Abstract
Intrathecal production of neopterin, a pteridine produced by interferon (IFN)-γ-stimulated monocyte-derived macrophages, is associated with neurological disorders and infections. We investigated whether IFN-α/β, IFN-γ, or human immunodeficiency virus (HIV) induce neopterin production by human astroglioma cells. IFN-α/β and IFN-γ, but not HIV, induced neopterin. Interestingly, IFN-γ, but not IFN-α/β, increased expression and activity of the tryptophan-catabolizing enzyme indoleamine (2,3)-dioxygenase. In contrast, IFN-α/β, but not IFN-γ, reduced the uptake of three aromatic amino acids in U87MG and U138 astroglioma cells. Thus type I and type II IFN stimulate astrocyte-derived cells to produce neopterin and exert differential effects on amino acid metabolism.
Keywords: Astrocyte-derived cells; Neopterin; Indoleamine (2,3)-dioxygenase; Interferon; Amino acids; HIV
Introduction
Neopterin is a metabolite of guanosine triphosphate, produced by monocytic cells stimulated with interferon (IFN)-γ [13]. Plasma neopterin is a marker of immune activation during viral infection and inflammation [9, 22], and correlates with tryptophan (Trp)-degradation by the immunosuppressive enzyme indoleamine (2,3)-dioxygenase (IDO) [24]. High levels of neopterin are observed in several disorders of the central nervous system (CNS) [11]. Furthermore, increased IDO activity in certain neurologic conditions may result in the production of neurotoxic catabolites of the kynurenine (Kyn) pathway, such as quinolinic acid, and depress the serotonergic function by depleting trp, the substrate required for serotonin production [10]. Infection with the human immunodeficiency virus (HIV)-1 is associated with increased neopterin in plasma and cerebrospinal fluid (CSF) which correlate with the development of acquired immunodeficiency syndrome (AIDS)-associated dementia [7, 11].
It is unclear which cells produce neopterin in the CNS. IFN-γ failed to induce neopterin production in astrocytic, neuronal and microglial cell lines [19]. Identifying the cellular sources of neopterin may help understanding the pathways leading to its production in the CNS. In the present study we analyzed neopterin production by the human astroglioma cell line U87MG. We found that IFN-γ and IFN-α/β, but not HIV, induced neopterin production, suggesting that astrocytic cells may produce neopterin during inflammatory processes. While IFN-γ induced IDO expression and activity, IFN-α/β reduced the uptake of three aromatic amino acids in U87MG and U138 astroglioma cells, suggesting a negative effect of IFN-α/β on protein synthesis.
Materials and Methods.
Cell lines.
U87MG (AIDS Research and reference Reagent Program, Division of AIDS, NIAID, NIH) and U138 (American Type Culture Collection, Manassas, VA) cell lines were cultured in RPMI 1640 (Invitrogen, Gaithersburg, MD) with 10% fetal bovine serum (Hyclone, Logan, UT). Confluent cultures were stimulated with IFN-α/β (12 species of IFN- α (R&D Systems, Minneapolis, MN), 1000 U/ml; IFN-β 10000 U/ml) or IFN-γ (Peprotec, Rocky Hill, NJ; 1 μg/ml). Concentrations of IFN-α, IFN-β and IFN-γ were chosen based on previous reports describing their effectiveness in inducing IDO activity in monocyte-derived dendritic cells and freshly isolated human blood leukocytes [5, 6].
Incubation with aldrithiol-2-inactivated HIV.
All viruses were kindly provided by Dr. Jeffrey D. Lifson (AIDS Vaccine Program, SAIC Frederick, Inc., NCI-Frederick, Frederick, MD). Preparation of viruses and control microvesicles was previously described [2, 4, 8, 16]. U87MG, U87.CD4, U87.CD4.CCR5 and U87.CD4.CXCR4. lines were cultured with AT-2 HIV-1MN (X4-tropic) or AT-2 HIV-1Ada (R5-tropic) at 300 ng/mL p24CA. treatment with infectious, non AT-2-treated viruses generated comparable results.
Measurement of neopterin.
Neopterin concentrations were measured by enzyme-linked immunosorbent assay (BRAHMS Diagnostica, Hennigsdorf-Berlin, Germany) according to manufacturer’s instruction.
Quantitative real time PCR.
Total RNA was extracted using the guanidium thiocyanate-phenol-chloroform method, modified for TRIzol (Invitrogen, Carlsbad, CA). Reverse transcription and real-time PCR were performed as previously described [5].
Measurement of kynurenine (Kyn), tryptophan (Trp), phenylalanine (Phe) and tyrosine (Tyr).
Trp and Kyn concentrations were determined by high performance liquid chromatography on reversed phase as previously described [23]. Phe and Tyr concentrations were monitored using wavelength maxima of their natural fluorescence (excitation wavelength: 210nm; emission wavelength: 302nm) [14]
Statistical analysis.
All experiments were performed 5 times. Differences were assessed using a two-tailed paired Student’s t-test. P<0.05 was considered statistically significant.
Results
IFN-α/β and IFN-γ, but not HIV, induced neopterin production in U87MG cells.
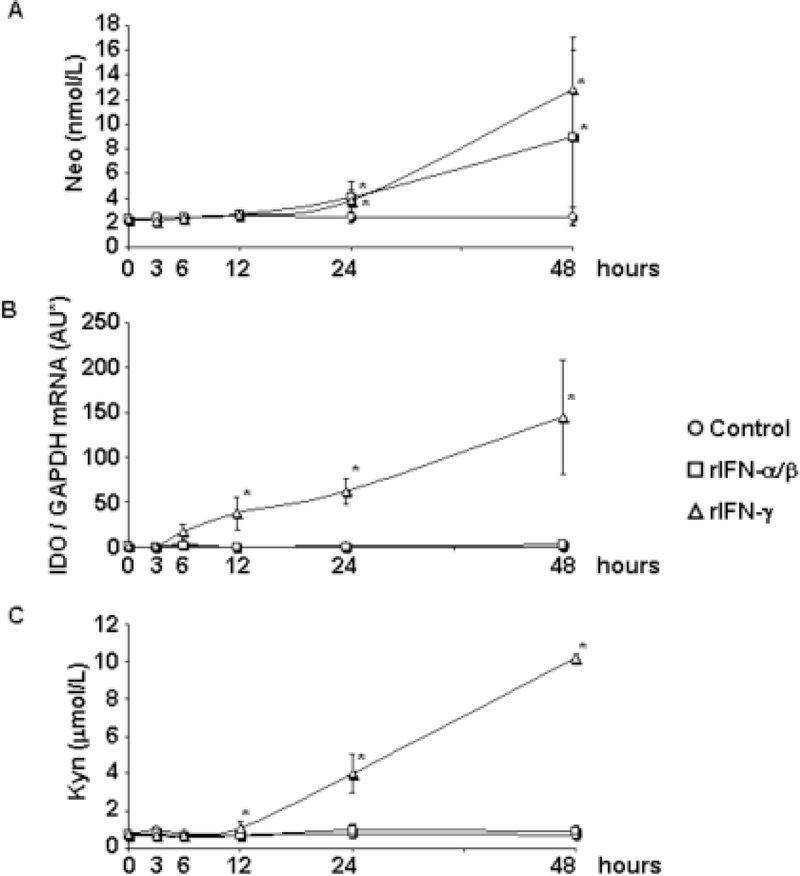
We cultured U87MG cells in presence or absence of recombinant IFN-α/β and IFN-γ and measured neopterin in culture supernatants during 48 hours of culture. Neopterin production was significantly increased by both IFN-α/β and IFN-γ after 24 and 48 hours compared to untreated cells (Fig. 1A).
Figure 1. Neopterin production, IDO expression and activity in IFN-treated U87MG.
U87MG human astroglioma cells were cultured to confluence and incubated for 48 hours in the absence (circles) or presence of rIFN-α/β (squares) or rIFN-γ (triangles); supernatants and cell aliquots were collected after 0, 3, 6, 12, 24 and 48 hours. A) Concentration of neopterin in supernatants was measured by ELISA. B) Cellular IDO mRNA expression was measured by real time PCR. C) Concentration of Kyn, the bioproduct of IDO-mediated Trp catabolism, was measured by HPLC in supernatants. *P<0.05 compared to control-treated cells.
Because high CSF neopterin correlates with the development of AIDS-associated dementia in HIV-infected patients [7], we tested whether exposure of astroglioma cells to reverse-transcription deficient (AT-2) HIV affected neopterin production. We used HIVMN (X4-tropic) or HIVAda (R5-tropic) to treat U87MG cells transfected or not with the HIV receptor CD4, and U87MG transfected with CD4 and one of the HIV coreceptors CXCR4 or CCR5. HIV exposure did not increase neopterin production in any cell lines (data not shown).
IFN-γ, but not IFN-α/β, induced IDO in astrocyte-derived cells.
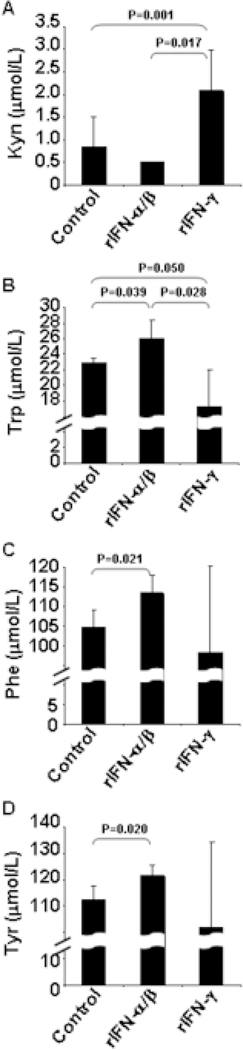
We tested the effect of IFN-α/β and IFN-γ on IDO expression and activity in U87MG and found that IFN-γ, but not IFN-α/β, induced IDO (Fig. 1B). The amount of Kyn, the bioproduct IDO-mediated Trp-catabolism, was also significantly increased after 12, 24 and 48 hours in supernatants of IFN-γ-treated cells compared to both control- and IFN-α/β-treated cells, which were indistinguishable from baseline (0h) (Fig. 1C). We obtained similar results for Kyn production using the U138 cell line, which has reduced proliferative rate compared to U87MG and is not tumorigenic in immunosuppressed mice (Fig. 3A).
Figure 3. Concentrations of Kyn, Trp, Phe and Tyr in supernatants of IFN-treated U138.
U138 human astroglioma cells were cultured to confluence and incubated for 48 hours in the absence or presence of rIFN-α/β or rIFN-γ; supernatants were collected after 48 hours (solid bars) of culture. Concentration of Kyn (A), Trp (B), Phe (C) and Tyr (D) in supernatants was measured by HPLC.
IFN-α/β reduced the consumption of three aromatic amino acids.
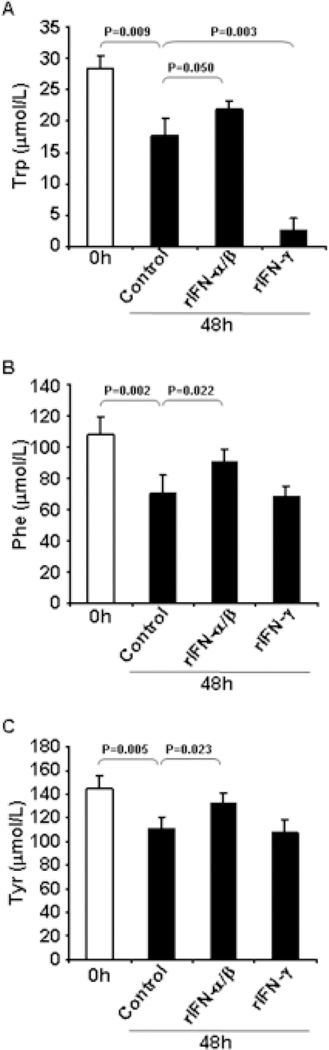
We measured Trp levels in supernatants of the astrocyte-derived cultures. Trp concentration was reduced by 38±7% in control U87MG cultures after 48 hours (Fig. 2A), compared to baseline levels (0h). IFN-γ reduced Trp in the supernatants compared to control (Fig. 2A), consistent with IFN-γ-induced IDO. Surprisingly, Trp concentration in the supernatant of IFN-α/β-treated cells was increased compared to control cultures (Fig 2A), suggesting that IFN-α/β may interfere with the uptake or usage of Trp by astrocytes.
Figure 2. Concentrations of Trp, Phe and Tyr in supernatants of IFN-treated U87MG.
U87MG human astroglioma cells were cultured to confluence and incubated for 48 hours in the absence or presence of rIFN-α/β or rIFN-γ; supernatants were collected before (open bars, 0h) and after 48 hours (solid bars) of culture. Concentration of Trp (A), Phe (B) and Tyr (C) in supernatants was measured by HPLC.
To verify whether other amino acids could be affected, we measured the concentration of the essential aromatic amino acid Phe and its derivative Tyr in the culture supernatants. Phe and Tyr concentrations were significantly reduced in control U87MG cultures after 48 hours (Fig. 2B and 2C), compared to baseline levels (0h). However, we detected significantly higher levels of Phe and Tyr in the supernatant of IFN-α/β-treated U87MG cells compared to control after 48 hours (Fig. 2A and 2B), suggesting that the uptake or usage of Phe and Tyr may be inhibited by IFN-α/β. Similar reduced consumption of Trp, Phe and Tyr was observed for IFN-α/β-treated compared to untreated U138 cells (Fig. 3B, 3C and 3D). Considered together, these observations suggest that the uptake or usage of aromatic amino acids may be inhibited by IFN-α/β, consistent with their antiproliferative activity.
Discussion
Alterations of neopterin levels are detected in CSF of patients with neurologic disorders [11]. In particular, increased neopterin is associated with inflammatory processes and/or infections. Neopterin production correlates with IDO activity, which catabolizes Trp into bioproducts of the Kyn pathway, some of which have neurotoxic activity [20]. Thus, neopterin levels may be predictive of events leading to neuronal damage, such as production of Trp-derived neurotoxin. It is still debated which cells produce neopterin in the CNS. In the present study we show that astrocyte-derived cells, particularly the human astroglioma cell line U87MG, produces neopterin upon IFN-α/β or IFN-γ stimulation, but only IFN-γ induced IDO expression and activity. These observations suggest that immunologic conditions can occur in which neopterin production is detached from, and thus may not correlate with, the rate of Trp catabolism. The apparent contrast of these findings with the previously reported lack of neopterin production by U87MG cells [19], might be explained by the higher concentration of cytokine used in this study (1μg/ml vs 5ng/ml). Furthermore, we found that IFN-α/β reduced the consumption of three aromatic amino acids, suggestive of inhibition of amino acid uptake or increased protein degradation.
Neopterin production is often associated with chronic viral infections [22, 24]. Viruses such as HIV activate innate and adaptive immunity, respectively associated with the production of IFN-α/β and IFN-γ, which may induce neopterin production by astrocytes and account for the high levels of neopterin detected in the CSF of HIV-infected patients. However, the fact that direct exposure to HIV did not affect neopterin production suggests that HIV-mediated activation of non-astrocytic cells is required to indirectly induce neopterin production in the CNS. The cellular sources of IFN-α/β and IFN-γ during HIV infection are likely to be plasmacytoid dendritic cells (pDC) and T lymphocytes, respectively. Indeed, HIV directly activates pDC to produce type I IFN and express IDO [3, 5, 12], and plasma levels of type II IFN are known to be increased and correlate with neopterin in HIV-infected patients [18]. Whether these cytokines are produced in lymphoid tissues and diffuse through the blood-brain barrier, or whether pDC and T cells infiltrate the CNS to produce type I and type II IFN locally is still to be determined. It is noteworthy that the induction of neopterin by both IFN-α/β or <di>IFN-γis</di> a characteristic that astrocyte-derived cells share with monocyte-derived dendritic cells, which also showed different responsiveness to IFN-γ and IFN-α/β with respect to IDO activity [25].
The differential effect of IFN-α/β or IFN-γ on IDO activity and amino acid metabolism may reflect the functions of these mediators in immune responses. Thus, IFN-α/β mediates innate anti-viral responses, during which a reduction of metabolic activity by astrocytes may limit the production of viral particles by infected cells. Conversely, in the setting of T cell responses, IFN-γ-induced IDO may on one side limit the replication of pathogens by depleting Trp, and on the other, exert an immunosuppressive function, inhibiting dangerous T cell responses, similar to that described for a murine model of multiple sclerosis [17]. These observations are consistent with the finding that herpes simplex virus replication is inhibited by both IFN-γ and IFN-α/β in astroglioma cells, but only the IFN-γ-mediated anti-viral effect is IDO dependent [1]. A direct role for IDO in antiviral immunity at the CNS level was also recently suggested for HIV-induced encephalitis [21]. The findings reported by Suh and colleagues differ from our observations in that they found that both IFN-β and IFN-γ induced IDO expression in primary human astrocyte cultures and that IDO expression induced by TLR3 stimulation was mediated by IFN-β [21]. Nevertheless, a negative effect of IDO-mediated Trp depletion on HIV replication was demonstrated [21]. It should be noted that, in the setting of HIV-induced encephalitis, IDO may on one side limit viral replication as reported by Suh et al [21], but on the other suppress efficient anti-HIV immune responses, thus preventing complete clearance of the virus, as demonstrated in a murine model [15].
The results reported herein describe, in an artificial in vitro system, how under certain conditions the production of neopterin may not correlate with the activation of immune-suppressive mechanisms, such as that mediated by IDO, which could contribute to the neuronal damage through the production of neurotoxic catabolites. The present work raises the possibility that astrocytic cells may be a source of neopterin under conditions in which IFN-α/β or IFN-γ production are increased. However, further investigation is required to clarify the relevance of these findings in vivo, and their importance in the pathogenesis of neurologic disorders. The different effects of pro-inflammatory and anti-viral immune mediators on astrocyte-derived cells metabolism may function to maintain the immunologic homeostasis of the CNS, but could turn into neuropathogenic mechanisms under conditions of chronic stimulation.
Acknowledgements
This work was supported by the Intramural Program of the Centers for Cancer Research, NCI, NIH,,by the government of the State of the Austrian Tyrol and by the “Stiftung Propter Homines”, Vaduz-Fürstentum Liechtenstein.
The authors thank Dr. Jeffrey D. Lifson, for kindly providing the AT-2 HIV virus. The following reagents were obtained through the AIDS Research and reference Reagent Program, Division of AIDS, NIAID, NIH: U87MG cells from Dr. Bruce Chesebro; U87.CD4, U87.CD4.CCR5 and U87CD4.CXCR4 cells from Dr. HongKui Deng and Dr. Dan R. Littman
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O. and Daubener W, Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections, J Virol, 78 (2004) 2632–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur LO, Bess JW Jr., Chertova EN, Rossio JL, Esser MT, Benveniste RE, Henderson LE and Lifson JD, Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine, AIDS Res Hum Retroviruses, 14 Suppl 3 (1998) S311–9. [PubMed] [Google Scholar]
- 3.Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD and Bhardwaj N, Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions, J Clin Invest, 115 (2005) 3265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bess JW Jr., Gorelick RJ, Bosche WJ, Henderson LE and Arthur LO, Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations, Virology, 230 (1997) 134–44. [DOI] [PubMed] [Google Scholar]
- 5.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D. and Shearer GM, HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells, Blood, 109 (2007) 3351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boasso A, Herbeuval JP, Hardy AW, Winkler C. and Shearer GM, Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells, Blood, 105 (2005) 1574–81. [DOI] [PubMed] [Google Scholar]
- 7.Brew BJ, Dunbar N, Pemberton L. and Kaldor J, Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin, J Infect Dis, 174 (1996) 294–8. [DOI] [PubMed] [Google Scholar]
- 8.Chertova E, Crise BJ, Morcock DR, Bess JW Jr., Henderson LE and Lifson JD, Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins, Curr Mol Med, 3 (2003) 265–72. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs D, Weiss G, Reibnegger G. and Wachter H, The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases, Crit Rev Clin Lab Sci, 29 (1992) 307–41. [DOI] [PubMed] [Google Scholar]
- 10.Grohmann U, Fallarino F. and Puccetti P, Tolerance, DCs and tryptophan: much ado about IDO, Trends Immunol, 24 (2003) 242–8. [DOI] [PubMed] [Google Scholar]
- 11.Hagberg L, Dotevall L, Norkrans G, Larsson M, Wachter H. and Fuchs D, Cerebrospinal fluid neopterin concentrations in central nervous system infection, J Infect Dis, 168 (1993) 1285–8. [DOI] [PubMed] [Google Scholar]
- 12.Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M. and Shearer GM, Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells, Proc Natl Acad Sci U S A, 102 (2005) 13974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, Reibnegger G, Swetly P, Troppmair J. and Wachter H, Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma, J Exp Med, 160 (1984) 310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neurauter G, Ledochowski M, Mayersbach P, Schennach H. and Fuchs D, HPLC determination of serum phenylalanine and tyrosine via monitoring of their natural fluorescence, Pteridines, In press (2007). [Google Scholar]
- 15.Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE and Persidsky Y, Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis, Blood, 106 (2005) 2382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW Jr., Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE and Lifson JD, Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins, J Virol, 72 (1998) 7992–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai K, Zou JP, Tschetter JR, Ward JM and Shearer GM, Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis, J Neuroimmunol, 129 (2002) 186–96. [DOI] [PubMed] [Google Scholar]
- 18.Schroecksnadel K, Zangerle R, Bellmann-Weiler R, Garimorth K, Weiss G. and Fuchs D, Indoleamine-2, 3-dioxygenase and other interferon-gamma-mediated pathways in patients with human immunodeficiency virus infection, Curr Drug Metab, 8 (2007) 225–36. [DOI] [PubMed] [Google Scholar]
- 19.Speth C, Stockl G, Fuchs D, Wirleitner B, Widner B, Wurzner R, Mohsenipour I, Lass-Florl C. and Dierich MP, Inflammation marker 7,8-dihydroneopterin induces apoptosis of neurons and glial cells: a potential contribution to neurodegenerative processes, Immunobiology, 202 (2000) 460–76. [DOI] [PubMed] [Google Scholar]
- 20.Stone TW, Mackay GM, Forrest CM, Clark CJ and Darlington LG, Tryptophan metabolites and brain disorders, Clin Chem Lab Med, 41 (2003) 852–9. [DOI] [PubMed] [Google Scholar]
- 21.Suh HS, Zhao ML, Rivieccio M, Choi S, Connolly E, Zhao Y, Takikawa O, Brosnan CF and Lee SC, Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response, J Virol, 81 (2007) 9838–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wachter H, Fuchs D, Hausen A, Reibnegger G. and Werner ER, Neopterin as marker for activation of cellular immunity: immunologic basis and clinical application, Adv Clin Chem, 27 (1989) 81–141. [DOI] [PubMed] [Google Scholar]
- 23.Widner B, Werner ER, Schennach H, Wachter H. and Fuchs D, Simultaneous measurement of serum tryptophan and kynurenine by HPLC, Clin Chem, 43 (1997) 2424–6. [PubMed] [Google Scholar]
- 24.Wirleitner B, Neurauter G, Schrocksnadel K, Frick B. and Fuchs D, Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects, Curr Med Chem, 10 (2003) 1581–91. [DOI] [PubMed] [Google Scholar]
- 25.Wirleitner B, Reider D, Ebner S, Bock G, Widner B, Jaeger M, Schennach H, Romani N. and Fuchs D, Monocyte-derived dendritic cells release neopterin, J Leukoc Biol, 72 (2002) 1148–53. [PubMed] [Google Scholar]