Abstract
Background/Aims
It has been largely accepted that dietary habits affect intestinal microbiota composition. In this pilot study, we hypothesized that time-restricted feeding, which can be regarded as a type of intermittent fasting, may have a distinct effect on intestinal microbiota. Ramadan fasting is an excellent model to understand how time-restricted feeding affect the microbiota.
Materials and Methods
A total of nine subjects were included in this study during Ramadan, consisting of 17 h of fasting/day during a 29-day period. Stool samples were collected at baseline and the day of the end of Ramadan. 16S rRNA qPCR assay has been performed for quantification of Akkermansia muciniphila, Faecalibacterium prausnitzii, Bifidobacterium spp., Lactobacillus spp., Bacteroides fragilis group, and Enterobacteriaceae. Blood samples were also collected to test for metabolic and nutritional parameters.
Results
A significantly increased abundance of A. muciniphila and B. fragilis group was observed in all subjects after Islamic fasting when compared with the baseline levels (p=0.004 and 0.008, respectively). Serum fasting glucose and total cholesterol levels were also significantly reduced in all of the subjects (p<0.01 and p=0.009, respectively).
Conclusion
Islamic fasting, which represents intermittent fasting, leads to an increase in A. muciniphila and B. fragilis group, which were considered as healthy gut microbiota members. Although this is a pilot study, which should be tested with larger sample size, there are a very limited number of studies in the literature on fasting and microbiota in human subjects. Thus, our present findings may contribute to the understanding of fasting-gut microbiota interaction.
Keywords: Gut microbiota, Akkermansia muciniphila, Bacteroides fragilis, Islamic fasting, intermittent fasting
INTRODUCTION
The gut microbiota plays a crucial role on host metabolic functions with its unique and diverse composition (1,2). This complex ecosystem has several features, such as nutrient absorption, regulation of the host immunity, and antagonistic effect against pathogen colonization (1,3,4). Thus, in recent decades, a tremendous amount of evidence indicated the crucial role of the gut microbiota in health and disease (1,3,5,6). Following the pioneer researches on the significant features of obesity-associated gut microbiome, several efforts have been made to enlighten the effect of dietary habits on gut microbiota composition (3,5–7). Akkermansia muciniphila, a mucin-degrading bacterium, may represent the 3%-5% of the microbial community in healthy individuals and is found to be significantly decreased in diet-induced obesity (7). High-fat diet also leads to increased Firmicutes levels in the gut without altering host metabolism (8). However, not only the dietary content but also the timing of the food consumption may have an impact on the gut microbiota by affecting the complex interactions between eating habits and circadian rhythms (9). Studies on mice revealed that a change in diet composition and/or fasting-feeding intervals has the capability to shift the gut microbiome (3,10–12).
Islamic fasting is a kind of intermittent fasting regimen, which includes a fasting period from sunrise to sunset during the holy month of Ramadan (13). Owing to the difficulties in experimental design, there are a very limited number of studies in the literature regarding intermittent fasting and gut microbiota in human subjects (14). To the best of our knowledge, there have been no reports evaluating the effect of Ramadan fasting on the gut microbiota. Thus, the aim of the present study was to determine whether Ramadan fasting has an impact on the abundance of certain microbiota members by using the 16S rRNA qPCR approach.
MATERIALS AND METHODS
Patients and sample collection
The study was conducted during Ramadan between June 18, 2015 and July 16, 2015, which was approximately 17 h of fasting/day from sunset to sunrise during a 29-day period. The study was approved by the institutional ethical committee (approval no.: 250, approval date: 06.01.2014). A total of nine (seven female and two male) healthy volunteers were included in the study. The age range of the subjects was between 31 and 56 (45.0±9.7) years. A short dietary questionnaire was used to determine habitual dietary intake and exclude high fat and/or high glucose food consumption. Informed consent was obtained from all healthy volunteers. All volunteers met the inclusion criteria (within the normal body mass index (BMI) range, no use of any antibiotics, and probiotic and/or prebiotic supplements within 2 months prior to sample collection). Stool and blood samples from nine volunteers were collected the day before the beginning of Islamic fasting and the day of the end of Ramadan. Blood samples were extracted for routine biochemical tests (alanine transaminase, aspartate aminotransferase, alkaline phosphatase, fasting glucose, high-density lipoprotein, low-density lipoprotein, total cholesterol, and triglyceride). Patients were carefully instructed as to collect stool samples. Stool samples were immediately stored at −80 °C after 200 mg aliquot was transferred into 1.5 mL collection tubes for DNA isolation. The DNA isolation from stool samples was performed by QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Input-output DNA amounts were calculated to convert the qPCR results as log10 copy/g feces.
Quantitative PCR analysis
Stool samples were extracted using QIAamp Stool Mini Kit (Qiagen) according to the manufacturer’s instructions. Purified DNA templates isolated from standard bacteria were used as internal controls for qPCR experiments. The DNA amount was measured using NanoDrop ND-1000 (Thermo Scientific, Wilmington, DE, USA). Copy number/μL was calculated, and DNA standards per bacterial group were prepared as previously described (15). Briefly, standard curves were constructed by using 10-fold dilution series of the standard DNA to determine the exact bacterial copy numbers in stool samples. In addition to the species-specific primer pairs for amplification of A. muciniphila and Faecalibacterium prausnitzii, genus-specific (Lactobacillus spp. and Bifidobacterium spp.), group-specific (Bacteroides fragilis group), and family-specific (Enterobacteriaceae) primers were also selected to target multiple species as previously used in the literature (16–18).
The amplification reaction was conducted in a total volume of 20 μL, consisting of 4 mM MgCl2, 0.25 μM of each primer, 2 μL of LightCycler FastStart DNA Master SYBR Green I (Roche), and 2 μL of DNA template. Amplification involved an initial denaturation at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at a specific annealing temperature (Table 1) for 5 s, and extension at 72 °C for 10 s. Melting curve analysis was also performed to determine the specificity of the PCR reactions.
Table 1.
Specific primer pairs used in the present study.
| Bacterium | Primer sequence (5′-3′) | bp | Ann. (°C) |
|---|---|---|---|
| Bifidobacterium spp. | CTCCTGGAAACGGGTGG | 550 | 56 |
| GGTGTTCTTCCCGATATCTACA | |||
| B. fragilis group | ATAGCCTTTCGAAAGRAAGAT | 495 | 50 |
| CCAGTATCAACTGCAATTTTA | |||
| Lactobacillus spp. | AGCAGTAGGGAATCTTCCA | 341 | 55 |
| CACCGCTACACATGGAG | |||
| A. muciniphila | CAGCACGTGAAGGTGGGGAC | 327 | 60 |
| CCTTGCGGTTGGCTTCAGAT | |||
| F. prausnitzii | GATGGCCTCGCGTCCGATTAG | 199 | 60 |
| CCGAAGACCTTCTTCCTCC | |||
| Enterobacteriaceae | CATTGACGTTACCCGCAGAAGAAGC | 195 | 63 |
| CTCTACGAGACTCAAGCTTGC |
Ann: annealing temperature.
Statistical analysis
GraphPad Prism software version 6.0 (GraphPad Software, Inc., CA, USA) was used to analyze data. Standard statistical methods were used for the calculation of means and standard deviations. Independent t-test was used to compare the mean values of BMI and biochemical laboratory parameters between patients and healthy controls. The bacterial DNA copy numbers determined before and at the end of Islamic fasting were statistically analyzed using Wilcoxon signed-rank test. A p value of <0.05 was used to establish significance.
RESULTS
Demographics and laboratory data
There was no significant difference between the volunteers with respect to BMI at baseline. Moreover, the before and after Islamic fasting results were not significantly different with respect to BMI. Liver enzyme, lipoprotein, and triglyceride levels did not differ between baseline and after the Islamic fasting period. However, fasting glucose and total cholesterol levels were significantly reduced after Islamic fasting (Table 2).
Table 2.
Laboratory data of the participants measured before and after Islamic fasting.
| Characteristic | Before IF (n=9) Mean±SD |
After IF (n=9) Mean±SD |
p* |
|---|---|---|---|
| BMI (kg/m2) | 22.9±1.1 | 22.8±1.1 | 0.810 |
| ALT (U/L) | 24.6±12.4 | 25.6±11.2 | 0.868 |
| AST (U/L) | 22.8±4.8 | 23.3±4.8 | 0.837 |
| ALP (U/L) | 79.3±22.8 | 77.9±22.6 | 0.904 |
| Total cholesterol (mg/dL) | 231.5±62.2 | 217.5±57.9 | 0.009 |
| HDL (mg/dL) | 52.9±11.7 | 52.0±10.3 | 0.876 |
| LDL (mg/dL) | 157.4±51.0 | 154.6±48.6 | 0.914 |
| Triglyceride (mg/dL) | 110.5±52.9 | 106.4±53.6 | 0.879 |
| Glucose (fasting; mg/dL) | 87.8±20.8 | 73.8±15.8 | 0.006 |
IF: Islamic fasting; BMI: body mass index; ALT: alanine transaminase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
p values were determined by independent t-test.
Bacterial count by qPCR analysis
The specificity of each primer pair was confirmed by both agarose gel electrophoresis after conventional PCR and by melting curve analysis.
Wilcoxon signed-rank test was used to compare the bacterial count at baseline and at the end of Islamic fasting for analysis of paired data. A. muciniphila and B. fragilis were significantly enriched after the end of Islamic fasting (p=0.0039 and p=0.0078, respectively). Lactobacillus spp. levels also showed an increasing trend after Ramadan (6.3±0.6 vs. 6.7±0.8 log10 copy/g feces), whereas F. prausnitzii (11.5±1.1 vs. 10.2±1.7 log10 copy/g feces), Bifidobacterium spp. (8.0±1.3 vs. 7.4±1.0 log10 copy/g feces), and Enterobacteriaceae (7.4±1.4 vs. 6.6±1.2 log10 copy/g feces) levels decreased without significance (Figure 1). The significant bacterial counts for each individual are also presented in Figure 2.
Figure 1.
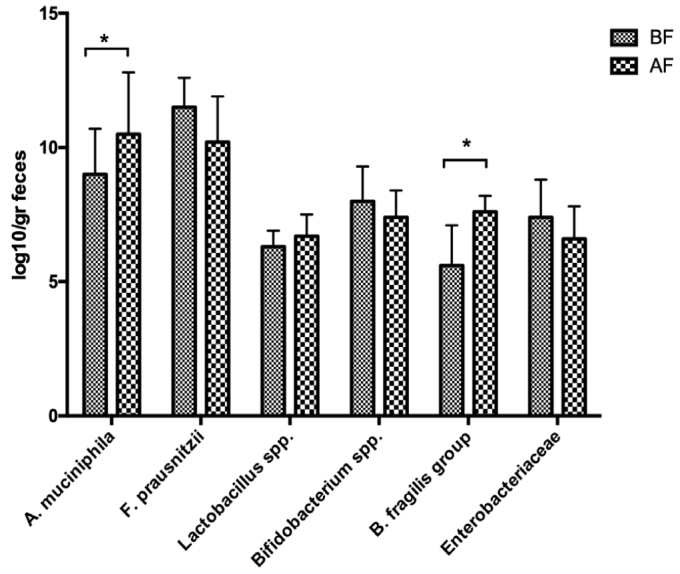
Comparison of mean counts for all bacteria tested between before Islamic fasting and after Islamic fasting samples. A. muciniphila, p=0.0039; F. prausnitzii, p=0.2382; Lactobacillus spp., p=0.9895; Bifidobacterium spp., p=0.9241; B. fragilis, p=0.0078; Enterobacteriaceae, p=0.7622.
Figure 2.
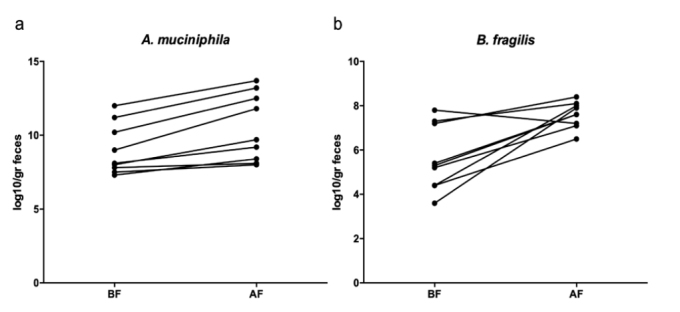
Significantly increased A. muciniphila (9.0±1.7 vs. 10.5±2.3 log10 copy/g feces, p=0.004) and B. fragilis group (5.6±1.5 vs. 7.6±0.6 log10 copy/g feces, p=0.008) at the end of Islamic fasting for individual subjects. Paired data were analyzed using Wilcoxon signed-rank test.
According to Pearson correlation test, there were no correlations between the bacterial counts and glucose or total cholesterol levels, except a weak negative correlation between A. muciniphila counts and fasting glucose determined after Ramadan fasting (r=−0.51, p=0.04).
DISCUSSION
In our present study, we aimed to determine the beneficial effects of Islamic fasting on the gut microbiota, which can be considered as an intermittent fasting model since it is similar to time-restricted feeding. Our main finding is a significantly increased abundance of A. muciniphila and B. fragilis group members after the Islamic fasting period.
In the present study, we also showed lower total cholesterol and fasting glucose levels after the Islamic fasting period than baseline levels. This result was not surprising and compatible with a previous report on Ramadan fasting using a large-scale study (19). A meta-analysis study on Ramadan fasting indicated a significant reduction in fat percentage and weight in overweight or obese individuals after Ramadan (20). Similar results were also shown in another study conducted on 240 adult subjects (21). We did not observe a significant reduction in BMI in our cohort. This might be due to our limited sample size or gender differences.
The gut microbiota continuously responds to various dietary compounds, thus changes in the content of the diet and feeding/fasting intervals may lead to minor differences in gut microbiota composition at species and genus levels. However, the core microbiome has the capability to resist environmental perturbations (22,23). Healthy gut microbiome has unique features including stability (relative stability of the microbial community over time), resilience (resistance to environmental perturbations and capacity to recover), and functional redundancy (protection of functionality despite the compositional changes) (22,23). Thus, in our present study, we mainly focus on the absolute quantification of bacteria in species, genus, and family levels by taking into account that the core microbiome is relatively stable.
In Islamic fasting, feeding/fasting periods range between 12 and 18 h/day. During our study period, the duration of fasting per day was 17 h. Islamic fasting differs from other experimental fasting regimens, since fasting occurs in a cycle from sunrise to sunset (24). However, as a nocturnal-feeding regimen, Ramadan fasting perturbs the human circadian rhythms (13). In addition to fasting/feeding intervals, time of eating has also an effect on bacterial abundance and function (25). It has been previously argued that the disruption of the circadian rhythms may have a negative effect on the gut microbiota. However, in our previous research, we determined that the beneficial microbiota members showed an increased abundance with Islamic fasting. Further researches addressing the effect of nocturnal eating on the gut microbiome may enlighten this issue.
There have been a very limited number of studies regarding the effect of fasting on the gut microbiota since the experimental design is challenging. Therefore, Islamic fasting is a perfect and accurate model since the period of fasting/feeding is fully controlled. Our present findings provide a better understanding of gut microbial changes during fasting by evaluating changes in bacterial counts after Ramadan.
According to our present results, A. muciniphila becomes more abundant after Ramadan fasting. A. muciniphila is a mucin-degrading bacterium which resides in the mucus layer, and its relative abundance is inversely correlated with body weight (7,26). Everard et al. (7) demonstrated the dramatically decreased levels of A. muciniphila in both diet-induced and genetically obese mice. One clinical study, in which 13 participants were subjected to a 1-week fasting program followed by a probiotic administration, showed an increased abundance of F. prausnitzii and A. muciniphila (14). This effect may be attributed to caloric restriction and subsequent depletion of some bacterial groups due to the decreased food intake. Another study by Dao et al. (27) showed an increased abundance of A. muciniphila after calorie restriction in obese patients along with the healthier metabolic outcomes. Apparently, A. muciniphila is particularly not affected by decreased energy intake and becomes more abundant by the decreased competition that occurred due to the depletion of other bacterial groups. A. muciniphila strongly adheres to the mucosal layer (28). Thus, it may remain relatively stable during the dietary changes and subsequent changes in intestinal passage/flow rates and changing defecation regimens in the gut. In another human clinical trial, which investigated the relationship between functional microbiota and eating behaviors, there was no correlation between A. muciniphila and overnight-fast duration (9). This controversial result may be due to the differences of fasting regimens. Another possible explanation is the type and fiber content of the diet, since A. muciniphila is highly responsive to diet change and fiber content (29). As shown in a previous report, A. muciniphila was enriched in the absence of fiber (29). This may be another explanation for increased A. muciniphila abundance after Ramadan fasting, since dietary patterns during Ramadan usually consist of low fiber intake when compared with before Ramadan (21). Moreover, relatively stable counts of Lactobacillus spp. and Bifidobacterium spp. during our study period may be related with low fiber intake, since their abundance is known to increase with high dietary fiber intake (30). It is also the major limitation of our present study since we do not have exact energy, macronutrient, and fiber intake data during Ramadan. Similar with our present findings, Remely et al. (14) also did not observe a significant change in Bifidobacterium levels after fasting program. Bifidobacterium levels were only increased after probiotic intervention when compared with baseline.
We also determined an increased abundance of B. fragilis group members at the end of Ramadan fasting. The primer pairs used in our study targeted all B. fragilis group members including B. fragilis, Bifidobacterium distasonis, Bifidobacterium vulgatus, and Bifidobacterium thetaiotaomicron, which were also important members of the gut microbiota. Santacruz et al. (31) reported an increased B. fragilis group in overweight adolescents after obesity treatment program, which consists of a reduction in energy intake. As a major member of healthy gut microbiota, Bacteroides genus has increased tolerance to changes in the intestinal tract and high capability to reduce oxygen levels in the gut and has high capability to metabolize complex polysaccharides and fatty acids (32). In the absence of polysaccharides and glycoproteins, such as the fasting periods, Bacteroides genus has the unique ability to switch their transcriptional profile to use host-derived glycans (33). High ability of survival and rapid adaptation to nutrient availability may contribute to the resistance of Bacteroides to time-restricted energy intake and may lead to increased dominancy after the depletion of the other bacterial groups during fasting. The tendency to healthy gut microbiota after fasting may be explained by the fact that the relative resistance of healthy microbiome members, such as Bacteroides and Akkermansia, to changes in the micro-environment with dietary changes.
Lactobacillus spp., Bifidobacterium spp., F. prausnitzii, and Enterobacteriaceae counts were not affected during Ramadan fasting. A decreasing trend was observed in F. prausnitzii and Enterobacteriaceae, but not significant. This finding may also be considered as a tendency to healthy microbial composition. F. prausnitzii as a member of Firmicutes is responsible for increased energy harvest from food and associated with obesity despite its anti-inflammatory and butyrate source features (3,34). Similarly, Enterobacteriaceae is also known as a source of endotoxin production, and its abundance is closely related with decreased gut permeability (35).
Our present study demonstrated the significant increase in A. muciniphila and B. fragilis group members after Ramadan fasting, which has been largely accepted as the major members of the healthy gut microbiome. In addition to our limited sample size, the results of this pilot study on Islamic fasting are of importance since there are a very limited number of human trials regarding the effect of fasting on the gut microbiota due to its difficulties in study design. Further studies with larger sample size may help to better understand the relationship between fasting and gut microbiota.
Footnotes
“See Editorial Comment 1008”
Ethics Committee Approval: Ethics committee approval was received for this study from the Institutional Review Board of Gazi University (Approval No.: 250, Approval Date: January 6, 2014).
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - C.Ö.K., M.Y., T.K.; Design - C.Ö.K., M.Y.; Supervision - C.Ö.K., M.Y., T.K.; Resources - T.K.; Materials - T.K.; Data Collection and/or Processing - C.Ö.K., M.Y., T.K.; Analysis and/or Interpretation - C.Ö.K., M.Y.; Literature Search - C.Ö.K., M.Y., T.K.; Writing Manuscript - C.Ö.K., M.Y., T.K.; Critical Review - M.Y., T.K.;
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 2.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 4.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy EF, Cotter PD, Healy S, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–42. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 9.Kaczmarek JL, Musaad SM, Holscher HD. Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr. 2017;106:1220–31. doi: 10.3945/ajcn.117.156380. [DOI] [PubMed] [Google Scholar]
- 10.Haas JT, Staels B. Fasting the Microbiota to Improve Metabolism? Cell Metab. 2017;26:584–5. doi: 10.1016/j.cmet.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Wei S, Han R, Zhao J, et al. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores beta cells and reconstructs gut microbiota in mice. Nutr Metab. 2018;15:80. doi: 10.1186/s12986-018-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014;20:1006–17. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Ann Rev Nutr. 2017;37:371–93. doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 14.Remely M, Hippe B, Geretschlaeger I, Stegmayer S, Hoefinger I, Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wiener klinische Wochenschrift. 2015;127:394–8. doi: 10.1007/s00508-015-0755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozkul C, Yalinay M, Karakan T, Yilmaz G. Determination of certain bacterial groups in gut microbiota and endotoxin levels in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol. 2017;28:361–9. doi: 10.5152/tjg.2017.17033. [DOI] [PubMed] [Google Scholar]
- 16.Maeda H, Fujimoto C, Haruki Y, et al. Quantitative real-time PCR using TaqMan and SYBR Green for Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, tetQ gene and total bacteria. FEMS Immunol Med Microbiol. 2003;39:81–6. doi: 10.1016/S0928-8244(03)00224-4. [DOI] [PubMed] [Google Scholar]
- 17.Matsuki T, Watanabe K, Fujimoto J, et al. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. ppl Environ Microbiol. 2002;68:5445–51. doi: 10.1128/AEM.68.11.5445-5451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartosch S, Fite A, Macfarlane GT, McMurdo MET. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol. 2004;70:3575–81. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larijani B, Zahedi F, Sanjari M, et al. The effect of Ramadan fasting on fasting serum glucose in healthy adults. Med J Malaysia. 2003;58:678–80. [PubMed] [Google Scholar]
- 20.Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analysis. Nutrients. 2019;11 doi: 10.3390/nu11020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norouzy A, Salehi M, Philippou E, et al. Effect of fasting in Ramadan on body composition and nutritional intake: a prospective study. J Human Nutr Diet. 2013;26(Suppl 1):97–104. doi: 10.1111/jhn.12042. [DOI] [PubMed] [Google Scholar]
- 22.Kang S, Ma W, Li FY, et al. Functional Redundancy Instead of Species Redundancy Determines Community Stability in a Typical Steppe of Inner Mongolia. PloS one. 2015;10:e0145605. doi: 10.1371/journal.pone.0145605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moya A, Ferrer M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016;24:402–13. doi: 10.1016/j.tim.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Alzoghaibi MA, Pandi-Perumal SR, Sharif MM, BaHammam AS. Diurnal intermittent fasting during Ramadan: the effects on leptin and ghrelin levels. PloS one. 2014;9:e92214. doi: 10.1371/journal.pone.0092214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaczmarek JL, Thompson SV, Holscher HD. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutrition reviews. 2017;75:673–82. doi: 10.1093/nutrit/nux036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santacruz A, Collado MC, Garcia-Valdes L, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 27.Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–36. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 28.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 29.Desai MS, Seekatz AM, Koropatkin NM, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016;167:1339–53.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke SF, Murphy EF, Nilaweera K, et al. The gut microbiota and its relationship to diet and obesity: new insights. Gut Microbes. 2012;3:186–202. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santacruz A, Marcos A, Warnberg J, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity. 2009;17:1906–15. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 32.Wexler AG, Goodman AL. An insider’s perspective: Bacteroides as a window into the microbiome. Nature Microbiol. 2017;2:17026. doi: 10.1038/nmicrobiol.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonnenburg JL, Xu J, Leip DD, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–9. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 34.Balamurugan R, George G, Kabeerdoss J, Hepsiba J, Chandragunasekaran AM, Ramakrishna BS. Quantitative differences in intestinal Faecalibacterium prausnitzii in obese Indian children. Br J Nutr. 2010;103:335–8. doi: 10.1017/S0007114509992182. [DOI] [PubMed] [Google Scholar]
- 35.Zhu LX, Baker SS, Gill C, et al. Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection between Endogenous Alcohol and NASH. Hepatology. 2013;57:601–9. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]


