Abstract
Background
Staphylococcus aureus is the bacterium cultured most often from respiratory secretions of people with cystic fibrosis (CF). S. aureus, both methicillin-susceptible and methicillin-resistant (MRSA), can adapt to form slow-growing, antibiotic-resistant isolates known as small-colony variants (SCVs) that are not routinely identified by clinical laboratories. S. aureus SCVs are further categorized into biochemical subtypes with different risk factors and clinical associations. We sought to determine prevalence, clinical significance, and risk factors for SCVs and their subtypes among children with CF.
Methods
The SCVSa study was a 2-year, observational, longitudinal study of 230 children at 5 US CF centers using culture methods sensitive for SCVs. We determined the prevalence of SCVs and their subtypes and assessed their independent associations with lung function (FEV1 % predicted, FEV1pp) and exacerbations in this population using linear mixed effects and GEE logistic regression models.
Findings
S. aureus SCVs were identified among approximately 28% of subjects. Children with SCVs, and particularly the thymidine-dependent subtype, had significantly lower lung function at baseline that remained lower throughout the study than subjects without SCVs (p=0.0068). Compared to subjects without SCVs, subjects with thymidine-dependent SCVs had significantly increased odds of respiratory exacerbations (OR=2.808; 95% CI 1.687,4.672, p=0.0001), even after adjusting for age, sex, race, CFTR mutation, and enrollment FEV1pp (OR=2.174; 95% CI 1.326,3.567, p=0.0021), while those with non-thymidine-dependent SCVs did not. In multivariate models with SCVs, P. aeruginosa, and MRSA, only SCVs were consistently associated with worse clinical outcomes.
Interpretation
Approximately 28% of CF children in a multicenter US population were infected with SCVs. Infection with SCVs, and particularly thymidine-dependent SCVs, was associated with lower lung function and increased exacerbation risk, while neither P. aeruginosa nor MRSA had similar associations in multivariate models including SCVs. These results support consideration for clinical laboratories to adopt SCV identification and subtyping methods and for CF registries to include SCV prevalence for ongoing surveillance and study.
Funding
The CF Foundation (HOFFMA14A0) and the NIH (K24HL141669).
Introduction
People with the genetic disease cystic fibrosis (CF) have chronic, polymicrobial airway infections. The resulting obstructive lung disease is the most important determinant of morbidity and mortality due to CF. Staphylococcus aureus is the bacterium most commonly cultured from CF respiratory specimens. In 2016, S. aureus was detected in over 70% of CF patients in the US, with a prevalence of over 80% in children 11–17 years old, coinciding with a period of declining average lung function1. However, compared with other common CF pathogens, including Pseudomonas aeruginosa and Burkholderia species, relatively little is known about S. aureus with respect to CF pathogenesis.
S. aureus isolates are often categorized by their susceptibility to the antibiotic methicillin; methicillin-resistant S. aureus (MRSA) has been associated with worse outcomes in diverse infectious diseases compared with methicillin-susceptible S. aureus (MSSA). In CF, MRSA infection has been associated with higher mortality and worse lung function2,3. However, there is a lack of consensus about whether this association is attributable to the organism itself or to its association with the higher antibiotic burden that usually accompanies worse underlying disease4–6.
Many bacteria undergo genetic changes during chronic CF infections as they adapt to the airway environment and to treatments such as antibiotics. While S. aureus adaptations have been less well-studied than those of other CF pathogens, slow-growing, antibiotic-resistant S. aureus mutants known as small-colony variants (SCVs) have been identified from chronically-infected CF patients and from other chronic infections, including those of bone, devices, and wounds7. SCVs can be further categorized according to the type of metabolic defect they carry (reviewed in detail by Kahl et al.8,9). For example, thymidine-dependent (THYD) SCVs have inactivating mutations in the folate biosynthetic pathway. These mutations are generally thought to be selected by exposure to antifolate antibiotics, especially trimethoprim-sulfamethoxazole, because thymidine monophosphate biosynthesis requires folate, and these metabolic mutations lead to sulfonamide resistance10,11. Hemin and menadione-dependent SCVs exhibit defective membrane electron transport and are known to be selected by both aminoglycosides and growth with P. aeruginosa12. Fatty acid-dependent SCVs have been selected in vitro with triclosan and other compounds13, and SCVs dependent on CO2 occur through as-yet unknown mechanisms. SCVs with no identified metabolic defects and auxotrophies have also been described7. SCVs can be formed at the same frequency by MRSA and MSSA14.
SCVs grow poorly on standard laboratory media, and, as a result, SCVs are not routinely identified by most clinical laboratories, precluding studying their prevalence and clinical associations from registry data or clinical records. Case reports and prevalence studies have therefore relied on sensitive culture techniques and experience with SCV detection. THYD SCVs were described in case studies of CF patients as early as the late 1970s15,16, and a subsequent study identified these SCVs among 10% of CF subjects17. Subsequent single-center European studies included all SCV subtypes known at the time, reporting prevalences of 8–33% of CF patients18–24. However, relatively few studies have examined the association of SCV detection with clinical characteristics. In those few studies, SCVs were found to be associated with lower lung function19,20, and SCV-positive patients had often received trimethoprim-sulfamethoxazole18–20,25. However, associations with pulmonary exacerbations were not assessed in these studies.
In a prospective, single-center, US study using culture methods sensitive for SCVs, we identified these variants in 24% of children with CF over a two-year period14. SCV detection was associated with lower lung function and recent treatment with sulfonamide antibiotics, suggesting antibiotics select for SCVs, and also that SCVs may either lead to worse lung disease outcomes or, alternatively, indicate higher antibiotic exposure due to worse pre-existing disease. Together, the above studies suggest that these findings may be generalizable to all children with CF. However, it was not known whether SCVs commonly infect CF patients throughout the US, or whether SCV infection is associated with worse lung function and increased risk of pulmonary exacerbations in the broader pediatric CF population, given that treatment strategies, such as antibiotic regimens, differ among providers, centers, and countries26.
Therefore, we studied the prevalence, risk factors and clinical associations of S. aureus SCVs in a large, multicenter US pediatric CF population. The larger and multicenter nature of this study, as well as new information about emerging SCV subtypes9,13, enabled a more thorough analysis of the risk factors and clinical associations of SCVs compared with prior studies. We hypothesized that S. aureus SCVs commonly infect children with CF in the US and are independently associated with worse lung disease indicators, including lung function and exacerbation frequency. The goal of this study was to define the burden and consequences of SCV infection in the US CF population to better inform ongoing laboratory and therapeutic strategies for managing CF lung disease.
Methods
Study Design
This report conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines27. This study was performed at five CF centers: Seattle Children’s Hospital (SCH), Boston Children’s Hospital (BCH), Texas Children’s Hospital (TCH), University of Pittsburgh Children’s Hospital (UPCH), and the University of Alabama Children’s Hospital (UABCH) between 2014–2017 and approved by the respective sites’ institutional review boards. Eligible patients at enrollment were aged 6–16 years, with documented CF diagnosis, minimum 2 CF clinic visits and 2 respiratory cultures in the 12 previous months, and written informed consent/assent. The CFTR mutations in the study population are listed in Table S1. The minimum age for inclusion was 6 years to increase the likelihood of obtaining reliable spirometric data. Patients were excluded for previous lung transplant or co-morbidities that would interfere with data interpretation (e.g., prematurity, non-CF immunodeficiency, or congenital or structural lung disease). Subjects provided informed consent and were observed prospectively for up to 2 years [mean 6.4 (SD 2.02) study visits per subject] during regularly-scheduled quarterly CF clinic visits (Figure S1). The mean and median interval between visits was 3.94 and 3.45 months, respectively (Range 3.0–4.4 months). Information was not collected on clinic schedule precluding comparison between scheduled and actual visit dates. Participants recorded their use of antibiotics in either written or online data logs. Lung function measurements and culture results were recorded by site research coordinators. Respiratory exacerbations were defined as in our previous study14 as the administration of new inpatient or outpatient antibiotics for respiratory symptoms while on study; because this definition required accurate antibiotic treatment data, we did not define pre-study exacerbation frequency. Sixty percent of the subjects received maintenance antibiotics during the study, with either inhaled tobramycin, aztreonam, or azithromycin.
Bacterial isolate culture and characterization
Respiratory tract specimens [sputum or oropharyngeal (OP) swabs (Figure S2)] were stored at 4°C after collection and shipped overnight on ice to the CF Foundation-funded Therapeutics Development Network Center for CF Microbiology (TDN-CCFM) at SCH. All respiratory specimens were cultured at the TDN-CCFM’s central laboratory within 48 hr after collection as described previously14. Culture results from the central study laboratory were not provided to treating physicians. SCVs were defined using a specialized method previously described14, but with the additional analysis of growth on a laboratory medium (i.e. Mueller-Hinton agar) on which SCVs grow very poorly. SCV auxotrophic testing was performed on Luria-Bertani agar plates with disks impregnated with thymidine (5 μg), hemin (Oxoid), menadione (1.5 μg), or tween 80 (10% solution). Auxotrophy for CO2 was assessed after growth of SCVs on blood agar plates and Luria-Bertani agar in air compared to 5% CO2 following 24 hr incubation at 35°C.
Statistical Methods
Baseline demographic, clinical and laboratory characteristics of study children were summarized for the whole group, and compared between children who ever had SCV S. aureus and those who never had SCV S. aureus detected during the study period (Table 1). Continuous variables were summarized using means (and standard deviations) and compared using the two-sample t-test if normally distributed; otherwise they were summarized using medians (and interquartile ranges) and compared using Wilcoxon-Mann–Whitney test. Categorical variables were summarized using counts and proportions and compared between study groups using Pearson’s chi-square tests or Fisher’s exact tests as appropriate. For each variable, where values are missing, the denominator will be stated in the respective table. Timing of visits was considered as a continuous variable (follow-up time in months since baseline) in our initial analyses; time had no impact on results in these analyses. Therefore, time was treated as a discrete variable in all analyses.
Table 1.
Demographics and clinical characteristics of subjects at enrollment.
| Demographics / Characteristics | Total Popul (N=230) | Never SCV Pos (N=166) | Ever SCV Pos (N=64) | p-value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 11.8 (3.1) | 11.5 (3.1) | 12.5 (3.0) | 0.0302 |
| Weight (kg) | 40.5 (14.4) | 40.6 (14.8) | 40.1 (13.5) | 0.82 |
| Height (cm) | 145.4 (17.1) | 144.9 (17.7) | 146.6 (15.5) | 0.51 |
| BMIa | 18.5 (3.0) | 18.6 (3.1) | 18.1 (3.0) | 0.22 |
| Gender | N (%) | N (%) | N (%) | 0.92 |
| Male | 109 (47.2%) | 79 (47.6%) | 30 (46.9%) | |
| Female | 122 (52.8%) | 87 (52.4%) | 34 (53.1%) | |
| Race | 0.78 | |||
| White | 220 (95.2%) | 158 (95.2%) | 61 (95.3%) | |
| Black/African American | 6 (2.6%) | 4 (2.4%) | 2 (3.1%) | |
| American Indian/Alaskan Native | 2 (0.9%) | 2 (1.2%) | 0 (0.0%) | |
| American Indian or Alaska Native | 1 (0.4%) | 1 (0.6%) | 0 (0.0%) | |
| Unknown | 2 (0.9%) | 1 (0.6%) | 1 (1.6%) | |
| Hispanic race | 0.70 | |||
| Hispanic | 10 (4.3%) | 8 (4.8%) | 2 (3.1%) | |
| Non-Hispanic | 220 (95.2%) | 157 (94.6%) | 62 (96.9%) | |
| Unknown | 1 (0.4%) | 1 (0.6%) | 0 (0.0%) | |
| Pancreatic status | 0.20 | |||
| Pancreatic Sufficient (PS) | 15 (6.5%) | 13 (7.8%) | 2 (3.1%) | |
| Pancreatic Insufficient (PI) | 216 (93.5%) | 153 (92.2%) | 62 (96.9%) | |
| Genotype | 0.41 | |||
| F508 homoz | 126 (54.5%) | 87 (52.4%) | 38 (59.4%) | |
| F508 heteroz | 80 (34.6%) | 62 (37.3%) | 18 (28.1%) | |
| Other | 25 (10.8%) | 17 (10.2%) | 8 (12.5%) | |
| Sweat chloride value | 101.6 (15.3) | 100.7 (17.0) | 103.6 (9.4) | 0.27 |
| Medications and Treatmentsb | N (%) | N (%) | N (%) | |
| Bronchodilator | 208 (90.4%) | 150 (90.4%) | 58 (90.6%) | 0.95 |
| Pancreatic Enzyme Supplement | 213 (92.6%) | 151 (91.0%) | 62 (96.9%) | 0.12 |
| Inhaled Steroid | 122 (53.0%) | 83 (50.0%) | 39 (60.9%) | 0.14 |
| Oral Steroid | 11 (4.8%) | 6 (3.6%) | 5 (7.8%) | 0.18 |
| Hypertonic Saline | 161 (70.0%) | 123 (74.1%) | 38 (59.4%) | 0.0290 |
| Dornase alfa | 193 (83.9%) | 133 (80.1%) | 60 (93.8%) | 0.0117 |
| CFTR Modulator | 17 (7.4%) | 17 (10.2%) | 0 (0.0%) | 0.0078 |
| Mean (SD) | Mean (SD) | |||
| GLI FEV1pp at enrollment | 90.4 (18.9) | 92.4 (18.6) | 85.5 (19) | 0.0145 |
Calculated using the 2006 and 2007 World Health Organization’s reference populations for children aged 6 months to <5 and children ≥5, respectively (de Onis, et al., Bulletin of the World Health Organization 2007; 85: 661–668)
Indicates medications or treatments subjects received
Potential sources of bias in this study included selection (e.g. center and subject), informational (e.g. recall), and confounding biases that can lead to misinterpretation of the data. We adjusted for confounding bias using multivariate analyses. The sample size was chosen a priori using data from previous studies for estimates and was targeted assuming a cumulative prevalence of SCVs of 25% that would provide sufficient power for exploring clinical associations with disease severity, including 80% power to detect significant differences in lung function.
Prevalence and correlates of infection with SCVs
We determined the prevalence of SCVs and used multivariate logistic regression models to assess the correlates of infections with SCV detection among CF children. In bivariate and multivariate analyses, we evaluated unadjusted associations between being ever infected with SCV S. aureus and several a priori defined predictors, including pre-enrollment culture findings. To identify independent predictors of SCV S. aureus detection, we adjusted for the following a priori selected confounders in the multivariate logistic regression; child’s age, sex, race genotype. We retained in multivariate analyses correlates that had strong plausible theoretical and clinical link with SCV S. aureus even if they were not significant in bivariate analysis.
Analysis of the association of SCVs, lung function, and pulmonary exacerbation risk
FEV1% predicted (FEV1pp) values were calculated using the reference equations for spirometry from the Global Lung Function Initiative (GLI)28. Means of FEV1pp at each visit were calculated based on the available data and tabulated for subjects for whom SCVs were never detected, and those for whom SCVs were ever detected, during the study (Table S5 and Figure 1). Visual exploratory and inspection of longitudinal FEV1pp data using profile and spaghetti plots suggested that a random intercept-only linear mixed effects regression model was more plausible than a model with both random intercept and slope for this data set. Therefore, associations of SCVs with differences and longitudinal changes in lung function as measured by FEV1pp were evaluated using linear mixed effect regression models with random intercept with exchangeable correlation matrix and a robust variance estimator. We assessed the association between infection with SCVs and occurrence of pulmonary exacerbations using generalized estimating equations logistic regression models with exchangeable correlation matrix and a robust variance estimator. Multivariate regression models were constructed based on carefully selected potential cofactors and confounders according to subject matter and prior knowledge from previously published studies and conceptual frameworks. For all analyses, two-sided tests and 5% level of significance were used. Analyses were conducted using Stata 15.1 (Stata Corp., College Station, TX). Because subjects had different numbers of study visits during their 2-year follow-up period, resulting in fewer subjects having Visit 8 data, all analyses were restricted to data from Visits 1–7.
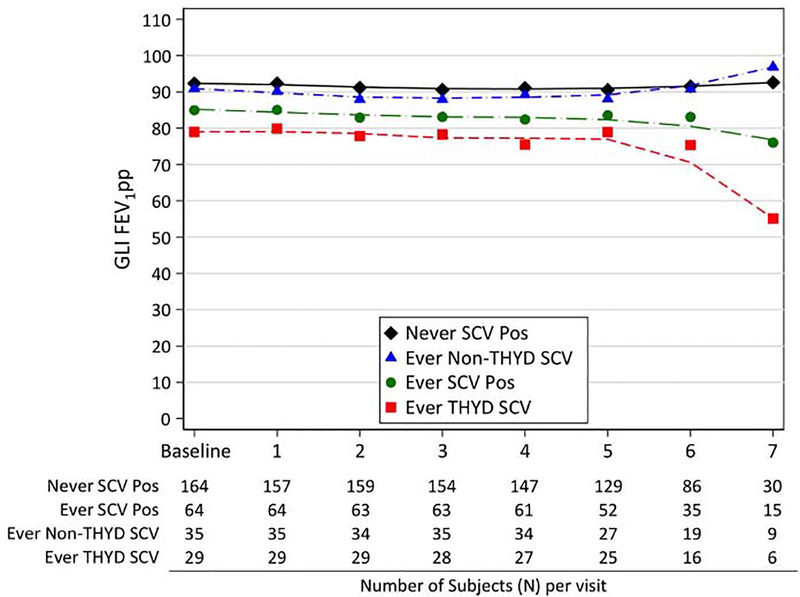
Figure 1.
Lung function measures of S. aureus SCV culture-negative subjects compared to SCV culture-positive subjects at each study visit. (Visit 8, which included relatively few subjects, is omitted for clarity). The number of subjects per culture status is indicated below each study visit.
Role of the Funding Source
The funding source had no role in the design of this study; data collection, analysis, or interpretation; or in the writing of this report. The corresponding author had full access to all of the study data and final responsibility for the decision to submit for publication.
Results
The study population comprised 230 CF subjects at 5 centers (Table S2). One center (Seattle) had fewer subjects than the others because children who participated in the prior single-center SCV study14 were excluded from participating in the current study. Subjects were followed for two years, with a mean of 6.4 visits per subject (range, 2–9 visits). Demographic characteristics are listed in Table 1. The mean age of study subjects was 11.8 years, and the majority (54.5%) of subjects were homozygous for the F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) protein (Table S1).
Sixty-four of the 230 (27.8%) study subjects were culture-positive during the study and after enrollment for SCVs (Table S2, Figure S2, and S3), as defined by poor growth of isolated S. aureus on indicator media. Fifty-five of these 64 subjects were culture negative for SCVs at enrollment (based on baseline cultures) but became SCV culture-positive, at least once during the study (Figure S3). Nine of these 64 subjects were positive for SCVs at enrollment and were SCV culture-positive at least once during the study (Figure S3). The overall prevalence of subjects with SCVs during the study (27.8%) was similar to those at baseline of canonical CF pathogens P. aeruginosa (28.7%) and MRSA (24.8%) in the study population (Table S3). Figure S2 demonstrates the overall prevalence of MSSA, MRSA and SCVs of each S. aureus category (MSSA-SCV and MRSA-SCV) during the study. SCV prevalence varied among the 5 study sites between 14 and 35.3% (Table S2). As shown in Table 1, subjects with SCVs detected during the study were older and more likely to receive dornase alfa at baseline, but less likely to receive hypertonic saline. The CFTR modulator ivacaftor, while uncommon among this pediatric population, was more commonly used at enrollment among SCV-negative subjects. Baseline lung function, measured in mean forced expiratory volume in 1 second % predicted (FEV1pp), was significantly lower among subjects with SCVs during the study (p=0.0145). Subjects with SCVs detected during the study were also more likely to be culture-positive for MSSA, MRSA, and/or MSSA-SCVs in their baseline respiratory cultures (Table S3). There were similar numbers of subjects with repeated detection of SCVs (> 1 positive culture during study, “persistent”) and subjects culture-positive for SCVs only once (“intermittent”) during the study (Figure S3 and Table S4). Subjects with persistent SCV infection did not differ in age, sex, or genotype from subjects with intermittent infections (Table S4). The vast majority of subjects with either persistent (29/30) or intermittent (31/34) SCV infections, received dornase alfa, suggesting that SCV persistence was not likely attributable to this medication.
To determine the relationship between SCVs and lung function, we first compared mean FEV1pp measures at each of the study visits for subjects with and without SCVs detected during the study. As shown in Figure 1 and Table S5, subjects with SCVs had consistently lower lung function than those without SCVs at all study visits. SCVs were associated with significantly lower FEV1pp in univariate (Coeff = −7.071, 95% CI −12.195,−1.948, p=0.0068) and multivariate (Coeff = −5.495, 95% CI −10.507,−0.483, p = 0.0316) analyses after adjusting for both patient demographics and P. aeruginosa culture status (Table S6). Regression analyses of the association between SCV detection and rate of change in FEV1pp did not demonstrate evidence of a significant interaction between SCV detection and follow-up time in either univariate or multivariate analyses (Table S7), indicating that subjects with and without SCVs had similar rates of decline in FEV1pp during the study. FEV1pp was lower for subjects with either intermittent and persistent SCV infections compared with SCV culture-negative subjects, but results were only significant for subjects with intermittent infections (Table S8).
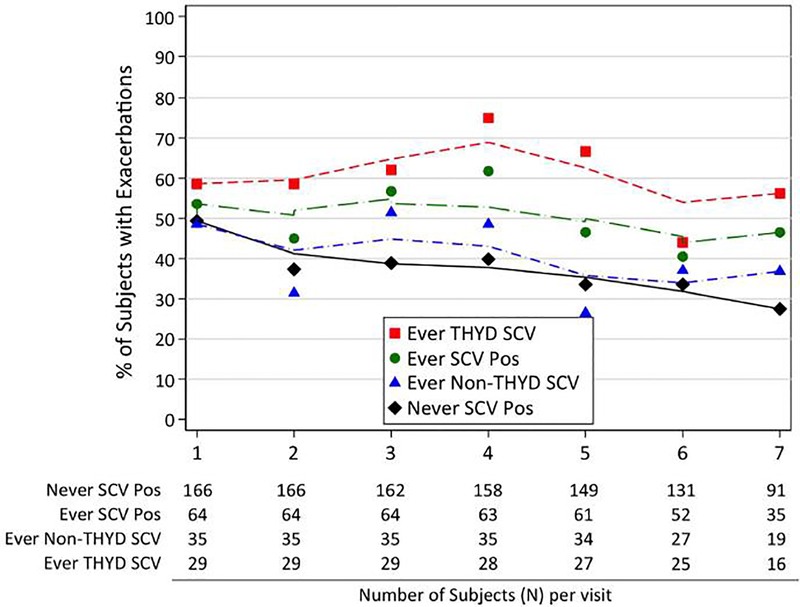
Figure 2 and Table S9 illustrate the prevalence of respiratory exacerbations (defined as a new antibiotic prescription for respiratory symptoms) among study subjects according to SCV detection. Subjects with SCVs had significantly more exacerbations during the study than subjects without SCVs at each study visit with sufficient data for analysis (Visits 1–7). As shown in Table S10, SCV detection was significantly associated with increased odds of pulmonary exacerbation in both unadjusted analyses (OR = 1.727, 95% CI 1.185,2.516, p = 0.004) and when adjusting (OR = 1.492, 95% CI 1.037,2.147, p = 0.031) for demographic variables and P. aeruginosa detection (which was associated with lower odds of exacerbation), but no longer reached significance when adjusting for lung function at enrollment. These results held even if Visit 7, which included data from fewer subjects than earlier visits, was also excluded in the analyses. Subjects with SCVs detected in multiple cultures had significantly higher odds of an exacerbation than subjects with SCVs detected once (Table S11).
Figure 2.
Prevalence of pulmonary exacerbations since the prior study visit for SCV culture-negative subjects compared with SCV culture-positive subjects at study visits 1–7 (Visit 8, which included relatively few subjects, is omitted for clarity). The number of subjects per culture status is indicated below each study visit.
To determine the relationship between SCV detection and prior antibiotic exposure, antibiotic treatment histories for the preceding 3 months were collected from subjects and families at each study visit. As shown in Table 2, only treatment with sulfonamides was significantly associated with SCV detection at the subsequent study visit by univariate analysis (OR=2.223, 95% CI 1.062,4.655, p=0.0338). Multivariate analyses confirmed this association (OR=1.892, 95% CI 1.026,3.488, p=0.0407) for all SCV subtypes (Table S12); this relationship was even stronger for THYD SCVs in multivariate analyses (OR=10.876, 95% CI 3.831,30.876, p=<0.0001). Subjects treated with dornase alfa also had significantly increased odds of SCV detection, while treatment with hypertonic saline was associated with lower odds in both univariate (Table 2) and multivariate analyses (Table S12).
Table 2:
Association between treatment and subsequent SCV detection in univariate analysis.
| Univariate |
|||
|---|---|---|---|
| Covariatea | Odds Ratio | 95% Conf. Interval | p-value |
| Aminoglycoside | |||
| No* | 1 | ||
| Yes | 1.283 | (0.644 – 2.556) | 0.48 |
| Beta-lactam | |||
| No* | 1 | ||
| Yes | 1.026 | (0.515 – 2.042) | 0.94 |
| Fluoroquinolone | |||
| No* | 1 | ||
| Yes | 1.105 | (0.514 –2.376) | 0.79 |
| Glycopeptide | |||
| No* | 1 | ||
| Yes | 1.218 | (0.442–3.354) | 0.70 |
| Lincosamide | |||
| No* | 1 | ||
| Yes | 0.858 | (0.225–3.275) | 0.82 |
| Macrolide | |||
| No* | 1 | ||
| Yes | 1.375 | (0.771–2.452) | 0.28 |
| Polymyxin | |||
| No* | 1 | ||
| Yes | 3.432 | (0.891–13.215) | 0.07 |
| Rifamycin | |||
| No* | 1 | ||
| Yes | 0.361 | (0.043–2.990) | 0.35 |
| Sulfonamide | |||
| No* | 1 | ||
| Yes | 2.223 | (1.062 – 4.655) | 0.0338 |
| Tetracycline | |||
| No* | 1 | ||
| Yes | 1.744 | (0.694 – 237) | 0.24 |
| Anti-fungal | |||
| No* | 1 | ||
| Yes | 5.323 | (0.474–59.746) | 0.18 |
| Hypertonic Saline | |||
| No* | 1 | ||
| Yes | 0.511 | (0.278–0.938) | 0.0304 |
| Inhaled Steroid | |||
| No* | 1 | ||
| Yes | 1.560 | (0.867–2.806) | 0.14 |
| Dornase alfa | |||
| No* | 1 | ||
| Yes | 3.722 | (1.262–10.976) | 0.0172 |
Baseline category
Treatment exposures were evaluated for the study quarter prior to the culture under consideration.
We categorized the biochemical subtype of each SCV isolate to further investigate their likely selective pressures and antibiotic resistance patterns. As shown in Table S13, SCVs of each auxotrophic type were detected in this study population, including fatty-acid dependent SCVs (15%), which have not previously been described in CF respiratory infections. The majority of all SCVs detected (55.7%) were THYD; a higher proportion of MRSA-SCVs (81%) than MSSA-SCVs (34.7%) were THYD (Table S13). Subjects with persistent SCV infections were more likely to have THYD SCVs than any other SCV subtype (Table S4).
We investigated whether different SCV types had different associated clinical outcomes. THYD SCVs appeared to account for the majority of the clinical associations observed for all SCVs in this population. For example, subjects with THYD SCVs had significantly lower FEV1pp (Figure 1, Tables S5 and S14) and a higher risk of pulmonary exacerbations (Figure 2, Tables 3 and S9), even after adjusting for patient demographics, P. aeruginosa culture status, and baseline lung function, than did subjects with all other SCV types. Subjects with MRSA-SCVs, which were predominantly THYD, had significantly lower lung function and increased odds of pulmonary exacerbations in both univariate and multivariate analyses compared with other subjects (Tables S15 and S16). MSSA-SCVs were also associated with lower lung function in univariate and multivariate analyses (Table S15).
Table 3.
Association between SCV subtypes and occurrence of pulmonary exacerbations in univariate and multivariate analyses.
| Univariate |
Multivariate |
Multivariate |
Multivariate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariatea | ORb | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value |
| SCV Culture Status | ||||||||||||
| Never SCV Pos* | 1 | 1 | 1 | 1 | ||||||||
| Ever Non-THYD SCV | 1.076 | (0.677–1.709) | 0.76 | 1.037 | (0.654–1.645) | 0.88 | 1.010 | (0.644–1.584) | 0.97 | 1.008 | (0.645–1.574) | 0.97 |
| Ever THYD SCV | 2.808 | (1.687–4.672) | 0.0001 | 2.497 | (1.513–4.121) | 0.0003 | 2.386 | (1.464–3.889) | 0.0005 | 2.174 | (1.326–3.567) | 0.0021 |
| Visit number | 0.890 | (0.833–0.951) | 0.0006 | 0.898 | (0.844–0.955) | 0.0007 | 0.898 | (0.843–0.955) | 0.0007 | 0.896 | (0.842–0.954) | 0.0005 |
| Age at enrollment | 1.078 | (1.022–1.137) | 0.0060 | 1.093 | (1.037–1.153) | 0.0010 | 1.081 | (1.025–1.140) | 0.0042 | |||
| Sex | ||||||||||||
| Male* | 1 | 1 | 1 | |||||||||
| Female | 1.416 | (1.021–1.965) | 0.0373 | 1.486 | (1.078–2.049) | 0.0155 | 1.437 | (1.045–1.976) | 0.0258 | |||
| Hispanic race | ||||||||||||
| Hispanic* | 1 | 1 | 1 | |||||||||
| Non-Hispanic | 0.854 | (0.378–1.927) | 0.70 | 0.743 | (0.334–1.649) | 0.47 | 0.765 | (0.349–1.674) | 0.50 | |||
| Genotype | ||||||||||||
| F508 homoz* | 1 | 1 | 1 | |||||||||
| F508 heteroz | 1.092 | (0.763–1.562) | 0.63 | 1.033 | (0.727–1.468) | 0.86 | 1.018 | (0.718–1.443) | 0.92 | |||
| Other | 0.770 | (0.438–1.353) | 0.36 | 0.765 | (0.441–1.328) | 0.34 | 0.727 | (0.420–1.258) | 0.25 | |||
| CFTR Modulator | ||||||||||||
| No* | 1 | 1 | 1 | |||||||||
| Yes | 0.547 | (0.280–1.067) | 0.08 | 0.532 | (0.277–1.022) | 0.06 | 0.565 | (0.296–1.075) | 0.08 | |||
| P. aeruginosa Cx Status | ||||||||||||
| Never PA Pos* | 1 | 1 | ||||||||||
| Ever PA Pos | 0.551 | (0.383–0.794) | 0.0014 | 0.519 | (0.359–0.749) | 0.0005 | ||||||
| GLI FEV1pp at enrollment | 0.992 | (0.984–1.001) | 0.10 | |||||||||
Baseline category
SCV categories are mutually exclusive.
Abbreviations: Odds Ratio, OR; Confidence Interval, CI; Culture, Cx; THYD, thymidine-dependent.
Because it was possible that detection of THYD SCVs was simply a marker for sulfonamide treatment, we analyzed whether sulfonamide treatment itself was associated with worse clinical outcomes. Sulfonamide treatment was not associated with lung function (difference −2.96, 95% CI −7.81,1.88, p = 0.23) (Table S17). Sulfonamides were associated with higher odds of pulmonary exacerbations (OR = 3.117, 2.344–4.145, p = <0.001), as were all antibiotics used in this study other than maintenance macrolides (Table S18), most likely reflecting their standard use in exacerbation treatment and the study definition of exacerbation. Sulfonamides were only the fifth most commonly prescribed antibiotic class in this study (Table S19). Therefore, the high prevalence of THYD SCVs could not be attributed to disproportionate use of this antibiotic. However, significantly more subjects with MRSA were treated with sulfonamides during the study than subjects with only MSSA (Table S20, 59.5% versus 40.5%; p=0.0079), likely explaining the higher frequency of THYD MRSA-SCVs than THYD MSSA-SCVs (Table S13).
To further investigate the relationship between medications and SCVs, we compared reported medication use and SCV detection frequencies among the 5 study sites. Sites with the highest SCV prevalences generally reported higher mean sulfonamide treatment frequencies than centers with lower SCV rates; for example, the sites with the highest and lowest SCV detection frequencies (35.3% and 14% of subjects, respectively, Table S2) reported the correspondingly highest and second lowest mean sulfonamide usage during the study (22.7% and 11.2% of subjects, respectively). Interestingly, the center with the lowest prevalence of SCVs also reported significantly more hypertonic saline usage (96%) than the other centers (range of 60–69%).
Discussion
In this multicenter US study, S. aureus SCVs were detected among approximately 28% of children with CF when using sensitive culture methods rarely used in clinical laboratories. SCVs were associated with multiple measures of worse lung disease during the study, including lower lung function and higher exacerbation frequency, even in multivariate models that adjusted for disease severity at baseline. By comparison, P. aeruginosa did not have consistent associations with these poor outcomes in models that included SCVs, and relationships between MRSA and poor outcomes were apparently attributable to MRSA-SCVs in this population. Risk factors for SCVs included age, treatment with dornase alfa at enrollment, and sulfonamide treatment during the prior quarter. More detailed analysis demonstrated that these observations were attributable primarily to THYD SCVs, which were the most common SCV subtype in this study and are known to be selected by sulfonamide treatment10; however, sulfonamide treatment was not a risk factor for worse outcomes in the absence of SCVs.
THYD SCVs were particularly common among MRSA isolates, an observation likely attributable to higher sulfonamide treatment of MRSA culture-positive subjects in this study; of note, trimethoprim-sulfamethoxazole is recommended as first-line therapy for MRSA, but not MSSA, CF infections in the US29,30. MRSA prevalence in the US CF population has also been shown to be higher than in many other countries31; therefore, one might expect sulfonamides to be used particularly often in the US. However, these antibiotics are commonly used in some countries continuously as antistaphylococccal prophylaxis or treatment for CF patients, neither of which are common in the US32. Considering our findings, the role of trimethoprim-sulfamethoxazole in management of S. aureus respiratory infection in CF merits discussion. We propose that this drug be avoided upon SCV detection; however, given our results, and because S. aureus CF isolates tend to be sulfonamide-susceptible33,34, we do not recommend removing this antibiotic from the CF antistaphylococcal armamentarium. Our observations also identified a new plausible candidate mechanism for SCV selection: treatment with dornase alfa (recombinant human DNase I). This enzyme cleaves free DNA near pyrimidines and may release free thymidine, which supports the growth of THYD SCVs. The importance of this mechanism in SCV selection will require further study.
The associations with worse lung disease measures is consistent with observations from a single-center US study14, and also from European studies that were smaller18,20–22, single-center18–21, and/or did not focus on SCVs or their associations with clinical outcomes22. In the US single-center study14, SCVs were associated with worse lung function decline that was not observed in this multicenter study. However, the prior study population was younger on average (mean age at enrollment 9 y, compared with 12 y in the current study), and the current study may therefore have been less likely to capture initial infection, making it difficult to determine whether lung function trajectory changes after this event. By comparison with SCVs, the canonical CF pathogen P. aeruginosa was actually associated with fewer exacerbations in multivariate analyses in this pediatric CF population, and in models that adjusted for MRSA SCVs, P. aeruginosa was not significantly associated with lower lung function. Because SCVs have rarely been considered in CF studies or clinical care, these results raise questions about the associations between P. aeruginosa, MRSA, and CF clinical outcomes identified in previous studies.
The causal relationship between SCV detection and clinical outcomes cannot be definitively established from this observational study. SCVs are known to be selected by antibiotics, which are used more frequently in people with more severe symptoms. Therefore, the associations established here may indicate either that SCVs worsen disease or are simply a marker of worse pre-existing disease. Both explanations may also be true. Similar controversy exists for clinical associations identified for MRSA5,6, multiresistant P. aeruginosa35, and for the gradual decrease in CF sputum microbial diversity observed to be associated with advancing age and disease36. Three observations could be interpreted to support the latter, marker hypothesis, regarding SCVs: First, SCVs were not associated with advanced lung function decline in the current study; second, SCVs were detected less often in patients treated with ivacaftor, which is predicted to decrease the need for antibiotics; and third, single detection (intermittent infection), but not repeated detection (persistent infection), was associated with worse pulmonary function. Alternatively, two observations support the possibility that SCVs may be particularly pathogenic: First, sulfonamide treatment, which can select SCVs, was not independently associated with worse lung function. Therefore, SCVs do not simply indicate sulfonamide exposure as a proxy of severity. Second, the regression models that identified associations between SCVs and both exacerbation risk and worse lung function adjusted for FEV1pp at enrollment, a measure of pre-existing disease severity. Future studies of SCV-preventive or treatment interventions may be able to address this question.
At least one prior study, performed in Australia, did not identify SCVs among bronchoalveolar lavage (BAL) samples from children with CF37. Here, most study specimens were oropharyngeal swabs. While it is possible that SCVs primarily infect the upper airways rather than lower airways, compared with the US, the CF S. aureus prevalence is markedly lower than in Australia, where antistaphylococcal antibiotic prophylaxis and treatment on detection are routine practices1,38. Therefore, SCVs may be less common, and their clinical impact lower, in populations where antistaphylococcal strategies are employed (although SCVs have been detected often in European countries where such strategies are common19). We previously identified SCVs in pediatric CF sputum14, which may more accurately reflect lower airway microbiology than do OP swabs. Another limitation of using OP swabs in this study was the inability to measure inflammatory markers, some of which, including neutrophil elastase, have been associated with both specific bacteria and with outcomes in CF39,40. A future sputum and/or BAL-based study may be able to address these questions.
This study was also limited by the quarterly respiratory sampling regimen, and by the fact that each culture identified only the most abundant morphologically distinct isolates, undersampling the diversity of infecting S. aureus cell populations. SCVs are known to revert to a normal colony phenotype during subculture, which can lead to an incorrect categorization. The study was also limited by relatively few observations at Visits 7 and 8 and lack of pulmonary exacerbation data at baseline, in addition to the inability to establish causality described previously. While the effects of potential confounders were adjusted for in multivariate analyses, selection (i.e. center and subject selection) and informational (e.g. recall) biases may reduce generalizability and accuracy of the findings.
SCVs are not routinely identified by most clinical laboratories, including many of those specifically dedicated to CF microbiology, and susceptibility testing is not usually possible due to their slow growth. However, methods have been developed to identify these variants and test their antibiotic susceptibilities that are not yet in widespread use33. The high prevalence of SCVs and their clinical associations identified in this and prior studies of other CF populations14,18–21 indicate that consideration should be made for the routine adoption of these methods by clinical microbiology laboratories, particularly for samples from chronic infections where SCVs are often present, including those of the CF airway, skin and skin structure, bone, and medical devices7,8. We also recommend that SCV epidemiologic and susceptibility data be recorded in registries, such as the US CF Patient Registry, which currently tracks P. aeruginosa, MRSA, and other “standard CF pathogens”1.
Conclusion
S. aureus SCVs commonly infected a multicenter US pediatric CF population and were associated with significantly worse lung disease measures. These variants are not routinely identified by most clinical laboratories. These results highlight the importance of SCVs in CF lung disease and support consideration of the routine adoption of clinical laboratory methods that are sensitive for SCV detection and that define SCV subtypes, in addition to their inclusion in registries. Future interventional studies may establish the causal relationship between SCV infection and disease outcomes.
Supplementary Material
Research in context.
Evidence before this study
Staphylococcus aureus is the bacterium most frequently cultured from the airways of people with cystic fibrosis (CF). S. aureus is known to adapt to form slow-growing, antibiotic-resistant variants, known as small-colony variants (SCVs), during many chronic human and animal infections, including those of the CF airway. S. aureus SCV infections have been hypothesized to result in worse clinical outcomes than normal-colony S. aureus infections. Identification of S. aureus SCVs requires special culture techniques that are not used by most clinical laboratories, and SCVs are therefore not included in registry data, limiting any research on SCV prevalence or clinical associations. To clarify what was known about the prevalence and clinical associations of S. aureus SCVs in CF, we searched PubMed for all studies, regardless of publication date, containing the keywords “aureus cystic fibrosis small colony”. The resulting studies indicated that S. aureus SCVs commonly infected CF patient airways in Europe and the US, with prevalences varying substantially, and none were detected in an Australian study. In some studies, SCVs were associated with lower lung function. However, these studies were generally short in duration, were usually single-center studies, did not focus on SCVs, did not investigate their independent associations with outcomes, and used different culture techniques, limiting generalizability. We sought to determine the prevalence, risk factors, and independent clinical associations of SCVs among a large, multicenter population of children with CF using a standardized laboratory protocol.
Added value of this study
This study identified S. aureus SCV infection among 28% of a multicenter, US population of children with CF during a two-year period, demonstrating a strong correlation between SCV detection and worse clinical outcomes, including consistently lower lung function and higher risk of respiratory exacerbations. In multivariate analyses that included SCVs, neither of the common CF pathogens Pseudomonas aeruginosa and MRSA had similar associations. The prevalence of S. aureus SCVs in this population was similar to those identified in prior, single-center studies in Europe and in the US. This observation, combined with the larger sample size and the multicenter nature of the current study population, indicate that the current findings are generalizable to the CF population, warranting consideration of the general adoption of SCV detection and subtyping methods among CF clinical laboratories and investigation of SCV-targeting treatments.
Implications of all the available evidence
The available evidence indicates that S. aureus SCVs commonly infect the respiratory tracts of children with CF and are associated with significantly worse respiratory outcomes. These results support considering the general adoption of clinical laboratory methods that detect SCVs and inclusion of these variants in registry data for future research, surveillance, and CF clinical care.
Acknowledgments
This study was supported by grants from the Cystic Fibrosis Foundation (BURNS03Y2, HOFFMA14A0, SINGH15R0) and the NIH (K24HL141669, P30DK089507). We are grateful to the subjects and families who participated in this study.
Conflict of Interests
DJW, RG, AU, JLB, and LRH report grants from Cystic Fibrosis Foundation during the conduct of the study. RG reports National Institutes of Health, other from Vertex Pharmaceuticals, outside the submitted work. JLB reports grants from Seattle Children’s Research Institute during the conduct of the study. LRH reports grants from the National Institutes of Health during the conduct of the study, and he is the director of a national resource center supported by the Cystic Fibrosis Foundation called the Center for CF Microbiology. In this role, LRH provides consultation to both private and public entities performing research in CF microbiology but does not accept direct compensation for this role; all financial arrangements are made with LRH’s institution. The other authors declared no conflicts of interests.
Footnotes
| Daniel J | Wolter |
| Frankline M | Onchiri |
| Julia | Emerson |
| Mimi R | Precit |
| Michael | Lee |
| Sharon | McNamara |
| Laura | Nay |
| Marcella | Blackledge |
| Ahmet | Uluer |
| David M | Orenstein |
| Michelle | Mann |
| Wynton | Hoover |
| Ronald L | Gibson |
| Jane L | Burns |
| Xuan | Qin |
| Anne Marie | Buccat |
| Alan | Genatossio |
| Nicoline | Schaap |
| Omalee | Lopez |
| Kathy | Doan |
| Robert | Fowler |
| Khadija | Iken |
| Kelsey | Little |
| Elizabeth | Hartigan |
| Kathryn | Little |
| Heather | Hathorne |
| Susan | Keeling |
| Katie | Slaten |
| Lucas R | Hoffman |
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2016 Annual Data Report to the Center Directors. 2017. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf (accessed Oct 9, 2018).
- 2.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010; 303: 2386–92. [DOI] [PubMed] [Google Scholar]
- 3.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med 2008; 178: 814–21. [DOI] [PubMed] [Google Scholar]
- 4.Ren CL, Morgan WJ, Konstan MW, et al. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol 2007; 42: 513–8. [DOI] [PubMed] [Google Scholar]
- 5.Sawicki GS, Rasouliyan L, Ren CL. The impact of MRSA on lung function in patients with cystic fibrosis. Am J Respir Crit Care Med 2009; 179: 734–5; author reply 735. [DOI] [PubMed] [Google Scholar]
- 6.Sawicki GS, Rasouliyan L, Pasta DJ, et al. The impact of incident methicillin resistant Staphylococcus aureus detection on pulmonary function in cystic fibrosis. Pediatr Pulmonol 2008; 43: 1117–23. [DOI] [PubMed] [Google Scholar]
- 7.Proctor RA, von Eiff C, Kahl BC, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 2006; 4: 295–305. [DOI] [PubMed] [Google Scholar]
- 8.Kahl BC. Small colony variants (SCVs) of Staphylococcus aureus--a bacterial survival strategy. Infect Genet Evol 2014; 21: 515–22. [DOI] [PubMed] [Google Scholar]
- 9.Kahl BC, Becker K, Löffler B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin Microbiol Rev 2016; 29: 401–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kriegeskorte A, Lorè NI, Bragonzi A, et al. Thymidine-Dependent Staphylococcus aureus Small-Colony Variants Are Induced by Trimethoprim-Sulfamethoxazole (SXT) and Have Increased Fitness during SXT Challenge. Antimicrob Agents Chemother 2015; 59: 7265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee I, Kriegeskorte A, Fischer A, et al. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J Bacteriol 2008; 190: 834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman LR, Déziel E, D’Argenio DA, et al. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 2006; 103: 19890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bazaid AS, Forbes S, Humphreys GJ, Ledder RG, O’Cualain R, McBain AJ. Fatty Acid Supplementation Reverses the Small Colony Variant Phenotype in Triclosan-Adapted Staphylococcus aureus: Genetic, Proteomic and Phenotypic Analyses. Sci Rep 2018; 8: 3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolter DJ, Emerson JC, McNamara S, et al. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin Infect Dis 2013; 57: 384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George RH, Healing DE. Thymidine-requiring Haemophilus influenzae and Staphylococcus aureus. Lancet 1977; 2: 1081. [DOI] [PubMed] [Google Scholar]
- 16.Sparham PD, Lobban DI, Speller DC. Thymidine-requiring Staphylococcus aureus. Lancet 1978; 1: 104–5. [DOI] [PubMed] [Google Scholar]
- 17.Gilligan PH, Gage PA, Welch DF, Muszynski MJ, Wait KR. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol 1987; 25: 1258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahl B, Herrmann M, Everding AS, et al. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis 1998; 177: 1023–9. [DOI] [PubMed] [Google Scholar]
- 19.Besier S, Smaczny C, von Mallinckrodt C, et al. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol 2007; 45: 168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider M, Mühlemann K, Droz S, Couzinet S, Casaulta C, Zimmerli S. Clinical characteristics associated with isolation of small-colony variants of Staphylococcus aureus and Pseudomonas aeruginosa from respiratory secretions of patients with cystic fibrosis. J Clin Microbiol 2008; 46: 1832–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagci S, Hascelik G, Dogru D, Ozcelik U, Sener B. Prevalence and genetic diversity of Staphylococcus aureus small-colony variants in cystic fibrosis patients. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2011; published online Nov 28. DOI: 10.1111/j.1469-0691.2011.03742.x. [DOI] [PubMed] [Google Scholar]
- 22.Junge S, Görlich D, den Reijer M, et al. Factors Associated with Worse Lung Function in Cystic Fibrosis Patients with Persistent Staphylococcus aureus. PLoS ONE 2016; 11: e0166220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morelli P, De Alessandri A, Manno G, et al. Characterization of Staphylococcus aureus small colony variant strains isolated from Italian patients attending a regional cystic fibrosis care centre. New Microbiol 2015; 38: 235–43. [PubMed] [Google Scholar]
- 24.Masoud-Landgraf L, Zarfel G, Kaschnigg T, et al. Analysis and Characterization of Staphylococcus aureus Small Colony Variants Isolated From Cystic Fibrosis Patients in Austria. Curr Microbiol 2016; 72: 606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahl BC, Duebbers A, Lubritz G, et al. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J Clin Microbiol 2003; 41: 4424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schechter MS, Regelmann WE, Sawicki GS, et al. Antibiotic treatment of signs and symptoms of pulmonary exacerbations: a comparison by care site. Pediatr Pulmonol 2015; 50: 431–40. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–7. [DOI] [PubMed] [Google Scholar]
- 28.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goss CH, Muhlebach MS. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros 2011; 10: 298–306. [DOI] [PubMed] [Google Scholar]
- 30.Zobell JT, Epps KL, Young DC, et al. Utilization of antibiotics for methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. Pediatr Pulmonol 2015; 50: 552–9. [DOI] [PubMed] [Google Scholar]
- 31.Akil N, Muhlebach MS. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol 2018; 53: S64–74. [DOI] [PubMed] [Google Scholar]
- 32.Hurley MN, Smyth AR. Staphylococcus aureus in cystic fibrosis: pivotal role or bit part actor? Curr Opin Pulm Med 2018; 24: 586–91. [DOI] [PubMed] [Google Scholar]
- 33.Precit MR, Wolter DJ, Griffith A, Emerson J, Burns JL, Hoffman LR. Optimized In Vitro Antibiotic Susceptibility Testing Method for Small-Colony Variant Staphylococcus aureus. Antimicrob Agents Chemother 2016; 60: 1725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns JL, Gibson RL, McNamara S, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 2001; 183: 444–52. [DOI] [PubMed] [Google Scholar]
- 35.Merlo CA, Boyle MP, Diener-West M, Marshall BC, Goss CH, Lechtzin N. Incidence and risk factors for multiple antibiotic-resistant Pseudomonas aeruginosa in cystic fibrosis. Chest 2007; 132: 562–8. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Schloss PD, Kalikin LM, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA 2012; 109: 5809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carzino R, Hart E, Sutton P, King L, Ranganathan S, AREST CF. Lack of small colony variants of Staphylococcus aureus from lower respiratory tract specimens. Pediatr Pulmonol 2017; 52: 632–5. [DOI] [PubMed] [Google Scholar]
- 38.Cystic Fibrosis Australia. Australian Cystic Fibrosis Data Registry Annual Report, 2016. 2017. https://www.cysticfibrosis.org.au/getmedia/a3b28200-caeb-4c5a-ad15-98c71a8c7dc8/ACFDR-2016-Annual-Report-Final-Copy-Single-Page-Version.pdf.aspx.
- 39.Sagel SD, Gibson RL, Emerson J, et al. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J Pediatr 2009; 154: 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gangell C, Gard S, Douglas T, et al. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis 2011; 53: 425–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.