Abstract
Background/Aims
Alcohol is the leading cause of liver cirrhosis, which results in portal hypertension and subsequently, culminates into esophageal varices and esophgeal variceal bleeding. Esophagogastroduodenoscopy is gold standard for diagnosis of varices. Non-invasive markers based on clinical, laboratory & ultrasonographic parameters can be utilised for prediction of risk of esophageal varices & variceal bleed in alcoholic cirrhosis from central India.
Materials and Methods
This was a cross sectional observational study. Child Turcot Pugh scores, MELD, AST ALT Ratio(AAR), AST Platelet Ratio Index(APRI), FIB-4 index and Platelet count-Spleen diameter(PC/SD) ratio were calculated for all patients and correlated with esophagogastroduodenoscopy findings. Short term follow up was done for variceal bleeding.
Results
Total 202 male patients were included with mean age of 43.77±9.95 years. 188(93%) patients had esophageal varices. 61(30.19%) patients had variceal bleeding. On univariate analysis platelet count, APRI, spleen bipolar diameter, and PC/SD ratio were significantly associated with varices. For prediction of esophageal varices, only PC/SD ratio was significant and showed area under the curve of 65.6% at cut-off of <997. CTP score, FIB-4, APRI, and PC/SD ratio were significant for variceal bleeding. At cut-off <985 PC/SD ratio had sensitivity of 82% and specificity of 63% with AUC of 78% for prediction of variceal bleeding. Also, FIB-4 and APRI had diagnostic accuracy of 64% and 61% with AUC of 74% and 72% respectively for bleed.
Conclusion
FIB-4 and PC/SD may be useful among armamentarium of non-invasive markers for predicting esophageal varices and risk of variceal bleeding in alcoholic liver cirrhosis.
Keywords: Non-invasive markers, alcoholic cirrhosis, esophageal varices, esophageal variceal bleeding, esophagogastroduodenoscopy
INTRODUCTION
Among different etiologies responsible for liver cirrhosis, alcohol is the most common cause of liver cirrhosis in India (1). This condition results in the development of portal hypertension (PH), which plays a cardinal role in the clinical manifestations of the disease. A hepatic venous pressure gradient (HVPG) >5 mmHg indicates the presence of PH. Clinically significant portal hypertension (CSPH) is defined as an HVPG ≥10 mm Hg. Variceal bleeding occurs when the HVPG is >12 mmHg (2). Esophageal varices (EV) develop due to an increase in the resistance of hepatic vasculature, which is secondary to hepatic fibrosis and regenerative nodules. Esophageal variceal bleeding (EVB) is a principal cause of morbidity and mortality in patients with liver cirrhosis. The mortality is 11%–40% due to EVB (3). The formation of varices occurs at a rate of 3%–12% per year, and conversion into large varices is 8%–12% per year (4,5). The severity of underlying chronic liver disease and the varices gauged on endoscopy help to predict future variceal bleeding (6). Recent Baveno VI consensus endorsed surveillance endoscopy in all patients with liver cirrhosis at the time of detection and every 1–3 years, depending on the findings of screening endoscopy and ongoing liver injury (7). Esophagogastroduodenoscopy (EGD) is the gold standard with a high sensitivity and specificity for diagnosis and grading of EV. Its invasiveness, requirement of conscious sedation (8), and its fairly high cost are demerits of EGD (9). Also, in resource-poor countries, EGD is not widely available. Many patients suffering from chronic liver disease will not have EV on EGD. The occurrence of EV depends on the degree of underlying PH, which is accurately graded using the HVPG measurement. But the availability and its invasiveness are limiting HVPG factors.
To overcome these obstacles, many non-invasive methods have been devised as an expedient marker for EV and prediction of EVB.
In cirrhotic patients, prediction of EV is based on these simple, non-invasive markers of PH, such as model for end-stage liver disease (MELD), platelet count (PC), aspartate-aminotransferase-to-platelet-ratio index (APRI), aspartate-aminotransferase-to-alanine-aminotransferase (AST-to-ALT) ratio (AAR), platelet-count-to-spleen-diameter (PC/SD) ratio, and fibrosis-4-index (FIB-4) (10–12). These markers are based on routine laboratory values that are often obtained for initial evaluation of patients with CSPH, making them easy and readily available for clinical implications (13). But some previous studies showed controversial results and doubtful utility of these markers in the prediction of varices. These studies included different population groups and different etiologies of cirrhosis (14–16).
Thus, we took a homogeneous cohort of patients with alcoholic liver cirrhosis from central India. We hypothesized that non-invasive markers of PH may be utilized for prediction of EV and/or EVB.
MATERIALS AND METHODS
This was a cross-sectional observational study carried out from December 2016 to May 2017. Institutional ethics committee approval was taken prior to the starting of study. Enrollment was done in a period of 6 months. Full written informed consent was taken from the study population.
Study population
Consecutive male patients with cirrhosis aged 18–70 years attending the Gastroenterology Department, with a clinically significant alcohol intake without variceal bleeding, were enrolled in the study. Alcoholic cirrhosis was diagnosed on the basis of history, clinical examination, and biochemical and imaging findings. The exclusion criteria were as follows: (1) past episodes of upper gastrointestinal bleeding; (2) etiology other than alcoholic cirrhosis; (3) previous portosystemic shunt; (4) history of gastrointestinal surgery; (5) liver metastasis and hepatocellular carcinoma (HCC); (6) portal, hepatic or splenic vein thrombosis; (6) affection of liver or spleen such as chronic myeloid leukemia, myeloproliferative diseases, tropical splenomegaly due to chronic malaria, etc.; (7) previous splenectomy; (8) transjugular intra-hepatic porto-systemic shunt (TIPSS).
Laboratory evaluation
All patients underwent a detailed laboratory assessment, which included hematological and biochemical work up including hemoglobin, PC, total leukocyte count, prothrombin time, and serum levels of bilirubin, alanine aminotransferase, aspartate aminotransferase, total protein, albumin, and blood urea. Enrolled patients were classified depending on the Child-Turcotte-Pugh (CTP) class. From these laboratory values, non-invasive markers such as MELD, AST/ALT ratio, APRI, PC/SD, and FIB-4 were calculated for each patient (10, 12). The MELD score was calculated using the United Network for Organ Sharing (UNOS) Internet site MELD calculator (www.unos.org).
The spleen bipolar diameter (BPD) was measured in millimeters with a high-resolution B-mode ultrasonography using PHILIPS HD 11 XE with a 5 MHz transducer. The PC was calculated using a Sysmex XS-1000i automated hematology analyzer 5 parts.
Calculation of non-invasive markers
| ( 17) |
| ( 18) |
| ( 19) |
Endoscopic evaluation and follow-up
EGD was performed using a Fujinon EG-250WR5 Video Gastroscope in all patients to detect the presence and grade of EV. Varices were classified into small and large by endoscopist who was blinded to values of non-invasive markers (20). All patients were given the standard-of-care treatment, according to the Baveno VI guidelines, in the form of non-selective beta-blockers and/or endoscopic band ligation(7).
All patients were followed-up for a period of 6 months personally/telephonically and any event of upper gastrointestinal (GI) hemorrhage was noted and correlated with the findings of AAR, APRI, FIB-4, and PC/SD ratio with EGD.
Statistical analysis
The statistical analysis was done using the Epi Info 7.2 and Statistical Package for Social Sciences version 20.0 (IBM Corp.; Armonk, NY, USA). The qualitative data were expressed in terms of percentages, and the difference between the proportions was tested using the chi-squared test. The quantitative data were expressed in terms of mean and standard deviations. The difference between two means of normal data was tested by Student’s t-test, and the difference between two means of non-normal data was tested by the Mann-Whitney’s U test. The binary logistic regression analysis was applied to find the independent predictors for the outcome variables. Receiver operating characteristic (ROC) curves were applied to define the best cut-off, and based on the area under curve, the best parameter to define the outcome variable. The sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood, and negative likelihood ratio were defined for the best cut-off. Every analysis was two tailed, and the significance value was set to 0.05.
RESULTS
Demographic characteristics of study population
A total of 202 male patients with alcoholic cirrhosis were enrolled in the study. The mean age was 43.77±9.95 years. Out of them, 133 (65.84%) had a large EV, 55 (27.23%) had a small EV, and 14 (6.93%) patients had no EV on EGD. The mean duration of alcohol intake was significant with 12.52±3.69 years in the EV and 10±2.72 years in the no EV group, respectively (p<0.005). The average alcohol intake in grams was significantly different when compared between the subgroups. In the large EV group, 96.16±48.26 grams of alcohol intake on a daily basis was seen. The majority of alcoholic patients [130(64.35%)] in our study were consuming country-made liquor. According to the CTP score, patients were classified into the Child-Pugh classes. The maximum number of patients was in the Child-Pugh Class C, i.e., 133 (65.84%) followed by 59 (29.2%) in the Child-Pugh Class B, and 10 (4.95%) in the Child-Pugh Class A.
Prediction of esophageal varices with non-invasive markers
On comparison of baseline characteristics (Table 1) of our alcoholic cirrhosis cohort in the EV and no EV group, the PC was significantly lower in the large EV group (p=0.0102). The BPD was significantly higher in the EV group in comparison with the no EV group (p=0.0034) In the large EV patients, majority were in the Child-Pugh C class (63.15%).
Table 1.
Baseline characteristics on univariate analysis with the presence of esophageal varices.
| Parameters | No Esophageal Varices (n=14) | Esophageal Varices (n=188) | p |
|---|---|---|---|
| Age (in years) | 42.93±7.93 | 43.84±10.10 | 0.6912 |
| Alcohol consumption per day (in grams) | 29 (29–58) | 58 (58–115) | 0.0559a |
| Duration of alcohol intake (in years) | 10.00±2.72 | 12.52±3.69 | 0.0050 |
| Hemoglobin (g/dL) | 8.93±2.37 | 8.26±2.25 | 0.3272 |
| Total leukocyte count (103/μL) | 8805 (5760–14000) | 7175 (5120–9630) | 0.2083a |
| Platelets (109/L) | 158.50 (112–307) | 134 (80–201) | 0.1477a |
| Total Bilirubin (mg/dL) | 2.70 (0.80–11) | 2.60 (1.4–4.5) | 0.4305a |
| Total protein (g/dL) | 6.46±0.55 | 6.55±0.81 | 0.5880 |
| Serum albumin (g/dL) | 2.86±0.56 | 2.80±0.44 | 0.6856 |
| Alanine aminotransferase (ALT) (U/L) | 32.50 (20–43) | 29 (22–46) | 0.2510a |
| Aspartate aminotransferase (AST) (U/L) | 64 (43–92) | 64 (43.5–94.5) | 0.6774a |
| Prothrombin time (sec) | 17.46±3.18 | 19.19±4.22 | 0.0727 |
| Creatinine (mg/dL) | 0.90 (0.7–1.2) | 1.00 (0.8–1.2) | 0.3185a |
| Spleen Bipolar Diameter (mm) | 11.26±2.13 | 13.32±2.45 | 0.0034 |
| CTP score | 9.79±2.49 | 10.31±2.05 | 0.4549 |
| MELD | 16.71±8.77 | 16.22±5.62 | 0.8281 |
| AAR | 2.24±0.98 | 2.21±1.22 | 0.9131 |
| APRI | 1.08±0.73 | 1.70±2.59 | 0.0263 |
| FIB-4 | 3.13 (1.59–5.05) | 3.93 (2.28–6.06) | 0.0825a |
| PC/SD ratio | 1221.50 (878–2743) | 974.50 (614–1655) | 0.0764a |
p-values from Mann-Whitney’s U test. All other p-values are from Student’s t-test.
CTP score: Child-Turcotte-Pugh score; MELD: model for end-stage liver disease; AAR: aspartate-aminotransferase-to-alanine-aminotransferase (AST-to-ALT) ratio; APRI: AST-to-platelet-ratio index; FIB-4: fibrosis-4-index; PC/SD: platelet-count-to-spleen-diameter ratio.
On the evaluation of non-invasive markers, APRI was significantly higher in the EV group (p=0.026). The PC/SD ratio was significantly lower in the large EV group in comparison with the small EV and no EV group (p=0.0014). AAR was not found to be significant on the univariate analysis. FIB-4 was showing a trend toward significance (p=0.082). On the binary logistic regression analysis, the spleen BPD was found to predict EV (R2=0.315, p=0.008).
On the application of ROC curve (Figure 1), only the PC/SD ratio was significant enough to predict EV with the area under the curve (AUC) of 0.656 with a 95% confidence interval (CI) (0.50–0.80) (p=0.05). At the cut-off <997, the PC/SD ratio had a sensitivity of 52.13% and specificity of 64.29% with diagnostic accuracy of 52.97%, with the odds ratio of 7.77 in the prediction of EV. Other non-invasive markers such as MELD, AAR, APRI, and FIB-4 were not found to be significant on the application of the ROC curve (Table 2).
Figure 1.
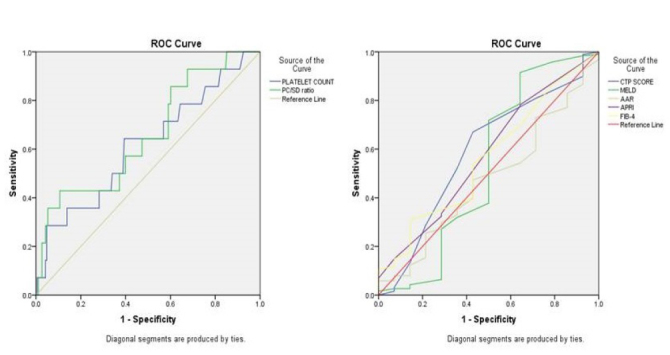
Receiver-operating characteristics (ROC) curve of non-invasive markers to predict the presence of esophageal varices (EV).
Table 2.
Performance of Non-invasive markers for prediction of esophageal varices (EV)
| Parameters | AUC1 | p | 95% Confidence Interval | |
|---|---|---|---|---|
|
| ||||
| Lower Bound | Upper Bound | |||
| CTP score | 0.587 | 0.278 | 0.423 | 0.751 |
| MELD | 0.532 | 0.687 | 0.327 | 0.738 |
| AAR | 0.473 | 0.733 | 0.322 | 0.623 |
| APRI | 0.575 | 0.348 | 0.418 | 0.732 |
| FIB-4 | 0.563 | 0.433 | 0.412 | 0.714 |
| Platelet count (109/L) | 0.616 | 0.148 | 0.454 | 0.778 |
| PC/SD ratio | 0.656 | 0.05 | 0.503 | 0.809 |
Area under the curve (AUC) obtained from the receiver operating characteristic.
CTP score: Child-Turcotte-Pugh score; MELD: model for end-stage liver disease; AAR: aspartate-aminotransferase-to-alanine-aminotransferase (AST-to-ALT) ratio; APRI: AST-to-platelet-ratio index; FIB-4: fibrosis-4-index; PC/SD: platelet-count-to-spleen-diameter ratio.
Prediction of esophageal variceal bleeding with non-invasive markers
During the follow-up, 61 (30.19%) patients had EVB. The history of daily alcohol intake was found to be significantly higher in patients with EVB (The mean daily alcohol intake in grams 101.93 vs. 76.39). On the analysis of baseline characteristics between EVB and no EVB in alcoholic cirrhosis, hemoglobin, the PC, total protein, and serum albumin were significantly lower in the EVB group (Table 3). On univariate analysis, the CTP score was significantly higher in the EVB group in comparison with the no EVB group (p=0.0001). On the comparison of non-invasive markers with EVB, the means of the APRI and FIB-4 values were significantly higher in the EVB group (APRI, 2.75 vs. 1.19; FIB-4, 7.98 vs. 3.9. respectively). The PC/SD ratio was found to be significantly lower in the EVB group in comparison to the no EVB group (p<0.001).
Table 3.
Baseline Characteristics on univariate analysis with esophageal variceal bleeding
| Parameters | No Esophageal Variceal Bleeding (n=141) | Esophageal Variceal Bleeding (n=61) | p |
|---|---|---|---|
| Age (in years) | 43.18±9.07 | 45.13±11.71 | 0.2503 |
| Alcohol consumption per day (in grams) | 76.39±44.6 | 101.93±51.75 | 0.0011 |
| Duration of alcohol intake (in years) | 12.14±3.85 | 12.80±3.23 | 0.2109 |
| Hemoglobin (g/dL) | 8.74±2.3 | 7.32±1.82 | <0.001 |
| Total leukocyte count (103/μL) | 7650 (5560–10060) | 6580 (4440–9600) | 0.1660 a |
| Platelets (109/L) | 160 (117–232) | 124 (53–80) | <0.001 a |
| Total Bilirubin (mg/dL) | 2.60 (1.3–4.9) | 2.60 (1.6–4) | 0.0720 a |
| Total protein (g/dL) | 6.66±0.71 | 6.26±0.92 | 0.0034 |
| Serum albumin (g/dL) | 2.85±0.46 | 2.70±0.43 | 0.0303 |
| Alanine aminotransferase (ALT) (U/L) | 30 (22–43) | 29 (21–50) | 0.4678 a |
| Aspartate aminotransferase (AST) (U/L) | 66 (44–91) | 63 (40–103) | 0.5281 a |
| Creatinine (mg/dL) | 1.11±0.74 | 1.21±0.65 | 0.3252 |
| Spleen Bipolar Diameter (mm) | 13.06±2.58 | 13.45±2.23 | 0.2329 |
| CTP score | 9.94±2.16 | 11.05±1.65 | 0.0001 |
| MELD | 16.06±6.1 | 16.69±5.3 | 0.4652 |
| AAR | 2.23±1.27 | 2.15±1.02 | 0.6533 |
| APRI | 1.19±1.26 | 2.75±3.96 | 0.0037 |
| FIB-4 | 3.90±3.35 | 7.98±7.24 | 0.0001 |
| PC/SD ratio | 1200 (878–1867) | 630 (443–910) | <0.001 a |
p-values from Mann-Whitney’s U test. All other p-values from Student’s t-test.
CTP score: Child-Turcotte-Pugh score; MELD: model for end-stage liver disease; AAR: aspartate-aminotransferase-to-alanine-aminotransferase (AST-to-ALT) ratio; APRI: AST-to-platelet-ratio index; FIB-4: fibrosis-4-index; PC/SD: platelet-count-to-spleen-diameter ratio.
A binary logistic regression analysis showed that the hemoglobin, PC, creatinine, CTP score, and MELD score were significant independent predictors of EVB (R2=0.507).
The ROC curve for the PC and PC/SD ratio showed an AUC of 0.79 with 95% CI (0.72–0.85) and 0.78 with 95% CI (0.71–0.85), respectively. The cut-off for PC was 134 (109/L) with a sensitivity of 80.33%, specificity of 62.41%, and diagnostic accuracy of 67.82% with a positive likelihood ratio (LR+) of 2.13. Similarly, the best cut-off for the PC/SD ratio was found to be 985 with a sensitivity of 81.97%, specificity of 63.12%, and diagnostic accuracy of 68.81% with a positive likelihood ratio (LR+) of 2.22. According to the ROC curve, a PC <134 (109/L) and PC/SD <985 have a good predictive value for the diagnosis of the upper gastrointestinal (UGI) bleeding (Figure 2).
Figure 2.

Receiver-operating characteristics (ROC) curve of non-invasive markers to predict the presence of esophageal variceal bleeding (EVB).
The ROC curve was also applied to other non-invasive markers such as AAR, APRI, MELD, and FIB-4. In these, only APRI and FIB-4 were found to be significant. The ROC curve for FIB-4 suggested the best cut-off value of 3.91. This cut-off reveals a sensitivity of 72.13%, specificity of 60.28%, and diagnostic accuracy of 63.86% with a positive likelihood ratio (LR+) of 1.81. An AUC of 0.74 with a 95% CI (0.66–0.81) suggests the FIB-4 value >3.91 has a good predictive value for the diagnosis of UGI bleeding. An APRI >1.05 cut-off had an AUC of 0.72 with a sensitivity of 68.85%, specificity of 58.16%, and diagnostic accuracy of 61.39% in the prediction of variceal bleeding (Table 4).
Table 4.
Performance of Non-invasive Markers for Prediction of Esophageal Variceal Bleeding (EVB).
| Parameters | MELDa | AARa | APRI | FIB-4 | Platelet Count (109/L) | PC/SD Ratio |
|---|---|---|---|---|---|---|
| AUC (95% CI)1 | 0.54 (0.46–0.62) | 0.50 (0.41–0.58) | 0.72 (0.64–0.80) | 0.74 (0.66–0.81) | 0.79 (0.72–0.85) | 0.78 (0.71–0.85) |
| Cut-off | 15.00 | 2.00 | 1.05 | 3.91 | 134 | 985 |
| Sensitivity | 52.46 | 47.54 | 68.85 | 72.13 | 80.33 | 81.97 |
| Specificity | 55.32 | 53.19 | 58.16 | 60.28 | 62.41 | 63.12 |
| PPV | 33.68 | 30.53 | 41.58 | 44 | 48.04 | 49.02 |
| NPV | 72.9 | 70.09 | 81.19 | 83.33 | 88 | 89 |
| Diagnostic accuracy | 54.46 | 51.49 | 61.39 | 63.86 | 67.82 | 68.81 |
| LR + | 1.17 | 1.01 | 1.64 | 1.81 | 2.13 | 2.22 |
| LR− | 0.85 | 0.98 | 0.53 | 0.46 | 0.31 | 0.28 |
| Odds ratio | 1.36 (0.74–2.49) | 1.02 (0.56–1.87) | 3.07 (1.62–5.80) | 3.92 (2.04–7.55) | 6.77 (3.30–13.89) | 7.77 (3.73–16.25) |
statistically not significant
Area under the curve (AUC) obtained from the receiver operating characteristic.
PPV: positive predictive value; NPV: negative predictive value; LR: likelihood ratio; CTP score: Child-Turcotte-Pugh score; MELD: model for end-stage liver disease; AAR: aspartate-aminotransferase-to-alanine-aminotransferase (AST-to-ALT) ratio; APRI: AST-to-platelet-ratio index; FIB-4: fibrosis-4-index; PC/SD: platelet-count-to-spleen-diameter ratio.
DISCUSSION
Cirrhosis is the final pathway in the stages of chronic liver disease, which is complicated by PH (21). EV develop in approximately 60%–80% of cirrhotic patients due to PH (22). EVB is one of the common and dreaded complication in patients with cirrhosis having a mortality rate of around 10%–20% (7). An appropriate management of EVB can be done using history, clinical examination, laboratory studies, and early EGD (23). The prognosis of acute UGI bleeding is more dismal in cirrhotic than non-cirrhotic patients (24). All patients upon the diagnosis of cirrhosis are recommended to screen for EV using endoscopy.
In the developing countries with limited resources, endoscopy is generally widely available. In cirrhotic patients, varices screening by annual or biannual endoscopies are a difficult and costly affair. Also, EGD is invasive and many times uncomfortable for the patient, resulting in the need for non-invasive methods to evaluate the presence of EV in these cirrhotic patients. PH develops from increased hepatic resistance secondary to liver fibrosis. By utilizing these non-invasive markers of liver fibrosis, development of EV can be predicted (25). These non-invasive markers can be used to differentiate between high- and low-risk patients. Patients with a high risk for EV can be planned for EGD and subsequent follow-up as needed. This could cut down on cost and also in need of endoscopies.
In our study, univariate analyses in all clinical and biochemical parameters, PC, spleen bipolar diameter, and APRI are significantly correlated with the presence of EV. The PC/SD ratio was significantly lower in patients with large EV in comparison with small or no EV. Several studies showed a similar significant correlation of the PC/SD ratio with a size of EV, and the Child-Turcotte-Pugh classification (26,27). On the application of binary logistic regression analysis, the spleen BPD was only variable to predict EV. FIB-4 values showed a trend toward significance in cirrhotic patients when compared with the presence of EV. Zhang et al. noted that the APRI and FIB-4 values were significantly higher in cirrhosis with PH on comparison with patients suffering from chronic liver disease (21).
The ROC curve and AUC were applied to assess the efficacy of these non-invasive markers for the prediction of large EV. Only the PC/SD ratio was found to be significant for varices prediction at a cut-off value <997 with a sensitivity of 52% and a specificity of 64% with an AUC of 0.65.
Other non-invasive markers such as AAR, APRI, and FIB-4 were not found to be significant on the ROC curve application. The primary non-invasive marker in our study for the prediction of EV is the PC/SD ratio. This ratio might be a factor of clinical utility in PH. Many studies have shown a significant association between the PC and SD with the occurrence of EV (28). Decrement in the PC/SD ratio in alcoholic cirrhosis can be explained by progressively increasing the spleen size and decrease in the PC (29). The etiology of thrombocytopenia in cirrhosis is multifactorial, which includes PH, a decreased mean platelet life span, or decrease in the production of thrombopoietin (30). In alcoholic cirrhosis, importantly thrombocytopenia could result from the myelotoxic effect of alcohol (31). Giannini et al. used the PC/SD ratio cut-off of <909 and found a positive predictive value of 96% and a negative predictive value of 100% for the presence of EV (29). Many studies also showed similar results, but in a different population subgroups and different etiologies of cirrhosis (10,14,32). A meta-analysis done by Chawla et al. showed similar results that included a total of 1275 patients and reported a sensitivity of 89% and specificity of 74% (33).
In our results, FIB-4 was found to be insignificant in the ROC curve application for the presence of varices. It is a marker of liver dysfunction and includes age, AST, ALT, and PC. A progression of liver fibrosis and PH are more frequently observed with advanced age (19). Patients from the EV group were relatively younger (mean age, 43.84 years) than the patients studied by Kraja et al. That could be an explanation for an insignificant value of FIB-4 in our study. In the study by Kraja et al., patients were older (mean age, 52.3 years) and showed a high sensitivity and specificity of FIB-4 for the diagnosis of EV (34). In a recent systematic review and meta-analysis summary, the AUCs of APRI, AAR, and FIB-4 scores for the prediction of varices were 0.68, 0.73, and 0.78, respectively. However, the APRI, AAR, and FIB-4 scores had a low to moderate diagnostic accuracy in prediction of varices and large varices in liver cirrhosis (35).
In our study, EVB was observed in 61 (30.19%) patients on the follow-up. For the prediction of variceal bleeding in alcoholic liver disease, we compared all clinical and laboratory parameters. The CTP score was significantly higher in patients with EVB. It reflects that patients with higher CTP scores are more prone to bleeding. On the application of the binary logistic regression analysis for UGI bleeding, hemoglobin, PC, and serum albumin were found to be significant predictors, which correlates with the severity of PH.
On applying the ROC curve, the PC, FIB-4, PC/SD, and APRI significantly correlated with UGI bleeding. The maximum AUC was for PC (79%) followed by the PC/SD ratio (78%) for the prediction of UGI bleeding. On retaining the cut-off of 985 for the PC/SD ratio, we found a diagnostic accuracy of 68.81% with an OR of 7.77 for EVB. This suggests that the PC/SD ratio <985 is a good predictor of EVB. In a retrospective study with a large cohort suggested varices as the basis of bleeding by using the PC with the cut-off of 69 (109/L)(36).
FIB-4 also appears to be a promising marker in the prediction of EVB with a diagnostic accuracy of 63.86% in our results. But in a recent study by Kraja et al., these non-invasive markers were not significant in the prediction of EVB (34).
This study may have a reasonable value in developing countries where resources are limited. Thus employing of these non-invasive markers have to be deduced. In our study, to decrease the etiological bias, we have included a homogenous cohort of alcoholic cirrhosis cases at our center. A limitation to our study is the short duration of follow-up. Also, we have taken the values of non-invasive markers at the time of enrollment in the study, but subsequent follow-up values were not taken into consideration.
In conclusion, the FIB-4 Index and PC/SD ratio are the most dependable among the armamentarium of non-invasive markers in the prognostication of EV and/or EVB in alcoholic liver cirrhosis. They can be used in the screening of EV, risk classification, future risk of EVB, and appropriate referral to higher centers. This non-invasive assessment of PH and liver fibrosis is especially useful in areas where the HVPG and EGD availability is sparse. Measuring of the PC/SD ratio and FIB-4 is simple, reliable, objective, and cost effective. However, these markers cannot replace EGD. Future studies involving large populations with a long-term follow-up along with a repetition of non-invasive markers at a timely interval may be planned to examine the exact utility of these non-invasive markers for prediction of EV and/or EVB.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Government Medical College & Superspeciality Hospital.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.G.K.; Design - H.G.K., N.R.G.; Supervision - S.J.G., N.R.G.; Resources - S.J.G.; Materials - H.G.J., A.R.S., T.H.S.; Data Collection and/or Processing - H.G.K., T.H.S.; Analysis and/or Interpretation - H.G.K., N.R.G.; Literature Search - S.J.G., N.R.G., A.R.S., T.H.S.; Writing Manuscript - H.G.K.; Critical Review - H.G.K., S.J.G., N.R.G., A.R.S.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Mukherjee PS, Vishnubhatla S, Amarapurkar DN, et al. Etiology and mode of presentation of chronic liver diseases in India: A multi centric study. PLoS One. 2017;12:e0187033. doi: 10.1371/journal.pone.0187033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar A, Sharma P, Sarin SK. Hepatic venous pressure gradient measurement: time to learn! Indian J Gastroenterol. 2008;27:74–80. [PubMed] [Google Scholar]
- 3.Burroughs AK, Triantos CK. Predicting failure to control bleeding and mortality in acute variceal bleeding. J Hepatol. 2008;48:185–8. doi: 10.1016/j.jhep.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266–272. doi: 10.1016/S0168-8278(02)00420-8. [DOI] [PubMed] [Google Scholar]
- 5.De Franchis R, Dell’Era A. Non-invasive diagnosis of cirrhosis and the natural history of its complications. Best Pract Res Clin Gastroenterol. 2007;21:3–18. doi: 10.1016/j.bpg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal haemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983–9. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein DR, Jagannath S, Baron TH, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815–26. doi: 10.1016/j.gie.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel BM, Targownik L, Dulai GS, Karsan HA, Gralnek IM. Endoscopic screening for esophageal varices in cirrhosis: Is it ever cost effective? Hepatology. 2003;37:366–77. doi: 10.1053/jhep.2003.50050. [DOI] [PubMed] [Google Scholar]
- 10.Giannini EG, Zaman A, Kreil A, et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. 2006;101:2511–9. doi: 10.1111/j.1572-0241.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 11.Sebastiani G, Tempesta D, Fattovich G, et al. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large scale study. J Hepatol. 2010;53:630–8. doi: 10.1016/j.jhep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Deng H, Qi X, Peng Y, et al. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, and King Scores for Diagnosis of Esophageal Varices in Liver Cirrhosis: A Retrospective Study. Med Sci Monit. 2015;21:3961–77. doi: 10.12659/MSM.895005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzenberger E, Meyer T, Golla V, Sahdala NP, Min AD. Utilization of platelet count spleen diameter ratio in predicting the presence of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2010;44:146–50. doi: 10.1097/MCG.0b013e3181a745ff. [DOI] [PubMed] [Google Scholar]
- 14.González-Ojeda A, Cervantes-Guevara G, Chávez-Sánchez M, et al. Platelet count/spleen diameter ratio to predict esophageal varices in Mexican patients with hepatic cirrhosis. World J Gastroenterol. 2014;20:2079–84. doi: 10.3748/wjg.v20.i8.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong WD, Zhu QH, Huang ZM, et al. Predictors of esophageal varices in patients with HBV-related cirrhosis: a retrospective study. BMC Gastroenterol. 2009;9:11. doi: 10.1186/1471-230X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agha A, Anwar E, Bashir K, Savarino V, Giannini EG. External validation of the platelet count/spleen diameter ratio for the diagnosis of esophageal varices in hepatitis C virus-related cirrhosis. Dig Dis Sci. 2009;54:654–60. doi: 10.1007/s10620-008-0367-y. [DOI] [PubMed] [Google Scholar]
- 17.Giannini E, Botta F, Borro P, et al. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200–5. doi: 10.1136/gut.52.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 19.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal haemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–2102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Wang L, Wang L, et al. Liver stiffness measurement, better than APRI, Fibroindex, Fib-4, and NBI gastroscopy, predicts portal hypertension in patients with cirrhosis. Cell Biochem Biophys. 2015;71:865–73. doi: 10.1007/s12013-014-0275-z. [DOI] [PubMed] [Google Scholar]
- 22.Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology. 2002;122:1620–30. doi: 10.1053/gast.2002.33419. [DOI] [PubMed] [Google Scholar]
- 23.Wysocki JD, Srivastav S, Winstead NS. A nationwide analysis of risk factors for mortality and time to endoscopy in upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2012;36:30–6. doi: 10.1111/j.1365-2036.2012.05129.x. [DOI] [PubMed] [Google Scholar]
- 24.Lecleire S, Di Fiore F, Merle V, et al. Acute upper gastrointestinal bleeding in patients with liver cirrhosis and in noncirrhotic patients: epidemiology and predictive factors of mortality in a prospective multicenter population-based study. J Clin Gastroenterol. 2005;39:321–7. doi: 10.1097/01.mcg.0000155133.50562.c9. [DOI] [PubMed] [Google Scholar]
- 25.Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20:16811–9. doi: 10.3748/wjg.v20.i45.16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahassadi AK, Bathaix FY, Assi C, et al. Usefulness of Noninvasive Predictors of Oesophageal Varices in Black African Cirrhotic Patients in Côte d’Ivoire (West Africa) Gastroenterol Res Pract. 2012;2012 doi: 10.1155/2012/216390. 216390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying L, Lin X, Xie ZL, Hu YP, Shi KQ. Performance of platelet count/spleen diameter ratio for diagnosis of esophageal varices in cirrhosis: a meta-analysis. Dig Dis Sci. 2012;57:1672–81. doi: 10.1007/s10620-012-2058-y. [DOI] [PubMed] [Google Scholar]
- 28.Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2002;34:81–5. doi: 10.1097/00004836-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Giannini EG, Botta F, Borro P, et al. Application of the platelet count/spleen diameter ratio to rule out the presence of oesophageal varices in patients with cirrhosis: a validation study based on follow-up. Dig Liver Dis. 2005;37:779–85. doi: 10.1016/j.dld.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14(Suppl D):60D–66D. doi: 10.1155/2000/617428. [DOI] [PubMed] [Google Scholar]
- 31.Zimbwa TA, Blanshard C, Subramaniam A. Platelet count/spleen diameter ratio as a predictor of oesophageal varices in alcoholic cirrhosis. Gut. 2004;53:1055. [PMC free article] [PubMed] [Google Scholar]
- 32.Amin K, Muhammad D, Anjum A, Jamil K, Hassan A. Platelet count to splenic diameter ratio as a predictor of esophageal varices in patients of liver cirrhosis due to hepatitis C virus. JUMDC. 2012;3:6–11. [Google Scholar]
- 33.Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R. Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. Eur J Gastroenterol Hepatol. 2012;24:431–6. doi: 10.1097/MEG.0b013e3283505015. [DOI] [PubMed] [Google Scholar]
- 34.Kraja B, Mone I, Akshija I, Koçollari A, Prifti S, Burazeri G. Predictors of esophageal varices and first variceal bleeding in liver cirrhosis patients. World J Gastroenterol. 2017;23:4806–14. doi: 10.3748/wjg.v23.i26.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng H, Qi X, Guo X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in Predicting the Presence of Esophageal Varices in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e1795. doi: 10.1097/MD.0000000000001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rockey DC, Elliott A, Lyles T. Prediction of esophageal varices and variceal hemorrhage in patients with acute upper gastrointestinal bleeding. J Investig Med. 2016;64:745–51. doi: 10.1136/jim-2015-000047. [DOI] [PubMed] [Google Scholar]


