Abstract
Minimal residual disease (MRD) was monitored in 52 patients with sustained CR (≥2 years) after frontline therapy using next-generation flow (NGF) cytometry. 25% of patients initially MRD- reversed to MRD+. 56% of patients in sustained CR were MRD+; 45% at the level of 10−5; 17% at 10−6. All patients who relapsed during follow-up were MRD+ at the latest MRD assessment, including those with ultra-low tumor burden. MRD persistence was associated with specific phenotypic profiles: higher erythroblasts’ and tumor-associated monocytes/macrophages’ predominance in the bone marrow niche. NGF emerges as a suitable method for periodic, reproducible, highly-sensitive MRD-detection at the level of 10−6.
Introduction
Multiple Myeloma (MM) is the second most common hematological malignancy, characterized by the accumulation of clonal plasma cells (PCs) in the bone marrow (BM) and excess production of monoclonal protein in the serum and/or the urine.1,2 Novel therapies including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs) or monoclonal antibodies have significantly increased rates of complete remission (CR) and improved the clinical outcome of patients.3–10 However, most myeloma patients will relapse and will succumb to the disease despite the achievement of CR.11–13 Therefore, the identification of biomarkers capable of predicting disease progression and relapse is of utmost importance in the clinical management of MM patients at CR with underlying MRD.
The assessment of minimal residual disease (MRD) in the BM of MM patients has emerged as an excellent prognostic tool during the course of the disease; MRD positivity has been correlated with shorter progression-free survival (PFS) and inferior overall survival (OS).14–21 Different approaches have been used for the evaluation of marrow MRD [eg, ASO-PCR, next-generation sequencing (NGS), flow cytometry,18,22–26 with multiparameter flow cytometry seeming advantageous in terms of applicability, time and cost.21,27–29 PET/CT imaging is also required for MRD evaluation out of the bone marrow.2 However, the sensitivity and reproducibility of the technique have raised serious concerns and previous studies have reported that non-standardized flow cytometry methods were less sensitive than ASO-PCR and NGS.18,19,22,30 Hence, there is a pressing need for highly sensitive flow cytometry techniques that would allow the identification of ultra-low numbers of residual clonal cells in the BM of myeloma patients, particularly those in CR to predict ahead of time an eventual relapse.
The importance of MRD positivity as a prognostic biomarker in MM is reflected by its inclusion in the novel IMWG response criteria, setting 10−5 as the minimum sensitivity level for MRD negativity. However, there is preliminary data suggesting that even persistent MRD at the level of 10−6 identifies a subset of patients with greater risk of relapse when compared to MRD-negative (MRD−) cases.31,32 On this basis, the primary aim of this prospective study was to evaluate the incidence of MRD positivity in MM patients who remained in CR for ≥ 2 years after frontline therapy, using NGF to investigate if the method allows for reproducible detection of clonal PCs at levels of 10−6. Moreover, as the biology, clinical parameters and type of treatment associated with persistent MRD amongst patients in CR remain unclear to-date, we performed a comprehensive analysis of major prognostic factors at the time of diagnosis and type of treatment leading to the achievement of CR, to define if these could predict the presence of MRD.
Materials and methods
Study design
Inclusion criteria
The inclusion criteria were: (i) MM patients who had received frontline therapy for symptomatic disease; (ii) achievement of CR (IMWG criteria) after frontline treatment; (iii) sustained CR for a minimum period of 24 months off-treatment; (iv) ability to provide an informed consent for BM aspiration for MRD testing.
Study endpoints
The primary endpoint was the estimation of MRD− and MRD-positive (MRD+) rates with a sensitivity of 10−6, in MM patients who were in sustained CR after initial therapy. The MRD assessment was conducted with NGF as described below and no imaging techniques were included for the purposes of this study. Secondary endpoints included: (i) evaluating the duration of MRD response, (ii) the concordance between 2 independent experts who analyzed the results of NGF in a blinded fashion; (iii) the evaluation of differences regarding clinical and laboratory characteristics between MRD− and MRD+ patients; (iv) the evaluation of major BM populations for each MM patient and correlation of these data with the presence of MRD as well as with other prognostic factors at the time of diagnosis.
Patients’ enrolment
All patients had been diagnosed as having Multiple Myeloma and had been treated in the Department of Clinical Therapeutics (Athens, Greece). The enrolment period was between January 2016 and May 2017. Patients were informed for the objectives and the details of the present study before giving their approval and signing the informed consent form. The study was conducted according to the principles defined by the 18th World Medical Association Assembly (Declaration of Helsinki, 1964) and all its future amendments. The study protocol was designed and executed according to the guidelines and regulations pertaining studies in Greece as well as the Good Clinical Practice Guidelines as defined by the International Conference of Harmonization. The study was approved by the local ethics committee.
Statistical analyses
Statistical analysis was performed with Statistical Package for the Social Sciences software v.20 (IBM SPSS Statistics, Inc., Chicago, IL). Differences in categorical variables were assessed with the Fisher exact test, while differences in continuous variables were evaluated with parametric (t test) or non-parametric tests (Mann-Whitney) depending on the form of the respective distribution of values. Prior to each statistical evaluation, the distribution of continuous variables was examined with D’Agostino & Pearson omnibus normality test and/or Kolmogorov-Smirnov test. All analyses were two-sided and statistical significance was assumed at p < 0.05.
MRD analysis using next-generation flow cytometry
MRD assessment was performed according to the EuroFlow guidelines for sample preparation, antibodies, cytometer settings and analysis of data. In detail, BM samples were collected in EDTA-anticoagulated tubes and treated according to the established bulk-lysis procedure comprising a FACS-lysing-fixation step with low (0.5%) concentration of bovine serum albumin, allowing for the osmotic lysis of erythrocytes and maximum leukocyte recovery. The number and viability of cells obtained was tested with Trypan Blue under the microscope. If viability of recovered cells was ≥90%, 20 million cells were stained with two independent 8-color panels (10 million each) both containing CD19-PEC7, CD27-BV510, CD38-FITC, CD45-PERCP, CD56-PE and CD138-BV421. The surface panel also comprised CD81-APCC750 and CD117-APC, while the intracytoplasmic panel comprised the markers CyIgκ-APC and CyIgλ-APCC750. Ten million cells were labeled in each tube suspended at a final volume of 200 μl/tube.
A minimum of 5 million events were recorded per tube in a FACSCantoII cytometer (BD Bioscience, San Jose, CA), with a set FSC threshold at 10,000, within a maximum of one hour after the final washing step of the preparation process. Data analysis was performed with the Infinicyt software (Cytognos S.L., Salamanka, Spain) that allowed merging of the two independent panels, based on their six common markers and thus allowing the analysis of at least ten million events per patient sample. Following exclusion of cell doublets and debris, the selection of PCs from the remaining BM cells was principally performed using CD138/CD38, CD45/CD38, and SSC/FSC bivariate dot plots. The optimal PMT voltages were determined according to the EuroFlow SOP for instrument set-up and daily performance status of the instrument was monitored with both CS&T (BD) and Rainbow beads (Spherotech Inc, Lake Forest, IL).
MRD evaluation was implemented with a manual analysis strategy by two independent experts in a blinded process to confirm the reproducibility of the results. The principal component analysis (PCA) tool of the automated population separator (APS) diagram of the Infinicyt software was used to determine the significance of each marker for the discrimination of normal from aberrant PCs for each MRD+ sample.
Phenotypic profiling of the bone marrow niche
The BM niche of each MM patient was profiled by utilizing the appropriate marker combinations of our panels. Therefore, apart from PCs, the available markers allowed for the identification of major BM subsets of erythroid, myeloid and lymphoid lineages and specifically for: erythroblasts (CD45− CD38− SSClo) and erythroid progenitors (CD117+ CD45−/dim CD38−/dim SSClo); tumor-associated monocytes/macrophages (TAMs) (SSCint CD45+ CD38+ CD81+), neutrophils (SSChi CD45dim/+ CD81−), basophils (CD45dim CD38+ CD81−), eosinophils (SSChi CD45bright CD81bright), mast cells (CD117bright CD45dim) and myeloid progenitors (CD117+ CD45dim CD38+ SSChi); B cells (CD19+ SSClo CD45dim/+), T cells (CD45+ SSClo CD56− CD19−) and a cluster of both natural killer (NK) and natural killer T (NKT) cells (CD45+ SSClo CD56+ CD19−). B cells were further subgrouped into B-cell precursors (CD45dim CD38bright CD27−), naïve B cells (CD45+ CD38−/dim CD27−) and memory B cells (CD45+ CD38−/dim CD27+) for a total enumeration of 14 different BM populations including PCs. Each subset was calculated as relative percentage of total BM nucleated cells, and all subsets in conjunction composed the individualized hematopoietic profile of each patient. The three B-cell subsets were also evaluated as a relative percentage of the total number of B cells. Analysis was conducted with the Infinicyt software analyzing the FCS file referring to the 8-color surface tube, though there were no significant variations between the two tubes in our internal control test enumerating lymphocytes (T, NK, B cells and their subsets) from 20 BM samples (data not shown).
Results
Patients’ characteristics
A total of 52 MM patients fulfilled the inclusion criteria and had one (n = 36) or two (n = 16) BM aspirates collected for MRD evaluation. In this prospective study, the first MRD assessment was not planned at defined time points but was conducted during standard patients’ visits in our clinic. The second MRD evaluation was planned between 12 and 18 months ahead the first MRD examination depending on patient's availability and agreement to provide an additional sample for the purposes of this study.
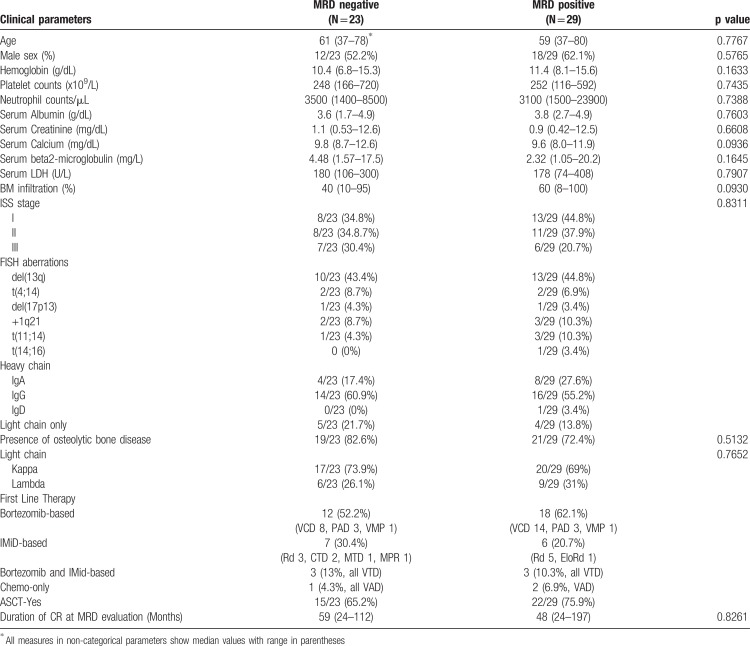
All patients had completed the frontline therapy, which contained bortezomib-based triplets (57.7%, mostly VCD), IMID-based regimens (25%), VTD as combinatory approach (11.5%) or chemotherapy only (5.8%). The majority of the patients (71.2%) received ASCT after high-dose melphalan. At the time of MRD assessment all patients had achieved CR and were off-treatment; no patients was under maintenance therapy. No patient received daratumumab combinations as part of the frontline therapy. The clinical characteristics of these patients at diagnosis and their frontline treatment are depicted in Table 1.
Table 1.
Clinical Characteristics of MM Patients who Achieved Sustained CR at the Time of Diagnosis.
Detection and clinical significance of persistent MRD in patients with sustained CR
With a median period of 59 months in CR (range: 24–197 months) after frontline treatment, 29/52 (56%) MM patients were found MRD+ at the latest MRD assessment. In several cases the number of aberrant cells detected by NGF was quite low, with 13/29 (45%) being MRD+ at the level of 10−5 and 5/29 (17%) at the level of 10−6; thus, persistent MRD at the level of 10−4 or higher was noted in less than half of patients (11/29; 38%). Sixteen MRD− patients at initial MRD testing were re-evaluated to investigate the duration of MRD− response in patients with sustained CR. With a median time-interval between the two consecutive analyses of 12 months (range: 12–18 months), 4/16 (25%) patients had their initial MRD− result reversing to an MRD+ status; the arising clonal cell populations in these cases were detectable at the level of 10−5 in three cases and at the level of 10−4 in the fourth. All these patients as well as all MRD− patients had normal involved FLC and normal FLCs ration at the time of their first MRD analysis.
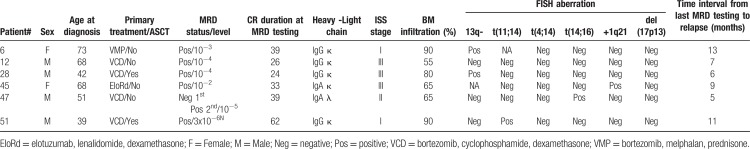
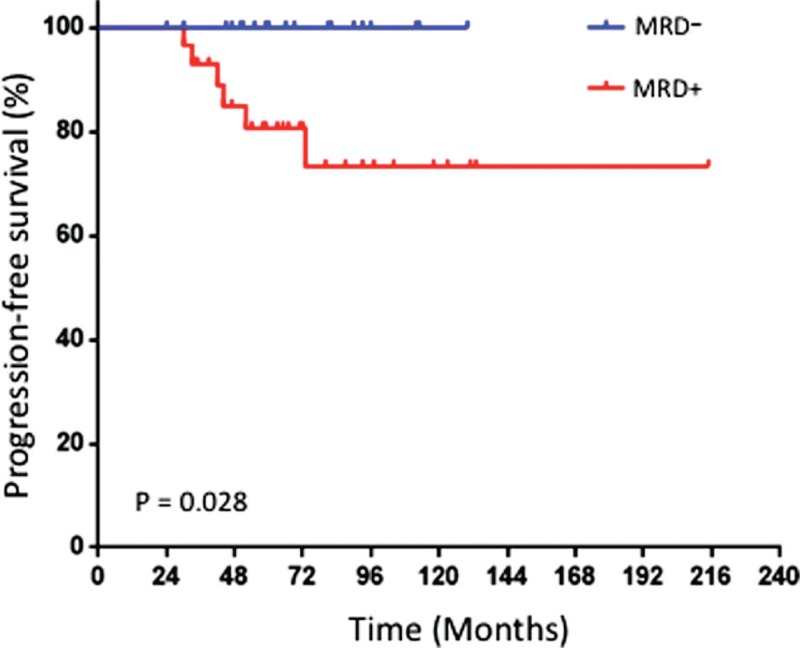
In clinical terms, 6/52 cases have relapsed to date and all 6 were MRD+ at the latest MRD assessment. One of these cases (Table 2, patient #47) was MRD− at initial testing and reversed to MRD+ status at follow-up testing, 5 months before disease progression (IMWG criteria), which implies that all relapses went through an MRD+ phase. The clinical features of all 6 patients are presented in Table 2. Despite the relative small number of relapsed cases, the presence of MRD conferred a significantly higher risk of disease progression when compared to a MRD− testing (HR: 6.48, 95% CI: 1.3–32.3 for MRD+ cases vs HR: 0.15, 95% CI: 0.03–0.77 for MRD− cases, p = 0.028; Fig. 1).
Table 2.
Clinical Characteristics of MM Patients that Relapsed after a Period of ≥2-years in Sustained CR
FIGURE 1.
Time to progression of MM patients in sustained CR after frontline therapy according to the presence (+) or absence (−) of MRD.
Dissociation between clinical parameters and persistent MRD in patients with sustained CR
The MRD status was not associated with the duration of CR (mean: 62.5 months in MRD− vs 60.4 months in MRD+) or with any particular feature at diagnosis such as age, gender, white blood cell count, heavy or light chain preference, creatinine or calcium levels in the serum, eGFR (by CKD-EPI), LDH, β2-microglobulin, and the incidence of thrombocytopenia or anemia, though MRD+ patients tended to have a higher BM infiltration at diagnosis. Interestingly, no correlation was observed between the presence of MRD and ISS stage or the cytogenetic profile of patients. Of note, only few patients had high-risk cytogenetics, as defined by the presence of del17p, t(4;14) and t(14;16) in either MRD group (13% of MRD− and 14% of MRD+), probably due to inability of patients with an adverse cytogenetic profile to achieve deep and sustained CR. That notwithstanding, none of the patients with high-risk cytogenetics displaying an MRD− testing have relapsed thus far. A similar percentage of patients were eligible for ASCT in the two MRD groups, and no differences were observed regarding the class of drugs used as frontline treatment (PI− vs IMiDs− vs PI+IMiD-based therapies).
Sensitivity and reproducibility of MRD evaluation by NGF
A minimum cluster of 20 clonal PCs was used for limit of detection (LOD) and a minimum cluster of 50 cells was used for limit of quantification (LOQ) in each sample, that were calculated by dividing these numbers to the total number of BM nucleated cells after excluding cell debris and doublets. Hence, the median LOD value reached by NGF in our samples was 2.2×10−6 (range, 2.1×10−6 − 2.8×10−6) and the median LOQ value reached was 5.5×106 (range, 5.3×10−6 – 7×10−6) (Supplementary Fig. 1A, Supplemental Digital Content). Importantly, the NGF-based quality control confirmed the presence of B-cell precursors, erythroblasts and mast cells in all samples analyzed, thus excluding potential false-negative results due to hemodilution.
The reproducibility of the analytical assessment of MRD was very high (51/52 patients’ samples; 98%), as there was only one disagreement between the two independent experts for one sample (MRD− vs MRD+ at the level of 10−6), which was considered as MRD− for the purposes of this study. Similarly, the MRD+ levels that were evaluated as the percentage of total BM nucleated cells were significantly correlated between the 2 independent researchers (r = 0.97, p < 0.0001; Supplementary Fig. 1B, Supplemental Digital Content).
For cases with MRD at the level of 10−6, detection of the aberrant clone was possible only by merging the two 8-color panels using the Infinicyt software, and thus by analyzing at least 10 million total events, as recommended by EuroFlow. Furthermore, the evaluation of MRD proved to be highly specific as the MRD result obtained by the two independent 8-color panels was fully concordant, both qualitatively and quantitatively in MRD+ cases (data not shown).
Phenotypic characterization of MRD+ clonal PCs
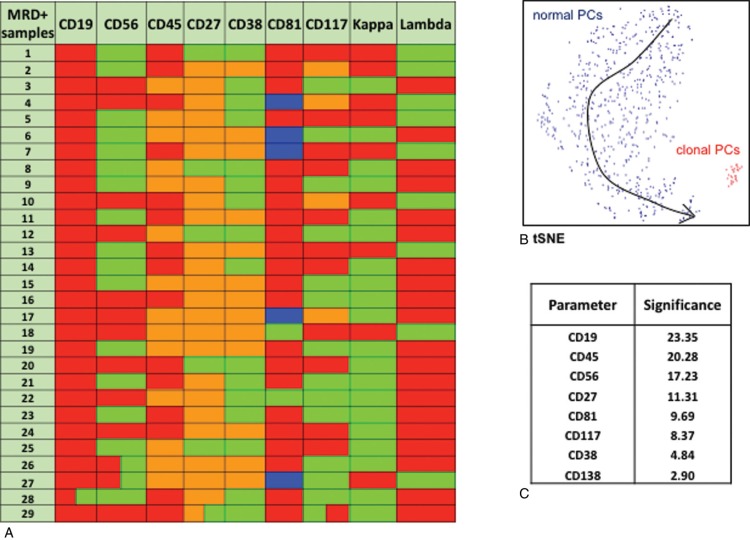
The high number of markers used in the current flow cytometry setting allowed for the detailed phenotypic characterization of aberrant PCs in MRD+ cases. As shown in Figure 2A, each MRD+ patient showed a unique phenotypic profile. Nevertheless, clonal PCs in all MRD+ cases were negative for CD19 and negative or weakly positive for CD45 expression, thus constituting the most informative markers for discriminating normal from abnormal PCs. In this setting, CD56 was the third most significant marker, but also CD27 proved a useful marker – especially in some cases with a low tumor burden (Fig. 2B), since the majority of MRD+ cases (24/29, 83%) showed a lower CD27 expression in their clonal PCs than in their residual normal counterpart. The relevant significance of each marker to discriminate between MRD and normal PCs by PCA is shown in Figure 2C.
FIGURE 2.
A. Phenotypic heterogeneity of clonal plasma cells among MRD+ cases. Each row depicts the unique phenotypic profile of aberrant cells in each case. Red: negative; Green: positive; Orange: dim expression; Blue: negative to dim expression. B. t-SNE representation of the phenotypic discrimination between normal (blue) and abnormal (red) cells in an exemplificative MRD+ case with low tumor burden. C. Relevant significance of each marker used for distinguishing clonal plasma cells. The table shows the cumulative mean value of each marker for all 29 MRD+ cases.
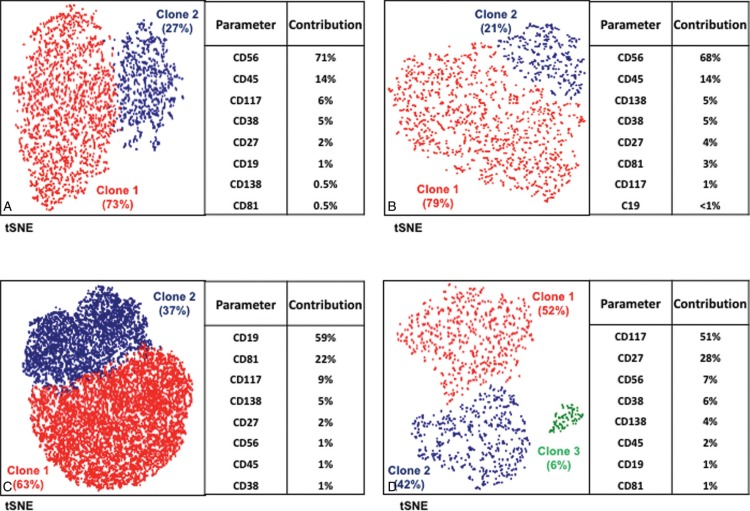
Besides the notorious phenotypic heterogeneity among MM patients, distinct clonal subsets were also observed within the same sample. In particular, 4/29 MRD+ cases showed 2 phenotypically different clonal subpopulations coexisting in the BM. In 2 biphenotypic cases, the distinct subsets (both kappa in one case, both lambda in the second) were discriminated upon differential CD56 expression; in the third case the concomitant kappa clonal subsets showed differential CD19 expression (the CD19− subset was predominant), and in the fourth case, the 2 kappa populations detected, had clearly different CD117 and CD27 expression (the principal clone, clone 1, was CD27dimCD117+, whereas clone 2 was CD27+CD117−) (Fig. 3 and Supplementary Fig. 2, Supplemental Digital Content).
FIGURE 3.
Biphenotypic MRD+ cases. t-SNE projection of clonal PCs from four different MRD+ patients displaying clonal heterogeneity on phenotypic grounds. The relative percentage of each clone within the total tumor PC compartment as well as the contribution of each marker for the automated identification of such clones are shown for each case.
The bone marrow niche in MRD-negative and MRD-positive patients
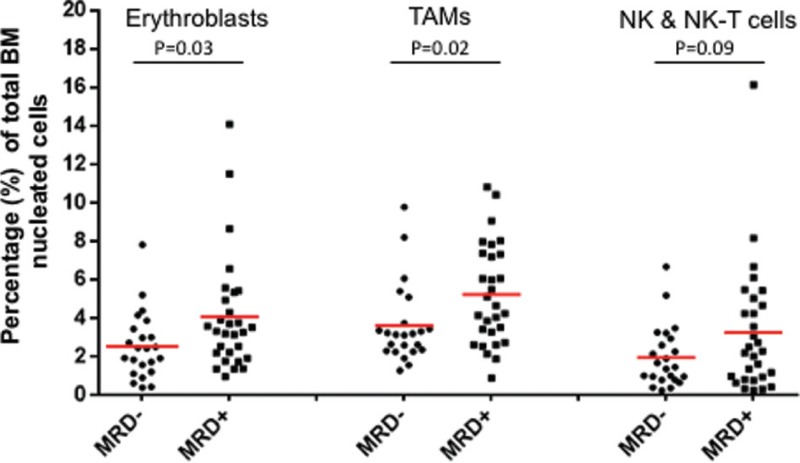
The multiparametric nature of NGF provided a unique opportunity for generating individualized BM niche profiles for each patient, and hence to evaluate whether the presence of MRD correlates with differences in the relative predominance of any BM subset. Thus, the presence of MRD was significantly correlated with higher percentages of erythroblasts (4.1% of total BM cells in MRD+ vs 2.6% in MRD− cases; p = 0.03) and TAMs (5.2% in MRD+ vs 3.6% in MRD− cases; p = 0.02), and to a lesser extend with an increased proportion of NK and NK-T cells (3.3% in MRD+ vs 2.0% in MRD− cases; p = 0.09) (Fig. 4). Notably, such relevant changes in the distribution of these subsets were also observed between the 2 consecutive BM examinations in patients that reversed to a MRD+ status with an initial MRD− result. In particular, erythroblasts showed a noticeable increase in 3/4 patients (remained stable in the fourth), as well as TAMs that also increased in 3 cases but decreased in the fourth, approximately by 2-fold compared with the initial examination (Supplementary Table 1, Supplemental Digital Content).
FIGURE 4.
Differences in the bone marrow niche profile among MRD+ and MRD− patients. Each dot represents the respective value of a single MM patient. The red line shows the mean value in each group. TAMs = tumor-associated monocytes/macrophages, NK = natural killer cells, NK-T = natural killer T cells.
Discussion
The presence of MRD in patients with MM has been associated with early relapses and inferior OS.14–21,33 Thus, we need highly sensitive and reliably quantitative techniques that allow the detection of very small numbers of MRD clones. In the present study, we applied NGF to assess MRD in a selective group of MM patients who, after frontline treatment, have remained in sustained CR for ≥2 years, and for whom there is no data. Thus, herein, we unveiled for the first time that all relapses after sustained CR go through a MRD+ phase. Importantly, unsustained CR was impossible to predict with any other biomarker except MRD.
Over a median period of 5 years in CR, we found that more than half of MM patients were MRD+. This observation seems at odds with a meta-analysis of the most relevant MRD studies showing an overall estimation of 32% MM patients in CR being MRD+ after frontline therapy (Supplementary Table 2, Supplemental Digital Content).14,16,17,19–21,34–40 In a recent study, Barlogie et al, evaluated the presence of MRD in long-term progression-free survivors, and reported that 4/68 (6%) of patients in sustained CR were MRD+.41 However, the evaluation of MRD in all these studies was performed using MRD approaches with a sensitivity level in the range of 10–4, thus possibly underestimating MRD+ cases with ultra-low tumor burden. In support of this notion, the aberrant clones detected in 18/29 (62%) MRD+ cases of our MM patient cohort were considerably small and would have been missed if a lower LOD had been used (ie, 10−4). On clinical grounds, six patients (12%) have relapsed to date, 5 of which were found MRD+ at initial examination and one at MRD re-evaluation 12 months after the first negative result. Two of these MRD+ cases would have been considered MRD− if a method of lower sensitivity had been applied. Collectively, these data uphold the application of highly sensitive yet affordable techniques for periodic MRD monitoring; they also imply that among patients achieving deep and sustained CR, being by itself an excellent goal of therapy for many MM patients, a significant number of cases may found MRD+, and thus may still be at higher risk for relapse. Such patients would benefit from continuous and closer monitoring of changes in their BM clonal PC load via MRD assessment.
There was a high concordance regarding MRD results between the 2 independent experts (IK and BP) suggesting that NGF is applicable and can be easily adopted by several centers that apply the EuroFlow protocols. Furthermore, the recent availability of automated analysis of NGF-based MRD monitoring will help standardizing MRD results across centers.21 Immunophenotypically, our results confirm previous observations regarding the existing phenotypic heterogeneity of PCs, and the occurrence of various disease-specific phenotypes that help discriminating normal from aberrant PCs,42,43 even if the latter comprise less than 0.1% of the total PC fraction. Moreover, the frequencies of aberrant expression profiles reproduced the ones recently reported by Flores-Montero et al, with CD19 and CD45 being negative in all of our MRD+ cases (100% vs 96% reported) and CD27 downregulation occurring in 83% cases (vs 89% reported).21 Interestingly, our analysis also revealed four cases with two co-existing aberrant clones with distinct phenotypic characteristics, which indicates a notable intratumor heterogeneity at the MRD level. Accordingly, NGF may prove helpful in our understanding of the biological processes occurring during the course of the disease. Thus, a phenotypic comparison between patient-paired diagnostic vs MRD samples would be highly recommended, as phenotypic discrepancies will highlight important biological/molecular aspects associated with disease relapse and also reveal phenotypic signatures linked to chemo-resistance.44,45
An important question was whether the MRD status can be associated with any of the prognostic parameters determined at diagnosis. We found no significant correlations whatsoever between age, LDH levels, cytogenetics, ISS stage, or any other prognostic parameter with MRD positivity. Similarly, no differences were observed in the prevalence of MRD negativity among patients who received ASCT and those who were not eligible, or among patients treated with different types of frontline therapy consisting of PIs or IMiDs or combinations of both. This lack of correlations suggests that baseline characteristics are not helpful to eventually predict the MRD status of MM patients. However, when we profiled patients’ BM niche using NGF, we noticed significant differences between the two groups. MRD+ samples were characterized by relatively higher predominance of erythroblasts and TAMs and to a lesser extent by elevated numbers of NK and NKT cells. Interestingly, the sequential analysis of paired-BM samples from patients with an initial MRD− status reversing to MRD positivity at re-evaluation, showed an accompanying increase in erythroblast levels, implying that this subset may somehow be involved in the underlying biology of MRD. Of note, Paiva et al, have reported that in a cohort of elderly treated MM patients, elevated levels of erythroblasts together with higher proportions of B cell precursors compose a unique immune signature that, independently from the MRD status, designates a dismal clinical course.20 The increased number of TAMs could be also reasonably associated with MRD positivity, as these are reportedly stimulated by myeloma cell-derived products and further support myeloma growth and survival by inflammatory cytokine release.46 Finally, increased percentages of effector cells, including NK, CD8+ T and NKT cells, in the BM of MM patients who achieve long-term disease control, have (similarly to the patients in our study) been suggested to reflect a competent mechanism of immune surveillance to control aberrant PC growth.47
In summary, our results show that NGF methodology developed by EuroFlow is a highly sensitive method for the detection of very small residual clonal cells (at levels reaching 10−6) that may remain in the BM of treated MM patients reaching sustained CR after frontline treatment. These patients with -even rare- residual aberrant cells are likely more prone to recur, despite deep and durable responses, and thus they may be managed differently, that is, with prolonged therapy.
Supplementary Material
Footnotes
Citation: Terpos E, Kostopoulos IV, Kastritis E, Ntanasis-Stathopoulos I, Migkou M, Rousakis P, Argyriou AT, Kanellias N, Fotiou D, Eleutherakis-Papaiakovou E, Gavriatopoulou M, Ziogas DC, Papanota AM, Spyropoulou-Vlachou M, Trougakos IP, Tsitsilonis OE, Paiva B, Dimopoulos MA. Impact of Minimal Residual Disease Detection by Next-Generation Flow Cytometry in Multiple Myeloma Patients with Sustained Complete Remission after Frontline Therapy. HemaSphere, 2019;3:6. http://dx.doi.org/10.1097/HS9.0000000000000300
Evangelos Terpos and Ioannis V. Kostopoulos contributed equally to this work.
Informed consent was obtained from all individual participants included in the study.
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.” IVK is an IKY scholar through “Strengthening Postdoctoral Researchers”, Operational Program “Human Resources Development, Education and Lifelong Learning”, Priority Axes 6,8,9, co-funded by the EC and Greece." BP has received an European Research Council (ERC) 2015 Starting Grant (MYELOMANEXT).
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. [DOI] [PubMed] [Google Scholar]
- 3.Gay F, Larocca A, Wijermans P, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood. 2011;117:3025–3031. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32:634–640. [DOI] [PubMed] [Google Scholar]
- 5.Mateos MV, Ocio EM, Paiva B, et al. Treatment for patients with newly diagnosed multiple myeloma in 2015. Blood Rev. 2015;29:387–403. [DOI] [PubMed] [Google Scholar]
- 6.Kumar SK, Anderson KC. Immune therapies in multiple myeloma. Clin Cancer Res. 2016;22:5453–5460. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. [DOI] [PubMed] [Google Scholar]
- 8.Avet-Loiseau H, Bahlis NJ, Chng WJ, et al. Ixazomib significantly prolongs progression-free survival in high-risk relapsed/refractory myeloma patient. Blood. 2017;130:2610–2618. [DOI] [PubMed] [Google Scholar]
- 9.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378:518–528. [DOI] [PubMed] [Google Scholar]
- 10.Siegel DS, Dimopoulos MA, Ludwig H, et al. Improvement in overall survival with carfizomid, lenalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2018;36:728–734. [DOI] [PubMed] [Google Scholar]
- 11.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385:2197–2208. [DOI] [PubMed] [Google Scholar]
- 12.Majithia N, Rajkumar SV, Lacy MQ, et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia. 2016;11:2208–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chim CS, Kumar SK, Orlowski RZ, et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia. 2018;32:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawstron AC, Davies FE, DasGupta R, et al. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood. 2002;100:3095–3100. [DOI] [PubMed] [Google Scholar]
- 15.Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627–1633. [DOI] [PubMed] [Google Scholar]
- 16.Paiva B, Gutierrez NC, Rosiñol L, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119:687–691. [DOI] [PubMed] [Google Scholar]
- 17.Rawstron AC, Child JA, de Tute RM, et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–2547. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawstron AC, Gregory WM, de Tute RM, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125:1932–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paiva B, Cedena MT, Puig N, et al. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood. 2016;127:3165–3174. [DOI] [PubMed] [Google Scholar]
- 21.Flores-Montero J, Sanoja-Flores L, Paiva B, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puig N, Sarasquete ME, Balanzategui A, et al. Critical evaluation of ASO RQ-PCR for minimal residual disease evaluation in multiple myeloma. A comparative analysis with flow cytometry. Leukemia. 2014;28:391–397. [DOI] [PubMed] [Google Scholar]
- 23.Mailankody S, Korde N, Lesokhin AM, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol. 2015;12:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai Y, Wong KY, Fung TK, et al. High applicability of ASO-RQPCR for detection of minimal residual disease in multiple myeloma by entirely patient-specific primers/probes. J Hematol Oncol. 2016;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silvennoinen R, Lundan T, Kairisto V, et al. Comparative analysis of minimal residual disease detection by multiparameter flow cytometry and enhanced ASO RQ-PCR in multiple myeloma. Blood Cancer J. 2014;4:e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrero S, Ladetto M, Drandi D, et al. Long-term results of the GIMEMA VEL-03-096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics’ impact on survival. Leukemia. 2015;29:689–695. [DOI] [PubMed] [Google Scholar]
- 27.Avet-Loiseau H. Minimal residual disease by next-generation sequencing: pros and cons. Am Soc Clin Oncol Educ Book. 2016;35:e425–e430. [DOI] [PubMed] [Google Scholar]
- 28.Rawstron AC, Paiva B, Stetler-Stevenson M. Assessment of minimal residual disease in myeloma and the need for a consensus approach. Cytometry B Clin Cytom. 2016;90:21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takamatsu H. Comparison of minimal residual disease detection by multiparameter flow cytometry, ASO-qPCR, droplet digital PCR, and deep sequencing in patients with multiple myeloma who underwent autologous stem cell transplantation. J Clin Med. 2017;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Lopez J, Sanchez-Vega B, Barrio S, et al. Analytical and clinical validation of a novel in-house deep-sequencing method for minimal residual disease monitoring in a phase II trial for multiple myeloma. Leukemia. 2017;31:1446–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paiva B, Puig N, Cedena MT, et al. Impact of next-generation flow (NGF) minimal residual disease (MRD) monitoring in multiple myeloma (MM): results from the Pethema/GEM2012 trial. Blood. 2017;130 suppl 1:905.(ASH abstract). [Google Scholar]
- 32.Avet-Loiseau H, Lauwers-Cances V, Corre J, et al. Minimal residual disease in multiple myeloma: final analysis of the IFM2009 trial. Blood. 2017;130 suppl 1:435.(ASH abstract). [Google Scholar]
- 33.Lahuerta JJ, Paiva B, Vidriales MB, et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35:2900–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.San Miguel JF, Almeida J, Mateo G, et al. Immunophenotypic evaluation of the plasma cell compartment in multiple myeloma: a tool for comparing the efficacy of different treatment strategies and predicting outcome. Blood. 2002;99:1853–1856. [DOI] [PubMed] [Google Scholar]
- 35.Paiva B, Vidriales MB, Cervero J, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112:4017–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roussel M, Lauwers-Cances V, Robilland N, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myélome. J Clin Oncol. 2014;32:2712–2717. [DOI] [PubMed] [Google Scholar]
- 37.Paiva B, Chandia M, Puig N, et al. The prognostic value of multiparameter flow cytometry minimal residual disease assessment in relapsed multiple myeloma. Haematologica. 2015;100:e53–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukumoto K, Fujisawa M, Suehara Y, et al. Prognostic impact of immunophenotypic complete response in patients with multiple myeloma achieving better than complete response. Leuk Lymphoma. 2016;57:1786–1792. [DOI] [PubMed] [Google Scholar]
- 39.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jelinek T, Bezdekova R, Zatopkova M, et al. Current applications of multiparameter flow cytometry in plasma cell disorders. Blood Cancer J. 2017;7:e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barlogie B, Mitchell A, van Rhee F, et al. Curing myeloma at last: defining criteria and providing the evidence. Blood. 2014;124:3043–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arroz M, Came N, Lin P, et al. Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytometry B Clin Cytom. 2016;90:31–39. [DOI] [PubMed] [Google Scholar]
- 43.Flores-Montero J, de Tute R, Paiva B, et al. Immunophenotype of normal vs. myeloma plasma cells: toward antibody panel specifications for MRD detection in multiple myeloma. Cytometry B Clin Cytom. 2016;90:61–72. [DOI] [PubMed] [Google Scholar]
- 44.Paiva B, Corchete LA, Vidriales MB, et al. Phenotypic and genomic analysis of multiple myeloma minimal residual disease tumor cells: a new model to understand chemoresistance. Blood. 2016;127:1896–1906. [DOI] [PubMed] [Google Scholar]
- 45.Arana P, Paiva B, Cedena MT, et al. Prognostic value of antigen expression in multiple myeloma: a PETHEMA/GEM study on 1265 patients enrolled in four consecutive clinical trials. Leukemia. 2018;32:971–978. [DOI] [PubMed] [Google Scholar]
- 46.Sponaas AM, Moen SH, Liabakk NB, et al. The proportion of CD16(+)CD14(dim) monocytes increases with tumor cell load in bone marrow of patients with multiple myeloma. Immun Inflamm Dis. 2015;3:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pessoa de Magalhães RJ, Vidriales MB, Paiva B, et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica. 2013;98:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.