Abstract
Background/Aims
The use of cholangioscopy for the diagnosis and treatment of hepatobiliary diseases is gradually becoming more common. We aimed to review our peroral cholangioscopy interventions, using the first-generation SpyGlass Direct Visualization System (SDVS) and summarize our experience in terms of procedures and results.
Materials and Methods
Forty-one patients who underwent this procedure at our Gastroenterology Clinic between February 2010 and October 2014 were included in this study. Patients were monitored for a median (IQR) of 44 (range 38–72) months. Demographic characteristics of these patients, results of the radiological and biochemical evaluation performed prior to the procedure, cholangioscopy findings together with the data relating to the procedure, histopathological diagnosis, clinical findings and results, and their effects on patient prognosis were assessed.
Results
In total, 41 patients underwent 46 cholangioscopy procedures. Of them, 21 (51.2%) were male. The most frequent clinical indications for cholangioscopy was the need to further investigate indeterminate stricture (n=16; 39%) and indeterminate filling defect (n=7; 17.1%). The procedure was considered successful in 39 patients with 41 (95.1%) receiving diagnostic and 33 (80.5%) receiving therapeutic benefits. The sensitivity and specificity for SVDS-guided biopsies and brush cytology were 80% and 87.5%; 26.6% and 75%, respectively. Complications related to the procedure occurred in a total of three patients (7.3%), two with cholangitis and one with perforation of gall bladder.
Conclusion
Our experience shows that cholangioscopy procedures, performed with SDVS, are clinically applicable and safe in the diagnosis and treatment of hepatobiliary diseases.
Keywords: Cholangioscopy, SpyGlass, indetermine biliary stricture, ERCP, visual diagnosis
INTRODUCTION
The standard examination technique for patients suspected with hepatobiliary duct disease is an evaluation of cholangiograms during endoscopic retrograde cholangiopancreatography (ERCP) if previous magnetic resonance cholangiopancreatography (MRCP) or other radiological techniques have been unsuccessful. However, the low diagnostic value of brush cytology or blind intraductal forceps biopsy, frequently used in the diagnosis of the etiology of biliary strictures, and the challenges in the assessment and management of biliary tree pathologies may make it obligatory to examine the bile duct with direct imaging using a cholangioscope in these patients.
Despite many improvements since the earliest cholangioscopy systems were developed, there are still many aspects of this methodology that could benefit from further improvement. Video-cholangioscopes, with their relatively more advanced technology and their striking visual quality, are now more widely available, but there are some associated problems. The SpyGlass Direct Visualization System (SDVS) was developed to overcome a range of methodological problems present in earlier systems, including complex equipment setup, relative fragility of system components, high rates of medical complications following use, lack of an adequate accessory channel, unsatisfactory image quality, and the need for two endoscopists. SDVS was the subject of an international study involving several centers with encouraging results (1). In 2009, The US Food and Drug Administration granted approval for the use of SDVS for endoscopic pancreatobiliary use, both for diagnosis and treatment (2). Since its introduction, further studies with varying sample sizes (n=5 to n=226) have shown the SDVS to be an effective therapeutic and diagnostic tool (3,4). However, to date, there are no studies describing the SDVS in terms of use, diagnostic and sampling abilities, and patient outcomes and making a comparison with other hepatobiliary diagnostic techniques in Turkey.
Our aim was to share our experience with the first generation of SpyGlass peroral cholangioscopy system, to present and analyze operating characteristics, and to review the details and outcomes of the procedures undertaken.
MATERIALS AND METHODS
Patients
All patients with an indeterminate biliary stricture or filling defect, referred from other centers for differential diagnosis, and those patients directly attending our clinics with the same indication, between February 2010 and October 2014, were included in our evaluation. During this period, 41 patients who underwent 46 cholangioscopy procedures at our Gastroenterology Clinic using the SDVS were included in the study. Patient data were extracted from the registry system, where all data and follow-up results relating to cholangioscopies performed are prospectively entered and evaluated.
Demographic characteristics of the patients (Table 1), results of the radiological and biochemical evaluations performed prior to the procedure, cholangioscopy findings, data relating each procedure, histopathological diagnosis, clinical findings and results, and their effects on patient’s prognosis and outcome were assessed.
Table 1.
Demographic characteristics of patient population.
| Parameter | n=41 |
|---|---|
| Age, mean (±SD), Y | 64.7 (11.9) |
| Body mass index, mean (±SD), kg/m2 | 29.3 (5.8) |
| Sex, no. (%) | |
| Male | 21 (51.2) |
| Female | 20 (48.8) |
| Inpatient, no. (%) | 41 (100) |
| Outpatient, no. (%) | 0 |
| Clinical presentations, no. (>1 may be applicable) | |
| Abnormal ERCP | 21 |
| Abnormal imaging | 33 |
| Asymptomatic | 8 |
| Abdominal pain | 18 |
| Itching | 6 |
| Jaundice | 21 |
| Cholangitis | 10 |
SD: Standard deviation.
Features of the SDVS
The SDVS (Microvasive Endoscopy, Boston Scientific Corp, Natick, MA, USA) (Figure 1) is a system intended both for diagnosis and for endoscopic therapy to evaluate pancreatobiliary pathologies (5). The components of the system are: I) an optical probe (SpyProbe); II) a SpyScope catheter protecting and guiding the optical probe and accessories; III) biopsy forceps (SpyBite); IV) light unit and the central computer unit creating the fiber-optic image; and V) a liquid irrigation unit (6).
Figure 1.

SpyGlass direct visualization capital components.
The SpyScope catheter contains four separate channels, one for the optical SpyProbe (Figure 2), one for the SpyBite forceps, and the remaining two channels dedicated to irrigation. The diameter of the whole SpyScope catheter is 3.3 mm. It is introduced through the 4.2 mm diameter working channel of the duodenoscope.
Figure 2.

SpyScope access and delivery catheter.
The SpyProbe optical component is 231 cm long and 0.9 mm wide. The SpyBite biopsy forceps is passed through the 1.2 mm working channel of the SpyScope, under direct visualization, if required. The distal biopsy forceps outer diameter is 1 mm and with forceps jaws fully opened 4.1 mm. The maximum opening angle of the jaws is 55° (7) (Figure 3).
Figure 3.
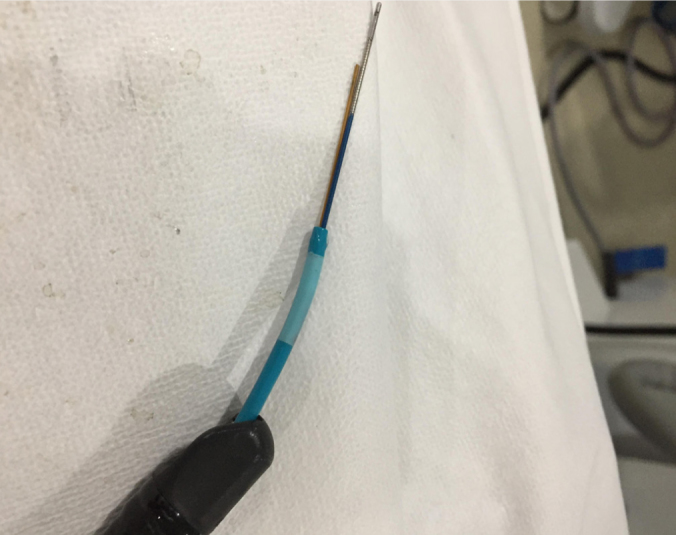
SpyBite biopsy forceps and spyglass direct visualization probe exiting through distal end of SpyScope access and delivery catheter (7).
Definition of success
Biochemical results, MRCP images, endoscopic ultrasonography (EUS), and other radiological examination imaging of all patients were reviewed. Cholangioscopy was performed in patients who could not be diagnosed despite ERCP, or for confirming a suspected diagnosis (diagnostic purpose) or for whom endoscopic therapy and interventions, appropriate to a confirmed final diagnosis, had not previously been successful (therapeutic purpose). The most frequent diagnostic indication was to investigate an indeterminate/suspected etiology of biliary stricture or filling defect, usually because of a suspicion of malignancy. The most common therapeutic indication was for bile duct stone management.
For this study, cannulation of the choledochal duct and advancement of the optical probe to the bile tree bifurcation point and optimal image capture was defined as “procedural success” whilst obtaining the expected clinical benefit from cholangioscopy was defined as “clinical success”. Clinical success was divided into two categories for evaluation: diagnostic success, which was optically identifying possible lesions or pathologies when compared to histopathological diagnosis; and therapeutic success.
Procedures
A single very experienced endoscopist performed all procedures. Patients were sedated with propofol given by the anesthesiology team. In all procedures, the duodenoscope used in combination with the SDVS was an ED-450XT5 (Fujinon Inc., Tokyo, Japan).
All patients underwent a routine ERCP, following a suitable fasting period, prior to the procedure, and a balloon cholangiogram was obtained. If not previously performed, an endoscopic sphincterotomy was carried out. Before the procedure, patients received antibiotic prophylaxis. This was followed by a peroral cholangioscopy session. If a stricture or a filling defect was identified on the cholangiogram, this was noted together with its location. The guide wire was advanced to the location of this pathology. After a choledochus cannulation with the SpyScope catheter, the fiber-optic probe was advanced through the biliary ducts, and an image was captured. After the endoscopist performing and monitoring the procedure approved that the optimal image was obtained, the catheter and the probe were advanced into the channel to the hepatic duct level or proximal part of the possible lesion area. The biliary ducts were examined during the retraction of the SDVS to the duodenum to assess the possible lesion, with repeated advancement and retraction for each duct.
If any neovascularization, suggesting the presence of a lesion, any apparent mass, or a tumor was observed, a minimum of three and a maximum of six biopsy samples were taken from different areas of the lesion by SpyBite forceps (8–10). In the absence of any obvious lesion, biopsies were taken randomly from the stricture site.
After taken biopsy by SpyBite and completion of cholangioscopy session, brush cytology was performed in all patients with malignancy suspicion, because if brush cytology was obtained firstly, a visual diagnosis could be negatively affected during SpyGlass procedure. In all cases, two experienced pathologist and cytologist, who were blinded to patient clinical information, evaluated and reported all biopsy and cytology materials. Rates of diagnosis obtained by visual examination or by taken biopsy during cholangioscopy and those for brush cytology after SDVS session were compared with final diagnosis one.
Any identified stones were removed using the visual function of the SDVS, by irrigation with sterile water controlled by foot pump or by using a balloon or a basket. The time between the cannulation of the choledochus using the SDVS and the retraction of the duodenoscope and complete withdrawal from the patient was calculated and defined as procedure time.
Following cholangioscopy with the SDVS, all patients were hospitalized for at least three days. During this time, they were followed both clinically and using laboratory and radiological findings to monitor for possible complications such as perforation, hemorrhage, cholangitis, and pancreatitis.
Statistical analysis
Descriptive statistics were reported as mean±standard deviation (SD) and median with interquartile range (IQR) values. For procedural success and clinical success, the positive (PPV) and negative predictive value (NPV) were calculated with sensitivity and specificity and their respective 95% confidence intervals (CI). Results were evaluated using the chi-square test, with the average values being compared using Student’s t-test and the median values with the Wilcoxon rank sum test. Values of p<0.05 were considered statistically significant.
RESULTS
During the study period, the number of patients who underwent ERCP at our center was 3342. Of these, 731 patients were assessed for suspicion of malignancy, and 41 patients were investigated using the SDVS cholangioscope. Thus, 1.2% of all ERCP patients and 3.1% of all patients with suspicion of malignancy were assessed using the SDVS.
The results of these 41 (male; 51%) patients undergoing peroral cholangioscopy using the SDVS system were evaluated. The median age was 57.1 (28–79) years and 56.8 (41–84) years for male and female patients, respectively. The basic characteristics of the patients undergoing the procedure, together with the indications based on the results of the previous ERCP and the data relating to the procedure, are given in Table 2. The most frequent indication for cholangioscopy was the need for further investigation, due to suspected biliary stricture and malignancy, and this was performed in 16 (39%) patients.
Table 2.
Characteristics and procedural data of 41 patients undergoing peroral cholangioscopy using the SDVS.
| Descriptive Information and Percentages | |
|---|---|
| Characteristics | |
| Number of patients | 41 |
| Age (average years) | 64.7 |
| Sex (M/F) | 21/20 |
| Number (%) of patients sphincterotomized | 21 (51.2) |
| Median (range) follow-up duration (months) | 44 (38–72) |
| Clinical Characteristics | n (%) |
| Cholelithiasis | 11 (26.8) |
| History of cholecystectomy | 15 (36.5) |
| Periampullary diverticulum | 8 (19.5) |
| Radiological Examination Data | |
| US, ERCP | 41 (100) |
| MRCP | 34 (82.9) |
| BT | 23 (56.1) |
| EUS | 12 (29.3) |
| SpyGlass Cholangioscopy Indications | |
| Indeterminate stricture | 16 (39.0) |
| Indeterminate filling defect | 7 (17.1) |
| Other | 4 (9.8) |
| Stone therapy management | 8 (19.5) |
| Cystic lesion | 2 (4.9) |
| Procedures Performed During the SpyGlass Peroral Cholangioscopy Examination | |
| Biopsy sampling | 23 (56.1) |
| Sphincterotomy | 20 (48.8) |
| Biliary sphincterotomy dilation | 6 (14.6) |
| - With sphincterotome | 4 (9.7) |
| - With dilation balloon | 2 (4.9) |
| Stent insertion | 12 (29.3) |
| Balloon dilation | 4 (9.7) |
| Brush cytology sampling | 23 (56.1) |
| Selective cystic duct cannulation | *2 (4.9) |
| Stone removal | 8 (19.5) |
Two patients with cirrhosis, both of whom had acute cholecystitis).
SDVS: SpyGlass direct visualization system.
During the procedure, the most frequent application was biopsy sampling (56.1%) and inserting a plastic stent (29.3%). A total of 46 cholangioscopy sessions were performed in the 41 patients. Thus, the mean intervention per patient was 1.12. The mean duration of the procedure was 42±11.2 min. To assess operator familiarity with the procedure, the series was chronologically divided into three groups; the first 15 procedures carried out, the next 16 procedures, and the final 15 procedures of the series. When the procedural durations were evaluated based on this division, a significant difference was observed between the average time to achieve hilus level intubation and optimal visual evaluation of the first 15 procedures compared with the final 15 procedures (23±9 versus 11±2 min; p<0.001). When total procedure time was examined in the same fashion also, the difference again was statistically significant (47±14.3 versus 37±10.8; p=0.032) (Table 3). Final diagnoses are shown in Figure 4, and visual diagnoses made with SDVS are summarized in Table 4.
Table 3.
Procedural features evaluated based on chronologically in three division as first 15, next 16, and last 15 procedures to assess operator familiarity with the procedure.
| Procedures (No. 1–15) | Procedures (No. 16–31) | Procedures (No. 32–46) | p (Between first and last 15 procedures) | |
|---|---|---|---|---|
| Average Time to Hilus Level Entubation (min) | 23±9* | 13±1 | 11±2* | p< 0.001 |
| Average Total Procedure Time (min) | 47±14.3* | 35±11.2 | 37±10.8* | p=0.032 |
| Procedures Success | 13/15** *Insufficient fibreoptic image quality (n=2) |
16/16 | 15/15 | N.A |
| Complications | 2*** Gall bladder perforation (n=1) Cholangitis (n=1) |
1*** Cholangitis (n=1) |
- | N.A |
N.A: Not available.
Figure 4.
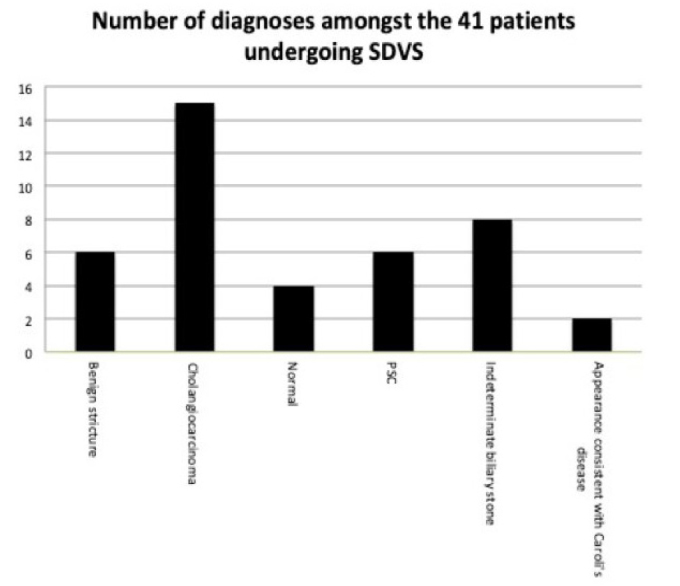
Final diagnosis with cholangioscopy (benign stricture (6), cholangiocarcinoma (CC) (15), normal (4), primary sclerosing cholangitis (PSC) (6), indeterminate biliary stone (8), and appearance compatible with Caroli’s disease (2)).
Table 4.
Visual diagnosis in 41 patients undergoing peroral cholangioscopy with the SDVS.
| Number of Procedures (%) | |
|---|---|
| Visual diagnosis with SDVS | |
| Benign/inflammatory changes | 6 (13) |
| Cholangiocarcinoma | 15 (32.6) |
| Extrinsic compression of the lumen | 2 (4.3) |
| Primary sclerosing cholangitis | 6 (13) |
| Stone not detected in the cholangiograms obtained with ERCP | *8 (17.4) |
| Insufficient fibreoptic image quality | 2 (4.3) |
| Choledochal cyst | 1 (2.2) |
| Caroli’s disease | *2 (4.3) |
| Normal | 4 (8.7) |
| Total | 46 (100) |
SDVS: SpyGlass Direct Visualization System.
Cholangiocarcinoma was diagnosed in 15 patients (Figure 5, 6). In eight of these patients, Ca 19.9 values were in normal range (20–800) (0–37 U/mL). Normal appearance in four patients and benign or inflammatory changes in six patients were reported. Primary sclerosing cholangitis (PSC) was detected in six patients (14.6%; Figure 7, 8). In two patients, the appearance was consistent with Caroli’s disease.
Figure 5.
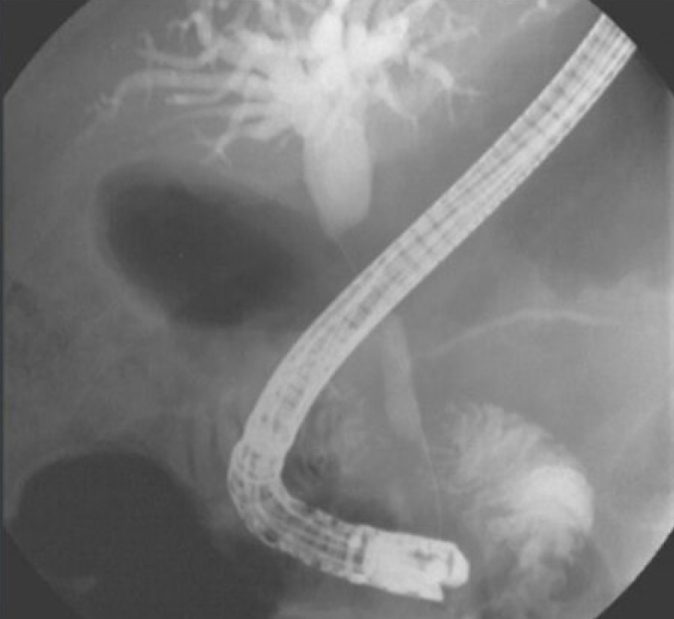
Patient with cholangiocarcinoma (cholangiographic view).
Figure 6.
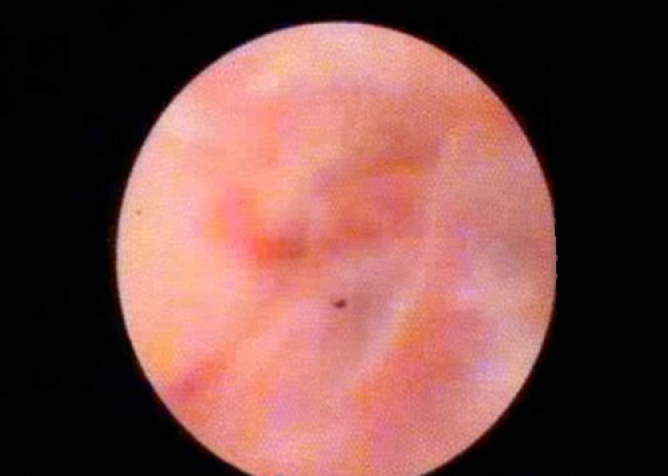
Patient with cholangiocarcinoma (cholangioscopic view).
Figure 7.
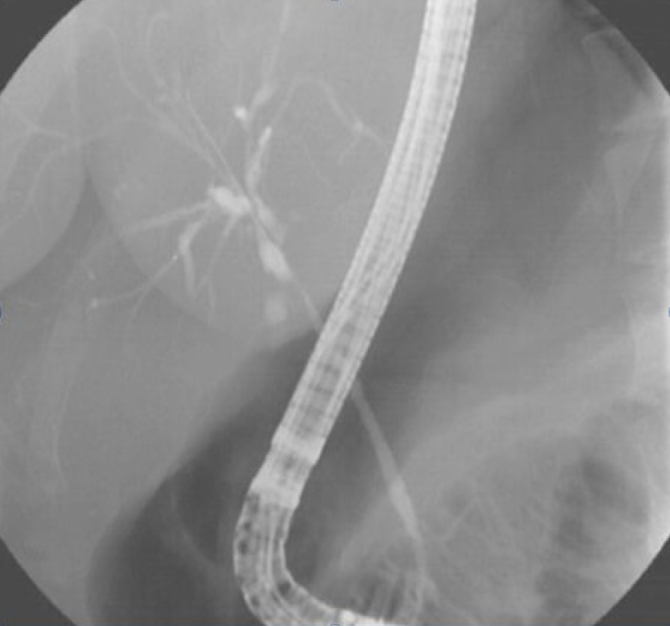
Patient with primary sclerosing cholangitis (cholangiographic view).
Figure 8.
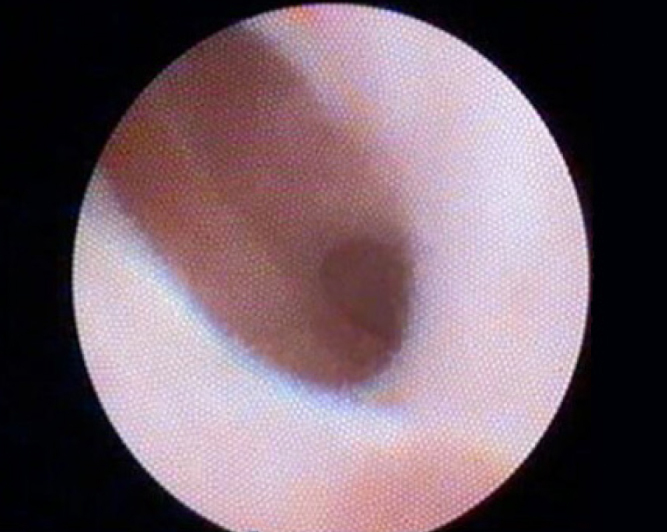
Patient with primary sclerosing cholangitis (cholangioscopic view).
Choledocholithiasis was observed in three patients with benign stricture, one patient with choledochal cyst, one patient with an appearance consistent with Caroli’s disease, and in eight further patients. Among these patients, five with previous ERCP did not have images consistent with biliary stones; while in two more, air bubbles had been reported. In six patients diagnosed with choledocholithiasis, the number and size of the biliary stones observed directly using the SDVS were more than those determined and reported with ERCP. During examination with SDVS, the biliary system of four patients was determined to be normal, in terms of the mucosa color and appearance and the lumen structure although preliminary diagnosis in these four was Klatskin tumor (n=2), benign stricture (n=1), and PSC (n=1).
In procedures performed with different indications, the incidence of malignancy, stone detection, or completely normal findings differed according to the indications (Figure 9).
Figure 9.
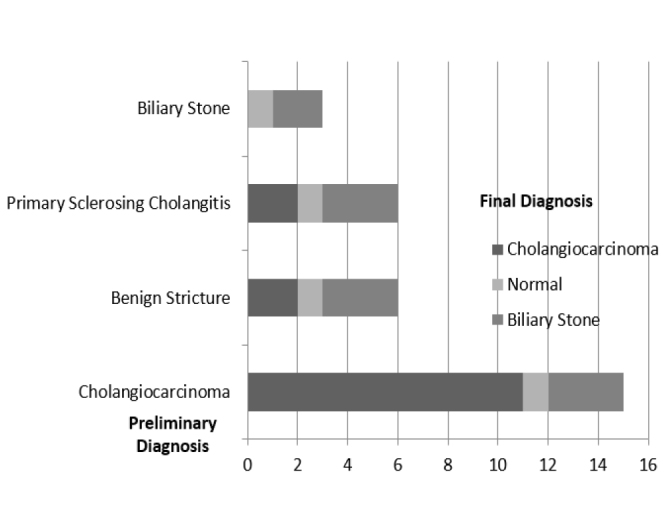
The frequency of cholangiocarcinoma, biliary stone, or normal appearance as final diagnosis obtained with SpyGlass in cholangioscopy procedures in different indications.
PSC: Primary Sclerosing Cholangitis
Biopsy sampling
Of the 23 patients with an SDVS-guided biopsy, 16 (69.6%) had an indeterminate stricture and 7 (30.4%) had an indeterminate filling defect. Following 106 biopsy attempts, 82 specimens (77.3%) were collected for pathological evaluation. The median (IQR) number of biopsy interventions and sufficient specimen collected per procedure was 4.6 (IQR, 3–8) and 3.5 (IQR, 2–6) respectively. Pathology confirmed that biopsy samples from 21 patients (91.3%) were sufficient for histological examination. However, in two patients, one with a stricture in the cystic duct-choledochus junction and another with a stricture in the pre-sphincteric area, biopsies were insufficient for histological examination. Final diagnosis in these two with biopsies obtained by ERCP was consistent with malignancy in one, while the other patient’s result was benign.
Two patients had false-negative biopsy results. In the first patient, biopsies were obtained from an area of partial irregularity, with an appearance consistent with extrinsic compression at the proximal of the choledochus. In the second patient, biopsy results obtained from the stricture area of the middle part of the choledochus were reported histologically as benign. These two patients were later reported to have pancreas carcinoma, based on the diagnosis from surgically obtained tissues.
From the 23 patients providing a biopsy, the results of 8 (34.8%) were of benign nature. These eight patients were monitored for a median (IQR) of 44 (38–72) months. Among the patients with benign histology, seven follow-up biopsies were taken from four patients (50%): one biopsy from two patients, two from the third patient, and three from the fourth. The median (IQR) time between the first SDVS biopsy and the last follow-up biopsy was 22 (13–72) months. During the follow-up period, the methods used for tissue sampling differed and included SDVS, ERCP-guided sampling, and EUS-FNA. Upon examination, all three patients’ biopsies were reported as benign; and malignancy was demonstrated with a biopsy taken at a 48th month in follow-up in the fourth patient who has PSC. No other patient developed malignancy during the monitored period.
The sensitivity and specificity for SDVS-guided biopsies were 80% and 87.5%, respectively (Table 5). Thus, the concordance rate between the SpyGlass visual examination results and the SpyGlass-guided biopsy-based diagnoses were 56% for malignancy and 43.4% for benign changes. However, with standard brush cytology performed in these patients, only four patients (26.6%) could be diagnosed with malignancy (sensitivity: 26.6%, specificity: 75%) (Table 5).
Table 5.
Overall technical success and clinical diagnostic outcome in our cohort, with the aggregated literature until 2017 (11).
| Technical Success and Clinical Diagnostic Outcome | Our Study | Laleman (11) |
|---|---|---|
| Diagnostic success n (%) | 39/41 (95.12) | 631/691 (91.3) |
| SpyBite biopsy success n (%) | 21/23 (91.3) | 485/515 (94.2) |
| Therapeutic success n (%) | 33/41 (80.5) | 213/244 (87.3) |
| Visual Diagnosis | ||
| Sensitivity | 93.3% (95% CI: 67.98–98.89) | 90.8 % (95 % CI 84.8–95) |
| Specificity | 87.5% (95% CI: 47.38–97.93) | 90.9 % (95 % CI 85.4–94.8) |
| PPV | 93.3% (95% CI: 67.98–98.89) | 89.5 % (95 % CI 83.3–94.0) |
| NPV | 87.5% (95% CI: 47.38–97.93) | 92.0 % (95 % CI 86.7–95.7) |
| Accuracy | 91% | 90.8% |
| Biopsy Diagnosis | ||
| Sensitivity | 80% (95% CI: 57.16–97.80 %) | 72.4 % (95 % CI 64.7–79.3) |
| Specificity | 87.5 ( 95% CI: 58.93–100) | 100 % (95 % CI 96.8–100) |
| PPV | 100% (95% CI: 73.35–100.00) | 100 % (95 % CI 96.8–100) |
| NPV | 77.7% (95% CI: 40.06–96.53) | 72.4 % (95 % CI 64.7–79) |
| Accuracy | 82.6% (19/23) | 84% |
| Brush Cytology Diagnosis | ||
| Sensitivity | 26.6%, | N.A |
| Specificity | 75% | N.A |
| PPV | 100% (95% CI: 40.23–100.00) | N.A |
| NPV | 42.8% (95% CI: 17.76–71.08 ) | N.A |
| Accuracy | 43.5% (10/23). | N.A |
| Visual/Biopsy Diagnosis Concordance | 56% (13/23) for malignancy 43.4% (10/23) for benign changes |
74 % (17/23), 85 % (41/48) (5,12) |
The location of the lesions and the procedural success rates are summarized in Table 6 for patients diagnosed with cholangiocarcinoma.
Table 6.
Location of cholangiocarcinoma and examination success.
| Location of the Stricture Area n=16 | Imaging Success n (%) | Diagnostic Success n (%) | Treatment Success n (%) | |
|---|---|---|---|---|
| Intra-hepatic duct | (4) | 4 (100) | 3 (75) |
*2 (50) 1: refused the surgery 1: exitus after surgery |
| Common hepatic duct | (6) | 6 (100) | 5 (83.3) | 4 (66.6) |
| Cystic duct | (1) | 1 (100) | 1 (100) | 1 (100) |
| Proximal choledochus | (2) | 2 (100) | 2(100) | 2 (100) |
| Middle choledochus | (2) | 2 (100) | 2(100) | 2 (100) |
| Distal choledochus | (1) | 1 (100) | --- | 1 (100) |
Biliary stone management and treatment
When SDVS was compared to ERCP, it provided additional information for new stones, more stones, and larger stones in 11 (26.8%) and detected new biliary stones in seven patients. This information enabled all patients to receive an endoscopic supplementary intervention, from which six patients had clinical benefit.
For patients with small biliary stones (1–3 mm), five biliary stones were removed from three patients using the irrigation function of the SDVS. If irrigation removal was unsuccessful, a biliary stone balloon was applied successfully in three patients, using the standard method in conjunction with a duodenoscope in two patients and in conjunction with the visualization provided with SDVS, by advancing the SpyProbe through the stone balloon catheter in one patient. Thus, six stones (2–5 mm), with one of these stones being in the hilar region, were removed from these patients.
The procedure was considered successful in 39 patients. In terms of clinical success, 39 patients (95.1%) received diagnostic and 33 (80.5%) received therapeutic benefits. In 35 patients (85.4%), patient management was improved by the use of SDVS.
Complications
Complications related to the procedure developed in three patients (7.3%). Two developed cholangitis, and one patient had a perforation of the gall bladder while undergoing a balloon dilatation due to a stricture located in the proximal of the choledochus and partially protruding into the common hepatic duct.
Cases with cholangitis were discharged after five to seven days of hospitalization and medical treatment. Following surgical therapy, the patient with perforation of the gall bladder was discharged without permanent sequelae. No patient deaths or sustained morbidity was associated with SDVS procedures. Procedure-related findings and complications are summarized in Table 7.
Table 7.
Procedure-related findings and complications.
| n=46 | |
|---|---|
| Total procedure time, mean (±SD) (min) | 42±11.2 |
| SpyBite biopsy (patient no.) | 23 |
| Brush cytology (patient no.) | 23 |
| Number of biopsy interventions (IQR)/sufficient Specimen collected per procedure (IQR) | 4.6 (3–8)/3.5 (2–6) |
| Adequacy of biopsy sample, No (%) | 21 (91.3) |
| Complications | |
| Mild pancreatitis | - |
| Postsphincterotomy bleeding | - |
| Perforation | * |
| Cholangitis | 2 |
| Procedures success (%) | |
| Diagnostic | 95.1 |
| Therapeutic | 80.5 |
DISCUSSION
A final differential diagnosis of biliary duct strictures is vital to plan therapy and prognostic value. This may prevent unnecessary surgical intervention in patients with benign stricture. In patients with a malignant stricture, it may prevent a delay in diagnosis and, thus, facilitate more rapid treatment and also may afford an opportunity to assess tumor resectability.
In our study using SDVS, 39 patients (95.1%) received diagnostic and 33 (80.5%) received therapeutic benefits. These results indicate that SDVS is an efficient and effective system for the diagnosis and treatment of hepatobiliary disorders. The system is predominantly used for two indications, namely the diagnosis of biliary stricture-indeterminate filling defects and the management of biliary stones.
We have shown that no problems were encountered in practice with SDVS procedures in patients with a cholangioscopy indication. We obtained a success rate of 95.1% (95% CI 75%–96%), which is in concordance with the published success rates that exceed 90%. In our hands, an additional benefit from using SDVS for hepatobiliary diagnosis was found in 85.4% of 35 patients. This proportion is at the upper end of the range (65%–95%) for additional benefit reported in the recent literature (11,12,14). Variability in additional benefit between studies may be due to differences in inclusion criteria, reasons for referral, final diagnoses, and experience of the endoscopists.
Differentiating between malignant and benign tissues in the bile duct epithelium is a challenge frequently encountered in cholangioscopy. However, direct visual assessment and visually guided biopsy have the potential to simplify this challenge (1,13).
The SDVS-guided biopsy samples were considered sufficient for histological diagnosis in 21 patients sampled (95.1%), confirming the assumption that direct visualization would increase the success rate of biopsies taken from the target region.
Specificity, calculated from the benign diagnosis accuracy rate, did not differ in our series, but the sensitivity, calculated from the malignancy accuracy rate, was higher for the visual diagnosis than for the biopsy diagnosis. Factors such as difficulty in collecting biopsies, particularly during the earliest procedures, or including patients with insufficient biopsy material for evaluation may have played a role in obtaining this result. The sensitivity of biopsies collected with SDVS was found to be 80% in a difficult patient population, which indicates the potential role of the tissue sampling technique in determining malignancies of the bile duct. Although the biopsy results were generally sufficient, in two patients with a false-negative result, there was an extrinsic compression in both patients; and the biopsy was taken with the suspicion of partial mucosal involvement. After retrospective evaluation for visual diagnosis, these patients were initially considered as being classifiable in the benign category. The total clinical yield of SpyGlass-assisted visual evaluation and tissue sampling in our series and the accumulated available literature indicate that using this methodology is superior to conventional brushing (45%), an intraductal biopsy (48.1%), or a combination of both (59.4%) (15). This superiority has been reported by Draganov et al., who made a direct comparison between SDVS and these conventional techniques (13).
In a recent systematic review, the overall rate of adverse events such as cholangitis, pancreatitis, and hemorrhage due to cholangioscopy was 10.5% (12). This number was no greater than the total rate of complications associated with ERCP. Major complications related to cholangioscopy were rarer in our study (3/41; 7.3%) than in the literature (1,12,13). Two patients developed cholangitis, which responded to medical therapy, and they were discharged without any complication. Perforation, which developed spontaneously after the procedure in the fundus section of the gall bladder in the second patient examined with SpyGlass, was considered to be a result of hydrops of the gallbladder with the contrast agent used for the cholangiogram. An additional cause of the perforation may have been the increased pressure in the gall bladder due to a stone partially located in the cystic duct of the patient, who also had to accompany recurring subacute cholecystitis. In our opinion, due to the low rate of complications even when the operator was inexperienced, cholangioscopy with SpyGlass can be defined as one of the safest interventions.
The relatively low number of patients and the fact that it was performed only by a very experienced endoscopist are the limitations of our study. However, in the literature, there is no other study in which biopsy taken under direct visualization was compared with brush cytology in such a long follow-up period. An upgraded digitalized version of SpyGlass, SpyGlass DS (SpyDS), which has better image recognition, has been recently introduced. This new system can significantly increase the diagnostic and therapeutic capacity of the first-generation system and should be re-evaluated in terms of feasibility and success. Such a study is currently running in our center.
In conclusion, this research has demonstrated how useful SDVS, operated by a single endoscopist, has been in the treatment of gall stones, and taking sufficient biopsy samples for histological diagnosis even when including “difficult” patients referred from other centers because of previous assessment problems. Our center, which was the first to use the SpyGlass system in Turkey, is a tertiary referral center; and to our knowledge, these results constitute the largest available national series. In our hands, the SpyGlass system has proven safe and effective and has provided additional clinical benefit over conventional diagnostic techniques.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Kocaeli University (KOU/GOAEK-2012/32).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – S.H., G.Ş., A.E.D., H.Y.; Design - S.H., G.Ş., A.E.D., H.Y.; Supervision - S.H., G.Ş., A.E.D., H.Y.; Data Collection and/or Processing - S.H., G.Ş., A.E.D., H.Y.; Analysis and/or Interpretation - S.H., G.Ş., A.E.D., H.Y.; Literature Search - S.H., G.Ş., A.E.D., H.Y.; Writing Manuscript - S.H., G.Ş., A.E.D., H.Y.; Critical Review - S.H., G.Ş., A.E.D., H.Y.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Chen YK, Parsi MA, Binmoeller KF, et al. Peroral cholangioscopy (PO) using a disposable steerable single operator catheter for biliary stone therapy and assessment of indeterminate strictures - A multicenter experience using SpyGlass®. Gastrointest Endosc; Abstracts of Digestive Disease Week, May 17–22, 2008 and the ASGE (American Society for Gastrointestinal Endoscopy) Postgraduate Course; May 21–22, 2008; San Diego, California, USA. 2008. p. AB103. [DOI] [Google Scholar]
- 2.Woo YS, Lee JK, Oh SH, et al. Role of SpyGlass peroral cholangioscopy in the evaluation of indeterminate biliary lesions. Dig Dis Sci. 2014;59:2565–70. doi: 10.1007/s10620-014-3171-x. [DOI] [PubMed] [Google Scholar]
- 3.Parsi MA. Peroral cholangioscopy in the new millennium. World J Gastroenterol. 2011;17:1–6. doi: 10.3748/wjg.v17.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon JH, Terheggen G, Choi HJ, Neuhaus H. Peroral cholangioscopy: diagnostic and therapeutic applications. Gastroenterology. 2013;144:276–82. doi: 10.1053/j.gastro.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 5.Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video) Gastrointest Endosc. 2007;65:832–841. doi: 10.1016/j.gie.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Chen YK. Preclinical characterization of the SpyGlass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc. 2007;65:303–11. doi: 10.1016/j.gie.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Chathadi KV, Chen YK. New Kid on the Block: Development of a Partially Disposable System for Cholangioscopy. Gastrointest Endosc Clin N. 2009;19:545–55. doi: 10.1016/j.giec.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000;52:635–8. doi: 10.1067/mge.2000.108969. [DOI] [PubMed] [Google Scholar]
- 9.Seo DW, Lee SK, Yoo KS, et al. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000;52:630–4. doi: 10.2307/3853285. [DOI] [PubMed] [Google Scholar]
- 10.Pereira P, Peixoto A, Andrade P, Macedo G. Peroral Cholangiopancreatoscopy with the SpyGlass® System: What do we Know 10 Years Later. J Gastrointestin Liver Dis. 2017;26:165–70. doi: 10.15403/jgld.2014.1121.262.cho. [DOI] [PubMed] [Google Scholar]
- 11.Laleman W, Verraes K, Van Steenbergen W, et al. Usefulness of the single operator cholangioscopy system SpyGlass in biliary disease: a single-center prospective cohort study and aggregated review. Surg Endosc. 2017;31:2223–32. doi: 10.1007/s00464-016-5221-2. [DOI] [PubMed] [Google Scholar]
- 12.Ramchandani M, Reddy DN, Gupta R, et al. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center prospective study. Gastrointest Endosc. 2011;74:511–9. doi: 10.1016/j.gie.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012;75:347–53. doi: 10.1016/j.gie.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Williamson JB, Draganov PV. The usefulness of SpyGlassTM choledochoscopy in the diagnosis and treatment of biliary disorders. Curr Gastroenterol Rep. 2012;14:534–41. doi: 10.1007/s11894-012-0287-z. [DOI] [PubMed] [Google Scholar]
- 15.Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168–76. doi: 10.1016/j.gie.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


