
| Hemiparesis | 0–4 | 0 = no weakness or posturing 1 = mild posturing intermittently 2 = fine motor impairments only 3 = significant fine and gross motor impairments 4 = severe fine and gross motor impairments, poor helper arm function, and walks with great difficulty or not at all |
| Seizure frequency | 0–4 | 0 = none ever 1 = one or more seizures, currently controlled 2 = breakthrough seizures 3 = monthly seizures 4 = at least weekly seizures |
| Cognitive function | 0–5 | Infant/preschooler: 0 = normal 1 = mild speech delay but comprehends well 2 = mild delay in speech and comprehension 3 = moderately delayed speech 4 = severely delayed speech 5 = profoundly delayed speech with little or no comprehension Child: 0 = normal 1 = school difficulties, regular classes 2 = resource help needed in school 3 = special education required 4 = trainable for activities of daily living 5 = full care Adult: 0 = normal 1 = lives and works independently 2 = works in community with parental support 3 = significant difficulty maintaining employment or satisfactory social relationships 4 = trainable (i.e. group home, supervised work setting) 5 = full care |
| Total | 0–15 | normal to severely impaired |
Acknowledgments
We would like to thank Catherine Bachur4 and Emma Kaplan4 (both supported by the funding sources above) for help coordinating the data collection process, and Gregory Kirkorian for his voluntary creation of Figure 1, A.
Supported by the National Institute of Neurological Disorders and Stroke (NINDS) (National Institutes of Health [NIH] U54NS065705) (to A.C.) and from Celebrate Cure Foundation (to A.C.). The Brain Vascular Malformation Consortium (U54NS065705) is a part of the NIH Rare Disease Clinical Research Network, supported through the collaboration between the NIH Office of Rare Diseases Research at the National Center for Advancing Translational Science and the NINDS. The authors declare no conflicts of interest.
Abbreviations
- SWS
Sturge-Weber syndrome
- PWB
port-wine birthmark
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Comi AM. Update on Sturge-Weber syndrome: diagnosis, treatment, quantitative measures, and controversies. Lymphat Res Biol. 2007; 5: 257–64. [DOI] [PubMed] [Google Scholar]
- 2.Waelchli R, Aylett SE, Robinson K, Chong WK, Martinez AE, Kinsler VA. New vascular classification of port-wine stains: improving prediction of Sturge-Weber risk. Br J Dermatol. 2014; 171: 861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013; 368: 1971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enjolras O, Riche MC, Merland JJ. Facial port-wine stains and Sturge-Weber syndrome. Pediatrics. 1985; 76: 48–51. [PubMed] [Google Scholar]
- 5.Tallman B, Tan OT, Morelli JG, Piepenbrink J, Stafford TJ, Trainor S, et al. Location of port-wine stains and the likelihood of ophthalmic and/or central nervous system complications. Pediatrics. 1991; 87: 323 7. [PubMed] [Google Scholar]
- 6.Piram M, Lorette G, Sirinelli D, Herbreteau D, Giraudeau B, Maruani A. Sturge-Weber syndrome in patients with facial port-wine stain. Pediatr Dermatol. 2012; 29: 32–7. [DOI] [PubMed] [Google Scholar]
- 7.Jansen FE, van Huffelen AC, Witkamp T, Couperus A, Teunissen N, Wieneke GH, et al. Diazepam-enhanced beta activity in Sturge Weber syndrome: its diagnostic significance in comparison with MRI. Clin Neurophysiol. 2002; 113: 1025–9. [DOI] [PubMed] [Google Scholar]
- 8.Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005; 20: 867–70. [DOI] [PubMed] [Google Scholar]
- 9.Kavanaugh B, Sreenivasan A, Bachur C, Papazoglou A, Comi A, Zabel TA. Intellectual and adaptive functioning in Sturge-Weber Syndrome. Child Neuropsychol. 2016; 22: 635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagtap S, Srinivas G, Harsha KJ, Radhakrishnan N, Radhakrishnan A. Sturge-Weber syndrome: clinical spectrum, disease course, and outcome of 30 patients. J Child Neurol. 2013; 28: 725–31. [DOI] [PubMed] [Google Scholar]
- 11.Pascual-Castroviejo I, Pascual-Pascual SI, Velazquez-Fragua R, Viano J. Sturge-Weber syndrome: study of 55 patients. Can J Neurol Sci. 2008; 35: 301–7. [DOI] [PubMed] [Google Scholar]
- 12.Peterman AF, Hayles AB, Dockerty MB, Love JG. Encephalotrigeminal angiomatosis (Sturge-Weber disease); clinical study of thirty-five cases. JAMA. 1958; 167: 2169–76. [DOI] [PubMed] [Google Scholar]
- 13.Haggstrom AN, Lammer EJ, Schneider RA, Marcucio R, Frieden IJ. Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics. 2006; 117: 698–703. [DOI] [PubMed] [Google Scholar]
- 14.Dutkiewicz AS, Ezzedine K, Mazereeuw-Hautier J, Lacour JP, Barbarot S, Vabres P, et al. A prospective study of risk for Sturge-Weber syndrome in children with upper facial port-wine stain. J Am Acad Dermatol. 2015; 72: 473–80. [DOI] [PubMed] [Google Scholar]