Abstract
Invasive fungal diseases (IFDs) remain a major clinical issue in patients with hematological malignancies (HMs). To confirm the efficacy and safety of the new azole isavuconazole (ISV) in a clinical care setting, we planned a multicenter retrospective study; we collected data on all possible/probable/proven IFDs in patients with HMs treated with ISV in 17 centers. Between July 2016 and November 2018, 128 patients were enrolled, and 122 were fully evaluable. ISV was employed as the 1st line therapy in 43 (35%) patients and as a subsequent therapy in 79 (65%) patients. The response rate was 82/122 patients (67.2%); it was similar when using ISV as a 1st or 2nd line treatment (60.5% vs 70.9%, respectively; p = 0.24). In multivariate analysis, both female sex (OR: 2.992; CI: 1.22–7.34) and induction phase of treatment (OR: 3.953; CI: 1.085–14.403) were predictive of a favorable response. At a median follow-up of 5 months, 43 (35.2%) patients were dead; the 1-year overall survival (OS) was 49.9%. In multivariate analysis, the response to ISV (OR: 0.103; CI: 0.041–0.262) and IFD refractoriness to previous antifungals (OR: 3.413; CI: 1.318–8.838) were statistically significant for OS. Adverse events (AEs) were reported in 15/122 patients (12.3%); grade 3–4 AEs were reported in 5 (4%) and led to ISV discontinuation. Our study confirms the safety and tolerability of ISV, also in diseases other than acute leukemia. Phase of hematological disease, gender and refractoriness to previous antifungals are the main predictive factors for the aforementioned response and outcome.
Introduction
The outcome of invasive fungal diseases (IFDs), particularly invasive pulmonary aspergillosis (IPA) in acute leukemia (AL) patients, has improved over the last years. However, IFD remains a major clinical issue in patients with hematological malignancies (HMs), both regarding the severity and toxicity of antifungal treatments as well as the potential drug interactions. Indeed, many studies have reported a negative prognostic impact of IFDs, particularly IPA, in AL patients1–3 and allogeneic stem cell transplantation (alloSCT) recipents.4 Heavy immunosuppression, potentially affecting the response of IFD, and the need for prolonged antifungal (AF) treatment, which could delay the correct chemotherapeutic delivery timing, are the main reasons for a dismal prognosis of this subset of patients. Moreover, the advent of new drugs, immunological and targeted therapies with small-molecule kinase inhibitors, has contributed to redefine the epidemiological scenario in HM patients, configuring new categories at risk for IFDs, such as lymphoproliferative disorders undergoing treatment with Bruton tyrosine-kinase (BTK) inhibitors.5
In the last decades, new antifungal drugs have been commercialized, allowing easier management of IFD treatment, with an improved outcome, at least in the non-alloSCT setting;6 however, potential toxicity and drug-drug interaction problems can lead to AF treatment discontinuation and can negatively impact the final outcome.7 Isavuconazole (ISV) is a new antifungal agent with a modest drug-drug interaction profile, reduced drug-related adverse events and efficacy similar to voriconazole, as demonstrated in a non-inferiority trial of IFD treatment.8 ISV demonstrated efficacy also against rare fungi and in patients with impaired renal function.9 Due to the broad spectrum of action and its favorable interaction profile, ISV has been proposed as a very promising AF drug and international guidelines highly recommend its use in HM patients with IPA.10,11 However, the experience evaluating the potential benefits of ISV in the real-life setting is still limited.12–14
The aim of this retrospective study was to describe the use of ISV as treatment for IFD in current clinical practice in hematology to define the setting of patients where ISV is employed, to investigate the timing of ISV use during IFD treatment, and to evaluate ISV tolerability and its impact on the clinical outcome.
Patients and methods
The present observational retrospective study was conducted from July 1, 2016 to November 30, 2018 at 17 hematology units of tertiary care centers or university hospitals located throughout Italy and participating in SEIFEM (Sorveglianza Epidemiologica InFezioni nelle EMopatie). All adult patients with associated HMs who received ISV as AF treatment were included in the registry and followed up. ISV was administered with a loading dose of 200 mg IV/po X 6 doses (48 hours) and then a maintenance dose of 200 mg IV/po once daily.
The data were entered into case report forms. The impact of the age, gender, neutropenia/lymphopenia, type and status of hematological disease (diagnosis [Dx], complete and partial remission [CR/PR], relapse/refractory [r/r]), phase of hematological treatment at IFD (induction, consolidation, salvage/best supportive care [BSC] and alloSCT), type of IFD, previous mould-active prophylaxis and timing of ISV treatment was evaluated.
The Ethics Committee of each participating site approved the use of the SEIFEM registry.
Definitions
IFD was diagnosed according to EORTC/MSG criteria and categorized as possible, probable, and proven.15
The response to treatment (success vs failure) was defined using clinical and radiological criteria. Assessment of the state of IFI and clinical outcome was based on the investigator's evaluation. The response was assessed after 4 weeks of treatment and after 8 and 12 weeks when available. The state of IFI was recorded at the last (12 week) assessment as follows:
-
–
Complete response: resolution of all clinical signs and symptoms of IFD
-
–
Partial response: improvement of clinical signs and symptoms of IFD and/or a major improvement in the radiographic findings
-
–
Stable response: no changes in the clinical and radiographic findings of IFD
-
–
Progressive or refractory disease: deterioration of the clinical or radiographic findings of IFD16
A “success” was defined as complete or partial resolution of all attributable clinical symptoms/physical findings associated with at least a stable radiological finding. Death before 4 weeks of treatment was defined as failure.
Statistical analysis
Univariate analysis was performed using χ2 test. Variables found to be significant (p < 0.1) in univariate analysis were tested in multivariate analysis, which was performed using a stepwise logistic regression model. Values of p < 0.05 were considered statistically significant. Survival was evaluated using the Kaplan–Meier method and was compared using log-rank tests. p values below 0.05 were considered statistically significant. Analyses were carried out using the SPSS statistical package version 22.
Results
Characteristics of patients and epidemiology of IFD
One hundred twenty-eight patients were enrolled. Epidemiologic, response and outcome data were fully available for 122 patients. The median age was 57.5 years (range: 19–80 years) and the M/F ratio was 72/50; Table 1 summarizes their characteristics. IFD was possible in 51 (42%), probable in 59 (48%) and proven in 12 (10%) cases. Aspergillus spp. was considered responsible for 66 (93%) of probable/proven IFI; in the remaining 5 cases, 1 Rhizomucor and 1 Fusarium spp. were isolated and no specific agent was identified microbiologically in 3 histologically proven mould infections.
Table 1.
Characteristics of the 122 evaluable patients
Timing of ISV employment
ISV was employed as 1st line therapy in 43 (35%) and as subsequent-line therapy in 79 (65%) patients. The median duration of previous treatments was 18 days (range: 8–111 d). ISV was chosen as the 1st line treatment for its large spectrum of action in 8/43 (19%) cases and for its safety and/or favourable drug-drug interaction profile in 35/43 (81%). The reasons for ISV use as 2nd line treatment include previous AF treatment failure in 40 (51%) and intolerance in 19 (24%) of 79 cases. In 16 cases (20%), ISV was chosen because of the need to switch to an oral antifungal agent and in 4 (5%) for a favorable drug-drug interaction profile.
Response rate
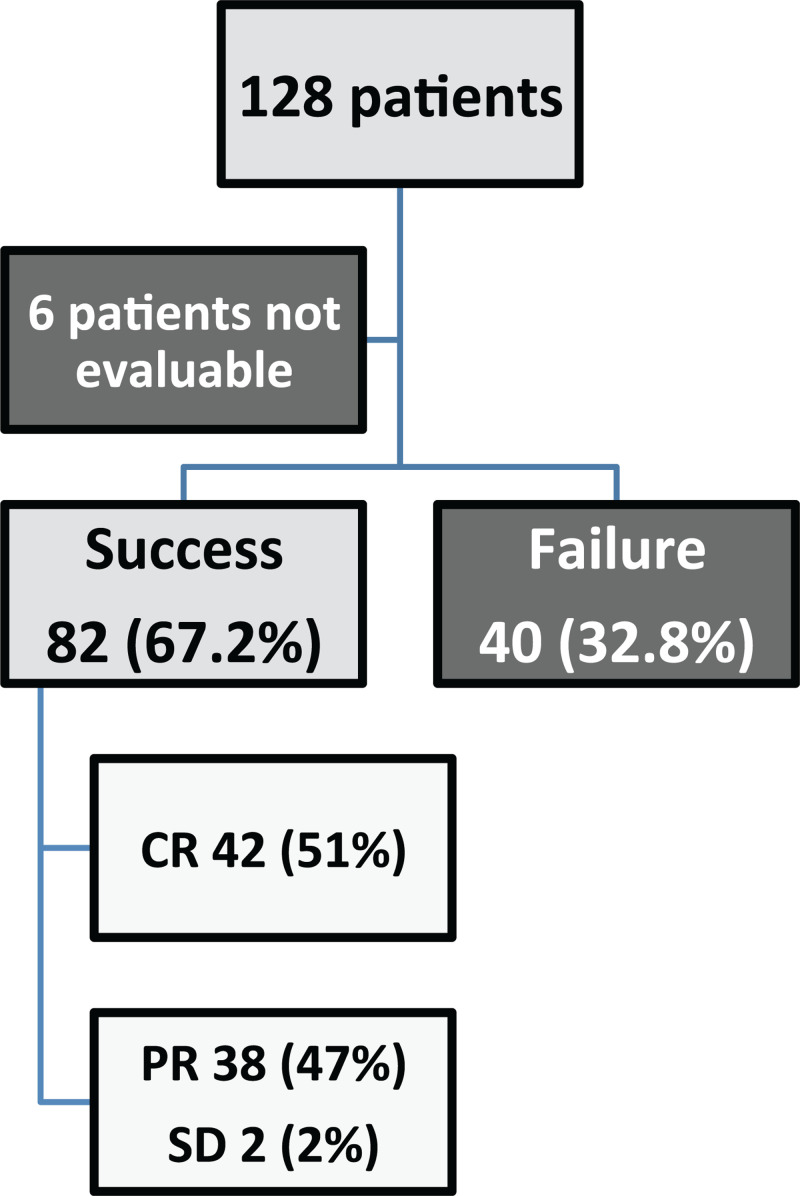
The clinical and radiological response rate (RR) was 67.2% (82/122 patients); the radiological response was complete in 42 (51%), partial in 38 (47%) and stable in 2 (2%) cases (Fig. 1). The RR was similar when using ISV as the 2nd or subsequent line compared with ISV as the 1st line (56/79, 70.9% vs 26/43, 60.5%; p = 0.24). When considering only patients treated with ISV as the 2nd line of treatment, the RR was higher in patients refractory to previous antifungal treatment IFD than those without IFD refractoriness (31/38, 81.6% vs 25/42, 59.6%, p = 0.03). Patients with refractory IFD had a similar probability of response when previous treatment was voriconazole or L-AmB, alone or in combination with other antifungals (RR, respectively: 12/17, 70.6% vs 14/26, 53.8%; p = 0.27). Female sex was associated with a better RR (40/50, 80% vs 42/72, 58.3%; p = 0.012). Considering the phase of hematological treatment, the RR was 23/27 (85.2%), during induction, 16/19 (84.2%) during consolidation, 19/35 (52.3%) during salvage/best supportive care (BSC) and 24/41 (58.5%) in alloSCT patients. Differences within the subgroups were statistically significant comparing induction vs salvage/BSC (p = 0.034), consolidation vs salvage/BSC (p = 0.049) and consolidation vs alloSCT (p = 0.049).
Figure 1.
Flow chart assessing the ISV response rate of the study population.
The response to ISV was higher in possible and probable IFD (35/51, 68.6%, and 42/59, 71.2%, respectively) than in proven IFD (5/12, 41.7%). ISV showed better activity in pulmonary IFD than in extra-pulmonary IFD (73/103, 70.9% vs 9/19, 47.4%; p = 0.045); in detail, 7 of 12 mycotic sinusitis cases showed a response to ISV. Moreover, none of the non-aspergillosis cases (1 Fusarium spp. fungaemia and 1 Rhizomucor spp. pulmonary infection) responded to ISV. No impact on the RR was observed for age, neutropenia, type of hematological disease, IFD type, and prior mold-active prophylaxis.
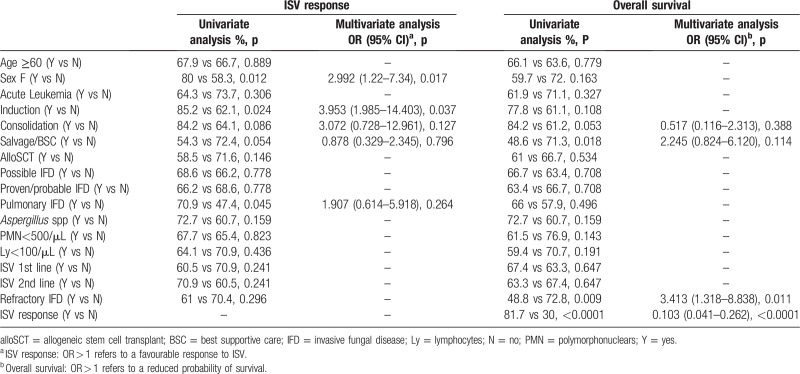
In multivariate analysis, both female sex (OR: 2.992; CI: 1.22–7.34) and ISV use during the induction phase of treatment (OR: 3.953; CI: 1.085–14.403) were predictive of a favorable ISV response. Table 2 summarizes univariate and multivariate analyses for the ISV overall response.
Table 2.
Univariate and multivariate analyses for the ISV response and overall survival
Overall survival
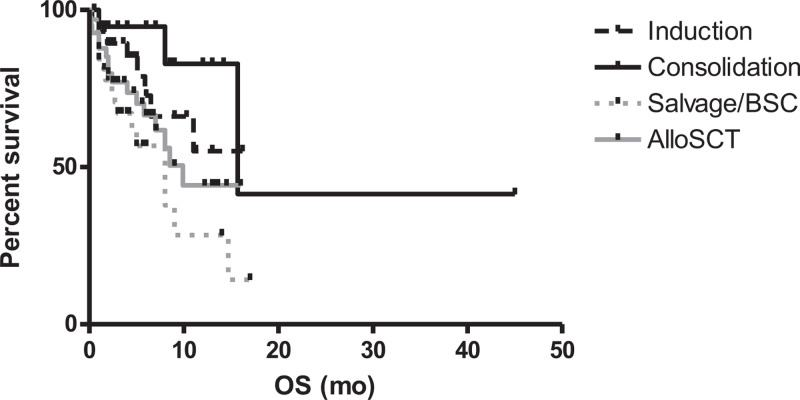
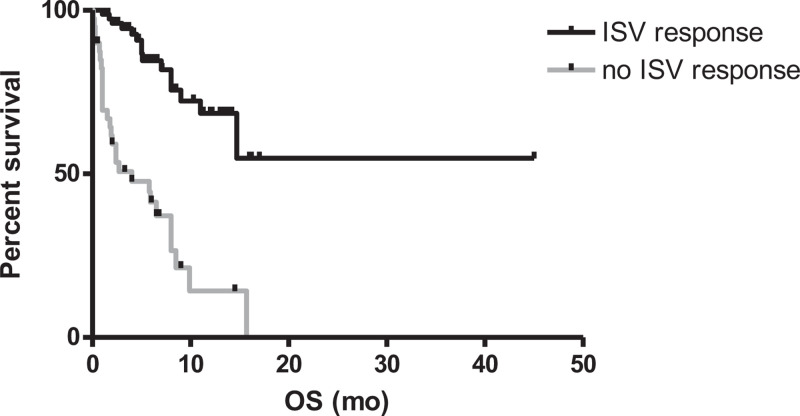
After a median follow-up of 5 months (range 0.5–45), 43/122 patients died (35.2%). The attributable mortality was 7/122 (5.7%). The estimated 1-year overall survival (OS) of the entire population was 49.9%. As observed for the RR, the phase of hematological treatment impacted the overall survival (OS): the 1-year OS rates were 55.1%, 82.9%, 25.3% and 44.2% in patients undergoing induction, consolidation, salvage/BSC and alloSCT, respectively. Differences within the subgroups were statistically significant comparing induction vs salvage/BSC (p = 0.025), consolidation vs salvage/BSC (p = 0.0029) and consolidation vs alloSCT (p = 0.046) (Fig. 2). The ISV 1-y survival rates were 68% in ISV-responding and 14.1% in ISV non-responding patients (p < 0.0001; Fig. 3). In patients receiving ISV as second-line antifungal therapy, having an IFD refractory to prior treatments negatively influenced survival (1-year OS: 36.7% for refractory IFI vs 65.4% for non-refractory IFI; p = 0.01).
Figure 2.
Overall survival according to the phase of treatment (The 1-year OS rates were 55.1%, 82.9%, 25.3%, and 44.2% using ISV during induction, consolidation, salvage/BSC and alloSCT, respectively).
Figure 3.
Overall survival according to the ISV response (1-year OS: 68% for an ISV response vs 14.1% for no ISV response; p < 0.0001).
In multivariate analysis, only the response to ISV (OR: 0.103; CI: 0.041–0.262) and IFD refractoriness (OR: 3.413; CI: 1.318–8.838) retained independent prognostic value. Table 2 summarizes univariate and multivariate analyses for OS.
Toxicity
Fifteen (12.3%) of the 122 patients experienced adverse events: 7 gastroenteric (nausea/vomiting), 5 hepatic (abnormal liver function tests), 3 cutaneous (skin rash) and 3 hypokalemia cases. Five patients (4%) receiving ISV as the 1st line of treatment discontinued the drug due to a ≥ grade 3 toxicity (3 abnormal liver function tests, 1 skin rash case, and 1 hypokalemia case). Only 2/41 (4.9%) patients undergoing alloSCT discontinued the drug, whereas none of those affected by acute lymphoblastic leukemia or lymphoma did.
Discussion
Although in recent years new antifungal drugs have become available, the management of HM patients with IFD remains a major problem. Dose-limiting toxicities, drug-drug interactions and intravenous formulations are responsible for the difficult management of fungal infection, early discontinuation of AF treatment and delay of hematological treatment. ISV has been presented as an efficacious and easily manageable AF treatment due to its safety profile and optimal bioavailability, even with the oral formulation.17
Real-life data regarding the ISV effect in HM patients are scarce and mainly monocentric.12–14 Indeed, ISV has been reported as a well-tolerated AF treatment.18 An efficacy similar to SECURE study has been reported by Hassouna et al,14 who described a monocentric study on 91 patients affected by hematological malignancies (mainly AL) or undergoing solid organ transplant and received ISV as treatment or prophylaxis. The safety of ISV as AF prophylaxis in neutropenic acute myeloid leukemia patients has been assessed in a phase 2, dose escalation study,19 but the actual efficacy was not fully confirmed in a recent study carried on high immunosuppressed patients, with a rate of 8/98 breakthrough IFD.20 Other real-life experiences concerning breakthrough IFD after prophylactic ISV have been reported,21,22 without evidence of a particular benefit, particularly during AL induction treatment where up to 13% of breakthrough IFD cases were reported.
In our real-life multicenter observational study, carried out only in the HM setting, almost half of the patients were affected by HM other than acute myeloid leukemia, confirming that ISV is widely used in many hematological diseases. ISV was well tolerated in most patients, including those undergoing alloSCT or affected by acute lymphoblastic leukemia and lymphoma receiving drugs with potential cross-interaction, such as immunosuppressants, vinca alkaloids and BTK inhibitors. Because ISV is a moderate inhibitor of CYP3A,23 interactions with other drugs that are substrates or inducers of CYP3A should be less pronounced. This aspect could be of particular interest in the management of patients with lymphoproliferative disorders who can now benefit from new drugs such as BTK inhibitors with known interaction with CYP450.
The RR of 67.2% is comparable to other antifungal treatments, as previously reported10 and is quite similar to the ORR of 62% observed at 84 days in the SECURE study.8 As expected, the phase of hematological treatment influenced the frequency of the response to ISV that was significantly lower in patients on salvage treatment/BSC or undergoing alloSCT. ISV response rates to ISV were similar during the induction and consolidation phase of treatment, confirming the improved outcome of patients affected by IFD during these disease phases.24 No difference of response for possible and proven/probable IFD were observed and this may be related to the more restrictive nature of the revised EORTC/MSG criteria in immunocompromised patients concerning the definition of “possible” IFD. Therefore possible IFD may be considered as a real infection in which the microbiological data could not be documented, probably due to the concomitant antifungal prophylaxis. ISV was less effective in subjects with extra-pulmonary IFD, as expected according to published observations,25 with the exception of fungal sinusitis, usually considered a favorable prognostic subgroup.26
The better response to ISV observed in female patients compared with male patients seems difficult to interpret and may be related to different distribution of clinical characteristics or disease subtype between the two sexes. Indeed, >60 year-old patients were more frequent in male than female population (54% vs 33%, p = 0.0417) and a diagnosis of AL was less frequent in male than female population (63% vs 78%, p = 0.07). However, a recent study27 showed a lower ISV clearance in women, particularly in elderly patients. This may be responsible for a higher plasma level of ISV in women, possibly indicating a more pronounced exposure to the drugs and better efficacy. However, no clear correlation between the pharmacokinetics and response to treatment have been demonstrated in the context of the SECURE study, and no specific data correlating the ISV plasma levels with gender and response to treatment are available so far. Indeed, ISV therapeutic drug monitoring (TDM) is not routinely recommended.28 Unfortunately, the ISV plasma levels were not analyzed in our retrospective study.
Presently, no strong recommendations are available for refractory IFD treatment,10 although a switch to a different class of antifungal is often suggested.29 In our real-life study, refractoriness to other antifungal treatments did not compromise the possibility of a response to ISV, which was more than 50% in patients receiving ISV as the 2nd line after failure of previous AF treatment. Moreover, the response was similar in patients previously exposed to L-AmB or voriconazole, alone or in combination with other antifungals. However, because the efficacy of ISV in refractory IFD was tested in a limited population, further studies are warranted to confirm these data.
In conclusion, our real-life study confirms the safety and tolerability for the treatment of IFI with ISV in HM patients, including those with diseases other than acute leukemia. The response rates were at least comparable to those observed in other studies. The phase of hematological disease, gender and refractoriness to previous antifungal treatments are the main predictive factors of the response and outcome.
Footnotes
Citation: Cattaneo C, Busca A, Gramegna D, Farina F, Candoni A, Piedimonte M, Fracchiolla N, Pagani C, Principe MI, Tisi MC, Offidani M, Fanci R, Ballanti S, Spolzino A, Criscuolo M, Marchesi F, Nadali G, Delia M, Picardi M, Sciumé M, Mancini V, Olivieri A, Tumbarello M, Rossi G, Pagano L. Isavuconazole in Hematological Patients: Results of a Real-Life Multicentre Observational Seifem Study. HemaSphere, 2019;3:6(e320). http://dx.doi.org/10.1097/HS9.0000000000000320
The authors have no conflicts of interest to disclose.
References
- 1.Even C, Bastuji-Garin S, Hicheri Y, et al. Impact of invasive fungal disease on the chemotherapy schedule and event-free survival in acute leukemia patients who survived fungal disease: a case-control study. Haematologica. 2011;96:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michallet M, Bénet T, Sobh M, et al. Invasive aspergillosis: an important risk factor on the short- and long-term survival of acute myeloid leukemia (AML) patients. Eur J Clin Microbiol Infect Dis. 2012;31:991–997. [DOI] [PubMed] [Google Scholar]
- 3.Girmenia C, Micozzi A, Piciocchi A, et al. Invasive fungal diseases during first induction chemotherapy affect complete remission achievement and long-term survival of patients with acute myeloid leukemia. Leuk Res. 2014;38:469–474. [DOI] [PubMed] [Google Scholar]
- 4.Girmenia C, Raiola AM, Piciocchi A, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20:872–880. [DOI] [PubMed] [Google Scholar]
- 5.Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. 2018;66:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dragonetti G, Criscuolo M, Fianchi L, et al. Invasive aspergillosis in acute myeloid leukemia: Are we making progress in reducing mortality? Med Mycol. 2017;55:82–86. [DOI] [PubMed] [Google Scholar]
- 7.Cornely OA. Isavuconazole: is there a need for a new antifungal? J Antimicrob Chemother. 2017;72 (suppl 1):i2–i4. [DOI] [PubMed] [Google Scholar]
- 8.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. [DOI] [PubMed] [Google Scholar]
- 9.Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. VITAL and FungiScope Mucormycosis Investigators. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–837. [DOI] [PubMed] [Google Scholar]
- 10.Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24 (suppl 1):e1–e38. [DOI] [PubMed] [Google Scholar]
- 12.Ordaya EE, Alangaden GJ. Real-life use of isavuconazole in patients intolerant to other azoles. Clin Infect Dis. 2016;63:1529–1530. [DOI] [PubMed] [Google Scholar]
- 13.DiPippo AJ, Kontoyiannis DP. Lack of toxicity with long term isavuconazole use in patients with hematologic malignancy. Clin Infect Dis. 2019;pii: ciz159. [DOI] [PubMed] [Google Scholar]
- 14.Hassouna H, Athans V, Brizendine KD. Real-world use-Isavuconazole at a large academic medical center. Mycoses. 2019;62:534–541. [DOI] [PubMed] [Google Scholar]
- 15.De Pauw B, Walsh TJ, Donnelly JP, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornely OA, Hoenigl M, Lass-Flörl C, et al. Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses. 2019;62:716–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt-Hoffmann A, Desai A, Kowalski D, et al. Isavuconazole absorption following oral administration in healthy subjects is comparable to intravenous dosing, and is not affected by food, or drugs that alter stomach pH. Int J Clin Pharmacol Ther. 2016;54:572–580. [DOI] [PubMed] [Google Scholar]
- 18.DiPippo AJ, Rausch CR, Kontoyiannis DP. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses. 2019;62:81–86. [DOI] [PubMed] [Google Scholar]
- 19.Cornely OA, Böhme A, Schmitt-Hoffmann A, et al. Safety and pharmacokinetics of isavuconazole as antifungal prophylaxis in acute myeloid leukemia patients with neutropenia: results of a phase 2, dose escalation study. Antimicrob Agents Chemother. 2015;59:2078–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen CD, Tallman GB, Hakki M, et al. Isavuconazole to prevent invasive fungal infection in immunocompromised adults: Initial experience at an academic medical centre. Mycoses. 2019;62:665–672. [DOI] [PubMed] [Google Scholar]
- 21.Rausch CR, DiPippo AJ, Bose P, et al. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin Infect Dis. 2018;67:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontana L, Perlin DS, Zhao Y, et al. Isavuconazole prophylaxis in patients with hematologic malignancies and hematopoietic-cell transplant recipients. Clin Infect Dis. 2019;pii: ciz282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson DT, Dimondi VP, Johnson SW, et al. Role of isavuconazole in the treatment of invasive fungal infections. Ther Clin Risk Manag. 2016;12:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nosari AM, Caira M, Pioltelli ML, et al. Hema e-Chart registry of invasive fungal infections in haematological patients: improved outcome in recent years in mould infections. Clin Microbiol Infect. 2013;19:757–762. [DOI] [PubMed] [Google Scholar]
- 25.Reuter S, Kern W, Zenz C, et al. Prognostic factors for invasive aspergillosis in patients with haematological malignancies. Scand J Infect Dis. 2009;41:483–490. [DOI] [PubMed] [Google Scholar]
- 26.Girmenia C, Pizzarelli G, Pozzi E, et al. Improving outcomes of acute invasive Aspergillus rhinosinusitis in patients with hematologic malignancies or aplastic anemia: the role of voriconazole. Haematologica. 2008;93:159–160. [DOI] [PubMed] [Google Scholar]
- 27.Desai AV, Han D, Kowalski DL, et al. No dose adjustment for isavuconazole based on age or sex. Antimicrob Agents Chemother. 2019;63:pii:e02629-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai A, Schmitt-Hoffmann AH, Mujais S, et al. Population pharmacokinetics of isavuconazole in subjects with mild or moderate hepatic impairment. Antimicrob Agents Chemother. 2016;60:3025–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girmenia C, Busca A, Candoni A, et al. Breakthrough invasive fungal diseases in acute myeloid leukemia patients receiving mould active triazole primary prophylaxis after intensive chemotherapy: An Italian consensus agreement on definitions and management. Med Mycol. 2019;57 (Supplement_2):S127–S137. [DOI] [PubMed] [Google Scholar]