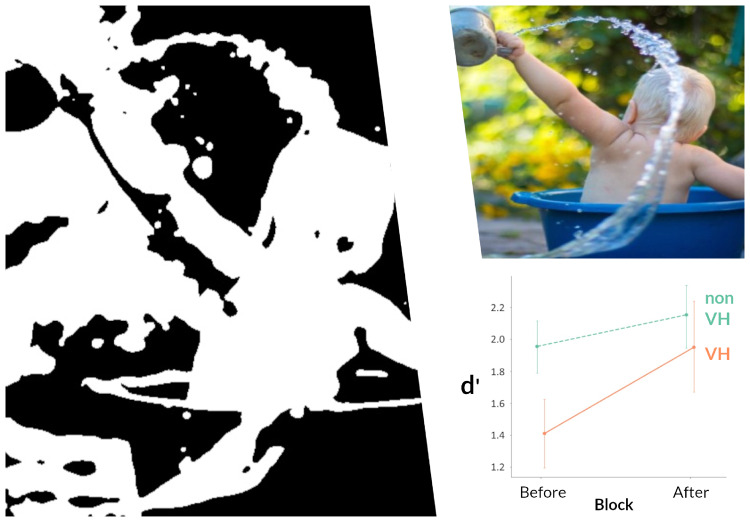
The mechanisms of visual hallucinations in Lewy body disease are unknown. Zarkali et al. use two-tone stimuli to show that Lewy body hallucinations are associated with altered integration of top-down predictions with sensory evidence. Specifically, results show an increased relative weighting of prior knowledge in Lewy body hallucinations providing important mechanistic insights.
Keywords: Visual hallucinations, Lewy body disease, predictive coding, visual perception, prior beliefs
Abstract
Hallucinations are a common and distressing feature of many psychiatric and neurodegenerative conditions. In Lewy body disease, visual hallucinations are a defining feature, associated with worse outcomes; yet their mechanisms remain unclear and treatment options are limited. Here, we show that hallucinations in Lewy body disease are associated with altered integration of top-down predictions with incoming sensory evidence, specifically with an increased relative weighting of prior knowledge. We tested 37 individuals with Lewy body disease, 17 habitual hallucinators and 20 without hallucinations, and 20 age-matched healthy individuals. We employed an image-based learning paradigm to test whether people with Lewy body disease and visual hallucinations show higher dependence on prior knowledge. We used two-tone images that are difficult to disambiguate without any prior information but generate a strong percept when information is provided. We measured discrimination sensitivity before and after this information was provided. We observed that in people with Lewy body disease who experience hallucinations, there was greater improvement in discrimination sensitivity after information was provided, compared to non-hallucinators and controls. This suggests that people with Lewy body disease and hallucinations place higher relative weighting on prior knowledge than those who do not hallucinate. Importantly, increased severity of visual hallucinations was associated with an increased effect of prior knowledge. Together these findings suggest that visual hallucinations in Lewy body disease are linked to a shift towards top-down influences on perception and away from sensory evidence, perhaps due to an increase in sensory noise. This provides important mechanistic insights to how hallucinations develop in Lewy body disease, with potential for revealing new therapeutic targets.
Graphical Abstract
Graphical Abstract.
Introduction
Hallucinations are commonly experienced in neurodegenerative diseases. They are particularly common and distressing in Lewy body disease (LBD: dementia with Lewy bodies and Parkinson’s disease), where they occur in up to 70% of patients and are associated with poorer outcomes (Fénelon et al., 2000; Aarsland et al., 2007; Weil et al., 2016). Their phenomenology and severity vary, from illusions and misperceptions in the early stages, to complex, detailed and even animated imagery as the disease progress (Fénelon et al., 2000; Gallagher et al., 2011). Insight into whether experienced images are real or not is initially preserved but often withers during the course of the disease (Fénelon et al., 2000; Weil et al., 2016). Despite their high prevalence and impact on patients and carers, the mechanisms of hallucinations in LBD remain unclear and treatment options limited (Fénelon et al., 2000; Weil et al., 2016).
An attractive framework to understand hallucinations is predictive coding; here perception is seen as approximating Bayesian inference, a process with two key ingredients: expectations from prior knowledge and sensory input (Cavanagh, 2011, O’Callaghan et al., 2017b; Parr et al., 2018). In this context, visual hallucinations (VH), or experiencing visual percepts that are not objectively there, can be thought of as false inference which arises when the integration of sensory input and prior knowledge is altered (Fletcher and Frith, 2009; Adams et al., 2013; Powers et al., 2016; Corlett et al., 2019). This framework has been applied to the study of hallucinations in non-neurodegenerative diseases. Hallucinations experienced by healthy individuals or in the context of psychiatric illness are associated with a shift towards prior knowledge and away from sensory evidence (Teufel et al., 2015; Alderson-Day et al., 2017; Powers et al., 2017; Davies et al., 2018). This relative increase in the weighting of prior knowledge in people who experience hallucinations has been linked with improved performance in disambiguating ambiguous visual and auditory signals (Teufel et al., 2015; Alderson-Day et al., 2017; Davies et al., 2018) as well as a propensity to hallucinate when stimuli are noisy (Powers et al., 2017).
Abnormal integration of priors with sensory information could also underlie hallucinations in neurodegeneration. In LBD, hallucinations are associated with visual-processing deficits (Diederich et al., 2005; Weil et al., 2016) and sensory evidence accumulation has been shown to be impaired in patients with Parkinson’s disease and VH (O’Callaghan et al., 2017a). However, hallucinations can also occur in patients without any lower or higher visual deficits (Gallagher et al., 2011; Weil et al., 2016) making a solely bottom-up explanation for the occurrence of hallucinations less attractive.
Here, we tested the hypothesis that hallucinations in LBD are associated with an increased weighting of prior knowledge relative to sensory evidence. Given that hallucinations in LBD are almost always visual (Fénelon et al., 2000; Aarsland et al., 2007), we used a visual learning paradigm to test our hypothesis. We tested patients with LBD with and without hallucinations and unaffected age-matched controls on a paradigm that uses ambiguous sensory stimuli viewed before and after additional knowledge was provided. This paradigm allowed us to keep the sensory evidence constant but manipulate prior knowledge by supplying participants with information and comparing performance before and after information was provided. We hypothesized (i) that patients with LBD and VH would show greater improvement in performance compared to patients with LBD who did not experience hallucinations; (ii) that this performance benefit would be higher in those patients with more severe hallucinations.
In summary, we explored how prior knowledge is used during visual processing in people with LBD and VH. In accordance with our hypotheses, we have shown, for the first time, that hallucinations in neurodegeneration are associated with an increased relative weighting of prior knowledge over incoming sensory evidence. Importantly, we show that the degree of this prior weighting is proportionate to the severity of hallucinations. These findings provide important insights into the mechanisms of hallucinations in LBD and wider neurodegeneration and could inform future research into therapeutic targets.
Materials and methods
Experimental design and procedure
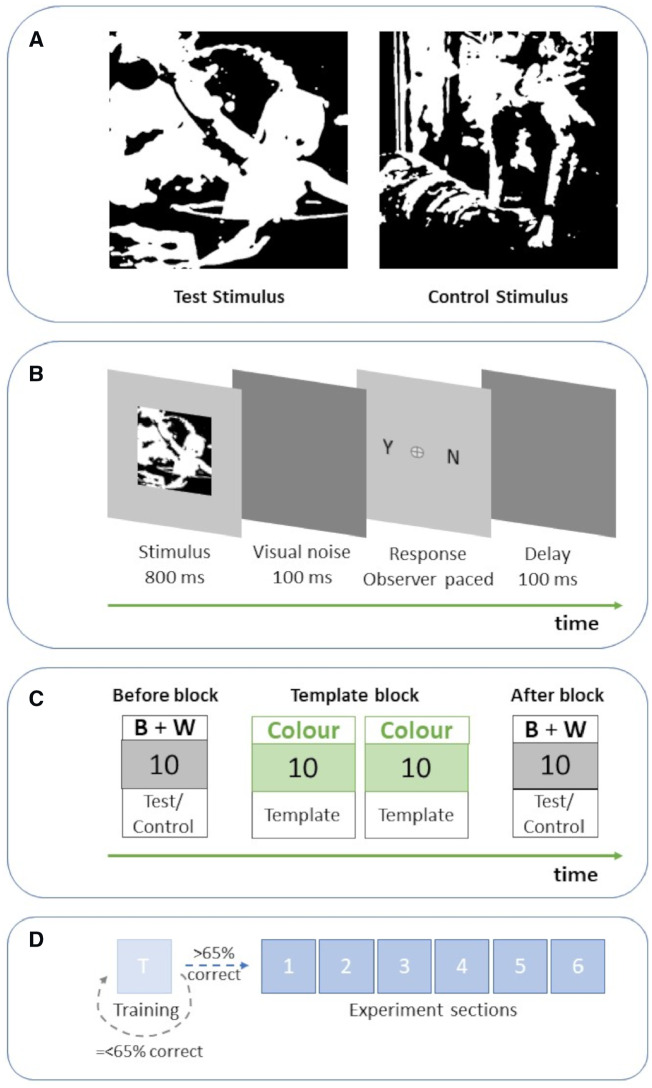
We used a visual learning paradigm with two-tone images as stimuli. When these are first seen without any prior knowledge, they appear as meaningless black and white patches (Fig. 1A) but when the template from which they were created is seen (Fig. 2) they generate a strong, coherent percept (Dolan et al., 1997; Moore and Cavanagh, 1998; Hegdé and Kersten, 2010; Hsieh et al., 2010; Cavanagh, 2011; Teufel et al., 2015). The ability to disambiguate two-tone images with the aid of prior knowledge is mediated by top-down influences from high-level processes onto low-level visual function. This is supported by both psychophysics and neuroimaging studies (Dolan et al., 1997; Hsieh et al., 2010; Teufel et al., 2015; Davies et al., 2018).
Figure 1.
Illustration of the experimental procedure. (A) Example of a test stimulus (Left) and control stimulus (Right). Both have similar characteristics but the test images contain a person. (B) Individual trial: participants are briefly presented with an image and then are asked to indicate whether the image contains a person or not. Response was observer-paced without time limit to ensure capture from our clinical groups. (C) Experiment section: first, in the Before Block, participants are presented with 10 two-tone images (5 test stimulus and 5 controls), back-to-back in random order. Then, participants are presented with a Template block of 20 colour images in random order. All templates for the two-tone stimuli shown in Before Block are included. After each presented template image participants are asked again to indicate the presence of a person. This facilitates participant compliance via task simplicity and also ensures that participants actively observe the template images to provide participants with prior knowledge of the two-tone image content. Finally, in the After Block participants are presented with the same two-tone images as in Before Block. (D) Experiment: the experiment consists of six sections and starts with a training section, identical to the experimental sections but with two-tone images that are easier to disambiguate. Only participants with >65% discrimination sensitivity proceed to the main experiment.
Figure 2.

Example of a template image. This image was used to create the test stimulus in Figure 1A.
To quantify the effect of prior knowledge in the different study groups, we compared how effectively the participants disambiguated the two-tone images before and after prior knowledge was supplied. We used a block design to supply participants with prior knowledge (Fig. 1C). First, in the ‘Before block’ participants were presented with 10 two-tone images, then in the ‘Template block’ participants were shown 20 coloured images (including the templates for the 10 two-tone images of the Before block to provide participants with prior knowledge of the two-tone image content) and finally in the ‘After block’ the 10 two-tone images of the Before block were repeated again. The order of specific images was randomized within each block. On each trial participants were presented with an image (two-tone or colour) for 800 ms. After each image presentation, we asked participants to indicate whether they could see a person in the image or not by keyboard press (Fig. 1B). Due to the expected prolonged reaction times of our clinical population the response screen was participant guided with no response time limit. We employed the same process during Template blocks, to ensure that participants were actively observing the template images thus receiving optimal prior information in preparation for the After block. In addition, keeping the same design across the whole experiment and including a training session at the onset ensured that our task was easy to understand and execute. This was of particular significance given the additional frailty and cognitive impairment in our cohort.
The experiment consisted of 6 sessions, each with the three blocks of trials described above, amounting to a total of 60 trials per participant all conducted on one study visit. The same set of 60 stimuli and 120 template images was used for each participant with order of presentation randomized in each block. The experiment started with a practice session identical to the experimental sessions; only participants achieving ≥65% discrimination sensitivity in the practice session proceeded to the experiment. This threshold was chosen to ensure that included participants were familiar with the procedure and understood the task. All of the invited participants in our study reached this threshold and were included in this analysis. After each experimental session participants were able to take a short break of maximum 5 min prior to continuing the experiment.
The experiment was conducted using a Dell XPS9570 15’ laptop with a 4k display at maximum brightness; stimuli were presented with a custom application made on Unity (version 2018.3.0b4) with participants seated at distance ∼60 cm from the screen. The experimental procedure is described in Fig. 1.
Stimuli
We used two-tone images that are difficult to disambiguate without any prior information but once the template from which they were created is seen, they give the strong experience of a coherent percept.
A total of 150 two-tone images were created with a custom Python script from coloured high-definition template images of people and animals downloaded from https://stocksnap.io and https://www.pexels.com/ under the Creative Commons License. Template images were resized to 500 × 500 pixels using cubic interpolation and converted to grayscale. Image noise was cleared through opening (erosion followed by dilation) using a 1 × 1 kernel. A Gaussian blur was then applied with a 9 × 9 kernel. Finally, a binary and Otsu threshold were applied to each image (Sezgin and Sankur, 2004) (Fig. 3).
Figure 3.
Illustration of the stimulus creation process.
Resulting two-tone images were piloted with 17 healthy volunteers to assess discrimination sensitivity before and after viewing the templates. Two-tone images that were too easy to disambiguate before viewing the template or too difficult after seeing the template were excluded. From the remaining two-tone images, 60 were selected (30 of people and 30 of animals) for this experiment. The presentation time of 800 ms chosen in our experiment was guided by reaction time data collected during pilot testing. (Seventeen healthy volunteers were asked to indicate the presence of a person in the two-tone stimuli using a keyboard press without any prior information and without presentation time limit. A keyboard press triggered the next stimulus to be presented. Decision time was recorded and 800 ms (mean + 2SD) was chosen for the main experiment.)
Participant recruitment
We recruited 37 patients with Lewy body disease (LBD: Parkinson’s disease and Dementia with Lewy bodies) from Parkinson’s disease clinics at the National Hospital for Neurology and Neurosurgery, London, UK. All patients satisfied the United Kingdom Parkinson’s Disease Society Brain Bank criteria for Parkinson’s disease, the Movement Disorder Society criteria for Parkinson’s disease dementia or the Dementia with Lewy Bodies Consortium Criteria for Dementia with Lewy Bodies (DLB) (Gibb and Lees, 1988; Emre et al., 2007; McKeith et al., 2017). Patients with LBD were classified as habitual hallucinators (VH) if they scored ≥1 on question two of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (UPDRS: ‘Over the past week have you seen, heard, smelled or felt things that were not really there?’; Goetz et al., 2008). Seventeen patients with LBD scored ≥1 and were classified as having hallucinations, whilst 20 patients did not.
Further qualitative and quantitative details on the experienced hallucinatory phenomena were collected with the University of Miami Parkinson’s Disease Hallucinations Questionnaire (Papapetropoulos et al., 2008). This quantifies severity (intensity) and frequency of hallucinations. Twenty unaffected controls were recruited from spouses and a volunteer database at our UK centre.
Motor and psychology assessments
Assessment of motor function was performed using the Unified Parkinson’s Disease Rating Scale (Goetz et al., 2008).
The Mini-Mental State Examination and Montreal Cognitive Assessment were used as measures of general cognition (Dalrymple-Alford et al., 2010; Creavin et al., 2016). To test specific cognitive domains, we used the following assessments (Lezak et al., 2004):
Attention: Digit span backwards (Wechsler, 2008), Stroop test: Naming subtest (Stroop, 1935)
Executive function: Stroop Interference (Stroop, 1935), Category fluency (Rende et al., 2002)
Memory: Word Recognition Task (Warrington, 1984), Logical Memory (immediate and delayed recall) (Wechsler, 2008)
Language: Graded Naming Task (Warrington, 1997), Letter fluency (Rende et al., 2002)
Visuospatial: Benton’s Judgment of Line (Benton et al., 1978), Visual Object and Space Perception Battery (Warrington and James, 1991), Hooper Visual Organization Test (Hooper, 1983).
Visual acuity was assessed using the 6-m Snellen chart. All participants had corrected bilateral acuity of ≥6/6. Colour vision was assessed using the D15 (Farnsworth, 1947) and contrast sensitivity using the Pelli-Robson test (Pelli et al., 1988). Smell was assessed using Sniffin’ Sticks (Hummel et al., 1997). The Hospital Anxiety and Depression Scale was used to assess mood (Zigmond and Snaith, 1983) and the REM Sleep Behaviour Disorder to assess sleep (Stiasny-Kolster et al., 2007).
All assessments and the experimental task were performed with patients in the ON state. This avoided potential distress associated with the OFF state, that may have impaired cognitive and perceptual performance and also ensured results were not influenced by between-subject differences in motor performance. Levodopa equivalent daily doses (LEDD) were calculated. The groups were matched for age, gender and time in education. Details of the three study groups are seen in Table 1.
Table 1.
Study group characteristics
| Attribute | Controls n = 20 | LBD non-VH n = 20 | LBD VH n = 17 | P-value* | |
|---|---|---|---|---|---|
| Demographics | Age in years | 69.7 (6.9) | 68.9 (7.1) | 68 (6.9) | 0.457b |
| Male (%) | 11 (55) | 13 (65) | 7 (41.2) | 0.079b | |
| Years in education | 15.9 (2.6) | 15.9 (2.3) | 15.4 (2.9) | 0.567a | |
| Mood (HADS) | Depression score | 2.2 (2.2) | 2.5 (2.5) | 4.5 (2.4) | 0.017 b |
| Anxiety score | 4.3 (2.6) | 4.5 (4.1) | 5.2 (2.9) | 0.212b | |
| Vision | Visual acuity (bilateral) | 1.1 (0.2) | 1.1 (0.2) | 1.0 (0.2) | 0.216b |
| Contrast sensitivity (Pelli-Robson) (log units) (bilateral) | 1.7 (0.2) | 1.7 (0.1) | 1.5 (0.2) | 0.017 b | |
| Colour vision (D15) | 0.2 (0.4) | 0.8 (1.3) | 0.4 (1.3) | 0.180b | |
| Neuropsychology | MMSE | 29.5 (0.7) | 29 (1.5) | 28 (2.1) | 0.057b |
| MOCA | 27.8 (1.2) | 26.8 (3.1) | 25.5 (3.7) | 0.131b | |
| Attention | Digit span backwards | 8.4 (2.4) | 7.2 (2.6) | 7.1 (2.2) | 0.860a |
| Stroop: naming (s) | 38.1 (6.5) | 35.8 (8.5) | 44.7 (14.5) | 0.007 b | |
| Executive function | Stroop: interference (s) | 69.1 (14.2) | 66.7 (22.9) | 83 (27.2) | 0.062b |
| Category fluency | 19.7 (4.4) | 19.8 (4.3) | 17.8 (5.6) | 0.219a | |
| Memory | Word recognition task | 24.8 (0.4) | 23.2 (1.9) | 23.6 (2.0) | 0.219b |
| Logical memory (delayed) | 12.4 (4.3) | 10.1 (3.9) | 11.1 (2.8) | 0.309a | |
| Language | Graded naming task | 24.4 (2.5) | 23.8 (3.9) | 22.3 (3.9) | 0.079b |
| Letter fluency | 16 (4.7) | 14.6 (5.5) | 12.8 (4.2) | 0.297a | |
| Visuospatial | VOSP | 56.2 (2.0) | 54.6 (3.3) | 52.8 (5.4) | 0.397b |
| Benton’s judgement of line orientation | 24.4 (4.3) | 25.4 (4.1) | 22.3 (4.4) | 0.071b | |
| Hooper’s visual organization test | 25.6 (2.4) | 23.2 (3.9) | 21.7 (4.9) | 0.187b | |
| Disease specific | Age at diagnosis | 64.4 (9.0) | 63.4 (7.3) | 0.719a | |
| Disease duration | 4.5 (4.6) | 4.6 (2.7) | 0.944a | ||
| RBDSQ | 4.2 (2.5) | 5.1 (3.0) | 0.190b | ||
| UPDRS total | 38.7 (13.4) | 52.8 (15.6) | 0.003 b | ||
| UPDRS question 1.2 | 1.76 (0.73) | ||||
| Miami hallucinations questionnaire | 5.2 (1.9) | ||||
| UPDRS part 3 (motor score) | 24.7 (7.5) | 30.5 (10.6) | 0.136b | ||
| LEDD (mg) | 401.9 (288.3) | 406 (244.8) | 0.500 | ||
| Smell test | 23.2 (3.9) | 21.6 (4.9) | 0.082a | ||
All data shown (except gender) are mean (SD).
HADS, Hospital anxiety and depression scale; LBD non-VH, patients without visual hallucinations; LBD VH, patients with Lewy body disease and visual hallucinations; LEDD, Total Levodopa equivalent daily dose; MMSE, Mini-mental state examination; MOCA, Montreal cognitive assessment; RBDSQ, REM sleep behaviour disorder screening questionnaire; UPDRS, Unified Parkinson’s disease rating scale; VOSP, Visual Object and Space Perception Battery.
Uncorrected P-values shown are for comparison between LBD/VH and LBD/non-VH (comparison of interest), in bold statistically significant values (P < 0.05, uncorrected).
Student t-test.
Mann-Whitney U test.
The study was approved by the Queen Square ethics committee and all participants provided written informed consent prior to taking part.
Statistical analysis
We used a two alternative forced choice task where participants were asked to indicate the presence of a person in each presented image. We measured correct responses and reaction times in the Before and After blocks. In addition, we used signal detection theory metrics for analysis. We derived hit rates and false alarm rates from the collected yes/no responses; this was done separately for the Before and After Block. We derived discrimination sensitivity (d′): a measure of the observer’s ability to distinguish ‘signal’ from ‘noise’ distributions (independent of any bias for one or the other), i.e. the separation between them normalized by their variance, and criterion (c): a measure of the bias the observer has to detect a signal (Macmillan and Creelman, 1991). d′ and c were derived from the following equations:
where h: hit rate, f: false alarm rate.
Independent t-samples and ANOVAs were used to compared normally distributed continuous variables and Mann-Whitney and Kruskall-Wallis tests for non-normally distributed ones. Visual inspection of variable distribution and the Shapiro Wilk test were used to test normality. Post hoc correction for multiple comparisons were performed with Tukey test for ANOVA and Nemeyni test for Kruskall-Wallis. In our main comparison of interest (LBD patients with and without hallucinations), we have also performed uncorrected t-tests and Kruskall-Wallis tests. This was chosen to avoid missing small yet potentially important differences between the two groups in an effort to minimize Type II error. Statistical significance was set at P < 0.05. All statistical analysis was performed in Python 3 using Jupyter Notebook version 5.5.0.
Data availability statement
The code used to create the two-tone stimuli as well as code used for data analysis is provided in the following repository: https://github.com/AngelikaZa/Increased-weighting-on-prior-knowledge-in-Lewy-Body-associated-visual-hallucinations.-BrainComms2019.git. The specific stimuli used for this experiment are also provided in the same repository. The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
We tested a total of 57 participants; 17 patients with LBD and visual hallucinations (LBD VH), 20 patients with LBD without hallucinations (LBD non-VH) and 20 unaffected, age-matched controls. Three patients with LBD VH had a diagnosis of Dementia with Lewy Bodies and the rest had a diagnosis of Parkinson’s disease, whilst two of the LBD non-VH patients had a diagnosis of Dementia with Lewy Bodies. Apart from diagnostic distinctions, there were no differences between patients with a diagnosis of Dementia with Lewy Bodies and those with Parkinson’s disease in disease duration, clinical or demographic measures. Demographics and clinical characteristics of patients with Dementia with Lewy Bodies and Parkinson’s disease are provided in Supplementary material.
Prior knowledge leads to greater performance improvement in patients who hallucinate
As expected by our experimental design, our cohort improved in performance in the After block compared to the Before block both in absolute percentage correct (t = 4.12, Hedge’s g = 0.77, P < 0.001) and discrimination sensitivity, d′ (t = 3.73, Hedge’s g = 0.69, P < 0.001). A between groups ANOVA revealed that this improvement differed between the three study groups in percentage correct: improvement mean ±SD in LBD VH was 6.9% ±4.4 (from 74.5% to 81.5%) compared to 2.7% ±4.4 (from 82% to 84.8%) and 4.7% ±3.5 (from 79.3% to 84%) in controls [F(2, 54) = 4.90, P = 0.011] and d′: 0.54 ± 0.41 improvement in d′ (from 1.41 to 1.95) in LBD VH compared to 0.2 ± 0.47 (from 1.96 to 2.15) in LBD non-VH and 0.4 ± 0.37 (from 1.92 to 2.32) in controls [F(2, 54) = 3.18, r2 = 0.11, P = 0.049]. The improvement in d′ across the three groups is shown in Fig. 4.
Figure 4.
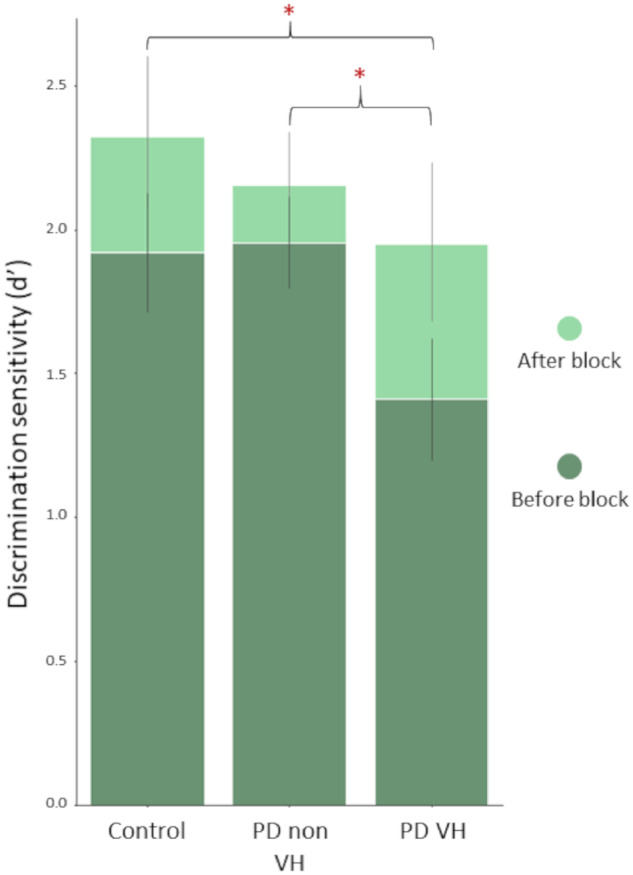
Improvement in performance in patients with LBD with and without hallucinations and controls. Discrimination sensitivity (d′) in the Before and After blocks across our three study populations.
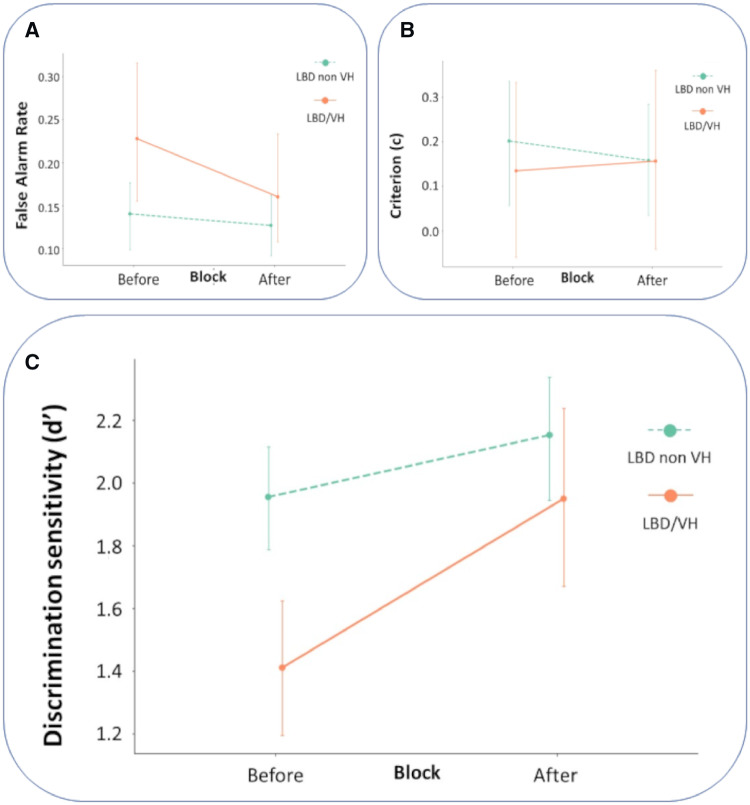
Importantly, planned post hoc testing showed that this difference was driven by a difference between patients with LBD with and without visual hallucinations (t-test and effect size between LBD non-VH and LBD/VH: t = 2.35, Hedge’s g = 0.75, P = 0.025). Hallucinators improved more than double the amount the non-hallucinators did after viewing the template images: improvement in d′ (d′ diff) mean ±SD = 0.20 ± 0.46 in LBD patients without hallucinations and 0.54 ± 0.41 in those with hallucinations (Fig. 5C). This improvement was seen despite the worse initial performance of hallucinators (d′ in the Before block between LBD non-VH and LBD/VH: t = 3.958, Hedge’s g = 1.27, P < 0.001), who in the After block reached the discrimination sensitivity of non-hallucinators (t = 1.16, P = 0.25). To ensure that the observed difference in improvement was not secondary to a ceiling effect in patients with LBD without hallucinations or controls performing at ceiling in the Before block, we tested the variance of d′ d′ in the Before block between the three groups. Variance was not significantly different in LBD VH compared to LBD non-VH or controls in the Before block (Levene’s test of variance W = 1.09, P = 0.344), confirming that neither of the three groups was performing at ceiling in the Before block. The same was true for the After block (W = 0.77, P = 0.469). In addition, participants without hallucinations who had better performance in the Before block (higher d′) were still able to improve in the After block; in fact, higher d′ in the Before block was associated with higher improvement in d′ (r2 = 0.260, df = 40, β = 0.1376, P = 0.001). Thus, while ceiling effects in non-hallucinating participants cannot be definitively excluded, the available evidence indicates that such effects are not driving the group differences.
Figure 5.
Improvement in performance in patients with LBD with and without hallucinations. (A) False alarm rates in patients with LBD with and without hallucinations (VH) in the Before and After blocks. LBD/VH showed a significant reduction from Before to After, but LBD non-VH did not (see text). (B) Criterion (c) in the Before and After blocks in patients with LBD. There were no group differences in the change from Before to After. Lower c values suggest a response bias to indicate the presence of a person independent of whether a test or control image is shown. (C) Discrimination sensitivity (d′) in Before and After blocks in patients with LBD: higher values suggest better participant ability to correctly disambiguate the two-tone images for the presence of a person. LBD/VH show greater improvement in performance from Before to After, compared with LBD non-VH. In all images confidence intervals 95% are shown.
Performance improvement is driven by a reduction in false alarm rates
The difference in performance improvement was primarily attributed to a greater reduction in false alarm rate in hallucinators: −0.07 ± 0.07 (from 0.23 to 0.16) in LBD VH compared to −0.01 ± 0.08 (0.14 to 0.13) in LBD non-VH (t = 2.19, Hedge’s g = 0.70, P = 0.035), Fig. 5A. LBD VH participants had higher false alarm rates in the Before block than LBD non-VH (Mann-Whitney U = 114, P = 0.043) but did not differ in false alarm rates in the After block (U = 150, P = 0.269). The improvement in hit rate did not significantly differ between LBD with and without VH (t = 1.35, P = 0.185), nor did the hit rates in the Before (U = 122, P = 0.073) or After block (U = 145, P = 0.221). Importantly, we did not find any group bias in responses, as criterion was not significantly different across the three groups in either the Before [F(2, 54)=0.902, P = 0.412] or After blocks [F(2, 54)=0.527, P = 0.593]. There was also no significant difference in criterion between the Before and After blocks in the whole cohort (t = 0.331, P = 0.742) or within the three individual groups, reflecting the fact that observers’ received information regarding both test and control stimuli leading to an improved performance in recognizing both the presence and the absence of a person in the After blocks (Fig. 5B).
Hallucinators and non-hallucinators did not significantly differ in cognitive function
Additionally, all participants underwent extensive neuropsychological, visual and motor assessments to minimize confounders that might influence experiment results. After corrections for multiple comparisons, the three groups did not significantly differ in demographics, time spent in education, low-level vision, general cognitive measures or within specific cognitive domains, including attention, executive functions or language. Of three measures of higher-level visuospatial perception, only one differed between groups: the Hooper test (Kruskal Wallis: H = 86, P < 0.001) with post hoc analysis attributing this difference to lower performance in LBD/VH compared to controls (U = 86, P = 0.005) with no difference seen between LBD/VH and LBD non-VH (U = 141, not significant); the other two visuo-perceptual measures were non-significant after correction for multiple comparisons. One of the memory measures differed: Word Recognition task (H = 88, P < 0.001) but post hoc analysis revealed that this was driven by a lower performance in LBD patients compared to controls (U = 100, P = 0.001) with no significant difference between LBD/VH and LBD non-VH patients (U = 145, P = 0.220). Depression scores were higher in LBD patients with hallucinations (H = 24.133, P < 0.001) but below the threshold for a diagnosis of depression (Bjelland et al., 2002; Mondolo et al., 2006).
In addition to post hoc testing, and to avoid missing out possible group differences, we also performed uncorrected tests in our main comparison of interest: LBD patients with and without hallucinations. The two groups did not differ in demographics, disease duration, age at diagnosis or levodopa equivalent dose. Four patients in our cohort were receiving anticholinesterase inhibitors; two LBD/VH and two LBD non-VH. None were receiving antipsychotic medications. LBD patients with hallucinations had lower contrast sensitivity (U = 106, P = 0.017) than those without hallucinations but there were no other statistically significant differences in performance between the two groups in all other visual and visuospatial tasks. Performance in cognitive tasks was also not significantly different, except for one measure of executive function (Stroop: Naming; U = 89, P = 0.007). Finally, LBD patients with and without hallucinations differed in total Unified Parkinson’s Disease Rating Scale (which measures motor and non-motor symptoms; U = 80.5, P = 0.003), with patients with hallucinations having higher scores compared to patients without hallucinations signifying higher levels of disability. However, the objective motor component of the Unified Parkinson’s Disease Rating Scale did not differ between the two groups (U = 1.867, P = 0.14). The demographics and results of clinical assessments in our cohort are seen in Table 1.
Performance improvement increases with hallucination severity
To better quantify the effect of prior knowledge in patients with LBD who hallucinate, we also collected qualitative and quantitative data on hallucination attributes and severity. Of the 17 patients with LBD and VH, 4 (23.5%) had provoked, 3 (17.3%) formed, and 10 (58.8%) animate hallucinations; on a validated quantitative scale of hallucination severity (Papapetropoulos et al., 2008) the mean ±SD score was 5.2 ± 1.9. Importantly, in people with LBD and hallucinations performance improvement was correlated with hallucination severity (r2 = 0.617, df = 16, P < 0.001). The correlation remained significant after correcting for the observed differences in contrast sensitivity, Stroop naming scores, depression scale scores and Unified Parkinson’s Disease Rating Scale as well as Mini-Mental State Examination, levodopa equivalent dose and Hooper (visuospatial measure) (df = 7, t = 2.549, P = 0.038).
Given that our group of participants included a subgroup with dementia with Lewy bodies, we performed an additional analysis, with those five patients excluded, to test whether effects were driven solely by that subgroup. Even with this subgroup of patients with DLB excluded, we found that d′ was significantly higher in hallucinators compared to non-hallucinators (improvement in d′: 0.62 ± 0.41 in Parkinson’s disease/VH compared to 0.27 ± 0.43 in Parkinson’s disease non-VH, t = 2.35, P = 0.025). This was still due to a higher reduction in false alarm rates in hallucinators than non-hallucinators (t = −2.33, P = 0.027) rather than a higher increase in hit rates (t = 0.95, P = 0.352). Finally, hallucination severity was still associated with higher improvement in d′ in the group of Parkinson’s disease hallucinators: r2 = 0.733, df = 13, t = 6.282, P < 0.001.
Discussion
Our study shows that patients with LBD VH place relatively higher weighting on prior knowledge in perceptual inference. Improvement in our visual disambiguation task was more than double in patients with LBD who hallucinate compare to those who do not, with hallucinators reaching the same discrimination sensitivity as non-hallucinators and controls despite worse initial performance.
Our findings provide mechanistic insights into LBD-associated hallucinations. Hallucinations in LBD are usually progressive, starting as minor illusions or misperceptions before complex, detailed or animate hallucinations emerge (Mosimann et al., 2004; Weil et al., 2016). Whilst initially patients have full insight into their symptoms, insight is often lost with disease progression and delusional ideas around hallucinations can develop (Weil et al., 2016). We found that severity of hallucinations was associated with greater improvement in performance and therefore with greater effects of prior knowledge. This may also provide some insights into how hallucinations may progress during the disease. Changes in the whole visual system, from the retina to the visual cortex, are seen in LBD with some evidence suggesting that, at least some, changes occur early in the disease course (Williams-Gray et al., 2009; Erskine et al., 2019). A possible explanation for hallucinations is that early damage in the low-level visual system could result in a loss of signal/noise, i.e. decreased sensory precision. Decreased sensory precision is consistent with drift diffusion modelling findings of a decreased drift rate (slower evidence accumulation) in LBD/VH relative to LBD (O’Callaghan et al., 2017a). That study did not directly assess the effects of visual priors but here, we have shown an increased relative weighting of prior knowledge. This was not due to bias (lower criterion) towards perceiving people in LBD/VH. Prior knowledge could be afforded higher weighting in LBD/VH to compensate for reduced sensory precision. We saw higher false alarms and lower d′ in LBD/VH patients at baseline, implying the ‘signal’ and ‘noise’ distributions are closer in LBD/VH but we found no evidence of bias (lower criterion) towards perceiving people in LBD/VH. Rather, we saw an increase in false alarms and lower d′, implying the ‘signal’ and ‘noise’ distributions are closer in LBD/VH. Strikingly, the benefit of viewing the colour pictures in LBD/VH was mostly realized as a reduction in false alarms rather than an increase in hits.
The observed reduction in false alarm rate in the After blocks with the same hit rate and criterion could be explained by an increase in signal strength combined with a change in the absolute cut-off LBD/VH participants use to make their decision (criterion shift). In an equal variance signal detection theory model, a simpler explanation is that prior knowledge leads to sensory sharpening or lowering of the noise distribution which has been previously suggested in a similar task (Teufel et al., 2018): this effect may be greater in those with least sensory precision. In addition, it is still unclear whether hallucinations in LBD/VH are uniquely ascribable to a loss of sensory precision is unclear: it could also be that the precision of these patients’ prior beliefs increases in absolute terms (alongside any sensory precision loss). We did not see strong evidence for sensory precision loss in the low-level visual tasks in LBD/VH, but to assess the relative contributions of priors and likelihoods one needs a task designed to measure both separately (Karvelis et al., 2018).
Importantly, we have shown that the striking performance improvement seen in LBD patients with hallucinations cannot be fully attributed to a ceiling effect in non-hallucinating groups. Hallucinators did not significantly differ from non-hallucinating groups in variance of discrimination sensitivity. A lower variance in the LBD non-VH and controls would be expected if the main driver in improvement was a ceiling effect across all groups. More importantly, individually good performance in the Before block was associated with higher improvement in discrimination sensitivity; should a ceiling effect be present we would expect the highest performers in the Before block being unable to improve as much in the After block. Despite this, it is still possible that the observed improvement is driven by worse disambiguation in the Before block in hallucinators compared to non-hallucinating groups, rather than a stronger effect of prior knowledge. However, within the group of hallucinators, worse hallucination severity was associated with higher improvement in d′; this could not be explained by group differences in disambiguation.
Interestingly, we observed wide variability in performance improvement in patients who did not experience hallucinations. In addition, performance improvement was significantly correlated with hallucination severity. This raises the question of whether patients with Parkinson’s disease with greater improvement in performance are on the cusp of developing hallucinations in the near future. Prospective studies of sensory accumulation and prior knowledge in people with LBD could shed further light on this question and evaluate the ability of tests of prior knowledge such as ours as potential markers of susceptibility to hallucinations.
Our LBD subjects were quite evenly matched in terms of their vision and cognitive performance. Low-level vision is affected in Parkinson’s disease with reduce visual acuity, contrast sensitivity and colour vision reported, whilst both pathological and optical coherence tomography studies confirm changes in the retina, the earliest part of the visual system (Hajee et al., 2009; Lee et al., 2014; Weil et al., 2016). Higher order visual processing is also affected in LBD (Mori et al., 2000; Mosimann et al., 2004; Weil et al., 2016). Both low- and high-level visual processing is more affected in patients with LBD who experience hallucinations (Diederich et al., 2005) although hallucinations can occur even in patients with equivalent visual performance (Gallagher et al., 2011). Hallucinations in LBD are also linked with worse cognitive impairment (Fénelon et al., 2000; Weil et al., 2016). However, in our cohort, cognition in LBD VH was not significantly worse than in LBD without hallucinations across all cognitive domains, with worse performance in LBD/VH in only one measure of executive function. In addition, LBD/VH were poorer than LBD patients without hallucinations in one low-level visual task (contrast sensitivity) but we found no difference between LBD/VH and LBD without hallucinations in other tests of visual function.
We studied patients with Parkinson’s disease and Dementia with Lewy Bodies together due to their indistinguishable end-phenotype and common pathological features; such an approach has been advocated in recent years (Jellinger, 2012; Postuma et al., 2016; Weil et al., 2017). Considering the two diseases together can provide useful insights into common mechanisms leading to the same symptom of hallucinations. In our cohort, within the group of VH and the group of non-VH participants, patients with an underlying diagnosis of Dementia with Lewy Bodies and those with a diagnosis of Parkinson’s disease did not significantly differ in any clinical or cognitive measure (beyond diagnostic characteristics) and we continued to find the same effects when we examined our group without the DLB patients. However, underlying pathological differences could potentially have different effects on top-down and bottom-up pathways in the two conditions. Further studies in a more homogenous pathological population might elucidate these.
We performed all assessments and the experimental task with patients in the ON state, with patients taking their usual medications, to prevent potential confounds arising from motor differences and the distress of being OFF. Although levodopa equivalent doses did not differ between patients with and without hallucinations, further studies could specifically test LBD patients ON and OFF medication to examine the effect of dopamine on sensory integration. This is particularly relevant given the potential higher risk of VH with high levodopa doses in LBD (Fénelon et al., 2000; Gallagher et al., 2011; Weil et al., 2016) and the link between striatal dopamine release and hallucinations (Cassidy et al., 2018). Indeed, in Parkinson’s disease (without hallucinations), levodopa has been shown to increase the weighting of sensory evidence in a visual decision-making task (Vilares and Kording, 2017). This apparent contradiction—of dopamine appearing to promote hallucinations (top-down) and sensory evidence (bottom-up)—might be resolved if one considers that the benefit of increasing sensory weighting depends on the quality of the data. If this weighting benefits precise sensory data, then sensory input will contribute more to inference (Vilares and Kording, 2017), but if it merely amplifies sensory noise, then hallucinations may increase.
Finally, our findings are consistent with studies in psychiatric illness and hallucination-prone individuals. People at risk of or with early psychosis also exhibit a shift towards prior knowledge and have a perceptual advantage in disambiguating a degraded visual scene or degraded speech (Teufel et al., 2015; Alderson-Day et al., 2017; Davies et al., 2018). Our study in combination with these findings suggests that hallucinations may share a computational mechanism across diseases with the same neural systems involved in generating hallucinations regardless of the pathophysiological diagnosis. This could have useful implications in translating advances and treatments across fields.
In summary, we show that VH in LBD are associated with an increased use of prior knowledge when viewing ambiguous visual stimuli, with increased hallucination severity predicting greater effects of prior knowledge. The shift from sensory evidence to prior knowledge in visual perception may be a useful marker for hallucination severity in LBD as well as other neurodegenerative and psychiatric illnesses where hallucinations are prominent.
Supplementary Material
Acknowledgments
We thank our participants for their time; Ribeya Mahmoud and Molly Cartlidge for their help with data collection and Stephen Fleming for his expertise on signal detection theory.
Funding
A.Z. is supported by an Alzheimer’s Research UK Clinical Research Fellowship (2018B-001). R.S.W. is supported by a Wellcome Clinical Research Career Development Fellowship (201567/Z/16/Z). R.A.A. is an MRC Skills Development Fellow (MR/S007806/1) and has also been supported by the Academy of Medical Sciences (AMS-SGCL13-Adams), the National Institute of Health Research (CL-2013-18-003) and the NIHR UCLH Biomedical Research Centre. G.R. is supported by the Wellcome Trust.
Competing interests
R.S.W. reports personal fees from GE Healthcare.
Author contributions
AZ, RA, GR and RW conceived and designed the study. AZ developed and piloted the stimuli. AZ and SP designed the testing application. AZ and LL recruited and tested the study participants. AZ and RW performed data analysis. All authors wrote and approved the final version of the manuscript.
Glossary
Abbreviations
- HADS
Hospital Anxiety and Depression Scale
- LBD
Lewy body disease
- LEDD
Levodopa equivalent daily dose
- MMSE
Mini-Mental State Examination
- MOCA
Montreal Cognitive Assessment
- RBDSQ
REM Sleep Behaviour Disorder
- UM-PDHQ
University of Miami Parkinson's Disease Hallucinations Questionnaire
- UPDRS
Unified Parkinson’s Disease Rating Scale
- VH
Visual hallucinations
References
- Aarsland D, Brønnick K, Ehrt U, De Deyn PP, Tekin S, Emre M.. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry 2007; 78: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ.. The computational anatomy of psychosis. Front Psychiatry 2013; 4: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B, Lima CF, Evans S, Krishnan S, Shanmugalingam P, Fernyhough C, et al. Distinct processing of ambiguous speech in people with non-clinical auditory verbal hallucinations. Brain J Neurol 2017; 140: 2475–89. [DOI] [PubMed] [Google Scholar]
- Benton AL, Varney NR, Hamsher KD.. Visuospatial judgment: a clinical test. Arch Neurol 1978; 35: 364–7. [DOI] [PubMed] [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, Neckelmann D.. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52: 69–77. [DOI] [PubMed] [Google Scholar]
- Cassidy CM, Balsam PD, Weinstein JJ, Rosengard RJ, Slifstein M, Daw ND, et al. A perceptual inference mechanism for hallucinations linked to striatal dopamine. Curr Biol 2018; 28: 503–14.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P. Visual cognition. Vision Res 2011; 51: 1538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR.. Hallucinations and strong priors. Trends Cogn Sci (Regul Ed) 2019; 23: 114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creavin ST, Wisniewski S, Noel‐Storr AH, Trevelyan CM, Hampton T, Rayment D, et al. Mini‐Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst Rev 2016; CD011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010; 75: 1717–25. [DOI] [PubMed] [Google Scholar]
- Davies DJ, Teufel C, Fletcher PC.. Anomalous perceptions and beliefs are associated with shifts toward different types of prior knowledge in perceptual inference. Schizophr Bull 2018; 44: 1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG, Stebbins GT.. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord 2005; 20: 130–40. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fink GR, Rolls E, Booth M, Holmes A, Frackowiak RS, et al. How the brain learns to see objects and faces in an impoverished context. Nature 1997; 389: 596–9. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 2007; 22: 1689–707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- Erskine D, Taylor J-P, Thomas A, Collerton D, McKeith I, Khundakar A, et al. Pathological changes to the subcortical visual system and its relationship to visual hallucinations in dementia with Lewy bodies. Neurosci Bull 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth D. The Farnsworth dichotomous test for color blindness, panel D-15: manual. New York: Psychological Corp; 1947. Available from: https://catalog.hathitrust.org/Record/102201930 (23 January 2019, date last accessed). [Google Scholar]
- Fénelon G, Mahieux F, Huon R, Ziégler M.. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain J Neurol 2000; 123: 733–45. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD.. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 2009; 10: 48–58. [DOI] [PubMed] [Google Scholar]
- Gallagher DA, Parkkinen L, O'Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain 2011; 134: 3299–309. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ.. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988; 51: 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23: 2129–70. [DOI] [PubMed] [Google Scholar]
- Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol 2009; 127: 737–41. [DOI] [PubMed] [Google Scholar]
- Hegdé J, Kersten D.. A link between visual disambiguation and visual memory. J Neurosci 2010; 30: 15124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper H. Hooper visual organization test (VOT) manual. Los Angeles: CA: Western Psychological Services; 1983. [Google Scholar]
- Hsieh P-J, Vul E, Kanwisher N.. Recognition alters the spatial pattern of FMRI activation in early retinotopic cortex. J Neurophysiol 2010; 103: 1501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G.. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997; 22: 39–52. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neurobiology of cognitive impairment in Parkinson’s disease. Expert Rev Neurother 2012; 12: 1451–66. [DOI] [PubMed] [Google Scholar]
- Karvelis P, Seitz AR, Lawrie SM, Seriès P.. Autistic traits, but not schizotypy, predict increased weighting of sensory information in Bayesian visual integration. eLife. 2018; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Ahn J, Kim TW, Jeon BS.. Optical coherence tomography in Parkinson’s disease: is the retina a biomarker? J Parkinsons Dis 2014; 4: 197–204. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS.. Neuropsychological assessment. 4th edn.New York, NY, US: Oxford University Press; 2004. [Google Scholar]
- Macmillan NA, Creelman CD.. Detection theory: a user’s guide. New York, NY, US: Cambridge University Press; 1991. [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor J-P, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondolo F, Jahanshahi M, Granà A, Biasutti E, Cacciatori E, Di Benedetto P.. The validity of the hospital anxiety and depression scale and the geriatric depression scale in Parkinson’s disease. Behav Neurol 2006; 17: 109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Cavanagh P.. Recovery of 3D volume from 2-tone images of novel objects. Cognition 1998; 67: 45–71. [DOI] [PubMed] [Google Scholar]
- Mori E, Shimomura T, Fujimori M, Hirono N, Imamura T, Hashimoto M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol 2000; 57: 489–93. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Mather G, Wesnes KA, O'Brien JT, Burn DJ, McKeith IG.. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology 2004; 63: 2091–6. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C, Hall JM, Tomassini A, Muller AJ, Walpola IC, Moustafa AA, et al. Visual hallucinations are characterized by impaired sensory evidence accumulation: insights from hierarchical drift diffusion modeling in Parkinson’s disease. Biol Psychiatry Cogn Neurosci Neuroimaging 2017a; 2: 680–8. [DOI] [PubMed] [Google Scholar]
- O’Callaghan C, Kveraga K, Shine JM, Adams RB, Bar M.. Predictions penetrate perception: Converging insights from brain, behaviour and disorder. Conscious Cogn 2017b; 47: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos S, Katzen H, Schrag A, Singer C, Scanlon BK, Nation D, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson’s disease. BMC Neurol 2008; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T, Rees G, Friston KJ.. Computational neuropsychology and bayesian inference. Front Hum Neurosci 2018; 12: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli D, Robson JG, Wilkins AJ.. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci 1988; 2: 187–99. [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. Abolishing the 1-year rule: how much evidence will be enough? Mov Disord off Disord 2016; 31: 1623–7. [DOI] [PubMed] [Google Scholar]
- Powers AR, Kelley M, Corlett PR.. Hallucinations as top-down effects on perception. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AR, Mathys C, Corlett PR.. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 2017; 357: 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rende B, Ramsberger G, Miyake A.. Commonalities and differences in the working memory components underlying letter and category fluency tasks: a dual-task investigation. Neuropsychology 2002; 16: 309–21. [DOI] [PubMed] [Google Scholar]
- Sezgin M, Sankur B.. Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging 2004; 13: 146–66. [Google Scholar]
- Stiasny-Kolster K, Mayer G, Schäfer S, Möller JC, Heinzel-Gutenbrunner M, Oertel WH.. The REM sleep behavior disorder screening questionnaire—a new diagnostic instrument. Mov Disord 2007; 22: 2386–93. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18: 643–62. [Google Scholar]
- Teufel C, Dakin SC, Fletcher PC.. Prior object-knowledge sharpens properties of early visual feature-detectors. Sci Rep 2018; 8: 10853.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C, Subramaniam N, Dobler V, Perez J, Finnemann J, Mehta PR, et al. Shift toward prior knowledge confers a perceptual advantage in early psychosis and psychosis-prone healthy individuals. Proc Natl Acad Sci USA 2015; 112: 13401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilares I, Kording KP.. Dopaminergic medication increases reliance on current information in Parkinson’s disease. Nat Hum Behav 2017; 1: 0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK. Recognition memory test: manual. Berkshire: UKNFER-Nelson; 1984. [Google Scholar]
- Warrington EK. The graded naming test: a restandardisation. Neuropsychol Rehabil 1997; 7: 143–6. [Google Scholar]
- Warrington EK, James M.. The visual object and space perception battery. Bury St. Edmunds, Suffolk: Thames Valley Test Company; 1991. [Google Scholar]
- Wechsler D. Wechsler adult intelligence Scale-Fourth edition. Fourth San Antonio, TX: NCS Pearson; 2008. Available from: https://www.pearsonclinical.com/psychology/products/100000392/wechsler-adult-intelligence-scalefourth-edition-wais-iv.html (6 February 2019, date last accessed). [Google Scholar]
- Weil RS, Lashley TL, Bras J, Schrag AE, Schott JM.. Current concepts and controversies in the pathogenesis of Parkinson’s disease dementia and dementia with Lewy bodies. F1000Research 2017; 6: 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR.. Visual dysfunction in Parkinson’s disease. Brain J Neurol 2016; 139: 2827.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain J Neurol 2009; 132: 2958–69. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP.. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code used to create the two-tone stimuli as well as code used for data analysis is provided in the following repository: https://github.com/AngelikaZa/Increased-weighting-on-prior-knowledge-in-Lewy-Body-associated-visual-hallucinations.-BrainComms2019.git. The specific stimuli used for this experiment are also provided in the same repository. The data that support the findings of this study are available from the corresponding author, upon reasonable request.