Cerebral cavernous malformations (CCM) or cavernomas are collections of structurally abnormal slow-flow capillaries predominantly in the central nervous system.1,2 These are multiple mulberry-like distended caverns of dilated thin-walled capillaries without the normal intervening brain parenchymal architecture. Often, individual cavernomas are surrounded by hemosiderin representing remote oozing due to the abnormal capillaries.3,4
CCMs have been reported to be the second most common vascular malformation of the central nervous system after developmental venous anomalies (DVA).1 The majority of CCM cases comprises a single lesion with or without associated DVA. These are called sporadic CCMs and are often asymptomatic and nonhereditary. The other type, called hereditary or familial CCM (FCCM), is due to autosomal dominant inherited genetic mutation, associated with multiple lesions. CCM can be found at multiple locations in the central nervous system with supratentorial lesions being more common than the infratentorial lesions.1 The purpose of this review is to summarize the basic and most updated understanding of FCCM and to enlighten the audience with the latest research insight and developments in this rapidly evolving field.
Epidemiology
While CCMs are a rare disease, they are the most prevalent form of cerebrovascular malformation after DVA.1,5,6 In recent literature, prevalence of CCM has been reported to be 0.1% to 0.8%.5,7 A population-based SIVMS (Scottish Intracranial Vascular Malformation Study) estimated an yearly incidence rate of 0.56 per 100 000 in individuals ≥16 years.8 Various other studies have also reported the prevalence of the disease to be 0.16% to 0.5%.2,9,10 Recently, a prospective population based imaging study by Mayo Clinic consisting of older adults between the ages 50 and 89 years found the prevalence of CCM in the population to be 0.46%, with men displaying a slightly higher incidence than women (0.51% and 0.41%, respectively).2 The estimated population prevalence of familial CCMs is 1/5000 to 1/10 000 (Orphanet), and a recent study from screening exome sequencing databases estimates the prevalence at 1/3300 to 1/3800 persons.11
The mean age of presentation for these vascular malformations has been reported to be around 37 years; however, they can present at any age.1 A study by the Mayo Clinic found that the prevalence of CCM associated with DVA increases with age.12 While there does not exist a plethora of information detailing the distribution of CCM patients in relationship to race or ethnicity, a significantly higher prevalence of FCCM in Hispanic populations (especially in the American Southwest) is the result of the common Hispanic mutation of CCM1.13 The Genome Aggregation Database, an extension of Exome Aggregation Consortium contains 123 136 exome and 15 496 whole-genome sequences of individuals from diverse ethnicities.11,14 In this database 13 of the 32 mutations in CCM1 (12/22), CCM2 (1/9), and CCM3 (0/1) have formerly been recognized and are already noted in the Human Gene Mutation Database as pathogenic (HGMD 2017.2). A common Hispanic mutation (CCM1: c.1363C>T, p.Gln455*) identified among the Latino cohort is prevalent in the Southwestern United States.15
Genetic Information
Three protein-encoding genes (CCM1, CCM2, and CCM3) are known to cause FCCM16,17 (Table 1). Mutation in just one of these genes is sufficient for causing disease, with changes most commonly resulting in a truncated protein, and in rare instances, missense mutations leading to protein misfolding.17 Research suggests that each of these 3 genes are part of a greater signaling pathway that regulates cell proliferation, network formation, and growth in the endothelial layer.17 Mutations in the CCM genes are thought to inhibit important surface interactions between these as well as with numerous binding partners.17 FCCM is inherited in an autosomal dominant fashion, with every affected parent having a 50% chance of passing down the mutation to their offspring.13 Although some research suggests the possibility of a fourth, as yet unidentified gene, a recent finding of a large genomic inversion of CCM2 challenges this idea.18
Table 1.
The FCCM Genes
| Locus Name | Gene | Chromosome Locus |
Protein | Function |
|---|---|---|---|---|
| CCM1 | KRIT1 | 7q21.2 | Krev interaction trapped protein 1 | Regulate heart and vessel formation and angiogenesis. Inhibits endothelial cells, apoptosis, migration, and angiogenesis |
| Alternative name(s): CCM 1 protein | ||||
| CCM2 | CCM2 | 7p13 | CCM 2 protein | Regulate heart and vessel formation and integrity |
| Alternative name(s): Malcavernin | Stabilize the endothelial cell junctions | |||
| CCM3 | PDCD10 | 3q26.1 | Programmed cell death protein 10 | Stimulate cell proliferation |
| Alternative name(s): CCM 3 protein or TF-1 cell apoptosis-related protein 15 | Regulates apoptotic pathway | |||
| Increase mitogen-activated protein kinase and STK26 activity | ||||
| Involved with KDR/VEGFR2 signaling | ||||
| Regulate cardiovascular development and required for angiogenesis, vasculogenesis and hematopoiesis during development |
CCM indicates cerebral cavernous malformation; FCCM, familial cerebral cavernous malformation; KDR/VEGFR2, vascular endothelial growth factor receptor 2 and KDR is the human gene encoding it; and STK26, serine/threonine kinase 26.
Since the identification of KRIT1, CCM2, and PDCD10 as disease-causing genes nearly 20 years ago, the molecular mechanism underlying CCM development has been intensely studied in the laboratory.19,20 How mutations in 3 distinct genes, coding for 3 nonhomologous proteins, cause a single clinical disease became clear when biochemical studies revealed that KRIT1, CCM2, and PDCD10 directly interact in a heterotrimeric adaptor complex (CCM complex).21,22 Early work on the role of the CCM complex in zebrafish, mice, and cultured endothelial cells uncovered numerous phenotypes and molecular pathways. In particular, gain of ROCK (Rho-kinase) signaling was hypothesized to be an important aspect of pathogenesis and has formed the basis for an National Institutes of Health–funded clinical trial for FCCM treatment (URL: https://www.clinicaltrials.gov. Unique identifier: NCT02603328).23–25
Clinical Symptomatology
In FCCM, a substantial number of cases (20%–50%) remain asymptomatic and are incidentally discovered during head imaging.26 Although reported in infancy and childhood, most of the FCCM patients present with symptoms during the second and fifth decades of their lives. These patients most commonly experience seizures (40%–70%), focal neurologic deficits (FND) without intracranial bleed (25%–50%), nonspecific headaches (10%–30%), and intracranial hemorrhage (ICH; 25%–32%) which might be either intralesional or extend beyond the lesion.16,27 The onset of symptoms in children with FCCM is usually earlier than in children with the sporadic form of the disease.
The recurrent microhemorrhages in CCM that result in nearby deposition of hemosiderin and gliosis and inflammation around the lesion are believed to be the cause of seizures in CCM patients.16 The risk of recurrent seizures after the first seizure is relatively high in patients of CCM compared with the general population.27 Both lesion site and number seem to correlate with risk of hemorrhage; deeper, infratentorial lesions are correlated with a higher risk than supratentorial lesions, and multiple lesions are also associated with an increased risk of bleeding.7
Studies have reported an annual ICH risk of 1.6% to 4.6% among the sample populations and estimated ICH risk of 0.1% to 1.4% for each lesion per year.28 An overall 5-year ICH risk of 15.8% has been estimated in CCM patients, with the yearly risk of recurring ICH decreasing with every passing year.29,30 This is an important factor clinically, which is taken into consideration when deciding the right treatment strategies for CCM patients. The 2 main risk factors identified for future re-bleed in CCM patients are the first episode of ICH and the brain stem lesion (hazard ratio, 5.6 and 4.4, respectively).30 Another study found that the most significant predictor of hemorrhage due to CCM was previous hemorrhage.7 When left untreated, brain stem CCMs have 2% to 60% higher rates of hemorrhages and can also lead to death from complications when treated surgically.16,27,30 Other factors include female sex, size of the cavernoma, and its number.29 Although age does not seems to have an impact, ICH occurrence and an increased lifetime hemorrhage risk has been detected in the younger age group of FCCM patients.27 A study of Hispanic FCCM patients found that obese patients had a statistically significant link with fewer lesions, that older patients had an increased risk of developing multiple lesions, and that hypertension was not a risk factor for multiple lesions.13
Management in Patients With FCCM
The management and treatment of CCMs is complex, and determining the right treatment approach depends on multiple factors (Figure 1). CCMs can be managed conservatively or may require microsurgical resection or stereotactic radiosurgery. Genetic testing and counseling play a vital role in the management of FCCM cases as discussed ahead. CCMs represent 5% to 15% of all cerebral vascular malformations, and therefore should be distinguished both clinically and on diagnostic imaging from other vascular anomalies such as capillary telangiectasias, venous malformations, arteriovenous malformations, vascular tumors (hemangioblastomas), and Sturge-Weber syndrome.16,27
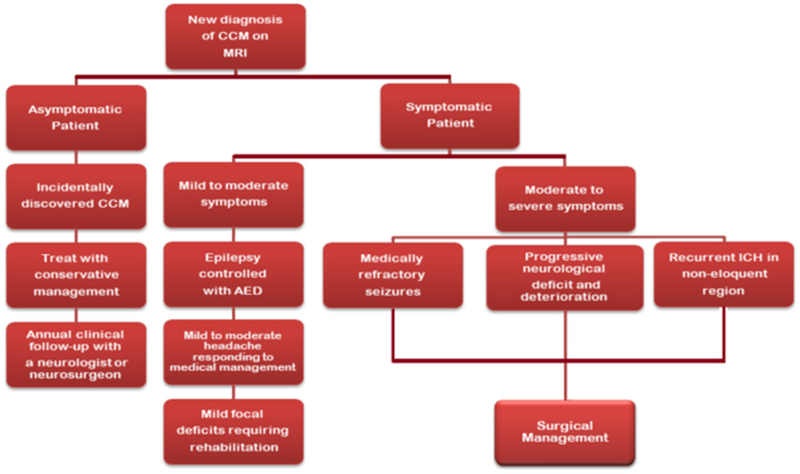
Figure 1.
Flow chart showing the management for the symptomatic and asymptomatic cerebral cavernous malformation (CCM) patients. AED indicates antiepileptic drug; and MRI, magnetic resonance imaging.
Imaging Modalities and Surveillance
Diagnosis of CCMs can be a challenge as compared with other vascular diseases. They are not detectable on cerebral angiography as catheter angiography can only identify potential abnormal venous flow associated with CCMs. Similarly, small lesions may not be detected on computed tomography scan.16,27 Hence, magnetic resonance imaging (MRI) is the modality of choice for evaluating CCMs. MRI sequences should include typical T1- and T2-weighted sequences including T2 FLAIR and T2* sequences, preferably including susceptibility-weighted imaging (SWI) or similar susceptibility-sensitive sequence. Other advanced imaging techniques such as diffusion tensor imaging and task-based functional MRI can have a role in surgical planning/navigation. Dynamic Contrast-Enhanced Quantitative Perfusion (DCEQP) and Quantitative Susceptibility Mapping (QSM) are developing imaging techniques that have recently been used in research studies to quantitatively measure CCM permeability (DCEQP) and iron content (QSM).
Conventional T1- and T2-Weighted MR Imaging
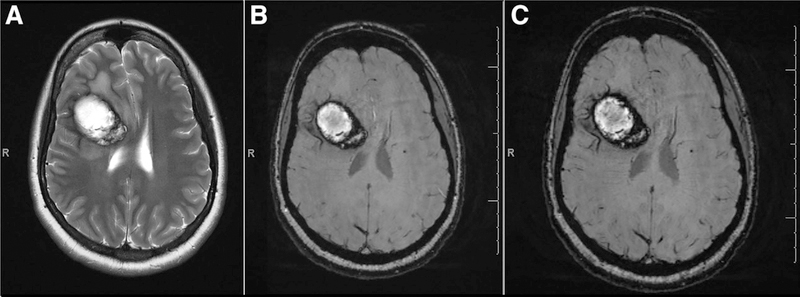
MRI has good sensitivity and specificity for CCMs and is considered to be the diagnostic modality choice.16,27,31 Larger CCMs often have the classic heterogeneous internal signal on conventional T1- and T2-weighted imaging (popcorn appearance), with a characteristic T2 hypointense ring, which is formed by the deposition of chronic blood breakdown products (hemosiderin) from prior hemorrhage/oozing31 (Figure 2). Based on their appearance on MRI and histopathology, the CCM lesions are divided into 4 characteristic types which are clinically useful in predicting hemorrhage32 (Table 2).
Figure 2.
A solitary cerebral cavernous malformation (CCM) with characteristic hypointense ring on (A) T2, (B) T2 *GRE, and (C) SWI. GRE indicates gradient recalled echo; and SWI, susceptibility-weighted imaging.
Table 2.
Classification of CCM by MRI and Histopathology
| Lesion | MR Signal | Histopathology |
|---|---|---|
| Type 1 | SE T1: Hyperintense core | Subacute hemorrhage, surrounding rim of hemosiderin-laden macrophages |
| SE T2: Hyperintense core or hypointense core | ||
| Type 2 | Most common type-classic popcorn lesion | Lesions with loculated hemorrhages and thromboses of varying ages enveloped by gliotic tissue, hemosiderin rim |
| SE T1: Mixed signal intensity centrally | ||
| SE T2: Mixed signal intensity centrally with surrounding hypointense/low signal rim with blooming | ||
| Type 3 | SE T1: Hypointense to isointense centrally | Chronic resolved hemorrhage with hemosiderin staining in and around lesion |
| SE T2: Hypointense lesion with hypointense rim with blooming/magnifying size of lesion | ||
| Type 4 | SE T1: Not seen or difficult to identify | Multiple punctate microhemorrhages |
| SE T2: Not seen or difficult to identify | Tiny CCM or telangiectasia | |
| T2* GRE: Punctate hypointense lesions, black dots with blooming | Small areas of hemosiderin deposition or possibly intravascular blood within telangiectasias or other small lesions | |
| SWI: Punctuate hypointense lesion (more sensitive than GRE) |
CCM indicates cerebral cavernous malformation; GRE, gradient recalled echo; MRI, magnetic resonance imaging; SE, spin echo MRI; and SWI, susceptibility-weighted imaging. Adapted and modified from Zabramski et al32 with permission.
T2* Gradient Recalled Echo MR Imaging and SWI
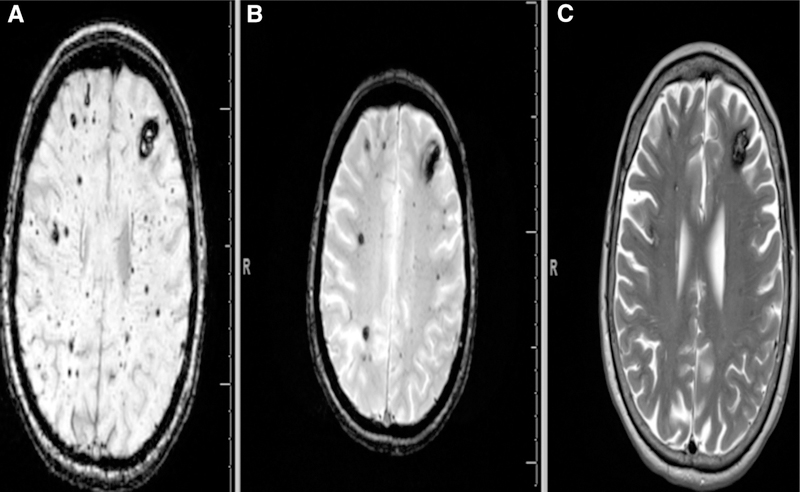
FCCM studies have demonstrated that T2*-weighted gradient recalled echo sequences are more sensitive for smaller CCMs than conventional T2 sequences33 (Figure 3; Figure 1 in the online-only Data Supplement). Ideally, imaging should include susceptibility-sensitive sequences such as SWI (Siemens) or similar sequences such as Susceptibility Weighted Angiogram (General Electric), which are even more sensitive for the detection of small (Type IV) CCMs than standard T2* gradient recalled echo and hence are considered a gold standard sequence for identifying and counting CCMs. A study on FCCMs by De Souza et al consisting of 15 patients demonstrated that SWI detected 1.7× more lesions than T2* gradient recalled echo34 (Figure 3). It is recommended that the MRI of suspected CCMs in brain and/or spinal cord should include SWI of the brain to confirm the lesion and evaluate for DVAs.27 If imaging studies reveal a solitary CCM associated with a DVA, there is more likelihood of a diagnosis of sporadic CCM, though, FCCM can rarely be associated with DVAs as well.3
Figure 3.
SWI is far more superior to T2*-weighted GRE MRI (B) and conventional T2 sequences (C) to detect smaller type-IV cerebral cavernous malformations (CCMs). Punctate hypointense lesions, black dots with blooming can be noticed both on SWI (A) and T2*-weighted GRE (B). The figure clearly demonstrates SWI to be more sensitive in identifying even small lesions in the same FCCM patient compared with T2*-weighted GRE which can identify most of the cavernomas except micro lesions. FCCM indicates familial cerebral cavernous malformation; GRE, gradient recalled echo; MRI, magnetic resonance imaging; and SWI, susceptibility-weighted imaging.
Dynamic Contrast-Enhanced Quantitative Perfusion and Quantitative Susceptibility Mapping
Two fundamental features of FCCM are vascular hyperpermeability and iron deposition at lesion site. DCEQP and QSM are developing imaging techniques that can be used for quantitative assessment of these pathophysiological phenomena of CCM. DCEQP is a T1-based perfusion technique, which can be used to measure increased vascular permeability in CCMs. QSM is a multiecho technique that can be used to quantify magnetic susceptibility, which is directly proportional to iron content and has been able to be applied to CCM.35–37 A 6% threshold increase in iron content as measured by QSM has been suggested as a biomarker in recently hemorrhagic CCMs.38 A strong correlation has been observed between the 2 techniques for measuring different end points of these 2 biological behaviors of CCMs, suggesting both to be interrelated.39 Therefore, DCEQP and QSM can potentially be used as objective and quantifiable biomarkers to monitor the disease course and the response to therapy and are promising imaging techniques currently being studied and applied in clinical trials.37–40
Follow-Up Imaging
Follow-up guidelines for CCMs are not well defined and are dependent upon multiple factors such as the patient’s insurance, patient preferences, and the treating neurologist or surgeons practice standards. The development of new neurological symptoms suggestive of ICH such as seizures, headache, changing neurological status, or FND warrant repeat imaging and should be performed as soon as possible to evaluate for any new CCM, hemorrhage, and edema.
Conservative Neurological Management
Patients with FCCM having small nonaggressive multiple lesions are usually managed conservatively, only alleviating the neurological symptoms, while the aggressive surgical approach is restricted for large solitary lesions found mostly in the sporadic form of disease.
In case of seizures, generally antiepileptic drugs after the first CCM-related seizure is indicated, while intractable epilepsy related to a specific cavernoma proven on prolonged EEG requires evaluation for surgical resection.16 If the seizures are secondary to ICH or in cases of noncompliant patients, surgery may be considered early to avoid further risk of future ICH.27 After the first diagnosis of CCM-related epilepsy, ≈50% to 60% of patients treated with antiepileptic drugs will become seizure free.41 A study comparing conservative management versus surgical treatment in 43 patients with nonrefractory seizures did not find any significant difference between the 2 approaches.42 The very low (<1% per year) risk of seizure in incidental CCM cases also justifies the adoption of conservative approach.1,26,41 However, the management is debatable between various experts (see the online-only Data Supplement).
For headaches including migraines, standard medical treatment is applied. In case of severe, persistent, or progressive headaches or when accompanying new or deteriorating FND, an urgent brain imaging is indicated and may require surgical intervention.16 An increased risk of hemorrhage has been reported with NSAIDs like ibuprofen, naproxen, and aspirin; therefore, patients with headaches and other acute or chronic pain should avoid these medications or substitute with appropriate alternatives.16 Because of addiction potential and association with rebound headaches, the use of narcotic analgesics is also discouraged.
Role of Genetic Testing and Counseling
As discussed, FCCM is the result of the functional loss in one of the 3 genes CCM1 (KRIT1), CCM2 (MGC4607), and CCM3 (PDCD10).5,16–18 These somatic mutations in the vascular endothelial cells include frameshift, nonsense, or splice site mutation, resulting in stop codon and dysfunctional mRNA. About 20% of all CCMs are familial and display an autosomal dominant inheritance pattern3,16,18 (Table I in the online-only Data Supplement). Incomplete penetrance and inconsistent presentation of the disease in families makes it difficult to accurately estimate the risks.5 FCCM cases mostly present with multiple lesions and a positive family history. When a new FCCM is diagnosed, family history must be enumerated up to third generation, with focus on new onset of clinical manifestations with evidence on diagnostic imaging.16,27 A consultation with a clinical geneticist and genetic counselor should be taken for further evaluation and counseling of the patient as well as the family. NextGen or Sanger sequencing analysis should be used along with deletion/duplication analysis for the CCM 1–3 genes.5,16,27
The CCM3 (PDCD10) mutations result in a more aggressive behavior as compared with CCM1 (KRIT1) and CCM2 (MGC4607) mutations. These mutations are more spontaneous, clinically associated with more severe disease course, and manifest at a younger age frequently accompanying ICH.43 They also seem to display close relation with other disease manifestations such as cutaneous vascular malformations, spinal cord cavernomas, scoliosis, and benign central nervous system tumor including meningioma, astrocytoma, and vestibular schwannoma.43 When it comes to genetic screening of asymptomatic individuals, all ethical issues and concerns must carefully be taken into consideration. In >5% of cases with multiple lesions or a positive family history, no pathogenic variant is identified in any of the 3 FCCM genes.16 In such cases an assumption of FCCM is made and MRI brain with gradient recalled echo or SWI should be offered to siblings, offsprings, and parents.
Considerations in Pediatric CCMs
Nearly a quarter of all familial and sporadic CCM cases occur in the pediatric population, with most of the literature from this age group generally reporting on particular location as brain stem, spinal cord, and basal ganglia.18,44 When doing diagnostic imaging in children usually <6 years or having developmental disorders, sedation can be given to obtain accurate results. However, this can expose them to some additional risk. Genetic testing should be done in children before selecting any approach for the treatment.
Considerations in Pregnancy
If a female FCCM patient with multiple lesions is planning a pregnancy, the medical practitioner should discuss preimplantation genetic testing, discuss prenatal testing for high-risk pregnancy, and offer counseling to discuss family planning, potential risks to offspring, and reproductive option. Even though the decisions regarding prenatal testing entirely depend on the choice of the couple, it is appropriate for the doctor to discuss all these issues with them in detail before the pregnancy. If the patient or couple decide to move ahead with the pregnancy, in such cases, a baseline MRI 1 year before birth (few months before conception) is recommended to determine the locations of the lesions.16,45
Compared with pregnant women without any seizure disorder, pregnant women with epilepsy require closer neurology follow-up.16 Appropriate antiepileptic drugs along with their risks and benefits and folic acid supplementation should be discussed in detail before conception. MRI without contrast should be considered if the patient experiences severe acute headache, migraine, recent ICH in brain or spinal cord, FND, or worsening of seizures during pregnancy.27 Such patients require very close observation during pregnancy. However, the risk of an untreated maternal epileptic disorder usually outweighs the adverse risk to the fetus making antiepileptic coverage during pregnancy, a recommended practice.16
Neurosurgical Management
Solitary lesions, found in sporadic cases, can be surgically removed when associated with refractory seizures or FND from recurrent bleeding or mass effect. However, surgical intervention in multifocal FCCM cases, though controversial, can be justifiable when these lesions become symptomatic.16 Generally, surgery is not recommended for asymptomatic lesions, particularly when present in eloquent region, in deep brain, or in the brain stem. However, an early surgical intervention of the epileptic lesion must be deliberated for medically refractory CCM-related epilepsy.27,41 At surgery, removal of the blood stained golden margin between the malformation and normal appearing brain must also be removed to lessen the seizure incidence. Similarly, surgery is not recommended for brain stem lesions after a single disabling bleed.27 However, surgical intervention can be considered following a second symptomatic bleed after carefully evaluating the risk of early postsurgical outcome influencing the quality of life because these lesions might display a more aggressive course in future.27
Recent Update on Research Approaches Molecular Pathogenesis
In the last few years, significant advances have been made toward understanding the molecular pathogenesis of CCM. It is now understood that the CCM complex binds MEKK3, a mitogen-activated protein-kinase essential in endothelial cells, through a direct interaction between CCM2 and MEKK3.46,47 Recently, mouse disease models have demonstrated that the CCM complex counteractively regulates MEKK3 signaling.48 Loss of CCM complex results in the gain of endothelial MEKK3 signaling, consequently resulting in pathological overexpression of downstream transcription factors KLF2 and KLF4 and an increase in ROCK activity, comprising a molecular pathway required for lesion formation.49,50 It remains unclear how gain of endothelial KLF2/4 expression causes lesion formation, although downstream effectors of the MEKK3-KLF2/4 pathway have been proposed such as THBS1 (thrombospondin-1), ADAMTS-metalloproteases, BMP6, and endothelial to mesenchymal transition.49–52 Continued investigation of the MEKK3-KLF2/4 pathway is required to fully leverage the translational potential of this causal disease mechanism.
Gut Microbiome
If the CCM complex negatively regulates MEKK3 signaling in endothelial cells to prevent disease, what are the upstream activators of MEKK3 in the context of disease? A recent study unexpectedly found that endothelial TLR4 (Toll-like receptor 4) is a potent driver of MEKK3 signaling in CCM disease and that brain endothelial TLR4 activation likely occurs through the leak of lipopolysaccharide, derived from gut commensal gram-negative bacteria, into blood circulation.53 A translational study involving FCCM patients is underway that will attempt to correlate variations in the gut microbiome with disease burden. These results will be critical toward the development of gut microbiome-based therapies for CCM (eg, fecal transplant) as a strategy to permanently alter disease risk.
Plasma Biomarkers
Recently, several blood biomarkers have been suggested for monitoring the disease severity and its course.54–56 These include plasma levels of calciferol (25-hydroxyvitamin D) and non-HDL (high-density lipoprotein) cholesterol; inflammatory cytokines including IL-2 (interleukin 2), INF-γ (interferon gamma), TNF-α (tumor necrosis factor alpha), and IL-1β (interleukin 1 beta); and molecules like matrix metalloproteinase-2 and −9, intercellular adhesion molecule-1, VEGF (vascular endothelial growth factor), and endoglin.54,55 These biomarkers were found to be associated with the disease symptoms like bleeding and seizures, as well as the lesion growth and new lesion formation.54,55 Similarly, it was also found that the weighted linear combination of soluble CD14 (cluster of differentiation 14), IL-1β, VEGF, and soluble ROBO 4 (round-about guidance receptor 4) can be used to predict a symptomatic ICH or lesional expansion.56 All these peripheral plasma biomarkers seem to express a relationship with chronic or acute disease activity and correlate with disease aggressiveness and therefore may prove helpful in prognostication and stratification of FCCM cases in future clinical trials.
Potential Imaging Biomarker
A recent study found small focal calcifications (SFCs) in the adrenal glands identified on computed tomography scans to be common in FCCM patients.57 These SFCs were found to be more common in the left adrenal gland, and FCCM patients with SFCs had significantly more brain lesions than did those without SFCs (P<0.001). It is suggested that these SFCs might be a clinically silent manifestation of disease and can be used as an imaging biomarker in FCCM patients.57
Atorvastatin Treatment Trial
Currently, a trial (URL: https://www.clinicaltrials.gov. Unique identifier: NCT02603328) is underway to investigate the long-term effects of statin (atorvastatin) on CCM lesions. It is aimed to evaluate the lesion development and bleeding (as measured by iron deposition) by using QSM-MRI. The study will also use the DCEQP-MRI to assess the vascularity of the brain and CCM lesions.
Conclusions
Although there is no cure for FCCM to date, the field is rapidly advancing because of collaborative partnership among physicians, patients, advocacy groups, and in conjunction with public and private research funding. These are exciting times in the field of FCCM as nationally funded trial readiness project has already been launched and the next collaborative National Institutes of Health–funded prospective trial is expected to start within the next 12 months.
Supplementary Material
Acknowledgments
Sources of Funding
The work by the following authors is supported by U01 NS10415701 to Dr Zafar; U54 NS065705–06 to Drs Kim, Morrison, Lawton, Hart, Zafar, and Mabray; F30NS100252 to A.T. Tang; R01NS100949, P01NS092521, and R01HL094326 to Dr Kahn.
Footnotes
Disclosures
Drs Kim, Morrison, Hart, Zafar, and Mabray are supported by National Institutes of Health (NIH) grant U54 NS065705; A.T. Tang is supported by NIH-National Institute of Neurological Disorders and Stroke Fellowship award. The other authors report no conflicts.
References
- 1.Dalyai RT, Ghobrial G, Awad I, Tjoumakaris S, Gonzalez LF, Dumont AS, et al. Management of incidental cavernous malformations: a review. Neurosurg Focus. 2011;31:E5. doi: 10.3171/2011.9.FOCUS11211 [DOI] [PubMed] [Google Scholar]
- 2.Flemming KD, Graff-Radford J, Aakre J, Kantarci K, Lanzino G, Brown RD Jr, et al. Population-based prevalence of cerebral cavernous malformations in older adults: mayo clinic study of aging. JAMA Neurol. 2017;74:801–805. doi: 10.1001/jamaneurol.2017.0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen TA, Morrison LA, Schrader RM, Hart BL. Familial versus sporadic cavernous malformations: differences in developmental venous anomaly association and lesion phenotype. AJNR Am J Neuroradiol. 2010;31:377–382. doi: 10.3174/ajnr.A1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison L, Akers A. Cerebral cavernous malformation, familial In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , eds. Genereviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993–2019. [Google Scholar]
- 5.Haasdijk RA, Cheng C, Maat-Kievit AJ, Duckers HJ. Cerebral cavernous malformations: from molecular pathogenesis to genetic counselling and clinical management. Eur J Hum Genet. 2012;20:134–140. doi: 10.1038/ejhg.2011.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batra S, Lin D, Recinos PF, Zhang J, Rigamonti D. Cavernous malformations: natural history, diagnosis and treatment. Nat Rev Neurol. 2009;5:659–670. doi: 10.1038/nrneurol.2009.177 [DOI] [PubMed] [Google Scholar]
- 7.Mouchtouris N, Chalouhi N, Chitale A, Starke RM, Tjoumakaris SI, Rosenwasser RH, et al. Management of cerebral cavernous malformations: from diagnosis to treatment. ScientificWorldJournal. 2015;2015:808314. doi: 10.1155/2015/808314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC, et al. ; Scottish Intracranial Vascular Malformation Study Collaborators. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke. 2003;34:1163–1169. doi: 10.1161/01.STR.0000069018.90456.C9 [DOI] [PubMed] [Google Scholar]
- 9.Al-Holou WN, O’Lynnger TM, Pandey AS, Gemmete JJ, Thompson BG, Muraszko KM, et al. Natural history and imaging prevalence of cavernous malformations in children and young adults. J Neurosurg Pediatr. 2012;9:198–205. doi: 10.3171/2011.11.PEDS11390 [DOI] [PubMed] [Google Scholar]
- 10.Morris Z, Whiteley WN, Longstreth WT Jr, Weber F, Lee YC, Tsushima Y, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegler S, Rath M, Paperlein C, Felbor U. Cerebral cavernous malformations: an update on prevalence, molecular genetic analyses, and genetic counselling. Mol Syndromol. 2018;9:60–69. doi: 10.1159/000486292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brinjikji W, El-Masri AE, Wald JT, Flemming KD, Lanzino G. Prevalence of cerebral cavernous malformations associated with developmental venous anomalies increases with age. Childs Nerv Syst. 2017;33:1539–1543. doi: 10.1007/s00381-017-3484-0 [DOI] [PubMed] [Google Scholar]
- 13.Choquet H, Nelson J, Pawlikowska L, McCulloch CE, Akers A, Baca B, et al. Association of cardiovascular risk factors with disease severity in cerebral cavernous malformation type 1 subjects with the common Hispanic mutation. Cerebrovasc Dis. 2014;37:57–63. doi: 10.1159/000356839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. ; Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahoo T, Johnson EW, Thomas JW, Kuehl PM, Jones TL, Dokken CG, et al. Mutations in the gene encoding KRIT1, a Krev-1/rap1a binding protein, cause cerebral cavernous malformations (CCM1). Hum Mol Genet. 1999;8:2325–2333. [DOI] [PubMed] [Google Scholar]
- 16.Morrison L, Akers A. Cerebral cavernous malformation, familial. 2016. [Google Scholar]
- 17.Li X, Fisher OS, Boggon TJ. The cerebral cavernous malformations proteins. Oncotarget. 2015;6:32279–32280. doi: 10.18632/oncotarget.5443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegler S, Rath M, Hoffjan S, Dammann P, Sure U, Pagenstecher A, et al. First large genomic inversion in familial cerebral cavernous malformation identified by whole genome sequencing. Neurogenetics. 2018;19:55–59. doi: 10.1007/s10048-017-0531-7 [DOI] [PubMed] [Google Scholar]
- 19.Bergametti F, Denier C, Labauge P, Arnoult M, Boetto S, Clanet M, et al. ; Société Française de Neurochirurgie. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am J Hum Genet. 2005;76:42–51. doi: 10.1086/426952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laberge-le Couteulx S, Jung HH, Labauge P, Houtteville JP, Lescoat C, Cecillon M, et al. Truncating mutations in ccm1, encoding krit1, cause hereditary cavernous angiomas. Nat Genet. 1999;23:189–193. [DOI] [PubMed] [Google Scholar]
- 21.Fisher OS, Boggon TJ. Signaling pathways and the cerebral cavernous malformations proteins: lessons from structural biology. Cell Mol Life Sci. 2014;71:1881–1892. doi: 10.1007/s00018-013-1532-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Zhang R, Zhang H, He Y, Ji W, Min W, et al. Crystal structure of CCM3, a cerebral cavernous malformation protein critical for vascular integrity. J Biol Chem. 2010;285:24099–24107. doi: 10.1074/jbc.M110.128470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenkar R, Shi C, Austin C, Moore T, Lightle R, Cao Y, et al. RhoA kinase inhibition with fasudil versus simvastatin in murine models of cerebral cavernous malformations. Stroke. 2017;48:187–194. doi: 10.1161/STROKEAHA.116.015013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH, et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 2012;43:571–574. doi: 10.1161/STROKEAHA.111.625467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010;207:881–896. doi: 10.1084/jem.20091258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore SA, Brown RD Jr, Christianson TJ, Flemming KD. Long-term natural history of incidentally discovered cavernous malformations in a single-center cohort. J Neurosurg. 2014;120:1188–1192. doi: 10.3171/2014.1.JNS131619 [DOI] [PubMed] [Google Scholar]
- 27.Akers A, Al-Shahi Salman R, A Awad I, Dahlem K, Flemming K, Hart B, et al. Synopsis of guidelines for the clinical management of cerebral cavernous malformations: consensus recommendations based on systematic literature review by the angioma alliance scientific advisory board clinical experts panel. Neurosurgery. 2017;80:665–680. doi: 10.1093/neuros/nyx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikoubashman O, Di Rocco F, Davagnanam I, Mankad K, Zerah M, Wiesmann M. Prospective hemorrhage rates of cerebral cavernous malformations in children and adolescents based on MRI aappearance. AJNR Am J Neuroradiol. 2015;36:2177–2183. doi: 10.3174/ajnr.A4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011;30:E24. doi: 10.3171/2011.3.FOCUS1165 [DOI] [PubMed] [Google Scholar]
- 30.Horne MA, Flemming KD, Su IC, Stapf C, Jeon JP, Li D, et al. ; Cerebral Cavernous Malformations Individual Patient Data Meta-analysis Collaborators. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol. 2016;15:166–173. doi: 10.1016/S1474-4422(15)00303-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell PG, Jabbour P, Yadla S, Awad IA. Emerging clinical imaging techniques for cerebral cavernous malformations: a systematic review. Neurosurg Focus. 2010;29:E6. doi: 10.3171/2010.5.FOCUS10120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabramski JM, Wascher TM, Spetzler RF, Johnson B, Golfinos J, Drayer BP, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994;80:422–432. doi: 10.3171/jns.1994.80.3.0422 [DOI] [PubMed] [Google Scholar]
- 33.Rivera PP, Willinsky RA, Porter PJ. Intracranial cavernous malformations. Neuroimaging Clinics. 2003;13:27–40. [DOI] [PubMed] [Google Scholar]
- 34.de Souza JM, Domingues RC, Cruz LC Jr, Domingues FS, Iasbeck T, Gasparetto EL. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with t2-weighted fast spin-echo and gradient-echo sequences. AJNR Am J Neuroradiol. 2008;29:154–158. doi: 10.3174/ajnr.A0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann A, Bredno J, Wendland MF, Derugin N, Hom J, Schuster T, et al. Validation of in vivo magnetic resonance imaging blood-brain barrier permeability measurements by comparison with gold standard histology. Stroke. 2011;42:2054–2060. doi: 10.1161/STROKEAHA.110.597997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage. 2011;54:2789–2807. doi: 10.1016/j.neuroimage.2010.10.070 [DOI] [PubMed] [Google Scholar]
- 37.Tan H, Liu T, Wu Y, Thacker J, Shenkar R, Mikati AG, et al. Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Invest Radiol. 2014;49:498–504. doi: 10.1097/RLI.0000000000000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeineddine HA, Girard R, Cao Y, Hobson N, Fam MD, Stadnik A, et al. Quantitative susceptibility mapping as a monitoring biomarker in cerebral cavernous malformations with recent hemorrhage. J Magn Reson Imaging. 2018;47:1133–1138. doi: 10.1002/jmri.25831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikati AG, Tan H, Shenkar R, Li L, Zhang L, Guo X, et al. Dynamic permeability and quantitative susceptibility: related imaging biomarkers in cerebral cavernous malformations. Stroke. 2014;45:598–601. doi: 10.1161/STROKEAHA.113.003548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girard R, Fam MD, Zeineddine HA, Tan H, Mikati AG, Shi C, et al. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg. 2017;127:102–110. doi: 10.3171/2016.5.JNS16687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenow F, Alonso-Vanegas MA, Baumgartner C, Blümcke I, Carreño M, Gizewski ER, et al. ; Surgical Task Force, Commission on Therapeutic Strategies of the ILAE. Cavernoma-related epilepsy: review and recommendations for management–report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2013;54:2025–2035. doi: 10.1111/epi.12402 [DOI] [PubMed] [Google Scholar]
- 42.Fernández S, Miró J, Falip M, Coello A, Plans G, Castañer S, et al. Surgical versus conservative treatment in patients with cerebral cavernomas and non refractory epilepsy. Seizure. 2012;21:785–788. doi: 10.1016/j.seizure.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Shenkar R, Shi C, Rebeiz T, Stockton RA, McDonald DA, Mikati AG, et al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with PDCD10 mutations. Genet Med. 2015;17:188–196. doi: 10.1038/gim.2014.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter RW, Detwiler PW, Spetzler RF, Lawton MT, Baskin JJ, Derksen PT, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg. 1999;90:50–58. doi: 10.3171/jns.1999.90.1.0050 [DOI] [PubMed] [Google Scholar]
- 45.Witiw CD, Abou-Hamden A, Kulkarni AV, Silvaggio JA, Schneider C, Wallace MC. Cerebral cavernous malformations and pregnancy: hemorrhage risk and influence on obstetrical management. Neurosurgery. 2012;71:626–630; discussion 631. doi: 10.1227/NEU.0b013e31825fd0dc [DOI] [PubMed] [Google Scholar]
- 46.Zawistowski JS, Stalheim L, Uhlik MT, Abell AN, Ancrile BB, Johnson GL, et al. CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum Mol Genet. 2005;14:2521–2531. doi: 10.1093/hmg/ddi256 [DOI] [PubMed] [Google Scholar]
- 47.Fisher OS, Deng H, Liu D, Zhang Y, Wei R, Deng Y, et al. Structure and vascular function of MEKK3-cerebral cavernous malformations 2 complex. Nat Commun. 2015;6:7937. doi: 10.1038/ncomms8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z, Tang AT, Wong WY, Bamezai S, Goddard LM, Shenkar R, et al. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature. 2016;532:122–126. doi: 10.1038/nature17178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Z, Tang AT, Wong WY, Bamezai S, Goddard LM, Shenkar R, et al. Corrigendum: cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling. Nature. 2016;536:488. doi: 10.1038/nature18311 [DOI] [PubMed] [Google Scholar]
- 50.Cuttano R, Rudini N, Bravi L, Corada M, Giampietro C, Papa E, et al. KLF4 is a key determinant in the development and progression of cerebral cavernous malformations. EMBO Mol Med. 2016;8:6–24. doi: 10.15252/emmm.201505433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–496. doi: 10.1038/nature12207 [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Ramirez MA, Fonseca G, Zeineddine HA, Girard R, Moore T, Pham A, et al. Thrombospondin1 (TSP1) replacement prevents cerebral cavernous malformations. J Exp Med. 2017;214:3331–3346. doi: 10.1084/jem.20171178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang AT, Choi JP, Kotzin JJ, Yang Y, Hong CC, Hobson N, et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature. 2017;545:305–310. doi: 10.1038/nature22075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girard R, Khanna O, Shenkar R, Zhang L, Wu M, Jesselson M, et al. Peripheral plasma vitamin D and non-HDL cholesterol reflect the severity of cerebral cavernous malformation disease. Biomark Med. 2016;10:255–264. doi: 10.2217/bmm.15.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girard R, Zeineddine HA, Fam MD, Mayampurath A, Cao Y, Shi C, et al. Plasma biomarkers of inflammation reflect seizures and hemorrhagic activity of cerebral cavernous malformations. Transl Stroke Res. 2018;9:34–43. doi: 10.1007/s12975-017-0561-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Girard R, Zeineddine HA, Koskimäki J, Fam MD, Cao Y, Shi C, et al. Plasma biomarkers of inflammation and angiogenesis predict cerebral cavernous malformation symptomatic hemorrhage or lesional growth. Circ Res. 2018;122:1716–1721. doi: 10.1161/CIRCRESAHA.118.312680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strickland CD, Eberhardt SC, Bartlett MR, Nelson J, Kim H, Morrison LA, et al. Familial cerebral cavernous malformations are associated with adrenal calcifications on CT scans: an imaging biomarker for a hereditary cerebrovascular condition. Radiology. 2017;284:443–450. doi: 10.1148/radiol.2017161127 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.