Abstract
The core objective of the current study was to evaluate the effect of microencapsulation on the viability and stability of probiotic bacteria in yogurt and simulated gastrointestinal conditions. For this purpose, probiotic bacteria were encapsulated with sodium alginate and carrageenan by encapsulator. Yogurt was prepared with the incorporation of free and encapsulated probiotic bacteria and was analyzed for physicochemical, microbiological, and sensorial attributes. Encapsulation and storage exhibited a significant (p < .05) effect on different parameters of yogurt. An increasing trend in syneresis and acidity while a decreasing trend in viscosity, pH, viability, and stability were observed. The value of syneresis increased from 2.27 ± 0.17 to 2.9 ± 0.14 and acidity from 0.48 ± 0.04 to 0.64 ± 0.01 during 4 weeks of storage. The value of viscosity decreased from 3.68 ± 0.21 to 2.42 ± 0.09 and pH from 4.88 ± 0.31to 4.43 ± 0.36 during 28 days of storage. Unencapsulated (free) cells exhibited poor survival. The viable cell count of probiotic bacteria in the free‐state in yogurt was 9.97 logs CFU/ml at zero‐day that decreased to 6.12 log CFU/ml after 28 days. However, encapsulation improved the viability of the probiotics in the prepared yogurt and GIT. The cell count of probiotics encapsulated with sodium alginate and carrageenan was 9.91 logs CFU/ml and 9.89 logs CFU/ml, respectively, at zero‐day that decreased to 8.74 logs CFU/ml and 8.39 log CFU/ml, respectively. Free cells (unencapsulated) showed very poor survival. Similarly, during in vitro gastrointestinal assay, the survival rate of encapsulated probiotic bacteria in simulated gastric solution and intestinal solutions was higher than that of free cells. In the case of encapsulated bacteria, only 3 logs while for free cells, 7 log reduction was recorded. Sodium alginate microcapsules exhibited better release profile than carrageenan. Conclusively, microencapsulation improved the survival of probiotic bacteria in carrier food as well as in simulated gastrointestinal condition.
Keywords: encapsulation, gastrointestinal conditions, probiotics viability, yogurt
Encapsulation was done by encapsulator using two coating materials and was incorporated in yogurt in free and encapsulated forms. To access the viability, unencapsulated and encapsulated probiotics were subjected to simulated gastrointestinal conditions. The results indicated that encapsulation provide the protection in carrier food and in gastrointestinal conditions.

1. INTRODUCTION
The functional foods improve human health apart from the native nutritional value. The traditional concept of food has changed and now, consumers demand therapeutic and curative foods. Owing to this, there is a consistent rise in the demand for functional foods supplemented with probiotics. There is a high demand for the manufacturing of modern food products owing to their improved taste and health impacts (Fito et al., 2001; Yağcı & Göğüş, 2008). The awareness of the functional foods has increased their marketing demands throughout the world (Agrawal, 2005). In previous millennia, dairy foods containing probiotic bacteria have been investigated extensively. “Probiotics are the live microorganisms which when administered in adequate amounts confer health benefit on the host” Hill et al., 2014. Various functional dairy foodstuffs are manufactured through fermented milk, beverages, and yogurts. The manufacturing of food with added prebiotics is a successful application as means of probiotic bacteria (Hashemi, Gheisari, and Shekarforoush (2015); Da Silva, Fátima, Olbrich dos Santos, & Pinto Correia, 2015). Probiotics confer certain important health benefits when consumed as food components or supplements (Sanders & Marco, 2010). Probiotics refer to the wide range of microorganisms especially bacteria as well as yeast. Most widely studied group is lactic acid bacteria (LAB), and Lactobacillus acidophilus and bifidobacteria are considered to be the most important probiotics with beneficial possessions on the human gastrointestinal (GI) pathway (Burgain, Gaiani, Linder, & Scher, 2011). Probiotics are found to be present in the wider range of food products especially in dairy products (Balthazar et al., 2018; Stella et al., 2005).
To draw health benefits from probiotics, these must be present in sufficient amounts in foods, the recommended level of probiotics in the carrier foods ranges from 106 to 107 CFU/ml (Champagne, Ross, Saarela, Hansen, & Charalampopoulos, 2011). Probiotic viability in all type of food products is affected by many intrinsic and extrinsic aspects such as oxygen, postacidification in fermented products, pH, storage temperature, production of hydrogen peroxide, and harsh processing conditions (Shah, 2000). Freezing injury and oxygen toxicity decrease the viability and stability of probiotic bacteria in dairy products (Vasilyevich & Shah, 2008). The endurance of probiotic bacteria in the gastrointestinal situation is very important for health‐promoting properties of the probiotics (Shi, Li, Zhang, et al., 2013).
Various protection methods are existing that can improve the viability of probiotic bacteria; however, still, the survival and success rate is lower. Currently, the encapsulation system is gaining much attention as it improves the endurance of probiotic bacteria in carrier foods as well as in the GIT system (Kanmani et al., 2011). Microencapsulation is the method/technique by which an ingredient (solid, liquid, and gas) is coated with a wall material (Muzzafar & Sharma, 2018). The bioactive ingredient can be a living (probiotic) or nonliving (vitamin, minerals) Encapsulation with different encapsulating materials has successfully shown to protect probiotic bacterial in many fermented dairy products (Abghari et al., 2011). Among the available materials for encapsulation, the sodium alginate and chitosan are widely utilized because these materials are nontoxic, economical, and well easy to handle (Krasaekoopt, Bhandari, & Deeth, 2006). Many researchers have reported that encapsulating materials (chitosan, alginate, xanthan, carrageenan, starch gelatin, and vegetable gum) have a significant protective effect on the endurance of Bifidobacteria bifidum and L. acidophilus within carrier foods and gastrointestinal system. Double‐layered chitosan–alginate beads encourage the endurance of the cell in the substantially acidic products like the juice of pomegranate (Nualkaekul, Lenton, Cook, Khutoryanskiy, & Charalampopoulos, 2012).
Bifidobacterium and Lactobacillus are considered as important probiotics owing to their beneficial impacts on human health (Saxelin, Tynkkynen, Mattila‐Sandholm, & Vos, 2005). The therapeutic characteristics of some probiotic foods show dependence on the viability of these probiotic bacteria. Significance and selection of carrier foods for probiotics are of great importance. Among the carrier foods, dairy products are highly accepted by consumers and play an important role in enhancing the target delivery (Gill & Prasad, 2008). Fermentation of milk with probiotic bacteria decreases the mineral loss and improves the nutritional status of the dairy products (Nadelman et al., 2017). The starter culture, particularly Lactobacillus spp. isolated from the camel milk, can have more benefits in terms of the nutritional profile for the dairy products (Ayyash, Al‐Nuaimi, Al‐Mahadin, & Liu, 2018). Due to the great diversification of dairy products, the milk industry possesses the potential to proportionate an additional benefit to consumers' health (Balthazar et al., 2018).
Yogurt predominantly probiotic yogurts endorse many health benefits by providing natural nutrients. Probiotic yogurt is also enriched with intestinal microbiota that has a positive impact on intestinal microflora. (Cruz et al., 2013). Yogurt among fermented product is a unique and significant source for the induction of probiotics and prebiotics in the human diet. Therefore, there is a great potential for the development of functional foods like probiotic yogurt. Keeping in view the importance of probiotics and yogurt, the present study was designed to probe the effect of encapsulation on the viability of probiotics in food and simulated conditions. The objectives of the present study were to encapsulate the probiotics with cost–effective coating materials and evaluate their survival under different conditions.
2. MATERIALS AND METHODS
2.1. Probiotic culture activation
A pure culture of probiotic bacteria (L. acidophilus ATTC‐4356) was obtained in freeze‐dried form National Institute of Food Science & Technology, University of Agriculture Faisalabad. The activation was done by inoculating the culture in the MRS‐broth at 37°C for 24 hr. The probiotic cells were centrifuged and thereafter, washed in sterile saline solution with the same centrifugation process. The obtained probiotic cells were used in the encapsulation process. The cell concentration was adjusted at more than 109CFU/ml.
2.2. Encapsulation of probiotics
Lactobacillus acidophilus was encapsulated with sodium alginate and carrageenan microgels by adopting the method of Yeung, (Yeung, Arroyo‐Maya, McClements, & Sela, 2016) with slight modification. Solutions of carrageenan (2.0% w/v) and sodium alginate (2.0% w/v) were prepared and sterilization was done at 121°C (15psi) for 15 min. The germ‐free solutions of polysaccharides were combined with 2 ml of 109 CFU/ml probiotic organisms present in a physiological saline solution. The beads were encapsulated aseptically by using an encapsulator (B‐390, Buchi‐Switzerland) under standard operating conditions. Liquors of encapsulating materials were injected into hardening solutions of 0.1 M calcium chloride solution. After an hour, beads were obtained by the filtration process and washed by sterile deionized water. The beads were stored in physiological saline solution at 4°C for future use.
2.3. Characterization of beads
The size or diameter of the encapsulated cells was determined by stage micrometer. For the determination of size and shape, the prepared beads were examined under a light microscope.
2.4. Entrapment efficiency
The efficiency of the encapsulation of probiotic bacteria was investigated according to the method followed by Chavarri (Chávarri et al., 2010) with slight modifications. Encapsulated probiotics were disintegrated in phosphate buffer by using stomacher (Seward, UK), and afterward, the amount of entrapped cells was calculated by the pour plate technique. The results were expressed as a number of colonies forming units/bead (CFU/bead).
The value of encapsulated efficiency was evaluated by using the following relationship:
where, N is the number of viable entrapped cells released from the encapsulated beads. N0 is the number of free cells added to the biopolymer mixture prior to encapsulation.
2.5. In vitro studies
Survival of free and encapsulated probiotics under simulated conditions.
The viability/survival of free and encapsulated probiotics was assessed by adopting the method of Damodharan (Damodharan, Palaniyandi, Yang, & Suh, 2017) with slight modification. Briefly, the simulated solution of gastric and intestinal was prepared. The simulated gastric juice was prepared by adding 0.03 M phosphate buffer and 10 mg/ml pepsin in test tube containing warmed water. The pH was adjusted to 2 by using I M HCL. The prepared solution was mixed with free and encapsulated probiotics and incubated at 37°C for a described period of interval. The preparation of simulated intestinal juice was carried out as previously described. In short, 0.03 M phosphate buffer, 10 mg/ml trypsin, and 0.3% bile salt were added to sterilized water. pH was adjusted to 7.5 by using 1 M NaOH. The prepared solution was mixed with free and encapsulated probiotics and incubated at 37°C. The survival of encapsulated and free suspending cells was recorded at predetermined time periods (0, 30, 60, 90, and 120 min).
2.6. Manufacturing of probiotic yogurt
Yogurt was manufactured by the following method as described by Schoina et al., (2015). Commercial pasteurized and homogenized milk was purchased from the local market. Milk analysis was carried out by following (AOAC 2006) methods. Milk was heated and cooled down to 45°C. The milk was poured in already germ‐free cups (100 ml each) and kept in incubator for fermentation at 42–45°C for 6–8 hr. Each sample was inoculated with 1:1 proportion of yogurt culture that is Streptococcus thermophiles and L. bulgaricus. The probiotic yogurt was manufactured by adding probiotics to unencapsulated (free) and encapsulated (sodium alginate and carrageenan). 0.1 g (approx10.45 CFU) of microbeads was added to yogurt sample. The following treatments were made T1. Control, T2: Yogurt containing free cells, T3: Yogurt containing encapsulated L. acidophilus with alginate, T4: Yogurt containing encapsulated L. acidophilus with carrageenan.
2.7. Product analysis
Probiotic yogurt was subjected to microbiological, physicochemical, and sensorial analysis. Yogurt was analyzed for pH, lactose, viscosity, viability, and storage stability.
2.8. Enumeration of storage stability and viability of free and encapsulated probiotics in yogurt
Enumeration of probiotic bacteria was performed following the protocol used by Pinto et al., (2012). Yogurt was stored at 4°C and was analyzed with an interval of 0, 7, 14, 21, and 28 days. Yogurt samples of all treatments were taken to calculate the viable cells. Yogurt samples of all treatments were diluted to 100 ml with saline solution and mixed with a stomacher and spread on MRS medium, incubated at 37°C for 48 hr. The viable count was calculated. Experiments were repeated for three times (AOAC, 2006).
2.9. pH
It was determined by using a digital pH meter. pH meter was calibrated by using a buffer solution (Granato, Araújo Calado, & Jarvis, 2014).
2.10. Viscosity
Viscosity was determined by using viscometer (Brookfield LVDVE‐230) by adopting a method of Amerinasab, Labbafi, Mousavi, & Khodaiyan, (2015). All readings were recorded in centipoise (cp) unit.
2.11. Syneresis
Syneresis was determined by the method described by Ayar & Gurlin, (2014).
2.12. Sensory analysis
Sensory evaluation of yogurt was carried out by adopting the protocol described by Sheu & Marshall, (1993). Sensory evaluation was done by expert panellists of Department of Food Sciences. All of the judges were nonsmokers and trained for evaluating dairy products by using a 9‐point hedonic scale for taste, flavor, color, body, texture, appearance, and overall acceptance.
2.13. Statistical analysis
The data obtained for each parameter were subjected to analysis of variance (ANOVA). Data were compared by a two‐way analysis of variance. All results are expressed as mean ± SD. Differences were considered significant at p\.05. Four replicates of each treatment were analyzed with predefined intervals.
3. RESULTS AND DISCUSSION
3.1. Encapsulation entrapment efficiency
It has been reported that coating with wall materials improves the survival and stability of probiotics in processing and in simulated gastrointestinal conditions (Iyer & Kailasapathy 2005). The effectiveness and survival of probiotics depend on the type of probiotic and the used wall materials. A variation in encapsulation yield was observed in sodium alginate and carrageenan as a coating material (Table 1). Statistically, the yield of the L. acidophilus cells encapsulated with sodium alginate was higher than those of encapsulated with carrageenan. In Table 1, a comparison of the encapsulation efficiency between two coatings can be observed that showed a significant difference (p < .05).
Table 1.
Encapsulation efficiency
| Type of matrix | Numbers before encapsulation | Numbers after encapsulation | Efficiency (%) |
|---|---|---|---|
| Sodium Alginate | 8.68 ± 0.06 | 8.47 ± 0.04 | 98% |
| Carrageenan | 8.44 ± 0.04 | 8.13 ± 0.05 | 96% |
3.2. Mean diameter of the encapsulated beads
The mean values for the diameter of the encapsulated beads were recorded, and the data showed that beads prepared with sodium alginate had a mean value of 714 µm while for carrageenan beads had mean diameter of 726 µm. The obtained results clearly showed that the diameter of beads is affected by the type of coating materials. A similar finding was found earlier by Shi et al., (2013). Encapsulating materials, as well as the diameter of the capsules, affect the viability of the probiotics. Undue reduction in diameter can remove the protective function of encapsulation, whereas, increasing capsule diameter decreases digestibility by pancreatic enzymes.
3.3. Stability of the encapsulated probiotic bacteria in simulated gastric conditions
Cell viability in the stomach and intestinal conditions is important in order to get the desired benefits of probiotics. Free and encapsulated cells were exposed to activated gastric juice. An instant decrease was examined in free cells in contrast to the cells encapsulated with sodium alginate and carrageenan (Figure 1 ). The sodium alginate capsules showed 3.57 log reductions while carrageenan capsules showed 4.28 log reduction as compared to unencapsulated cells that is 8.18 log. The encapsulation of the cells within either sodium alginate or carrageenan significantly affected (p < .05) on the survival of probiotic bacteria. The results demonstrated that encapsulation provides protection to probiotics in simulated gastric conditions. The results are also in accordance with Qi, Liang, Yun, & Guo, (2019) who found 60% increased survival rate of probiotics under simulated gastrointestinal conditions compared to free cells (25%) and concluded that the encapsulation by using biopolymers offers an effective way to protect probiotics in adverse processing and in vitro conditions.
Figure 1.
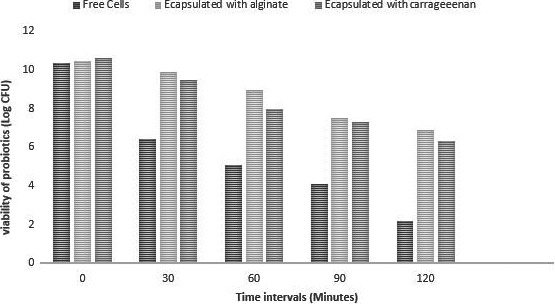
Survival of free and encapsulated probiotics under simulated gastric conditions. Probiotic survival (Log 10 CFU/ml) of free (unencapsulated) and encapsulated with (sodium alginate and carrageenan) in simulated gastric conditions in time interval (0, 30, 60, 90, 120 min)
3.4. Stability of the encapsulated probiotic in intestinal conditions (pH 7.5)
Different encapsulating materials showed a protective effect on probiotics when exposed to intestinal conditions. In the current study, free and encapsulated cells were exposed to the simulated intestinal artificial solution for a specific interval of time. A rapid reduction in unencapsulated cells was observed as compared to the encapsulated probiotics at pH 7.5 (Figure 2). The encapsulation of the cells with either sodium alginate or carrageenan had a statistically significant effect (p < .05) on cell survival. The sodium alginate and carrageenan capsules showed slow log reduction as compared to free cells. The present findings are also in accordance with Shah and Ravula, (2000), who reported that alginate coating improves the release and survival in GIT conditions. The results demonstrate that encapsulation with biopolymers is a useful tool for the longevity of sensitive ingredients. The results are also in accordance with Qi et al., (2019) who found 60% increased survival rate of probiotics under simulated gastrointestinal conditions compared to free cells (25%) and concluded that the encapsulation by using biopolymers offers an effective way to protect probiotics in adverse processing and in vitro conditions.
Figure 2.
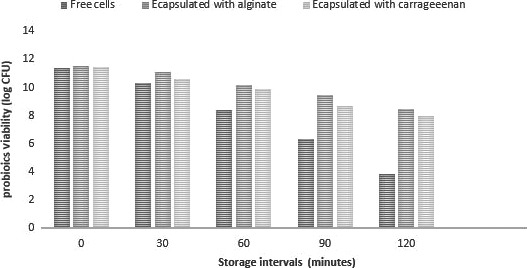
Survival of free and encapsulated probiotics under simulated intestinal conditions. Probiotic viability (Log 10 CFU/ml) of free (unencapsulated) and encapsulated with (sodium alginate and carrageenan) in simulated intestinal conditions in time interval (0, 30, 60, 90, 120 min)
3.5. Product analysis
Prepared yogurt was subjected to various physicochemical and microbiological analyses to assess the effect of coating materials on different attributes.
3.6. The viability of free (unencapsulated) and encapsulated probiotic bacteria in yogurt
The probiotic bacteria were encapsulated with alginate and carrageenan and incorporated into yogurt to evaluate their stability in this dairy product. The added probiotics were enumerated from day one and continued with an interval of 7 days until 28 days of storage (Figure 3). Free/unencapsulated probiotic bacteria showed a rapid drop in log reduction from day one to 28 days of storage at −20°C; whereas, probiotic bacteria encapsulated in alginate as well as carrageenan showed slow log reduction. The obtained results demonstrated that the numbers of viable probiotic bacterial cells decreased during storage. Further, it has been observed that the death rate of encapsulated cells was statistically lower as compared to free cells in yogurt. Similar results were found by Homayouni, Azizi, Ehsani, Yarmand, & Razavi, (2008) that encapsulation improves the survival of probiotics in the frozen dairy products. The results are also in accordance with Qi et al., (2019) who found 60% increased survival rate of probiotics under simulated gastrointestinal conditions compared to free cells (25%) and concluded that the encapsulation by using biopolymers offers an effective way to protect probiotics in adverse processing and in vitro conditions. These results are also in accordance with findings of Iqbal et al. (2018) who reported survival of probiotics in yogurt. According to Andleeb and Vasudha (Muzzafar & Sharma, 2018) who used different coating materials, used and reported a better survival of encapsulated probiotics in carrier food as compared to unencapsulated cells. Another study reported by Qi et al., (2019) showed the better survival of encapsulated probiotics in yogurt. The number of viable cells in case of encapsulation was decreased by just 1.76 log CFU/g while in case of free were decreased by 4.82 log CFU/g. In a study conducted by Pradeep Prasanna & Charalampopoulos, (2019), the results revealed that significant loss (3.67 log CFU/g) in the survival of unencapsulated probiotics was recorded as compared to encapsulated cells over a period of 28 days.
Figure 3.
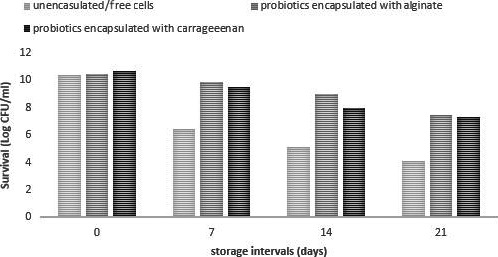
Viability of free and encapsulated probiotics in yogurt. Viability (Log 10 CFU/ml) of free (unencapsulated) and encapsulated (with Sodium alginate and carrageenan) probiotic bacteria (Lactobacillus acidophilus) in yogurt during storage intervals (0, 7, 14, 21 days). Each bar represents mean value for the viability of probiotics
3.7. Effect of free and encapsulated probiotic bacteria on the pH of the yogurt
The pH of probiotic carrier food has a direct relationship with the survival of the probiotic bacteria. The low pH causes a decrease in the survival rate of the probiotics. A decline in pH was observed during storage in all types of yogurt over a period of 28 days of storage (Figure 4). Encapsulated probiotic bacteria cause slower postacidification as compared to unencapsulated cells (Muzzafar & Sharma, 2018).
Figure 4.
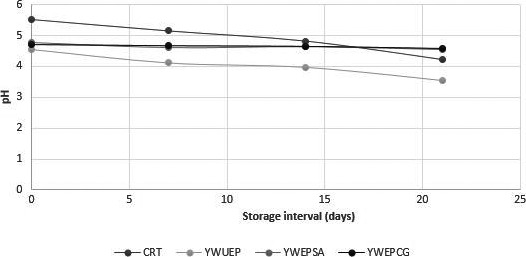
Effect of Free & encapsulated probiotics on pH of yogurt. Effect of free (unencapsulated) and encapsulated (with sodium alginate and carrageenan) on pH of yogurt during storage intervals (0, 7, 14, 21 days) compared with control. Each bar represents mean value for pH of treatments. CRT‐Control; YWUEP‐yogurt with unencapsulated probiotics; YWEPSA‐yogurt with encapsulated probiotics Sodium alginate YWEPCG‐yogurt with encapsulated probiotic carrageenan
3.8. Effect of free and encapsulated probiotic bacteria on the viscosity of yogurt
A decreasing trend in viscosity of all types of yogurt was observed. The decrease in viscosity could be due to an increase in syneresis during storage. A significant difference in viscosity was observed in all treatments. The highest viscosity was observed in treatment containing carrageenan beads. This could be due to the thickening and stabilizing properties of the biopolymers.
3.9. Effect of free and encapsulated probiotic bacteria on the syneresis of yogurt
An increasing trend in syneresis of all types of yogurt was observed. The increase in syneresis could be due to the conversion of solid contents of yogurt into different metabolites by probiotic bacteria during storage. A significant difference in syneresis was observed in all treatments (Figure 5). The highest syneresis value was observed in treatment containing free or unencapsulated probiotics.
Figure 5.
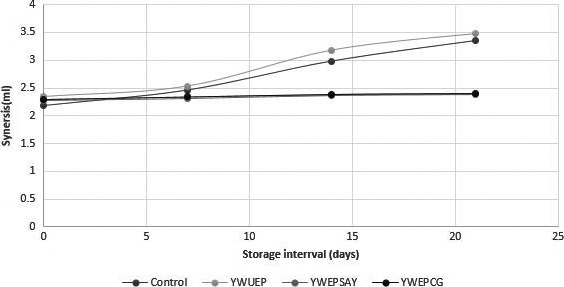
Effect of free and encapsulated probiotics on synersis of yogurt. Effect of free (unencapsulated) and encapsulated (with sodium alginate and carrageenan) on synersis of yogurt during storage intervals (0, 7, 14, 21 days) compared with control. Each bar represents mean value for viscosity of treatments. CRT‐Control; YWUEP‐yogurt with unencapsulated probiotics; YWEPSA‐Yogurt with encapsulated probiotics Sodium alginate YWEPCG‐Yogurt with encapsulated probiotic carrageenan
3.10. Sensory analysis
Sensorial characteristics of the products critically affect the acceptance of the newly developed product, and it has become a big challenge for the food industry. The overall acceptability of the prepared yogurt in terms of color, texture, and taste of free and encapsulated probiotic yogurt was noted (Figure 6). From the sensory analysis, it was concluded that the incorporation of free and encapsulated probiotics had significantly affected the sensory properties of yogurt.
Figure 6.
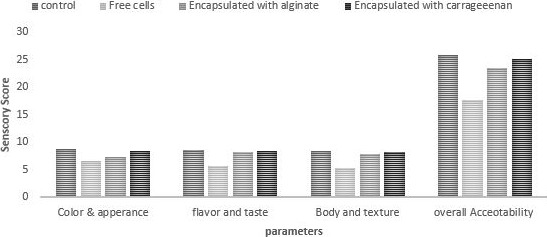
Effect of free and encapsulated probiotics on sensory attributes of yogurt. Effect of free (unencapsulated) and encapsulated (with sodium alginate and carrageenan) on sensory attributes (color, appearance, flavor, taste, body, texture and overall acceptability) of yogurt during storage intervals compared with control. CRT‐Control; YWUEP‐yogurt with unencapsulated probiotics; YWEPSA‐Yogurt with encapsulated probiotics Sodium alginate; YWEPCG‐yogurt with encapsulated probiotic carrageenan
4. CONCLUSIONS
The findings of the recent study show that encapsulation with both type of polymers has a significant effect on the probiotic viability under simulated gastrointestinal conditions and in carrier food (yogurt). A milder postacidification in case of yogurt containing encapsulated probiotics was observed. Encapsulation had significantly affected the physicochemical and sensory attributes of the yogurt. The results revealed that encapsulation with sodium alginate and carrageenan could be used to enhance the survival of probiotics. Microencapsulation is useful technology to ensure the recommended therapeutic level (106–108 CFU/g) of probiotics in carrier food. Microencapsulation must be adopted by the food industry to ensure the viability of probiotics in functional foods.
5. RECOMMENDATIONS
The microencapsulation using sodium alginate and carrageenan must be adopted by food industry for large‐scale production of microbeads for incorporation of functional foods.
CONFLICT OF INTEREST
Authors declare that they have no conflict of interest.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
ACKNOWLEDGMENTS
The authors are thankful to University of Gambia and Institute of Home & Food Sciences Government College University Faisalabad, Pakistan. The Authors also extend their appreciation to the International Scientific Partnership Program (ISPP) at King Saud University from Project No. 0023.
Afzaal M, Khan AU, Saeed F, et al. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci Nutr. 2019;7:3931–3940. 10.1002/fsn3.1254
Contributor Information
Farhan Saeed, Email: f.saeed@gcuf.edu.pk.
Faqir Muhammad Anjum, Email: dranjum@utg.edu.gm.
REFERENCES
- Abghari, A. ,Sheikh‐Zeinoddin M., & Soleimanian‐Zad, S. (2011). Nonfermented ice cream as a carrier for Lactobacillus acidophilus and Lactobacillus rhamnosus . International Journal of Food Science & Technology, 46(1), 84–92. [Google Scholar]
- Agrawal, R. (2005). Probiotics: An emerging food supplement with health benefits. Food Biotechnology, 19(3), 227–246. 10.1080/08905430500316474 [DOI] [Google Scholar]
- Amerinasab, A. , Labbafi, M. , Mousavi, M. , & Khodaiyan, F. (2015). Development of a novel yogurt based on date liquid sugar: Physicochemical and sensory characterization. Journal of Food Science and Technology, 52(10), 6583–6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC (2006). Official Methods of Analysis of Association of Official Analytical Chemists International. In: Horwitz, W. (Ed.), 18th ed. AOAC Press, Washington, DC. USA. Karimi, R., Mortazavian, A.M. and Amiri‐Rigi, A., 2012. Selective enumeration of probiotic microorganisms in cheese. Food microbiology, 29(1), pp.1‐9 [DOI] [PubMed] [Google Scholar]
- Ayar, A. , & Gurlin, E. (2014). Production and sensory, textural, physico‐chemical properties of flavored spreadable yogurt. Life Science Journal, 11(4), 58–65. [Google Scholar]
- Ayyash, M. , Al‐Nuaimi, A. K. , Al‐Mahadin, S. , & Liu, S. Q. (2018). In vitro investigation of anticancer and ACE‐inhibiting activity, α‐amylase and α‐glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chemistry, 239, 588–597. [DOI] [PubMed] [Google Scholar]
- Balthazar, C. F. , Silva, H. L. , Esmerino, E. A. , Rocha, R. S. , Moraes, J. , Carmo, M. A. , … Franco, R. M. (2018). The addition of inulin and Lactobacillus casei 01 in sheep milk ice cream. Food Chemistry, 246, 464–472. 10.1016/j.foodchem.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Balthazar, C. F. , Silva, H. L. , Esmerino, E. A. , Rocha, R. S. , Moraes, J. , Carmo, M. A. , & Franco, R. M. (2018). The addition of inulin and Lactobacillus casei in sheep milk ice cream. Food Chemistry, 246, 464–472. [DOI] [PubMed] [Google Scholar]
- Burgain, J. , Gaiani, C. , Linder, M. , & Scher, J. (2011). Encapsulation of probiotic living cells: From laboratory scale to industrial applications. Journal of Food Engineering, 104(4), 467–483. 10.1016/j.jfoodeng.2010.12.031 [DOI] [Google Scholar]
- Champagne, C. P. , Ross, R. P. , Saarela, M. , Hansen, K. F. , & Charalampopoulos, D. (2011). Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. International Journal of Food Microbiology, 149(3), 185–193. 10.1016/j.ijfoodmicro.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Chávarri, M. , Marañón, I. , Ares, R. , Ibáñez, F. C. , Marzo, F. , & del Carmen, V. M. (2010). Microencapsulation of a probiotic and prebiotic in alginate‐chitosan capsules improves survival in simulated gastro‐intestinal conditions. International Journal of Food Microbiology, 142(1), 185–189. [DOI] [PubMed] [Google Scholar]
- Cruz, A. G. , Cadena, R. S. , Castro, W. F. , Esmerino, E. A. , Rodrigues, J. B. , Gaze, L. , … Bolini, H. M. A. (2013). Consumer perception of probiotic yogurt: Performance of check all that apply (CATA), projective mapping, sorting and intensity scale. Food Research International, 54(1), 601–610. [Google Scholar]
- Da Silva, P. D. L. , de Fátima, B. M. , Olbrich dos Santos, K. M. , & Pinto Correia, R. T. (2015). Potentially probiotic ice cream from goat’s milk: Characterization and cell viability during processing, storage and simulated gastrointestinal conditions. Food Science and Technology, 62(1), 452–457. 10.1016/j.lwt.2014.02.055 [DOI] [Google Scholar]
- Damodharan, K. , Palaniyandi, S. A. , Yang, S. H. , & Suh, J. W. (2017). Co‐encapsulation of lactic acid bacteria and prebiotic with alginate‐fenugreek gum‐locust bean gum matrix: Viability of encapsulated bacteria under simulated gastrointestinal condition and during storage time. Biotechnology and Bioprocess Engineering, 22(3), 265–271. [Google Scholar]
- Fito, P. , Chiralt, A. , Betoret, N. , Gras, M. , Cháfer, M. , Martínez‐Monzó, J. , … Vidal, D. (2001). Vacuum impregnation and osmotic dehydration in matrix engineering. Journal of Food Engineering, 49(2‐3), 175–183. 10.1016/S0260-8774(00)00220-X [DOI] [Google Scholar]
- Gill, H. , & Prasad, J. (2008). Probiotics, immunomodulation, and health benefits in bioactive components of milk (pp. 423–454). New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- Granato, D. , de Araújo Calado, V. M. , & Jarvis, B. (2014). Observations on the use of statistical methods in food science and technology. Food Research International, 55, 137–149. [Google Scholar]
- Hashemi, M. , Gheisari, H. R. , & Shekarforoush, S. (2015). Preparation and evaluation of low‐calorie functional ice cream containing insulin, lactulose and Bifidobacterium lactis . International Journal of Dairy Technology, 68, 183–189. [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G. R. , Merenstein, D. J. , Pot, B. , & Calder, P. C. (2014). Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews. Gastroenterology & Hepatology, 11(8), 506. [DOI] [PubMed] [Google Scholar]
- Homayouni, A. , Azizi, A. , Ehsani, M. R. , Yarmand, M. S. , & Razavi, S. H. (2008). Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chemistry, 111(1), 50–55. [Google Scholar]
- Iqbal, R. , Zahoor, T. , Huma, N. , Jamil, A. , & Ünlü, G. (2018). In‐vitro GIT tolerance of microencapsulated Bifidobacterium bifidum ATCC 35914 using polysaccharide‐protein matrix. Probiotics Antimicrob Proteins 1–10. [DOI] [PubMed] [Google Scholar]
- Iyer, C. , Kailasapathy, K. (2005). Effect of co‐encapsulation of probiotics with prebiotics on increasing the viability of encapsulated bacteria under in vitro acidic and bile salt conditions and in yogurt. Journal of Food Science, 70(1), 18–23. [Google Scholar]
- Kanmani, P. , Kumar, R. S. , Yuvaraj, N. , Paari, K. A. , Pattukumar, V. , & Arul, V. (2011). Cryopreservation and microencapsulation of a probiotic in alginate‐chitosan capsules improve survival in simulated gastrointestinal conditions. Biotechnology and Bioprocess Engineering, 16(6), 1106–1114. [Google Scholar]
- Krasaekoopt, W. , Bhandari, B. , & Deeth, H. C. (2006). Survival of probiotics encapsulated in chitosan‐coated alginate beads in yogurt from UHT‐and conventionally treated milk during storage. LWT‐ Food Science and Technology, 39(2), 177–183. [Google Scholar]
- Muzzafar, A. , & Sharma, V. (2018). Microencapsulation of probiotics for incorporation in cream biscuits. Journal of Food Measurement and Characterization, 12(3), 2193–2201. [Google Scholar]
- Nadelman, P. , Frazao, J. V. , Vieira, T. I. , Balthazar, C. F. , Andrade, M. M. , Cruz, A. G. , & Maia, L. C. (2017). The performance of probiotic fermented sheep milk and ice cream sheep milk in inhibiting enamel mineral loss. Food Research International, 97, 184–190. 10.1016/j.foodres.2017.03.051 [DOI] [PubMed] [Google Scholar]
- Nualkaekul, S. , Lenton, D. , Cook, M. T. , Khutoryanskiy, V. V. , & Charalampopoulos, D. (2012). Chitosan coated alginate beads for the survival of microencapsulatedLactobacillus plantarum in pomegranate juice. Carbohydrate Polymers, 90(3), 1281–1287. [DOI] [PubMed] [Google Scholar]
- Pinto, S. S. , Fritzen‐Freire, C. B. , Muñoz, I. B. , Barreto, P. L. , Prudêncio, E. S. , & Amboni, R. D. (2012). Effects of the addition of microencapsulated Bifidobacterium BB‐12 on the properties of frozen yogurt. Journal of Food Engineering, 111(4), 563–569. [Google Scholar]
- Pradeep Prasanna, P. H. , & Charalampopoulos, D. (2019). Encapsulation in an alginate–goats’ milk–inulin matrix improves survival of probiotic Bifidobacterium in simulated gastrointestinal conditions and goats’ milk yoghurt. International Journal of Dairy Technology, 72(1), 132–141. [Google Scholar]
- Qi, W. , Liang, X. , Yun, T. , & Guo, W. (2019). Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. Journal of Food Science and Technology, 56(3), 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, M. E. , & Marco, M. L. (2010). Food formats for effective delivery of probiotics. Annual Review of Food Science and Technology, 1(1), 65–85. 10.1146/annurev.food.080708.100743 [DOI] [PubMed] [Google Scholar]
- Saxelin, M. , Tynkkynen, S. , Mattila‐Sandholm, T. , & de Vos, W. M. (2005). Probiotic and other functional microbes: From markets to mechanisms. Current Opinion in Biotechnology, 16(2), 204–211. 10.1016/j.copbio.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Schoina, V. , Terpou, A. , Angelika‐Ioanna, G. , Koutinas, A. , Kanellaki, M. , & Bosnea, L. (2015). Use of Pistacia terebinthus resin as immobilization support for Lactobacillus casei cells and application in selected dairy products. Journal of Food Science and Technology, 52(9), 5700–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, N. P. (2000). Probiotic bacteria: Selective enumeration and survival in dairy foods. Journal of Dairy Science, 83(4), 894–907. 10.3168/jds.S0022-0302(00)74953-8 [DOI] [PubMed] [Google Scholar]
- Shah, N. P. , & Ravula, R. R. (2000). Microencapsulation of probiotic bacteria and their survival in frozen fermented dairy desserts. Australian Journal of Dairy Technology, 55(3), 139. [Google Scholar]
- Sheu, T. Y. , & Marshall, R. T. (1993). Micro entrapment of lactobacilli in calcium alginate gels. Journal of Food Science, 58(3), 557–561. [Google Scholar]
- Shi, L. E. , Li, Z. H. , Li, D. T. , Xu, M. , Chen, H. Y. , Zhang, Z. L. , & Tang, Z. X. (2013). Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. Journal of Food Engineering, 117(1), 99–104. [Google Scholar]
- Shi, L. E. , Li, Z. H. , Zhang, Z. L. , Zhang, T. T. , Yu, W. M. , Zhou, M. L. , & Tang, Z. X. (2013). Encapsulation of Lactobacillus bulgaricus in carrageenan‐locust bean gum coated milk microspheres with double layer structure. LWT‐Food Science and Technology, 54(1), 147–151. 10.1016/j.lwt.2013.05.027 [DOI] [Google Scholar]
- Stella, M. , Alzamora, D. S. , María, S. , Tapia, A.‐M. , Welti‐Chanes, J. , & Pedro, F. (2005) Novel functional foods from vegetable matrices impregnated with biologically active compounds. Journal of Food Engineering, 67, 205–214. [Google Scholar]
- Vasilyevich, T. , & Shah, N. P. (2008). Probiotics—from Metchnikoff to bioactives. International Dairy Journal, 18(7), 714–728. 10.1016/j.idairyj.2008.03.004 [DOI] [Google Scholar]
- Yağcı, S. , & Göğüş, F. (2008). Response surface methodology for evaluation of physical and functional properties of extruded snack foods developed from food‐by‐products. Journal of Food Engineering, 86, 122–132. [Google Scholar]
- Yeung, T. W. , Arroyo‐Maya, I. J. , McClements, D. J. , & Sela, D. (2016). A microencapsulation of probiotics in hydrogel particles: Enhancing Lactococcus lactis subsp. cremorisLM0230 viability using calcium alginate beads. Food & Function, 7(4), 1797–1804. [DOI] [PubMed] [Google Scholar]


