The bone marrow is the production site of the ‘progeny’ of hematopoietic stem cells (HSCs) leading to the multilineage cellular constituents of the blood. The hematopoietic system works throughout our lifespan to provide a constant output of red blood cells to supply tissues with oxygen, as well as white blood cells and platelets to ensure immunity and maintain homeostasis. Besides HSCs, the bone marrow microenvironment is composed of heterogeneous cell populations of stromal cells with complex phenotypes and poorly defined trajectories of maturation, whose main function is to provide signals that regulate and support the daily production of billions blood cells. The bone marrow stroma contains many different cell types, including osteolineage cells, mesenchymal stem cells, arteriolar and sinusoidal endothelial cells, and neurons, which contribute to the formation of specific niches within which HSCs reside and which govern their fate. Our understanding of the hematopoietic system has immensely improved during the past few decades with increasing evidence indicating a crucial interdependency of HSCs and their surrounding bone marrow microenvironment.1 Whereas the adult hematopoietic system is the most widely studied adult stem cell system, our knowledge of the bone marrow microenvironment and its cell composition and functions is still partial. The nature and function of the stromal and vascular niches in the bone marrow still remain controversial, and how exactly their activity influences hematopoietic stem and progenitor cell (HSPCs) maturation and functions has to be clarified.
Due to technical limitations and low cell frequencies, resolving cellular subsets within the bone marrow has remained a major challenge until the advent of advanced sequencing techniques which allow the resolution of cell populations at single-cell levels.2 Multiple studies over the last few months have tried to define discrete stroma subpopulations by sequencing non-hematopoietic bone marrow cells at single-cell resolution, with the aim to resolve bone marrow cellular heterogeneity and define cell sources of pro-hematopoietic factors.3–5
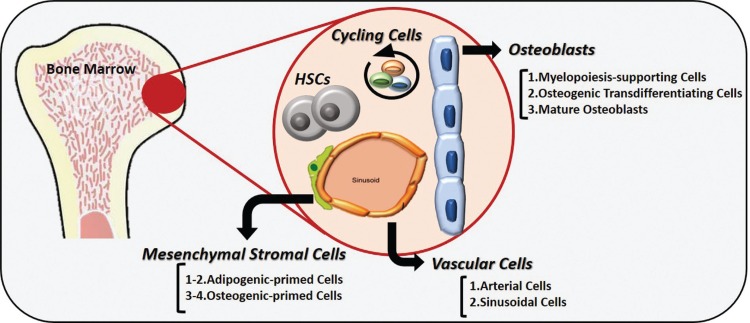
Tikhonova et al3 collected transcriptomic data of mesenchymal stromal cells, osteoblasts, and vascular cells isolated from the bone marrow of lineage-specific reporter mouse strains, in order to characterize these three major niche cellular components. T-distributed stochastic neighbor embedding (t-SNE) analysis supported a clear separation of the three input niche populations based on significantly different transcriptomic profiles. In addition, multiple subpopulations were identified within each niche lineage: 2 endothelial, 4 perivascular and 3 osteo-lineage clusters (Figure 1). Within the 2 vascular subpopulations, a major sinusoidal cluster and a second smaller arterial cluster can be distinguished based on elevated expression of specific signature genes. Among the four mesenchymal populations, 2 expressed adipogenesis-related genes and the other 2 expressed osteogenesis-related markers, suggesting differentiation into these respective lineages. Mature osteoblasts, myelopoiesis-supporting cells, and osteogenic-transdifferentiating cells fell within the osteo-lineage clusters. These findings show how novel single-cell sequencing technology provided previously undescribed details and helped to uncover the complexity of the bone marrow architecture.
Figure 1.
Novel subclusters identified in the bone marrow microenvironment by single-cell RNA sequencing.
Schematic representation of the bone marrow niche showing the main stroma populations: osteoblasts, vascular cells and mesenchymal stromal cells. RNA sequencing of bone marrow niche cells at single-cell resolution leads to the identification of: (1) specific subpopulations within the three main populations showing definite transcriptional skewing and trajectories of maturation; (2) a cycling-cell cluster, which includes cells of all three niche populations and is significantly expandend upon myeloablation.
Importantly, transcriptome analysis allowed the identification of specific sets of pro-hematopoietic factors produced by each niche cluster/subcluster, which provide cell extrinsic support to HSCs within the bone marrow microenvironment. The authors defined an additional small cluster of cycling cells expressing cell-cycle associated genes. This cluster represents 0.7% of all three main niche populations, suggesting that the majority of adult niche cells are resting. Interestingly, this ‘cycling-cell cluster’ was suddenly expanded to 5.4% of niche cells upon chemotherapy-induced myeloablation, which affects vascular and stromal cell populations. Upon stress erythropoiesis, a new adipocytic-primed cluster was identified. In addition, mesenchymal stromal cells underwent transcriptional reprogramming biased towards adipocytic skewing, leading to an overall reduction of the osteolineage cells. Changes in pro-hematopoietic factors were also observed in response to chemotherapy, with relevant alterations in the Wnt and Notch signaling pathways. Specifically, the Notch ligand Dll4 was reduced in vascular niche cells after myeloablation. To uncover the role of vascular Dll4 in hematopoiesis, the authors generated a mouse model of vascular-specific inducible deletion of Dll4. Dll4 deletion in the bone marrow vascular component lead to an overall change in hematopoietic progenitor frequencies, with significant expansion of the myeloid compartment and a decrease of lymphoid populations. Single-cell RNA sequencing of HSPCs from these mice confirmed a premature upregulation of myeloid genes responsible for the myeloid bias, loss of lymphoid differentiation and reduction in HSC enrichment associated with vascular Dll4 deficiency.
Along the same line, another recent work has mapped distinct bone marrow niche populations and their differentiation paths from mesenchymal stromal cells into adipocytes, osteoblasts and chondrocytes using single-cell RNA techniques and lineage-specific reporter mice.4 A detailed characterization was uncovered of 3 distinct paths in a hierarchy of differentiation originating from mesenchymal stem cells. Two major branching pathways were identified, originating from mesenchymal stem cells towards the adipocyte lineage or the osteocyte and chondrocyte lineages respectively. For each of these unique subpopulations, gene signatures and unique transcription factors which influence fate decisions to specific bone marrow lineages were characterized.
Through single cell interrogation and resolution, these studies uncovered unique details about the heterogeneity of the bone marrow microenvironment and provided a deeper understanding of its complexity, by revealing differentiation hierarchies for maturing stromal cells, determining key transcription factors, defining cellular sources of hematopoietic factors and delineating the functional consequences of niche transcriptional remodeling. These studies highlight the utility and reliability of single-cell RNA data for resolving complex cellular architectures as that of the bone marrow niche, hallmarked by a highly dynamic and diverse molecular landscape. Finally, these findings pave the way to the exploration of how malignancies affect the bone marrow microenvironment as well as the contribution of dysfunctional bone marrow niche populations to the development of malignancies. A first work in this direction showed that acute myeloid leukemia impairs osteogenic differentiation of mesenchymal stem cells which disturbs the production of major pro-hematopoietic factors necessary for normal hematopoiesis.5 These findings lay the groundwork for future generations of novel stromal-targeted therapies directed to modulating niche-associated factors or specific niche populations to counteract hematological diseases.
Footnotes
Citation: Vinchi F, Mendelson A, Yazdanbakhsh K, An X. Uncovering the Bone Marrow Microenvironment Cell by Cell. HemaSphere, 2019;3:6. http://dx.doi.org/10.1097/HS9.0000000000000299
The authors do not have conflicts of interest.
References
- 1.Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol. 2019;20:303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson NK, Göttgens B. Single-cell sequencing in normal and malignant hematopoiesis. HemaSphere. 2018;2:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tikhonova AN, Dolgalev I, Hu H, et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolock SL, Krishnan I, Tenen DE, et al. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 2019;28:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baryawno N, Przybylski D, Kowalczyk MS, et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019;177:1915–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]