Hereditary hemochromatosis (HH) is a frequent iron overload disorder hallmarked by severe tissue iron accumulation. If untreated, patients experience serious clinical consequences, such as liver cirrhosis, cardiac dysfunction, diabetes and growth retardation.1 To date, phlebotomy remains the most effective treatment, in particular for patients that have not yet developed organ failure. Adverse effects include malaise, tiredness, fainting and phlebitis. Phlebotomy strongly impacts on the patient's quality of life since an ambulatory care facility needs to be attended often on a weekly base.2,3 Pharmacological strategies such as iron chelation are indicated only with very severe iron overload or in the presence of anemia. Iron chelation is associated with a wide range of adverse effects spanning from blurred vision, dizziness and diarrhea to neutropenia, arthralgia and liver damage.2,3 Therefore, alternative treatment options that are effective and long-lasting are sought after.
HH is caused by an inappropriately low expression of hepcidin, a peptide hormone produced predominantly by hepatocytes. Hepcidin binding to the iron exporter ferroportin triggers its degradation and thus controls dietary iron absorption and iron release from splenic macrophages. Hepcidin expression is mainly controlled by the BMP-SMAD signaling pathway, which is activated in a paracrine manner by the bone morphogenetic proteins BMP2 and BMP6 produced by liver sinusoidal endothelial cells (LSEC). The BMP receptor on hepatocytes consists of a heterotetramer of BMP type I (ALK2 and ALK3) and type II (BMPR2 and ACVR2A) receptors that act together with the BMP co-receptor hemojuvelin (HJV) to activate SMAD1,5,8 phosphorylation, and hepcidin transcription. Accessory proteins such as TfR2 and HFE form a protein complex to sense transferrin-bound iron and fine tune BMP receptor activity.4 HFE binds ALK3 inhibiting its ubiquitination and proteasomal degradation thus stabilizing the receptor and increasing its activity.5 TMPRSS6, a serine protease is a repressor of hepcidin transcription, which is mutated in patients with iron refractory iron deficiency anemia (IRIDA).6 It cleaves HJV thus avoiding the over-activation of the BMP/SMAD pathway.
More than 80% of the patients affected by HH are homozygous for the p.C282Y mutation of the Hfe gene. This particular form of HH, defined as HH type 1, is the most frequent inherited autosomal recessive disorder in Caucasian people. Hfe-ko mice recapitulate the major hallmarks of the human disease, including decreased hepcidin expression, systemic iron overload and iron deposition in parenchymal tissues.7 Genetic Tmprss6 ablation in Hfe-ko mice is able to restore hepcidin levels and ameliorate the iron parameters.8 This opened the possibility to use TMPRSS6 targeting strategies as therapeutic approach for the treatment of iron overload disorders such as HH type 1. Recent studies have indeed demonstrated that suppression of hepatic Tmprss6 by applying siRNAs complexed with lipid nanoparticles or by using antisense oligonucleotides (ASO) increased hepcidin expression in Hfe-ko mice.9,10 However, these studies show two major limitations: the half-life of the drug and the lack of a selective hepatocyte targeting strategy. It is of note that Tmprss6 is not only expressed in hepatocytes but also in other tissues such as pituitary gland and testis (https://www.gtexportal.org/home/gene/TMPRSS6). The use of untargeted pharmacological strategies could thus generate adverse effects in these organs and/or reduce the efficacy of the drug.
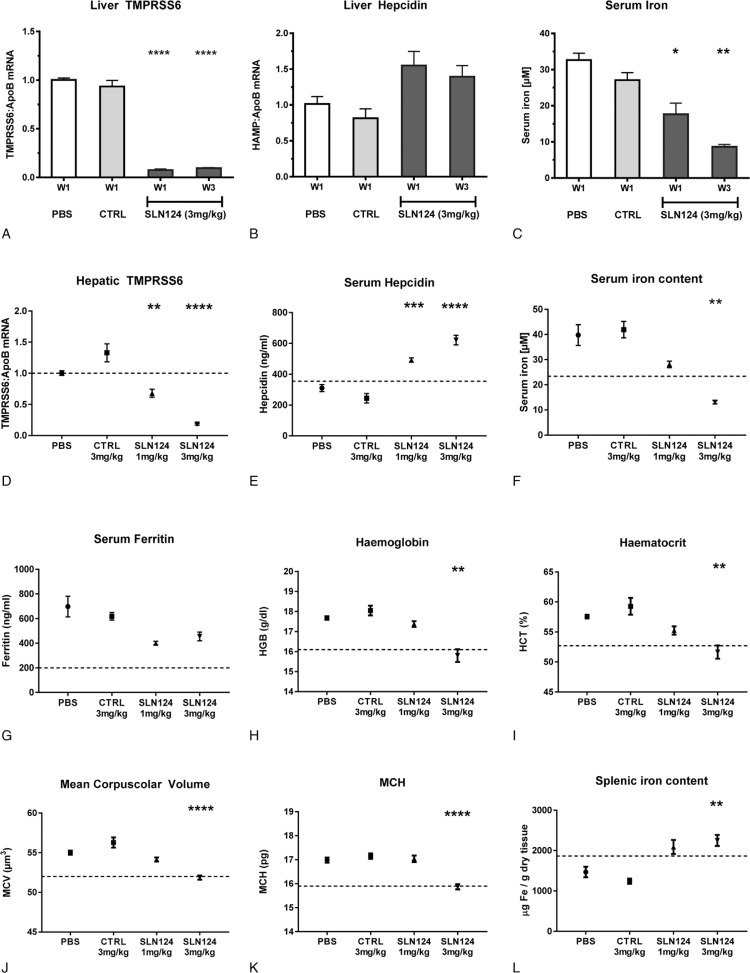
Here we report the pharmacological characterization of SLN124, a GalNAc-siRNA conjugate targeting Tmprss6 expression exclusively in hepatocytes. siRNAs conjugated to a GalNAc ligand bind to the asialoglycoprotein (ASGP) receptor expressed predominantly by hepatocytes thereby providing a potentially safe, specific and efficient delivery technology for therapeutic molecules.11 As shown in Figure 1 (A–C), a single subcutaneous injection of SLN124 (3 mg/kg body weight) is sufficient to achieve significant reduction of Tmprss6 gene expression in wild type mice for at least 3 weeks (Fig. 1A). Mice injected with PBS or with 3 mg/kg GalNAc-conjugated siRNA targeting Luciferase (CTRL) were used as controls. SLN124-mediated TMPRSS6 suppression correlates with a persistent increase in hepatic hepcidin mRNA expression and elevated hepcidin protein levels in the plasma causing hypoferremia (Fig. 1B–C). TMPRSS6 suppression was shown to stabilize the BMP co-receptor HJV, which increases expression of BMP/SMAD target genes such as hepcidin.12 Increased hepatic mRNA expression of SMAD6 and SMAD7 serve as indicators for an activated BMP/SMAD signaling pathway in SLN124-injected mice, whereby non targeted BMP2 and BMP6 gene expression is not increased (Fig. S1A–D). Hepcidin is an acute phase protein and its expression can be activated upon inflammation to restrict iron availability to pathogens.13 To exclude that hepcidin upregulation upon SLN124 administration is induced by inflammation, we measured mRNA expression of alpha-2-macroglobulin (A2M), an additional hepatocyte-specific acute phase protein, which remained unchanged (Fig S1E). Altogether, these experiments establish the in vivo efficacy of SLN124 in targeting TMPRSS6 to increase serum hepcidin levels and reduce systemic iron availability in wild type mice.
Figure 1.
SLN124 increases hepcidin levels and induces hypoferremia in wild type and Hfe-ko mice. A–C: 7–8-week old C57BL/6N female mice (4 per group) were treated with a single subcutaneous injection of PBS or with 3 mg/kg body weight GalNAc-Luciferase siRNA (CTRL) or SLN124, as indicated. Mice were sacrificed after 1 or 3 weeks (W1, W3) and blood and tissue samples collected. A-B: qPCR analysis of hepatic TMPRSS6 and Hepcidin mRNA. C: Serum iron measurement. D-L: Six-week old female Hfe-ko mice (6–7 animals per group) were subcutaneously injected with PBS, with 3 mg/kg body weight of GalNAc-Luciferase siRNA (CTRL) or with two different doses of SLN124, as indicated. Mice were analyzed 3 weeks after treatment. D: Gene expression analysis of hepatic TMPRSS6 mRNA levels. E: Quantification of hepcidin levels in the serum. F-G: Measurement of systemic iron parameters: serum iron content (F) and serum ferritin levels (G). H-K: Characterization of the erythroid parameters Haemoglobin (H), Haematocrit (I), Mean Corpuscolar Volume (J) and Mean Corpuscolar Haemoglobin (K). L: Non-heme splenic iron quantification. Data are reported as mean ± S.E.M. The dashed line indicates the average value of the parameter measured on age, gender and genetic background (C57BL/6J) matched wild type animals. Welch-ANOVA with Dunnett T3 multiple comparisons test against PBS control group. P values <.05 (∗), <.01 (∗∗), <.001 (∗∗∗) and <.0001 (∗∗∗∗) are indicated.
To explore the therapeutic potential of SLN124 for the treatment of HH type 1, we administered two different doses of the drug (1 mg/kg and 3 mg/kg) to 6-week old Hfe-ko female mice. Three weeks after SLN124 application, Hfe-ko animals show a dose-dependent suppression of TMPRSS6 mRNA levels (Fig. 1D), and increased serum hepcidin (Fig. 1E). Already at low doses, SLN124 reduces both serum iron levels and transferrin saturation close to physiological levels (Fig. 1F, S2A). Additionally, serum ferritin, a marker for body iron stores is strongly decreased (Fig. 1G). This indicates prevention of iron accumulation in hepatocytes (Fig. S3B) in growing mice with reduced systemic iron availability (Fig. 1F, S2A). Despite this, the overall hepatic iron content remains unchanged (Fig S3A) and redistribution of hepatic iron to Kupffer cells does not occur (Fig S3B). At higher doses, SLN124 turns iron overload into systemic iron deficiency (Fig. 1F, S2A). Patients with hereditary hemochromatosis type 1 show altered erythroid parameters, whereby the number, size and hemoglobin content of red blood cells is increased.14 The same erythroid alterations are preserved in Hfe-ko mice (Fig. 1H–K). A low dose of SLN124 reduces the density (hematocrit - HCT) and the size (mean corpuscular volume - MCV) of red blood cells (Fig. 2I, J) in Hfe-ko mice. The administration of a higher dose of SLN124 further decreases MCV and HCT, but also significantly reduces the levels of hemoglobin and the erythrocyte hemoglobin content (MCH). Analysis of the spleen showed that low dose SLN124 treatment significantly increases iron content (Fig. 1L). Histological analysis revealed iron accumulation in the red pulp, specifically in reticuloendothelial macrophages (Fig. S2B). This finding is consistent with the function of hepcidin in inhibiting iron export causing iron retention in splenic macrophages. Overall, these results show that SLN124 is a potent inducer of hepcidin and that it is able to normalize systemic iron indices and hematological parameters in a murine disease model of HH type 1.
Figure 2.
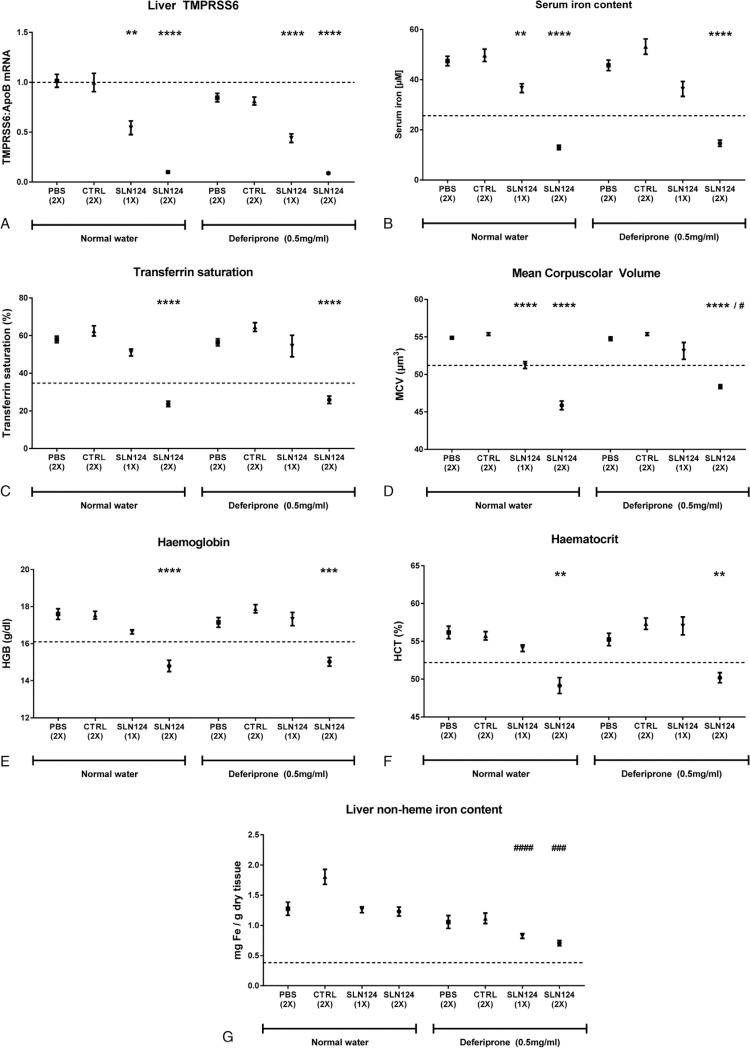
Combination treatment of SLN124 and deferiprone for the treatment of HH type 1. Fifteen-week-old Hfe-ko female mice (8 animals per group) were injected once (day 1) or twice (day 1 and day 22) over a period of 6 weeks with PBS or with 3 mg/kg body weight of GalNAc-Luc (CTRL) or SLN124 and treated with or without the iron chelator deferiprone in drinking water (0.5 mg/ml), as indicated. A: Gene expression analysis of hepatic TMPRSS6 mRNA levels. B–C: Measurement of serum iron levels (B) and transferrin saturation (C). D–F: Characterization of the erythroid parameters Mean Corpuscular Volume (D), Haemoglobin (E) and Haematocrit (F). G: Non-heme iron measurement of liver (G). Data are reported as mean ± S.E.M. The dashed line indicates the average value of the parameter measured on age, gender and genetic background (C57BL/6J) matched wild type animals. Welch-ANOVA with Dunnett T3 multiple comparisons test: (∗) against PBS control group as well as between corresponding deferiprone treated and deferiprone untreated groups (#). P values <.05 (∗/#), <.01 (∗∗/##), <.001 (∗∗∗/###) and <.0001 (∗∗∗∗/####) are indicated.
HH type 1 is commonly diagnosed at middle-to-late age, when patients are referred to a specialist as a consequence of organ damage secondary to iron overload. Phlebotomy is the first line treatment of HH type 1. The only pharmacological strategy available to date is to mobilize and remove excess iron from organs by treatment with iron chelating agents, like deferiprone.15 We next evaluated the long term efficacy of SLN124 in aged Hfe-ko mice (15-week old females), a time point when tissue iron accumulation becomes more severe. We further compared the efficacy of SLN124 with deferiprone and investigated the possibility to apply both strategies in combination. Hfe-ko mice were subjected to a single or to two subsequent doses of SLN124 (3 mg/kg), which were administered once every 3 weeks. Mice were analyzed 6 weeks thereafter. For comparison, Hfe-ko mice were treated with the iron chelator deferiprone supplied in drinking water (0.5 mg/ml) with or without an additional injection of SLN124 (Fig. 2).
Six weeks after application of a single dose of SLN124, Hfe-ko mice showed an impressive reduction of TMPRSS6 levels (Fig. 2A) as well as diminished levels of serum iron and transferrin saturation (Fig. 2B, C), demonstrating that a single injection of SLN124 is able to reduce the amount of circulating iron up to 6 weeks in aged mice with HH type 1. Similar to our observations in young mice, a single dose of SLN124 restored the erythroid parameters (MCV, HCT, and Hb) to values comparable to wild type animals (Fig. 2D–F). Mice treated with two doses of SLN124 showed an even more dramatic reduction of systemic iron-related indices. Serum iron content, transferrin saturation and the erythroid parameters were reduced beyond those of wild-type mice (Fig. 2B–F). Interestingly, 6 weeks of deferiprone treatment of Hfe-ko mice did not alter systemic and hematological iron-related parameters when compared to untreated animals. Importantly, when used in combination with SLN124, deferiprone did not enhance the therapeutic effect of SLN124 (Fig. 2B–F).
The liver is a central organ for maintaining iron homeostasis. It stores iron to provide this essential micronutrient in case of deficiency. If systemic iron levels are increased, its high storage capacity protects other tissues such as pancreas, heart and kidney from iron-mediated injuries. However, also in the liver chronic exposure to excess iron will cause tissue injury, such as fibrosis, cirrhosis and cancer. Iron massively accumulates in the liver of Hfe-ko mice, consistent with that observed in HH patients (Fig. 2G). Treatment with one or two doses of SLN124 over a 6 week period did not significantly reduce hepatic iron levels (Fig. 2G). By contrast, deferiprone treatment reduced liver iron content moderately (Fig. 2G). Interestingly, a stronger reduction of hepatic iron levels almost to wild-type level was observed when Hfe-ko mice were treated by a combination of deferiprone and SLN124. This finding confirms that the biological effects of iron restriction by SLN124 and iron removal by chelation therapy follow different modes of action and thus combination of treatments may be beneficial for improving red blood cell parameters and for the reduction of tissue iron overload.
Taken together, this preclinical study demonstrates the efficacy of SLN124 as a potent and long-term effective drug for the treatment of HH type 1. SLN124 can be used as monotherapy or in combination with deferiprone, an oral iron chelator, over an extended treatment period. Alone, SLN124 reduces systemic iron levels and normalizes erythropoiesis in a dose-dependent and long-lasting manner. Used in combination with the iron chelator deferiprone, SLN124 is well tolerated and enhances the iron-chelation effect in reducing hepatic iron stores. Moreover, no pathological on target side effects are expected in case of an excessive SLN124 administration since human patients affected by TMPRSS6 mutations present exclusively with iron deficient anemia. Based on its mechanism of action, SLN124 holds great promise as an effective and safe treatment for hereditary hemochromatosis type 1, particularly in those patients who cannot tolerate phlebotomy or have complications such as arthropathy or liver damage as a consequence of iron over-accumulation in the body.
Supplementary Material
Footnotes
Citation: Altamura S, Schaeper U, Dames S, Löffler K, Eisermann M, Frauendorf C, Müdder K, Neves J, Muckenthaler MU. SLN124, a GalNAc-siRNA Conjugate Targeting TMPRSS6, Efficiently Prevents Iron Overload in Hereditary Haemochromatosis type 1. HemaSphere, 2019;3:6(e301). http://dx.doi.org/10.1097/HS9.0000000000000301
The authors acknowledge the pathology institute and Nikon center (University of Heidelberg) for granting us the access to their facilities and equipment. Grants: EHA Advanced non-clinical research fellowship to SA. MUM acknowledges funding from the Deutsche Forschungsgemeinschaft (SFB1036, SFB1118) and from Silence Therapeutics.
US, SD, KL, ME, and CF are employed by Silence Therapeutics GmbH.
Supplemental Digital Content, http://links.lww.com/HS/A48
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.hemaspherejournal.com).
References
- 1.Pietrangelo A. Hereditary hemochromatosis. Ann Rev Nutr. 2006;26:251–270. [DOI] [PubMed] [Google Scholar]
- 2.Brissot P, Pietrangelo A, Adams PC, et al. Haemochromatosis. Nat Rev Dis Primers. 2018;4:18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brissot P, Ball S, Rofail D, et al. Hereditary hemochromatosis: patient experiences of the disease and phlebotomy treatment. Transfusion. 2011;51:1331–1338. [DOI] [PubMed] [Google Scholar]
- 4.Silvestri L, Nai A, Dulja A, et al. Hepcidin and the BMP-SMAD pathway: an unexpected liaison. Vitam Horm. 2019;110:71–99. [DOI] [PubMed] [Google Scholar]
- 5.Wu XG, Wang Y, Wu Q, et al. HFE interacts with the BMP type I receptor ALK3 to regulate hepcidin expression. Blood. 2014;124:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40:569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrmann T, Muckenthaler M, van der Hoeven F, et al. Iron overload in adult Hfe-deficient mice independent of changes in the steady-state expression of the duodenal iron transporters DMT1 and Ireg1/ferroportin. J Mol Med (Berl). 2004;82:39–48. [DOI] [PubMed] [Google Scholar]
- 8.Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. 2011;117:4590–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S, Casu C, Gardenghi S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. J Clin Invest. 2013;123:1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt PJ, Toudjarska I, Sendamarai AK, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(-/-) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood. 2013;121:1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Springer AD, Dowdy SF. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018;28:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestri L, Pagani A, Nai A, et al. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. [DOI] [PubMed] [Google Scholar]
- 14.Feeney GP, Carter K, Masters GS, et al. Changes in erythropoiesis in hereditary hemochromatosis are not mediated by HFE expression in nucleated red cells. Haematologica. 2005;90:180–187. [PubMed] [Google Scholar]
- 15.Mobarra N, Shanaki M, Ehteram H, et al. A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Oncol Stem Cell Res. 2016;10:239–247. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.